Abstract
Previously we have extensively characterized Salmonella enterica serovar Typhi (S. Typhi)-specific cell-mediated immune responses (CMI) in volunteers orally immunized with the licensed Ty21a typhoid vaccine. In this study we measured Salmonella-specific multifunctional (MF) CD8+ T cell responses to further investigate whether Ty21a elicits cross reactive CMI against S. Paratyphi A and S. Paratyphi B, which also cause enteric fever. Ty21a elicited cross-reactive CMI against all three Salmonella serotypes were predominantly observed in CD8+ T effector/memory (TEM) and, to a lesser extent, in CD8+CD45RA+ TEM (TEMRA) subsets. These CD8+ T cell responses were largely mediated by MF cells co producing IFN-γ, MIP-1β and expressing CD107a with or without TNF-α. Significant proportions of Salmonella-specific MF cells expressed the gut homing molecule integrin α4β7. In most subjects similar MF responses were observed to S. Typhi and S. Paratyphi B, but not to S. Paratyphi A. These results suggest that Ty21a elicits MF CMI against Salmonella which could be critical in clearing the infection. Moreover, because S. Paratyphi A is a major public concern and Ty21a was shown in field studies not to afford cross-protection to S. Paratyphi A, these results will be important in developing a S. Typhi/S. Paratyphi A bivalent vaccine against enteric fevers.
Keywords: Ty21a, Cross-reactive CD8 T cells, Multifunctional, Salmonella, Human
Introduction
Typhoid fever caused by Salmonella enterica serover Typhi (S. Typhi), is responsible for an estimated 21.7 million cases and 200,000 deaths per year worldwide.1, 2 Other significant causative agents of enteric fevers are S. Paratyphi A and S. Paratyphi B, and rarely S. Paratyphi C.3 Recent reports indicate that the incidence of paratyphoid A fever is on the rise in areas of endemicity (e.g., South and Southeast Asia, China) and among travelers returning from those areas.1, 4-7 The emergence of multiple antibiotic-resistant Salmonella strains has further increased the health risks posed by these infections.8
To prevent typhoid fever, three licensed vaccines are available, i.e., live attenuated oral vaccine Ty21a (Ty21a), parenteral polysaccharide Vi-vaccine (Vi vaccine) and Vi conjugated vaccine. In contrast, no vaccines are available against paratyphoid fevers. Although a high degree of homology at the DNA level exists among S. Typhi, S. Paratyphi A, and S. Paratyphi B, a critical virulence factor, the S. Typhi Vi polysaccharide, is not expressed either by S. Paratyphi A or S. Paratyphi B.9 Therefore, the parenteral Vi vaccines are not expected to provide cross-protection against paratyphoid A and B fevers. The possibility that the live attenuated Ty21a confers cross-protection against S. Paratyphi A and/or S. Paratyphi B has been studied in several field studies.10-12 Those studies indicate that Ty21a does not protect against S. Paratyphi A, while it does confer a moderate degree of protection against S. Paratyphi B disease.13
Because of the recent increased incidence of enteric fever caused by S. Paratyphi A, the need for an effective vaccine against paratyphoid A fever has been emphasized.14 However, the need for an effective vaccine against S. Paratyphi A, as well as more effective vaccines to typhoid fever, requires a better understanding of the immunological basis of the cross-reactive and cross-protective responses induced by Ty21a. This is complicated by the fact that S. Typhi is a human host-restricted pathogen and animal models do not faithfully recapitulate human disease. Nevertheless, the S. Typhimurium “typhoid” mouse model has led to important insights into the role that various innate and adaptive effector mechanisms might play in protection from Salmonella infection. For example, resistance to virulent challenge with S. typhimurium by immunized mice, requires production of interferon (IFN)-γ and tumor necrosis factor (TNF)-α by both CD4+ and CD8+ T cells.15-18
In humans, humoral, and most importantly cell-mediated immune (CMI) responses that are induced following vaccination of healthy volunteers with Ty21a and other live oral candidate vaccine strains (i.e., CVD 908, CVD 908-htrA, CVD 909, MZH09) have been studied extensively by us and others.19-30 Typically we observed that following immunization with live oral S. Typhi vaccines, both CD4+ and CD8+ T cell responses, including cytotoxic T cells (CTL), were observed depending on the nature of the stimulant used in in-vitro or ex-vivo experiments.23, 24, 27, 29-35 While typically CD4+T cells responses were more pronounced to soluble antigens (e.g., flagella), CD8+T cells were the predominant responders against S. Typhi-infected targets. 24, 27, 30, 32
Depending on the expression of defined markers, T memory (TM) cell subsets have been broadly divided into T central memory (TCM: CD45RA-CD62L+), T effector/memory (TEM; CD45RA-CD62L), and RA+TEM (TEMRA; CD45RA-62L-).36,37 Of note, TEMRA, considered to be “terminally differentiated TEM cells”,36, 37 were found to expresses high levels of perforin and granzyme and have been implicated in protection against viral ( e.g., HIV, Cytomegalovirus and Epstein-Barr virus) and bacterial (M. tuberculosis) infections. 38-42 Regarding immunity to S. Typhi, we have shown that oral immunization with attenuated typhoid vaccines elicits S. Typhi-specific CD8+ T cell responses, mostly involving the TEM and TEMRA subsets, although lower magnitude responses were also observed in the CD8+ TCM subset. 22, 25, 26, 30 Of interest, we showed that a significant portion of S. Typhi-specifc T cells co-expressed the gut homing molecule integrin α4β7, suggesting their potential to migrate to the primary site of infection.25, 30, 43 Taken together, these observations strongly suggest that live oral typhoid vaccines elicit CD8+ CTL and other CMI responses likely to be the primary mediator(s) of protective immunity, both in clearing acute infection, as well as in providing long-term protection against S. Typhi.32, 44 Recent studies further showed that antigen specific multifunctional (MF) T cells (cells producing two or more cytokines and/or expressing CD107a (a marker of cytotoxic activity),45 induced in response to various vaccines, 46 including Ty21a, 22, 26 might play a key role in long-term protective immunity.
However, in spite of the considerable progress in uncovering S. Typhi specific responses, very limited information is available on the cross reactive responses induced against S. Paratyphi A or S. Paratyphi B by live oral typhoid vaccines. Recently, we and others have described cross-reactive humoral responses induced by Ty21a against S. Typhi, S. Paratyphi A and B. 47-49 Humoral responses induced following immunization with Ty21a were directed predominantly against S. Typhi; however, cross-reactive responses were also recorded against S. Paratyphi A and B. We further observed the induction of cross-reactive functional vaccine induced antibodies which were, nevertheless, not sufficient to clear Salmonella infections once they become intracellular. 21, 48 Taken together, these observations support the notion that in addition to humoral immunity, CMI might be critical for the efficient control of S. Typhi, 22-27, 29, 30, 32, 44 as well as for S. Paratyphi A or S. Paratyphi B infections. 47, 48
To address the gaps in knowledge regarding the mechanisms of cross-protective immunity among enteric fevers, we compared the ability of Ty21a to induce cross-reactive CMI responses among S. Typhi, S. Paratyphi A and B. We observed, for the first time, that the predominant cross-reactive Salmonella specific responses were observed in the CD8+ TEM subset, whilst lower magnitude responses were also observed in CD8+ TEMRA cells. Moreover, we identified the dominant subsets of MF cells that mediate cross-reactive Salmonella-specific responses and show that Salmonella-specific CD8+ TM populations are composed of cells that express, or not, the gut-homing molecule integrin α4β7. Finally, of importance, we observed that Ty21a-elicited CMI responses against S. Typhi were found to be similar to those observed against S. Paratyphi B-, but not S.-Paratyphi A-infected targets. These observations provide a plausible immunological explanation for the observations of cross-protection between typhoid and paratyphoid B fever in Ty21a vaccinated subjects in field trials.
