Abstract
Heightened neural responsiveness of alcoholics to alcohol cues and social emotion may impede sobriety. To test mesocorticolimbic network responsivity, 10 (8 men) alcohol use disorder (AUD) patients sober for 3 weeks to 10 months and 11 (8 men) controls underwent fMRI whilst viewing pictures of alcohol and non-alcohol beverages and of emotional faces (happy, sad, angry). AUD and controls showed similarities in mesocorticolimbic activity: both groups activated fusiform for emotional faces and hippocampal and pallidum regions during alcohol picture processing. In AUD, less fusiform activity to emotional faces and more pallidum activity to alcohol pictures were associated with longer sobriety. Using graph theory-based network efficiency measures to specify the role of the mesocorticolimbic network nodes for emotion and reward in sober AUD revealed that the left hippocampus was less efficiently connected with the other task-activated network regions in AUD than controls when viewing emotional faces, while the pallidum was more efficiently connected when viewing alcohol beverages. Together our findings identified lower occipito-temporal sensitivity to emotional faces and enhanced striatal sensitivity to alcohol stimuli in AUD than controls. Considering the role of the striatum in encoding reward, its activation enhancement with longer sobriety may reflect adaptive neural changes in the first year of drinking cessation and mesocorticolimbic system vulnerability for encoding emotional salience and reward potentially affecting executive control ability and relapse propensity during abstinence.
INTRODUCTION
Emotional information is present in every social interaction, and such skill fosters interpersonal bonds promoting well-being (Carton et al., 1999). Faces expressing emotion are motivationally salient stimuli and automatically capture attention (Smith et al., 2013). Deficits in processing facial emotion occur in alcohol use disorder (AUD) (Maurage et al., 2008) and are detectable to varying degrees over different lengths of sobriety (Foisy et al., 2007; Montagne et al., 2006; Townshend and Duka, 2003). These deficits are valence independent, in that they can be found for negative and positive emotions (Maurage et al., 2011). Noteworthy, facial emotion processing deficits in AUD are independent of general cognitive impairment and endure even when cognitive ability is statistically controlled (Foisy et al., 2007; Maurage et al., 2008).
Affective flattening and lack of emotional responses towards socially rewarding stimuli occur in AUD (Schmidt et al., 2001). By contrast, with repeated alcohol use, alcohol-related cues gain emotional relevance through their associations with the psychotropic rewarding effects of alcohol ingestion (Houben and Wiers, 2006). Alcohol-related stimuli can then act as predictors of reward and as incentive stimuli motivating and controlling behavior (Flagel et al., 2011). Indeed, even in the absence of a significant pharmacological effect, conditioned cues can elicit striatal dopamine release in humans (beer flavor cues: Oberlin et al., 2013) and dopaminergic striatal activity (alcohol picture cue: Grüsser et al., 2004; Heinz et al., 2004; Vollstädt-Klein et al., 2010). In heavy drinkers, enhanced attentional biases toward conditioned alcohol cues have been linked to alcohol craving and alcohol seeking behavior (Dickter et al., 2013). Further, the attentional and cognitive mechanisms engaged by alcohol-related cues and facial emotion processing appear to differ between AUD and controls (Marinkovic et al., 2009; Maurage et al., 2011; Noel et al., 2007). Specifically, AUD patients exert deficits in disengaging attention from alcohol cues (Noel et al., 2007) and have difficulties to differentiate emotional facial expressions (Clark et al., 2007; Foisy et al., 2007; Philippot et al., 1999; Townshend and Duka, 2003).
At the functional level, activation of a mesocorticolimbic pathway has been implicated in emotional processing promoting alcohol-seeking behavior (Vollstädt-Klein et al., 2012), and this pathway was found to be suppressed in alcoholic patients when viewing aversive emotional stimuli (Charlet et al., 2013). A recent fMRI study showed that while viewing faces expressing fear, patients and controls activated the OFC, anterior cingulate cortex (ACC), and insula; however, attenuated OFC activation was observed in AUD patients with history of multiple relapses, together with poorer performance at recognizing ambiguous fearful expressions compared with controls (O’Daly et al., 2012). This pattern is consistent with reports of blunted temporo-limbic responses to emotional faces and lack of specificity in the neural response to emotional in contrast to neutral faces in AUD patients (Gilman et al., 2012; Marinkovic et al., 2009).