Results
The PBMC samples used in this study were collected from volunteers before (day 0) and after (days 42/84) immunization with Ty21a as described in Methods. Routine Complete Blood Counts (CBC) performed in these blood specimens were used to estimate the absolute numbers of lymphocytes and CD3+CD8+ cells. We observed that the percentages and absolute lymphocyte counts were similar (i.e., not statistically different, p>0.3) when pre-vaccination (day 0) and post-vaccination (days 42/84) were compared (Fig. S1). Furthermore, we measured the percentages of CD3+CD8+ T cells in PBMC by flow-cytometry and converted these percentages into approximate absolute counts of CD8+T cells using available absolute lymphocyte counts from CBC analyses. Again, no statistically significant differences (p>0.15) were observed in the calculated absolute counts for CD8+T cells among specimens collected at days 0, 42 or 84 (Fig. S1)
To measure Salmonella-specific responses, PBMC were stimulated ex-vivo with S. Typhi-, S. Paratyphi A- and B-infected autologous EBV-B cells (Fig. S2) as described in Methods. Activated CD8+ T cells (i.e., CD8+CD69+ cells) produced IFN-γ (CD69+INF γ+) and/or expressed CD107a (Fig. S3). Activated cells resided predominantly in the CD62L- TM-sub populations, i.e., TEM and TEMRA (Fig. S3). A similar phenomenon was also observed in TNF-α-producing cells (data not shown). Based on these observations, subsequent analyses were focused in the CD8+ TEM and TEMRA T cell subsets.
Evaluation of Salmonella-specific multifunctional CD8+T cells
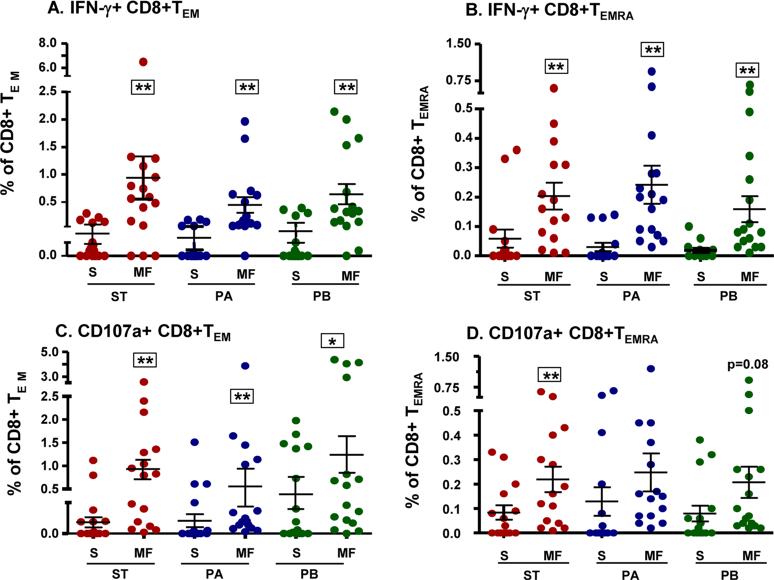
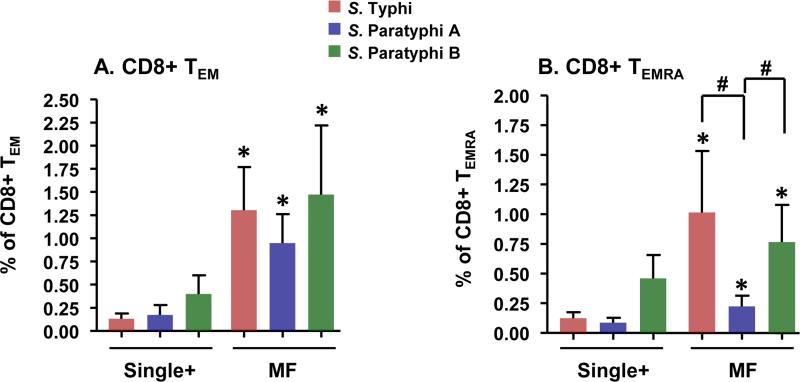
In response to S. Typhi-specific stimulation, activated effector CD8+ T cells from Ty21a vaccinees are capable of producing single cytokines or expressing CD107a only (single positives) or concommitantly producing two or more cytokines and/or expressing CD107a (Multifuntional, MF) 22, 26 (Fig.S4). We observed that Ty21a immunization elicited increases in CD8+ T cells that produce IFN-γ and/or express CD107a following stimulation with S. Typhi-, as well as with S. Paratyphi A- or B-infected targets (Fig. 1). A significantly higher proportion of these Salmonella-specific IFN-γ producing cells were MF when compared to single positive IFN-γ+ cells in both TEM (Fig. 1A) and TEMRA (Fig. 1B) subsets. Similarly, significantly higher percentages of Salmonella-specific MF cells expressing CD107a were also observed in CD8+ TEM subsets (Fig. 1C). However, in CD8+ TEMRA subsets, significant increases in Salmonella–specific MF CD107a responses were only observed after stimulation with S. Typhi-infected targets (p<0.01), while a trend was observed with S. Paratyphi B (p=0.08). No dominance of MF CD107a responses in the TEMRA subset was observed for S. Paratyphi A (p=0.23) (Fig. 1D). The above described post-vaccination increases in Salmonella-specific MF CD8+ TEM and CD8+TEMRA TM subsets for each individual volunteer are shown in Fig. S5.
Figure 1. Induction of multifunctional cells in Ty21a vaccinees.
PBMC collected from Ty21a vaccinees (n=16) were stimulated with S. Typhi-infected targets and the data analyzed using FCOM (described in the text). Shown are the peak post-vaccination increases in single positive (S) and IFN-γ+ (panels A,B) and CD107+ (panels C,D) total multifunctional (MF, the sum of all multifunctional subsets) cells in CD8+TEM (panel A,C) and CD8+TEMRA (panels B,D) subpopulations, specific for S. Typhi (ST)-, S. Paratyphi A (PA) or S. Paratyphi B (PB)-infected targets.
Post-vaccination peaks: Peak of the responses at days 42 or 84 minus pre vaccination [day 0] levels
Horizontal bars represent mean ± SEM.
**p<0.01. *p<0.05 compared to corresponding Single positive cells by Wilcoxon signed rank test, 2-tail
Characterization of Salmonella-specific multifunctional CD8+ TEM cells
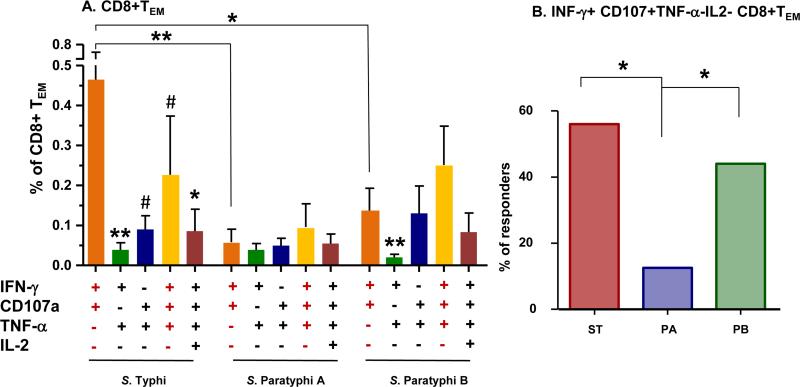
As described above, results indicated that vaccination with Ty21a elicits cross-reactive, predominantly MF CD8+TEM and TEMRA CMI responses against S. Typhi-, S. Paratyphi A- and S. Paratyphi B-infected targets. To further characterize these MF responses, we first categorized these MF cells into double (2+), triple (3+) or quadruple (4+) positives subsets, based on whether they produce IFN-γ, TNF-α and/or IL-2, and/or express CD107a. Results showed that among CD8+ TEM cells the percentages of Salmonella-specific MF cells followed the hierarchy 2+=4+>3+ (Fig. S6 A,B,C). In contrast, among CD8+TEMRA cells, the hierarchy of Salmonella-specific MF cells was 2+>3+>4+ (Fig. S6 D,E,F). We next evaluated whether unique MF profiles (e.g., production of particular cytokines and/or expression of CD107a combinations) were elicited by Ty21a against S. Typhi-, S. Paratyphi A- and S. Paratyphi B-infected targets. Of all possible combinations (16 for the 4 parameters evaluated), we focused our studies on the 5 dominant MF subsets in CD8+TEM and TEMRA, all showing net increases of >0.05% positive cells (Figs. 2 and 3). Of note, when combined, these 5 selected “high frequency” MF subsets typically represented >80% of all MF cells within both, the TEM and TEMRA TM subsets. In CD8+TEM subsets, post-vaccination increases showed a dominance of S. Typhi-specific IFN-γ+CD107a+TNF-α-IL-2 cells over the next 4 most frequent S. Typhi-specific MF subsets, i.e., IFN-γ+CD107a-TNF-α+IL-2 (p<0.01), IFN-γ+CD107a+TNF-α+IL 2 (p=0.07), IFN γ+ CD107a+TNF-α+ IL-2 (p=0.09) and IFN-γ+CD107a+TNF-α+IL-2+ (p=0.02) (Fig. 2A). Moreover, following Ty21a immunization, the induction of S. Typhi specific CD8+ TEM IFN-γ+CD107a+TNF-α-IL-2 cells (0.46 ± 0.18), was significantly higher than those specific to S. Paratyphi A (0.06 ± 0.03, p=0.01) or S. Paratyphi B (0.13 ± 0.06, p=0.04) (Fig. 2A). Of importance, the percentages of subjects (n=16) who were considered responders for IFN-γ+CD107a+TNF-α-IL-2-CD8+ TEM specific to S. Typhi (56.3%) were similar to those responding to S. Paratyphi B (43.8%, p=0.5) and both were significantly higher than the 12.5% of volunteers responding to S. Paratyphi A (p<0.01 and p<0.05 as compared to S. Typhi and S. Paratyphi B, respectively) (Fig. 2B).
Figure 2. Post-vaccination increases in multifunctional (MF) CD8+TEM cells in PMBC from Ty21a vaccinees (n=16).
Induction of multiple cytokine (IFN-γ, TNF-α and/or IL-2)-producing and/or expressing CD107a CD8+TEM cells following stimulation with targets infected with S. Typhi (ST), S. Paratyphi A (PA) or S. Paratyphi B (PB). Data was analyzed using FCOM as described in the text. The post-vaccination peak increases (peak level at days 42 or 84 post-vaccination minus the corresponding pre-vaccination levels) in dominant subpopulations are shown as mean ± SEM (panel A). The percentage of responders was calculated as: (number of volunteers with peak post vaccination increases ≥0.1% in IFN-γ+CD107a+TNF-α-IL-2 subsets)/(total volunteers [n=16])X100 (panel B).
Horizontal bars represent mean ± SEM
**p<0.01; *p<0.05; #p<0.1 compared to IFN-γ+CD107a+TNF-α-IL-2- MF cells for each corresponding Salmonella-infected target. Other significance values relate to the indicated sets. Wilcoxon signed rank test, 2-tail (panel A). *p<0.05, by Chi square test 2-tail (panel B)
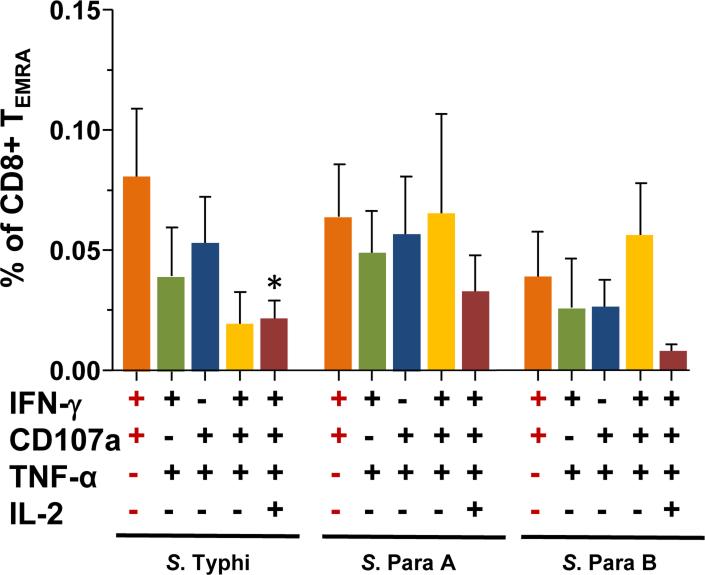
Figure 3. Post-vaccination increases in Multifunctional (MF) CD8+ TEMRA subsets in PMBC from Ty21a vaccinees (n=15).
Shown are post-vaccination peak increases (peak at days 42 or 84 post-vaccination minus the corresponding pre-vaccination levels) in the 5 dominant MF subpopulations following stimulation with targets infected with S. Typhi, S. Paratyphi A (S. Para A) or S. Paratyphi B (S. Para B). Bars indicate mean ± SEM.
*p<0.05, compared to IFN-γ+CD107a+TNF-α-IL2- MF cells for S. Typhi-infected targets. Wilcoxon signed rank test, 2-tail.
Characterization of Salmonella-specific multifunctional CD8+ TEMRA cells
A similar analysis to the one described above for TEM cells was used to characterize the Ty21a-induced cross-reactive MF responses in CD8+ TEMRA subsets (Fig. 3). The specific responses observed in CD8+ TEMRA subsets were generally of lower magnitude; however, their MF profiles were similar to those observed in TEM subsets. The post-vaccination increase of S. Typhi-specific CD107a+IFN-γ+TNF-α-IL-2-cells were moderately higher than the other subsets among CD8+TEMRA MF cells, although unlike CD8+TEM cells, these differences did not reach statistical significance (Fig. 3).
Comparison of MF cells between CD8+TEM and CD8+TEMRA subsets
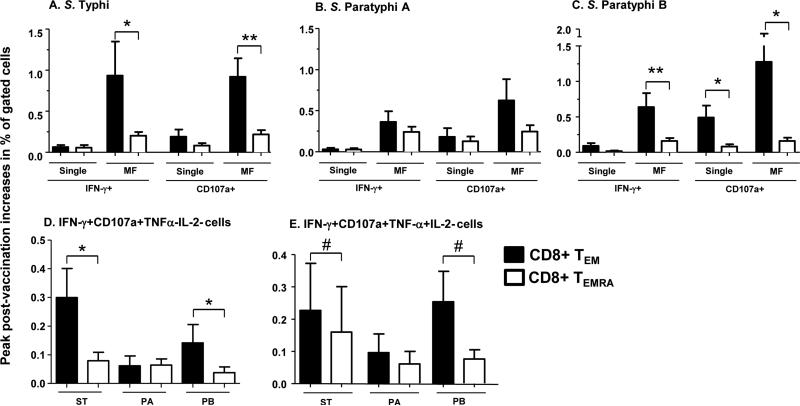
We have described above that Ty21a immunization elicited increases in Salmonella-specific responses in CD8+T cells in both TEM and TEMRA subsets (Figs. 1-3). We next compared those responses induced in these two TM subsets of CD8+ T cells (Fig. 4, S5). These comparative analyses showed that IFN-γ+ and CD107a+ MF responses specific to S. Typhi (Fig. 4A) and S. Paratyphi B (Fig. 4C), but not those to S. Paratyphi A (Fig. 4B), were significantly higher in TEM than the corresponding increases in CD8+ TEMRA subsets. Interestingly, increased percentages of single CD107a expressing cells in TEM over TEMRA were observed in S. Paratyphi B-, but not S. Typhi- and S. Paratyphi A-infected targets (Fig. 4 A,B,C). As described above, Salmonella-specific MF cells can be categorized according to their “functional” characteristics into 2+, 3+ and 4+ subsets. A comparative analysis showed that the 4+ MF cells specific to all three Salmonella strains were elicited at a significantly (p<0.01) higher percentage in CD8+TEM as compared to TEMRA subsets (Fig. S6). In contrast, 2+ MF CD8+TEM cells specific to S. Typhi (p=0.04) and S. Paratyphi B (p=0.08), but not S. Paratyphi A (p=0.2) were induced in lower percentages than the corresponding 2+ MF CD8+TEMRA cells (Fig. S6). Moreover, post vaccination increases observed in S. Typhi- (p=0.04) and S. Paratyphi B- (p=0.02) specific IFN-γ+CD107a+TNF-α-IL-2- MF cells were higher in TEM compared to TEMRA CD8+ subsets (Fig. 4D). Although similar effects were also observed with S. Typhi and S. Paratyphi B- specific IFN-γ+CD107a+TNF-α+IL-2- CD8+ TEM MF cells, these increases did not reach statistical significance (p<0.1) (Fig. 4E). Of importance, while induction of S. Typhi- or S. Paratyphi B-specific MF cells were higher in TEM compared to the corresponding responses in CD8+TEMRA subsets, no such differences were observed with S. Paratyphi A (Figs. 4B,D, E and S6). Taken together, these comparative analyses between CD8+ TEM and TEMRA subsets revealed that the response patterns elicited to S. Typhi were remarkably similar to those to S. Paratyphi B, but different than those to S. Paratyphi A.