The mesocorticolimbic pathway underlying emotional processing overlaps with the corticostriatal pathways for reward processing implicated in addictive behaviors (Volman et al., 2013). Nodes of the mesocorticolimbic and reward system process salience through dynamic interactions among them. For example, during alcohol cue viewing, AUD, relative to controls, exhibited decreased functional connectivity between dorsal and ventral striatal and posterior brain regions (Lee et al., 2013). By contrast, during emotional face processing, reduced functional connectivity between the amygdala and the orbitofrontal cortex was observed in social drinkers following acute ingestion of alcohol relative to placebo (Gorka et al., 2013), and also between the insula and PFC, and between amygdala and pallidum in AUD relative to controls (O’Daly et al., 2012). Taken together, these findings imply low emotionally-driven behavior together with enhanced alcohol-driven behavior in AUD. Specifically, it has been proposed that compromised interactions within the mesocorticolimbic system can result in dampening salience of emotional stimuli and diminished perception of affective signals (Marinkovic et al., 2009; O’Daly et al., 2012). The decoupling of mesocorticolimbic structures when processing emotional faces appears greater in those with multiple rather than single detoxifications (O’Daly et al., 2012), again implicating an effect of illness chronicity on brain function.
Accordingly, the duration of chronic alcohol consumption and abstinence may affect dynamic brain network adaptations. Cognitive status of AUD patients generally improves with longer sobriety (Rather and Sherman, 1989; Rosenbloom et al., 2004; Starcke et al., 2013). Moreover, abstinent AUD with longer sobriety are likely to remain abstinent, because extended sobriety (> 1 year) has been linked to reduced alcohol expectations toward the reinforcing effects of alcohol and propensity to relapse (Brown, 1985; Rather and Sherman, 1989). However, it remains unclear how functional brain networks in AUD respond to both alcohol cues and emotional facial expressions in early abstinence (< 1 year).
The present research aimed to test whether AUD patients differ from social drinking controls in their neural response to viewing emotional faces and alcohol-related visual cues, and how the hemodynamic response to these stimuli is affected by sobriety length. We hypothesized that both groups would engage mesocorticolimbic brain regions during emotional face and alcohol picture processing. In addition, we expected to observe greater mesolimbic activation to alcohol-related stimuli based on the assumption that alcohol stimuli gain affective salience in alcoholism and less activation to emotional faces in AUD than controls (e.g., Lee et al., 2013; O’Daly et al., 2012; Yang et al., 2013). At the brain network level, we hypothesized that relative to controls, AUD would exhibit greater mesocorticolimbic network efficiency when processing alcohol pictures but diminished network efficiency when processing emotional faces, reflecting increased emotional salience for alcohol-related cues associated with a dampened response to social-emotional stimuli in AUD patients. Lastly, we expected that mesocorticolimbic activation during alcohol pictures processing in AUD would relate to length of sobriety and craving intensity.