Figure 4. Comparison of the induction of cross/reactive multifunctional cells between CD8TEM and CD8TEMRA subpopulations.
Post-vaccination peak increases (peak level at days 42 or 84 post-vaccination minus the corresponding pre-vaccination levels) in IFN-γ+ and CD107a+ single and MF subsets in CD8TEM and CD8TEMRA subsets, in response to S. Typhi (ST), S. Paratyphi A (PA) and S. Paratyphi B (PB) were compared in panels A, B and C, respectively. Similar comparisons with IFN-γ+CD107a+TNF-α-IL-2- and IFN-γ+CD107a+TNF-α+IL-2- MF subsets are shown in panels D and E, respectively.
Bars indicate mean ± SEM.
*p<0.05, #p<0.10 compared to corresponding CD8 TEM subsets. Wilcoxon signed rank test, 2-tail.
Cross-reactive Salmonella-specific MIP-1β and IL-17 responses
MIP-1β and IL-17 are two critical chemokines/cytokines which have been recently implicated in protection against infections.50, 51 Therefore, we evaluated the induction of MIP-1β and IL-17 production in response to Salmonella-infected targets by PBMC obtained from Ty21a vaccinees (n=8). To this end, we used an optimized 14-color flow cytometry panel (described in Methods) that included additional mAbs against MIP-1β and IL-17. Similar to the results described above regarding induction of IFN-γ+ or CD107a+ MF (Fig. 1), Ty21a immunization elicited Salmonella-specific, predominantly MF, MIP-1β+ cells in CD8+ TEM (Fig. 5A) and TEMRA (Fig. 5B) subsets. Results from individual volunteers are shown in Fig. S7.
Figure 5. Post-vaccination increases in MIP-1β producing CD8+ cells in response to S Typhi-, S. Paratyphi A- and S. Paratyphi B-infected targets.
Post-vaccination peak increases in MIP-1β production by CD8+TEM (panel A) and CD8+ TEMRA (panel B) in single positive (single+) for MIP-1β (MIP-1β+IFN-γ-TNF-α-IL-2-IL-17-) and all other MIP-1β+ that are multifunctional (MF) were analyzed by FCOM in PBMC obtained from Ty21a vaccinees (n=8) Bars indicate mean ± SEM.
*p<0.05, MF compared to the corresponding single positive cells. #: P<0.12. Wilcoxon signed rank test, 2-tail.
To further our understanding of the MF capabilities of MIP-1β+ cells we evaluated their ability to concomitantly produce other cytokines/chemokines and/or express CD107a. Ty21a elicited MIP-1β+ specific MF cells consisted of 6 dominant MF subsets, which were identified as those exhibiting 2+ (MIP-1β+IFN-γ+CD107a-TNF-α-IL-2-IL-17- and MIP-1β+IFN-γ-CD107a+TNF-α-IL-2-IL 17-), 3+ (MIP-1β+IFN-γ+CD107a+TNF-α-IL-2-IL-17-), 4+ (MIP-1β+IFN-γ+CD107a+TNF-α+IL-2-IL-17- and MIP-1β+IFN-γ+CD107a+TNF-α-IL-2+IL-17-) or 5+ (MIP-1β+IFN+γ+CD107a+TNF-α+IL-2+-IL-17-) functional responses (data not shown). Interestingly, the common characteristics among these different CD8+TEM MIP-1β+ MF subsets was that, besides MIP-1β, all of them concomitantly produced/expressed one or both of the two key markers of CTL, i.e., IFN-γ and/or CD107a. Virtually identical profiles of MIP-1β+ MF cells were induced following stimulation with S. Typhi- or S. Paratyphi B infected targets, both in CD8+TEM and in CD8+ TEMRA subsets. However, as described above (Fig. 2), there was a trend (no statistically significant) towards lower magnitude responses to S. Paratyphi A-infected targets (data not shown). Of note, overall, the magnitude of all Salmonella-specific MF subsets in CD8+TEM cells responses were higher than those observed in TEMRA cells (data not shown).
We also included a mAb against IL-17 in our flow cytometry panel to evaluate its role in Ty21a elicited cross-reactive immunity. Post-vaccination increases in total IL-17 producing cells above 0.1% in CD8+TEM or TEMRA subsets following stimulation with Salmonella–infected targets were observed in 3 out of 8 volunteers (37.5%) (data not shown). However, because of the low magnitude of IL-17 responses, these data was not deemed adequate for detailed analyses for MF properties of IL-17 producing cells.
Characterization of the gut/homing potential of Salmonella–specific MF CD8+TEM cells
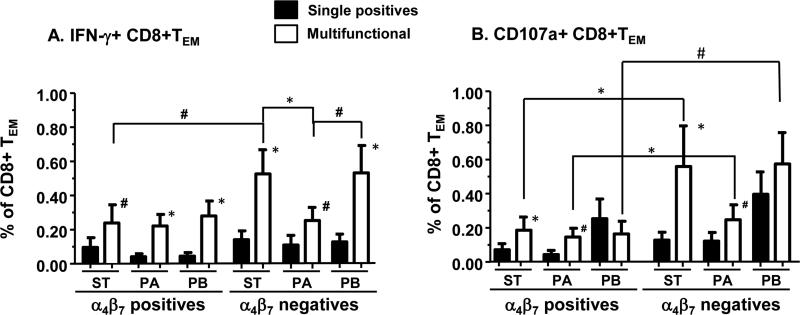
Mucosal immunity elicited in the gut microenvironment following immunization with Ty21a is thought to be an important component of the protective immune response against enteric fevers.30, 43, 44, 52 Specific effector T cells with the potential to migrate to the gut mucosa can be measured by evaluating the expression of integrin α4β7. Thus, we examined integrin α4β7 expression by Salmonella-specific IFN-γ+, CD107a+, and MIP-1β+ single and MF cells in PBMC from 12 volunteers immunized with Ty21a. We focused our studies on the gut homing patterns of cells in the CD8+ TEM subset since this was found to be the dominant subset of Salmonella-specific CD8+ T cells responding to Ty21a immunization. We observed that Ty21a immunization elicited increases in both single and MF Salmonella-specific CD8+ TEM cells expressing, or not integrin α4β7 (Fig. 6). Integrin α4β7+ co expressing IFN-γ+ (Fig. 6A) and CD107a+ (Fig. 6B) CD8+ TEM MF cells elicited by Ty21a immunization were equally responsive to S. Typhi-, S. Paratyphi A , or B infected targets. In contrast, integrin α4β7 negative IFN-γ+ MF cells specific to S. Typhi (p<0.05) or S. Paratyphi B (p=0.12) showed higher post-vaccination increases compared to those specific to S. Paratyphi A (Fig. 6A). Similarly, although the magnitude of integrin α4β7 CD107a MF cells specific to S. Typhi (p=0.26) or S. Paratyphi B (p=0.14) showed higher post vaccination increases than those specific to S. Paratyphi A, these differences did not reach statistical significance (Fig. 6B). Of note, trends were also observed for integrin α4β7 negative IFN-γ+ MF cells specific to S. Typhi (p=0.12) and S. Paratyphi B (p=0.2), but not to S. Paratyphi A (p=0.8), to exhibit higher post-vaccination increases compared to the corresponding integrin α4β7+ subsets (Fig. 6A). On the other hand, in response to all three Salmonella-infected targets, integrin α4β7- cells co-expressing CD107a, showed an higher post-vaccination increase compared to corresponding cells expressing integrin α4β7 (Fig. 6B). Specific MIP 1β responses were also observed in integrin α4β7 negative and positive cells (data not shown).
Fig. 6. Concomitant expression of the gut homing molecule integrin α4β7 by Salmonella-specific single and multifunctional CD8+TEM cells in Ty21a vaccinees.
PBMC collected from Ty21a vaccinees were stimulated with S. Typhi (ST), S. Paratyphi A (PA) and S. Paratyphi B (PB)-infected targets and the data were analyzed using FCOM (described in the text). Shown are the peak post-vaccination increases in antigen-specific IFN-γ+ (panel A; n=12), CD107a+ (panel B; n=12), single positive (closed bars) and the sum of all multifunctional (open bars) cells in CD8+ TEM subpopulations expressing integrin α4β7 (α4β7 positives) or not (α4β7 negatives). Post-vaccination peaks: Peak responses at days 42 or 84 minus pre vaccination [day 0] levels Bars indicate mean ± SEM.
*p<0.05, #p≤ 0.1 compared to corresponding Single positive cells by Wilcoxon signed rank test, 2-tail. Other significance values relate to the indicated sets.
We then investigated integrin α4β7 expression by the dominant Salmonella–specific CD8+TEMMF subsets described in Fig. 2 (e.g., IFN-γ+CD107a+TNF-α-IL-2 and INF-γ+CD107a+TNF-α+IL-2-). We observed that although a significant proportion of those also expressed integrin α4β7, most cells were α4β7 negative (data not shown). This dominance of integrin α4β7 negative MF cells was not observed in MIP-1β+ subsets.
Discussion
Ty21a and other attenuated S. Typhi oral vaccine strains elicit a wide array of CMI responses in immunized volunteers 23,26, 32, 33, 44, 53 including the induction of S. Typhi-specific multifunctional CD8+ T cells. 22, 26 In this study we investigated whether Ty21a immunization elicits cross-reactive CMI responses against two closely related Salmonella enterica serovars, i.e., S. Paratyphi A and S. Paratyphi B. In addition, by comparing the CD8+ T cell responses to these three Salmonella serovars following Ty21a immunization we explored whether defined effector CMI responses might help explain field observations showing that Ty21a provides significant cross-protection against S. Paratyphi B, but not against S. Paratyphi A.13 We used PBMC samples collected from healthy volunteers before (day 0) and after (day 42 and/or day 84) immunization with the live oral typhoid vaccine Ty21a. Measurements of the absolute numbers of lymphocytes and CD3+CD8+ cells based on CBC counts and the proportions of these cells obtained by flow cytometry revealed that immunization with Ty21a did not significantly affect the levels of these cells in circulation. Thus, it is unlikely that the observed post-vaccination increases in the percentages of Salmonella-specific CD8+ T cell subsets have been influenced by fluctuations in absolute cell counts following vaccination.
We have recently reported that healthy subjects who have neither a previous history of exposure to S. Typhi, including vaccination, nor have travelled to endemic areas, have variable background immune responses to this organism.54 These background responses are thought to be the result of the presence of cross-reactive T cells acquired during previous infections with other gram negative enteric pathogens or by natural exposure to other gram negative organisms which form part of the normal gut microbiota. Similar pre-vaccination responses were observed in the present studies. Thus, as in previous studies, we determined Ty21a elicited specific “recall” responses by subtracting the background responses before immunization in individual subjects from each post-vaccination time point (days 42/84).24, 27, 30, 53
Post-vaccination increases in specific CD8+ T cell responses were observed against all three Salmonella-infected targets (i.e., S. Typhi, S. Paratyphi A or S. Paratyphi B), predominantly in the TEM and TEMRA subsets. In contrast, very low Salmonella-specific responses were observed in TCM and, as expected, almost no responses in T naïve cells. These and previous studies 22, 25, 26, 30 provided the rationale for focusing most our current studies on multifunctional TEM and TEMRA CD8+ T subsets.
CD8+ T cells mediate effector functions by producing various cytokines (e.g., IFN-γ, TNF-α, IL-2, IL-17), chemokines (e.g., MIP-1β) or by releasing perforin and/or granzymes (indirectly measured by the expression of CD107a).45, 55 At the single cell level, T cells are capable of producing single cytokines/chemokines or simultaneously producing 2 or more cytokines/chemokines and/or expressing CD107a. The latter have been termed multifunctional (MF) cells. It has been shown that these MF T cells produce higher levels of individual cytokines, exhibit enhanced function and are more likely to correlate with protection from disease when compared to single cytokine producing cells.56, 57 In fact, induction of MF T cells at a higher magnitudes than single cytokine secreting cells have also been shown in other disease models, i.e., HIV, CMV, vaccinia and EBV infections,58 including the evaluation of candidate vaccines against M. tuberculosis 46, 59 and Ebola virus in humans.60, 61
MF cells that produce IFN-γ together with other critical cytokines (e.g., TNF-α, IL-2) and/or express CD107a can enhance the killing of intracellular bacteria more efficiently than single cytokine producing T cells.58, 62 Moreover, specific MF responses by TEM, as well as by TEMRA CD8+ T cells are thought to be associated with protection against various viral and bacterial infections.63, 64 Therefore, the quality of T cell responses, as measured by their MF capabilities, have the potential to provide a more revealing assessment of vaccine induced immune responses than single parameter functional measurements (e.g., only IFN-γ production). 58 In the present study we found that the dominant subsets of specific MF cells were 2+ S. Typhi-specific cells, which were largely comprised of IFN-γ+CD107a+TNF-α-IL-2-; IFN-γ+CD107a-TNF-α+IL-2 and IFN-γ-CD107a+TNF-α+IL-2- subsets. However, a significant proportion of 3+ MF cells were also induced. Of note, most the CD8+ MF cells produced IFN-γ, co-producing/expressing CD107a+ or TNF-α, whilst a smaller subset also co produced IL-2 (4+). These results markedly extend those reported in other infectious diseases showing that antigen specific MF TEM or TEMRA CD8+T cells that produce IFN-γ also contained subsets co-producing TNF-α, but very few co producing IL-2.58, 65 Recently, it has been proposed that during antigen specific memory cell proliferation and differentiation TNF-α and IL-2 producing clones may fade earlier than those secreting IFN-γ. Thus, terminal effector CD8+ memory subsets are comprised of mostly IFN-γ secreting cells with less functional heterogeneity.58 In this context, our observations of a dominance of S. Typhi-specific 2+ (IFN-γ+CD107a+TNF-α-IL02-) and 3+ (IFN-γ+CD107a+TNF-α+IL-2-) CD8+ TEM cells may indicate that Ty21a immunization elicits a heterogeneous population of activated CD8+ TEM that secrete IFN-γ and TNF-α with cytolytic activity (CD107a+), which subsequently become terminal effector cells, maintaining their ability to produce IFN-γ and express CD107a in the absence of TNF-α production. 58 Interestingly, we observed that post vaccination increases in S. Paratyphi A-specific IFN-γ+CD107a+TNF-α-IL-2- MF cells were less pronounced than those observed to S. Typhi or S. Paratyphi B. However, the significance of this observation is unclear since the exact role of the IFN-γ+CD107a+TNF-α-IL-2- CD8+T cells in protection remains undefined.
The observations that S. Typhi and S. Paratyphi B-specific IFN-γ+ and CD107a+ MF cells, as well as double and triple positive MF subsets were induced at a higher magnitude in TEM subsets than in TEMRA cells, are similar to our previous observations in Ty21a vaccinees. 22, 25, 26, 30, 32, 44 In contrast, MF cells specific to S. Paratyphi A were induced at lower magnitudes in most subjects and without such predominance of responses in TEM subsets. In the absence of known correlates of protection or knowledge on the functional role of MF cells in protection from S. Typhi infection, the significance of these differences observed between S. Paratyphi A and S. Paratyphi B at present is unclear. However, it is reasonable to speculate, the similarities between S. Typhi and S. Paratyphi B specific CMI responses, as well as the differences with S. Paratyphi A, may help explain field trials with Ty21a reporting cross protection against S. Paratyphi B but not from S. Paratyphi A.13 Of note, although similar immune responses were observed in the majority of participants, these responses were, to a certain extent, heterogeneous, with a few volunteers exhibiting different dominant patterns. These results highlight the importance of considering cumulative responses, as well as those from individual volunteers, when interpreting data derived from human studies. Further studies are needed to fully understand the role of these TM subsets in protection from enteric fevers. Production of β-chemokines (i.e., RANTES, MIP-1α, MIP-1β) by CD8+ T cells has been shown, among others, to play an important role in CTL activity. 66 For example, HIV antigen specific CD8+ MIP-1β+ cells co producing IFN-γ were related with non-progressors suggesting that they might play a role against the infection. 50, 67 A recent report also showed that in response to S. Typhi-antigens, PBMC obtained from S. Typhi infected convalescent patients produced MIP 1β. 68 We have previously shown that MIP-1β is co-produced with other cytokines, i.e., IFN-γ, TNF-α and IL-2 following vaccination with Ty21a.22 In this study, we further characterized Ty21a elicited CD8+ MF MIP-1β T cells specific to S. Typhi, and provide the first evidence that these cells are cross-reactive to S. Paratyphi A and S. Paratyphi B. We observed that the majority of these Salmonella-specific MIP-1β+ MF cells co produced/expressed IFN-γ and CD107a, suggesting that Ty21a elicited MF cells co-producing MIP 1β are likely an important component of a protective CTL response against enteric fevers.