METHODS AND MATERIALS
Participants
Groups comprised 10 abstinent AUD participants and 11 age-matched controls (CTL) (Table 1). AUDs were recruited from local rehabilitation programs; CTL were volunteers from the local community. All subjects were screened with the Structural Clinical Interview for DSM-IV (American Psychiatric Association, 1994) and a clinical examination to rule out other Axis I diagnoses. In the alcoholic group, all participants met DSM-IV criteria for alcohol dependence; 8 were in early remission (met criteria within the past 12 months), and 2 were in sustained remission (>12 months). The median number of weeks since AUD patients last met dependence criteria was 10.5 weeks (mean ± SD 26.73 ± 33.14 weeks). The last time AUD participants had a drink varied from 2 weeks to 10 months before testing (mean ± SD 97.9 ± 95.5 days). The median age of AUD onset was 18 years (mean 21.1 years, range 13-33 years). In all, 80% of AUD patients and no CTL met DSM-IV criteria for any type of drug abuse or dependence (Table 1). In no case was drug dependence more recent than alcohol dependence. Four AUD subjects reported use of over the counter drugs (1 pain non-narcotic, 1 antidepressant, 1 antihypertensive, 1 cholesterol) and one AUD subject reported use of marijuana. The control group consisted of social drinkers and reported having the last drink on average 11 days (SD=18) before scan, ranging from 1 day to 47 days. For all participants, history of head injury was an exclusion criterion. Subject groups matched in age and did not differ in visual acuity and body mass index. The AUD had lower socioeconomic status (Hollingshead and Redlich, 1958) than CTL (Table 1). Before the fMRI experiment, AUD patients completed the Alcohol Craving Questionnaire (ACQ) (Love et al., 1998; Singleton et al., 1994) to measure alcohol craving at the time of the experiment. All participants gave written informed consent to participate in this study, which was approved by the Institutional Review Boards at SRI International and Stanford University School of Medicine.
Table 1.
Demographic characteristics of the study groups: Alcohol use disorder patients (AUD), controls (CTL).
|
AUD (n=10, 8 men) Mean (SD) |
CTL (n=11, 8 men) Mean (SD) |
Significance (p) |
|
|---|---|---|---|
| Age | 40 (12) | 41 (15) | ns |
| Handedness (L/R) | 2/8 | 0/11 | 0.001** |
| Visual acuity | 1.5 (0.8) | 1.5 (1.0) | ns |
| BMI | 26.79 (3.54) | 25.82 (2.77) | ns |
| SES | 45 (17.37) | 23 (10.44) | 0.047* |
| Lifetime alc. (kg) | 1238 (935) | 70 (125) | 0.001** |
| Age at AUD onset | 21.1 (7.37) | - | - |
|
| |||
| Number (n) of subjects meeting drug abuse or dependency criteria | |||
| Cocaine (n) | 7 (70%) | - | |
| years since last used | 2.45 (3.57) | - | |
| Amphetaminesa (n) | 2 (20%) | - | |
| years since last used | 4.3 (1.59) | - | |
| Nicotinea,b (n) | 7 (70%) | 2 (18%) | |
Group comparisons: t-tests, Chi-square. Abbreviations: Standard deviation (SD), body mass index (BMI), lifetime alcohol consumption measured in kg (Lifetime alc.), Socioeconomic status (SES).
One AUD subject reported both nicotine and amphetamine abuse.
None of the participants smoked cigarettes in 48 hours previous to the scanning session.
Alcohol-Emotion-Picture fMRI task paradigm
Subjects viewed pictures of faces and beverages while being scanned in the 3T GE whole-body scanner. In the fMRI task paradigm, stimuli were presented in blocks of six pictures per condition. Conditions of interest were emotional faces, alcoholic beverages, and non-alcoholic beverages. Emotional face blocks contained pictures of happy, angry, or sad faces. Faces of adult men and women were selected from the MacBrain Face Stimulus Set (www.macbrain.org/resources.htm) and balanced in emotional face blocks. Beverages blocks contained pictures of alcoholic (e.g., wine, beer, whiskey) and non-alcoholic (e.g., juice, water, milk) drinks. Subjects were instructed to press a button every time they saw a picture. Each run consisted of six experimental blocks, each 33 seconds long. Three blocks of emotional faces (pictures of angry, sad and happy faces) were pseudo-randomly mixed with two blocks of beverage pictures (alcoholic and non-alcoholic) and blocks of resting (fixation); the block order was counterbalanced across runs. Each run started with a short countdown and scanning of two ‘dummy’ brain volumes. Three runs were performed. Each picture was presented for 4 sec at the center of the screen and parsed by inter-stimulus intervals of 500 ms. The inter-block interval was 4 seconds and the block order was counterbalanced across runs. Stimuli were presented using an Apple Macintosh laptop with PsyScope stimulus delivery software (http://psy.cns.sissa.it/) using a back-projection system during the fMRI session, participants viewed stimuli via a mirror attached to the head coil. Prior to entering the scanner, participants were given a description of the task and a brief practice session.