Effector immune responses in the gut microenvironment are expected to be important in protecting the host against S. Typhi and other enteric infections, including those caused by S. Paratyphi A and S. Paratyphi B. In previous studies we have demonstrated that, as expected, a substantial component of the S. Typhi-specific IFN-γ+ CD4+ and CD8+ T cells elicited by Ty21a and CVD 909 had the potential to home to the gut as measured by expression of the integrin α4β7 gut homing molecule. 25, 30 In this study we extended these observations by demonstrating that S. Typhi-specific CD8+ MF T cells producing/expressing IFN-γ+, CD107a+ and/or MIP-1β+ elicited by Ty21a immunization consisted of cells expressing, or not, integrin α4β7 and are cross reactive with S. Paratyphi A and S. Paratyphi B. Of note, Salmonella-specific integrin α4β7+ CD8+ MF TEM cells were present in circulation at a lower magnitude than integrin α4β7- cells. This observation is likely the consequence of the migration of Salmonella–specific integrin α4β7+ cells to the gut mucosa, resulting in a decrease in circulation.
The present studies have a few limitations. These include the relatively limited number of volunteers studied and the availability of only two time points after vaccination (Days 42 and 84). The latter might have limited our ability to detect post vaccination increases Salmonella– specific IL17+ cells in the majority of individuals.
In summary, the present investigations provide insights into the immunological basis underlying the observed cross-protection against S. Paratyphi B, but not S. Paratyphi A, observed in Ty21a field studies.13 Overall, these observations support the notion that a bivalent S. Typhi/S. Paratyphi A vaccine might be required to protect against enteric fevers.
Methods
Subjects, immunizations and isolation of peripheral blood mononuclear cells (PBMC)
Sixteen healthy adult volunteers (median age 42 years, range 23 to 52 years) from the Baltimore, MD/Washington, DC, area and the University of Maryland Baltimore (UMB) community, who had no history of typhoid fever were recruited for the study with the approval of UMB Institutional Review Board. They received four recommended spaced doses of Ty21a vaccine (Vivotif enteric coated capsules [Crucell].47 Blood samples were drawn pre-vaccination (day 0) and 42 (day 42) or 84 (day 84) days post-vaccination. PBMC were isolated immediately after blood draws by density gradient centrifugation and were cryopreserved in liquid nitrogen.33, 53
Target/stimulator cell preparation
Epstein Barr virus (EBV)-transformed B-LCL (EBV-B cells) were generated from PBMC obtained from Ty21a vaccinees as previously described.27, 53 Salmonella strains, i.e., wild-type S. Typhi strain (ISP-1820, Vi+, a clinical isolate from Chile), S. Paratyphi A (CV 223, ATCC# 9150), and S. Paratyphi B (CV 23, a clinical isolate from Chile) were obtained from the Center for Vaccine Development, University of Maryland, USA (CVD) reference stocks. EBV-B cells were incubated with Salmonella strains, at an MOI of 10:1 (bacteria:cell) as previously described and rested overnight.27, 53 Infected cells were gamma-irradiated (6,000 rad) before being used as “targets” for ex vivo PBMC stimulation. To confirm the adequacy of the infection with S. Typhi, S. Paratyphi A or S. Paratyphi B, infected EBV-B cells were stained with anti-Salmonella common structural Ag (CSA-1)-FITC (Kierkegaard & Perry, Gaithersburg, MD) and analyzed by flow cytometry using a customized LSR-II instrument (BD, Franklin Lakes, NJ, USA). The percentage of cells infected with S. Typhi was recorded for each experiment. Infected targets were only used if the infection was detected (CSA-1 positive) in >40% of viable cells (Fig. S2).
Ex-vivo PBMC stimulation
Frozen PBMC were thawed, rested overnight and stimulated with autologous S. Typhi-, S. Paratyphi A- or B- infected targets at a ratio of 10:1 (PBMC:target). After 2 hours, the protein transport blockers Monensin (1 μg/ml, Sigma) and Brefeldin A (2 μg/ml; Sigma) were added to the PBMC and cultures were continued overnight at 37°C in 5% CO2. Media alone and uninfected autologous EBV-B cells were used as negative controls. Staphylococcal enterotoxin B (SEB) (10 μg/mL; Sigma) was used as a positive control.
Surface and intracellular staining
Surface and intracellular staining was performed as described previously. 22 Briefly, following ex-vivo stimulation, PBMC were first stained for live/dead discrimination using LIVE/DEAD fixable violet dead cell stain kit (Invitrogen, Carlsbad, CA) and then surface stained with a panel of fluorochrome conjugated monoclonal antibodies (mAbs) that included CD14-Pacific Blue (TuK4, Invitrogen), CD19-Pacific Blue (SJ25-C1, Invitrogen), CD3-Qdot 655 (UCHT1, BD), CD4- PerCP-Cy5.5 (SK3, BD), CD8-Qdot 705 (HIT8A, Invitrogen), CD45RA- biotin (HI100, BD), CD62L- APC-EF780 (Dreg 56, Invitrogen), integrin α4β7-Alexa 488 (clone ACT-1; conjugated in house) and CD107a-A647(eBioH4A3, eBiosciences, San Diego, CA). Of note, to maximize the detection of anti-CD107a mAb was added during the overnight ex-vivo stimulation. The cells were then fixed and permeabilized with Fix & Perm cell buffers (Invitrogen) according to the manufacturer's recommendations and was followed by intracellular staining with mAbs against IFN-γ-PE Cy7 (B27, BD), TNF-α-Alexa 700 (MAb11, BD), IL-2-PE (5344.111, BD) and CD69-ECD, (TP1.55.3, Beckman Coulter, CA, USA). For some experiments a modified panel of mAbs (14 colors) was used to concomitantly detect two additional cytokines, i.e., MIP-1β, and IL-17. This modified panel of mAbs included surface staining with Live/DEAD fixable yellow dead-cell staining kit (Invitrogen), CD14-Brilliant violet (BV) 570 (TuK4, Invitrogen), CD19- BV570 (HIB19, Biolegend, San Diego, CA, USA), CD3- BV650 (OKT3, Biolegend), CD4- PE Cy5 (RPA T4, BD), CD8 PerCP Cy5.5 (SK1, BD), CD45RA-biotin (HI100, BD), CD62L-APC-EF780 (Dreg 56, eBioscience), CD107a-FITC (H4A3, BD) and integrin α4β7-A647(ACT 1; conjugated in house). Secondary staining was performed with streptavidin Qdot 800 (Invitrogen), followed by intracellular staining with IFN-γ-PE-Cy7 (B27, BD), TNF-α-Alexa 700 (MAb11, BD), IL-2 BV605 (MQ1 17H12, Biolegend), IL 17A BV421 (BL168, Biolegend), MIP-1β-PE (24006, R&D, Minneapolis, MN, USA) and CD69 ECD or PE (TP1.55.3, eBioscience). After staining cells were fixed in 1% paraformaldehyde and stored at 4 °C until analyzed. Flow cytometry was performed using a customized LSRII flow cytometer (BD) and data were analyzed using WinList version 7 (Verity Software House, Topsham, ME, USA). Of note, in preliminary experiments we optimized the multichromatic panels used in these studies by performing titration of mAbs alone or in combination, as well as fluorescence minus one (FMO) staining, to minimize spectral overlap and compensation (data not shown).
Gating protocol
T cell responses in different live CD8+ (CD3+, CD8+ CD4−) T cell memory (TM) subsets were evaluated by their expression of CD45RA and CD62L into T central memory (TCM; CD62L+ CD45RA-), T effector memory (TEM; CD62L- CD45RA-) and T effector memory CD45RA+ (TEMRA; CD62L- CD45RA+). Naïve T cells (TN) were defined as CD62L+ CD45RA+ (Fig. S2). The FCOM analysis tool (WinList version 7) was used to classify events based on combinations of selected gates in multidimensional space (i.e., whether cells express single or multiple intracellular cytokines and/or CD107a alone or in all possible combinations) for the detection of single or MF cells. Flow cytometric analyses were performed in 300,000 500,000 events collected for each sample, of which 161,700 (128,023 - 208,752) (median and interquartile range in parenthesis) were within the live lymphocyte gate (Fig. S3 panel A1).
Statistical analyses
The statistical tests used to analyze each set of experiments are indicated in the Figure Legends. P values of <0.05 were considered significant.
Supplementary Material
Acknowledgments
We are indebted to the volunteers who allowed us to perform this study. We also thank Robin Barnes and the staff from the Recruiting Section of Center for Vaccine Development for their help in collecting blood specimens; Regina Harley, Catherine Storrer, Haiyan Chen and Shah Zafar for excellent technical assistance. This paper includes work funded, in part, by NIAID, NIH, DHHS grants R01-AI036525 (to M.B.S.), U19 AI082655 (Cooperative Center for Translational Research in Human Immunology and Biodefense [CCHI], to M.B.S.), and U54-AI057168 (Regional Center for Excellence for Biodefense and Emerging Infectious Diseases Research Mid-Atlantic Region [MARCE] and U19-AI109776 (Center of Excellence for Translational Research [CETR], to M.M.L. and M.B.S).
Footnotes
Disclosures. The authors declare no conflict of interest. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
References
- 1.Crump JA, Mintz ED. Global trends in typhoid and paratyphoid Fever. Clin.Infect.Dis. 2010;50:241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull.World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 3.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N.Engl.J.Med. 2002;347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 4.Ochiai RL, et al. Salmonella paratyphi A rates, Asia. Emerg.Infect.Dis. 2005;11:1764–1766. doi: 10.3201/eid1111.050168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arndt MB, et al. Estimating the burden of paratyphoid a in Asia and Africa. PLoS neglected tropical diseases. 2014;8:e2925. doi: 10.1371/journal.pntd.0002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meltzer E, Stienlauf S, Leshem E, Sidi Y, Schwartz E. A Large Outbreak of Salmonella Paratyphi A Infection Among Israeli Travelers To Nepal. Clinical Infectious Diseases. 2014;58:359–364. doi: 10.1093/cid/cit723. [DOI] [PubMed] [Google Scholar]
- 7.Teh CS, Chua KH, Thong KL. Paratyphoid fever: splicing the global analyses. International journal of medical sciences. 2014;11:732–741. doi: 10.7150/ijms.7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parry CM, Threlfall EJ. Antimicrobial resistance in typhoidal and nontyphoidal salmonellae. Curr.Opin.Infect.Dis. 2008;21:531–538. doi: 10.1097/QCO.0b013e32830f453a. [DOI] [PubMed] [Google Scholar]
- 9.McClelland M, et al. Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat.Genet. 2004;36:1268–1274. doi: 10.1038/ng1470. [DOI] [PubMed] [Google Scholar]
- 10.Levine MM, et al. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine. 1999;17(Suppl 2):S22–S27. doi: 10.1016/s0264-410x(99)00231-5. [DOI] [PubMed] [Google Scholar]
- 11.Black RE, et al. Efficacy of one or two doses of Ty21a Salmonella typhi vaccine in enteric coated capsules in a controlled field trial. Chilean Typhoid Committee. Vaccine. 1990;8:81–84. doi: 10.1016/0264-410x(90)90183-m. [DOI] [PubMed] [Google Scholar]
- 12.Simanjuntak CH, et al. Oral immunisation against typhoid fever in Indonesia with Ty21a vaccine. Lancet. 1991;338:1055–1059. doi: 10.1016/0140-6736(91)91910-m. [DOI] [PubMed] [Google Scholar]
- 13.Levine MM, et al. Ty21a live oral typhoid vaccine and prevention of paratyphoid fever caused by Salmonella enterica Serovar Paratyphi B. Clin.Infect.Dis. 2007;45(Suppl 1):S24–S28. doi: 10.1086/518141. [DOI] [PubMed] [Google Scholar]
- 14.Fangtham M, Wilde H. Emergence of Salmonella paratyphi A as a major cause of enteric fever: need for early detection, preventive measures, and effective vaccines. J.Travel.Med. 2008;15:344–350. doi: 10.1111/j.1708-8305.2008.00237.x. [DOI] [PubMed] [Google Scholar]
- 15.Hess J, Ladel C, Miko D, Kaufmann SH. Salmonella typhimurium aroA-infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. Journal of immunology. 1996;156:3321–3326. [PubMed] [Google Scholar]
- 16.Mastroeni P, Villarreal-Ramos B, Hormaeche CE. Role of T cells, TNF alpha and IFN gamma in recall of immunity to oral challenge with virulent salmonellae in mice vaccinated with live attenuated aro- Salmonella vaccines. Microbial pathogenesis. 1992;13:477–491. doi: 10.1016/0882-4010(92)90014-f. [DOI] [PubMed] [Google Scholar]
- 17.Mastroeni P, Villarreal-Ramos B, Hormaeche CE. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect Immun. 1993;61:3981–3984. doi: 10.1128/iai.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dougan G, John V, Palmer S, Mastroeni P. Immunity to salmonellosis. Immunol Rev. 2011;240:196–210. doi: 10.1111/j.1600-065X.2010.00999.x. [DOI] [PubMed] [Google Scholar]
- 19.Kantele A. Antibody-secreting cells in the evaluation of the immunogenicity of an oral vaccine. Vaccine. 1990;8:321–326. doi: 10.1016/0264-410x(90)90088-4. [DOI] [PubMed] [Google Scholar]
- 20.Kirkpatrick BD, et al. Evaluation of Salmonella enterica serovar Typhi (Ty2 aroC-ssaV-) M01ZH09, with a defined mutation in the Salmonella pathogenicity island 2, as a live, oral typhoid vaccine in human volunteers. Vaccine. 2006;24:116–123. doi: 10.1016/j.vaccine.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Lindow JC, Fimlaid KA, Bunn JY, Kirkpatrick BD. Antibodies in action: role of human opsonins in killing Salmonella enterica serovar Typhi. Infect.Immun. 2011;79:3188–3194. doi: 10.1128/IAI.05081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McArthur MA, Sztein MB. Heterogeneity of multifunctional IL-17A producing S. Typhi-specific CD8+ T cells in volunteers following Ty21a typhoid immunization. PLoS.One. 2012;7:e38408. doi: 10.1371/journal.pone.0038408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salerno-Goncalves R, Fernandez-Vina M, Lewinsohn DM, Sztein MB. Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J.Immunol. 2004;173:5852–5862. doi: 10.4049/jimmunol.173.9.5852. [DOI] [PubMed] [Google Scholar]
- 24.Salerno-Goncalves R, Pasetti MF, Sztein MB. Characterization of CD8(+) effector T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J.Immunol. 2002;169:2196–2203. doi: 10.4049/jimmunol.169.4.2196. [DOI] [PubMed] [Google Scholar]
- 25.Salerno-Goncalves R, Wahid R, Sztein MB. Immunization of volunteers with Salmonella enterica serovar Typhi strain Ty21a elicits the oligoclonal expansion of CD8+ T cells with predominant Vbeta repertoires. Infect.Immun. 2005;73:3521–3530. doi: 10.1128/IAI.73.6.3521-3530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salerno-Goncalves R, Wahid R, Sztein MB. Ex Vivo kinetics of early and long term multifunctional human leukocyte antigen E-specific CD8+ cells in volunteers immunized with the Ty21a typhoid vaccine. Clin.Vaccine Immunol. 2010;17:1305–1314. doi: 10.1128/CVI.00234-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salerno-Goncalves R, et al. Concomitant induction of CD4+ and CD8+ T cell responses in volunteers immunized with Salmonella enterica serovar typhi strain CVD 908-htrA. J.Immunol. 2003;170:2734–2741. doi: 10.4049/jimmunol.170.5.2734. [DOI] [PubMed] [Google Scholar]
- 28.Wahid R, et al. Oral priming with Salmonella Typhi vaccine strain CVD 909 followed by parenteral boost with the S. Typhi Vi capsular polysaccharide vaccine induces CD27+IgD-S. Typhi-specific IgA and IgG B memory cells in humans. Clin.Immunol. 2011;138:187–200. doi: 10.1016/j.clim.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wahid R, Salerno-Goncalves R, Tacket CO, Levine MM, Sztein MB. Cell mediated immune responses in humans after immunization with one or two doses of oral live attenuated typhoid vaccine CVD 909. Vaccine. 2007;25:1416–1425. doi: 10.1016/j.vaccine.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wahid R, Salerno-Goncalves R, Tacket CO, Levine MM, Sztein MB. Generation of specific effector and memory T cells with gut- and secondary lymphoid tissue- homing potential by oral attenuated CVD 909 typhoid vaccine in humans. Mucosal.Immunol. 2008;1:389–398. doi: 10.1038/mi.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkpatrick BD, et al. The novel oral typhoid vaccine M01ZH09 is well tolerated and highly immunogenic in 2 vaccine presentations. J.Infect.Dis. 2005;192:360–366. doi: 10.1086/431605. [DOI] [PubMed] [Google Scholar]
- 32.Sztein MB, Salerno Goncalves R, McArthur MA. Complex adaptive immunity to enteric fevers in humans: lessons learned and the path forward. Frontiers in immunology. 2014;5:516. doi: 10.3389/fimmu.2014.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sztein MB, et al. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J.Infect.Dis. 1994;170:1508–1517. doi: 10.1093/infdis/170.6.1508. [DOI] [PubMed] [Google Scholar]
- 34.Tagliabue A, et al. Cellular immunity against Salmonella typhi after live oral vaccine. Clin.Exp.Immunol. 1985;62:242–247. [PMC free article] [PubMed] [Google Scholar]
- 35.Wyant TL, Tanner MK, Sztein MB. Potent immunoregulatory effects of Salmonella typhi flagella on antigenic stimulation of human peripheral blood mononuclear cells. Infect.Immun. 1999;67:1338–1346. doi: 10.1128/iai.67.3.1338-1346.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annual review of immunology. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 37.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 38.Hess C, et al. HIV-1 specific CD8+ T cells with an effector phenotype and control of viral replication. Lancet. 2004;363:863–866. doi: 10.1016/S0140-6736(04)15735-8. [DOI] [PubMed] [Google Scholar]
- 39.Northfield JW, et al. Human immunodeficiency virus type 1 (HIV-1) specific CD8+ T(EMRA) cells in early infection are linked to control of HIV-1 viremia and predict the subsequent viral load set point. J Virol. 2007;81:5759–5765. doi: 10.1128/JVI.00045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Champagne P, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 41.Faint JM, et al. Memory T cells constitute a subset of the human CD8+CD45RA+ pool with distinct phenotypic and migratory characteristics. Journal of immunology. 2001;167:212–220. doi: 10.4049/jimmunol.167.1.212. [DOI] [PubMed] [Google Scholar]
- 42.Bruns H, et al. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119:1167–1177. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lundin BS, Johansson C, Svennerholm A-M. Oral Immunization with a Salmonella enterica Serovar Typhi Vaccine Induces Specific Circulating Mucosa-Homing CD4+ and CD8+ T Cells in Humans. Infection and Immunity. 2002;70:5622–5627. doi: 10.1128/IAI.70.10.5622-5627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sztein MB. Cell-mediated immunity and antibody responses elicited by attenuated Salmonella enterica Serovar Typhi strains used as live oral vaccines in humans. Clin.Infect.Dis. 2007;45(Suppl 1):S15–S19. doi: 10.1086/518140. [DOI] [PubMed] [Google Scholar]
- 45.Betts MR. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. Journal of Immunological Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 46.Thakur A, Pedersen LE, Jungersen G. Immune markers and correlates of protection for vaccine induced immune responses. Vaccine. 2012;30:4907–4920. doi: 10.1016/j.vaccine.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 47.Wahid R, Simon R, Zafar SJ, Levine MM, Sztein MB. Live oral typhoid vaccine Ty21a induces cross-reactive humoral immune responses against Salmonella enterica serovar Paratyphi A and S. Paratyphi B in humans. Clin.Vaccine Immunol. 2012;19:825–834. doi: 10.1128/CVI.00058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wahid R, et al. Live oral Salmonella enterica serovar Typhi vaccines Ty21a and CVD 909 induce opsonophagocytic functional antibodies in humans that cross react with S. Paratyphi A and S. Paratyphi B. Clin.Vaccine Immunol. 2014;21:427–434. doi: 10.1128/CVI.00786-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pakkanen SH, Kantele JM, Kantele A. Cross-reactive gut-directed immune response against Salmonella enterica serovar Paratyphi A and B in typhoid fever and after oral Ty21a typhoid vaccination. Vaccine. 2012;30:6047–6053. doi: 10.1016/j.vaccine.2012.07.051. [DOI] [PubMed] [Google Scholar]
- 50.Makedonas G, Betts M. Polyfunctional analysis of human t cell responses: importance in vaccine immunogenicity and natural infection. Springer Semin Immun. 2006;28:209–219. doi: 10.1007/s00281-006-0025-4. [DOI] [PubMed] [Google Scholar]
- 51.Khader SA, Gopal R. IL-17 in protective immunity to intracellular pathogens. Virulence. 2010;1:423–427. doi: 10.4161/viru.1.5.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pasetti MF, Simon JK, Sztein MB, Levine MM. Immunology of gut mucosal vaccines. Immunological Reviews. 2011;239:125–148. doi: 10.1111/j.1600-065X.2010.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sztein MB, Tanner MK, Polotsky Y, Orenstein JM, Levine MM. Cytotoxic T lymphocytes after oral immunization with attenuated vaccine strains of Salmonella typhi in humans. J.Immunol. 1995;155:3987–3993. [PubMed] [Google Scholar]
- 54.Salerno-Goncalves R, Rezwan T, Sztein MB. B cells modulate mucosal associated invariant T cell immune responses. Frontiers in immunology. 2014;4:511. doi: 10.3389/fimmu.2013.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukuda M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. Journal of Biological Chemistry. 1991;266:21327–21330. [PubMed] [Google Scholar]
- 56.Darrah PA, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 57.Betts MR. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 59.Smaill F, et al. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Science translational medicine. 2013;5:205ra134. doi: 10.1126/scitranslmed.3006843. [DOI] [PubMed] [Google Scholar]
- 60.Ledgerwood JE, et al. Chimpanzee Adenovirus Vector Ebola Vaccine — Preliminary Report. New England Journal of Medicine. 2014 doi: 10.1056/NEJMc1505499. [DOI] [PubMed] [Google Scholar]
- 61.Rampling T, et al. A Monovalent Chimpanzee Adenovirus Ebola Vaccine - Preliminary Report. The New England journal of medicine. 2015 doi: 10.1056/NEJMoa1411627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sandberg JK, Fast NM, Nixon DF. Functional Heterogeneity of Cytokines and Cytolytic Effector Molecules in Human CD8+ T Lymphocytes. The Journal of Immunology. 2001;167:181–187. doi: 10.4049/jimmunol.167.1.181. [DOI] [PubMed] [Google Scholar]
- 63.Harari A, et al. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunological Reviews. 2006;211:236–254. doi: 10.1111/j.0105-2896.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 64.Rozot V, et al. Mycobacterium tuberculosis specific CD8+ T cells are functionally and phenotypically different between latent infection and active disease. European Journal of Immunology. 2013;43:1568–1577. doi: 10.1002/eji.201243262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prezzemolo T, et al. Functional Signatures of Human CD4 and CD8 T Cell Responses to Mycobacterium tuberculosis. Frontiers in immunology. 2014;5:180. doi: 10.3389/fimmu.2014.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim JJ, et al. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. The Journal of Clinical Investigation. 1998;102:1112–1124. doi: 10.1172/JCI3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kutscher S, et al. The intracellular detection of MIP-1beta enhances the capacity to detect IFN gamma mediated HIV-1-specific CD8 T-cell responses in a flow cytometric setting providing a sensitive alternative to the ELISPOT. AIDS Research and Therapy. 2008;5:22. doi: 10.1186/1742-6405-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhuiyan S, et al. Cellular and Cytokine Responses to Salmonella enterica Serotype Typhi Proteins in Patients with Typhoid Fever in Bangladesh. The American Journal of Tropical Medicine and Hygiene. 2014;90:1024–1030. doi: 10.4269/ajtmh.13-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.