Image acquisition
Whole-brain structural and functional MRI data were acquired with an 8-channel head coil at a 3T GE whole body scanner. Subject motion was minimized by following best practices for head fixation, and structural image series were inspected for residual motion. Whole-brain fMRI data were acquired with a T2*-weighted gradient echo-planar pulse sequence (2D axial, TE=30ms; TR=2200ms; flip angle=90°; in plane resolution=3.75mm; thick=5mm; skip=0mm; locations=36; FOV=240mm; 1 NEX). The start of the fMRI scan was triggered automatically from PsyScope software. Test instructions were reviewed with the subject by the examiner via the scanner intercom system before the onset of each run. A dual-echo FSE (2D axial; TR=5000ms; TE=17/99ms; thick=5 mm; skip=0mm mm; xy matrix=256; flip angle=90°; locations=36; FOV=240mm; 1 NEX) was used for spatially registering the fMRI data. A field map for correction of spatial distortions in the echo-planar images was generated from a gradient-recalled echo sequence pair (TR=460ms, TE=3/5ms, thickness=5mm, skip=0mm, locations=36).
Image preprocessing and statistical analyses
Image preprocessing and statistical analyses were performed using the SPM8 software package (Wellcome Department of Cognitive Neurology). The functional images were subjected to geometric distortion correction and motion correction. The FSE structural images were co-registered to the mean unwarped and motion-corrected functional image for each subject and segmented into gray and white matter images. Functional and structural gray matter images were normalized to Montreal Neurological Institute (MNI) space, and volumes were smoothed with a Gaussian kernel of 8mm (FWHM). Individual statistics were computed using a general linear model approach (Friston et al., 1995) as implemented in SPM8. Statistical preprocessing consisted of high-pass filtering at 132s, low-pass filtering through convolution with the canonical hemodynamic response function, and global scaling. A random effects analysis was conducted for group averaging and population inference. One image per contrast was computed for each subject from a design matrix that included estimated individual movement parameters as regressors in addition to Alcohol-Emotion Picture task conditions as explanatory variables.
For definition of network ROIs participating in the processing of alcoholic beverages and emotional faces, individual first level F-contrasts of interest were computed. To test for activation specificity to emotion and alcohol content of the identified network ROIs, MRI BOLD signal for each ‘emotion network’ ROI was extracted for each condition using MarsBaR (http://marsbar.sourceforge.net) and entered into repeated measures ANOVAs (SPSS 21) with emotion (happy, angry, sad) as within-subjects factor, and similarly for each ‘alcohol network’ ROI, extracted BOLD signal was entered into paired t-tests testing for alcohol-specificity (alcohol vs. non-alcohol). To explore regions specifically activated to alcohol beverage and facial emotion and content, t-contrast analyses (SPM8) were performed comparing ‘alcoholic vs. non-alcoholic beverages,’ ‘angry vs. happy faces,’ ‘sad vs. happy faces,’ and ‘angry vs. sad faces’ and presented in Supplementary Table 1. For second-level group analyses we spatially restricted the analysis to an anatomically defined mesocorticolimbic mask including bilaterally the dorsolateral prefrontal cortex (DLPFC), anterior prefrontal cortex, inferior orbitofrontal gyrus (OFG), superior frontal gyrus (SFG), insula, hippocampus and parahippocampal gyrus, thalamus, pallidum, striatum and fusiform gyrus, to focus our analysis on a circuitry known to be relevant to emotion and reward processing (Adolphs et al., 1996; Becerra et al., 2001; Müller-Oehring et al., 2013; Volkow and Li, 2004). To compare processing of alcohol-related pictures with that of emotional faces, first-level individual contrast images were subjected to a second-level factorial model with group (alcoholics vs. controls) as independent between-subject factor and condition (alcoholic beverages, happy faces, angry faces, sad faces) as dependent within-subject factor. The second-level factorial model allowed analysis of group and condition main effects and interactions. Group conjunction analyses were used to identify areas of spatial overlap between the statistical maps from the two groups (Friston et al., 1995; Nichols et al., 2005). In addition, an independent sample model tested for group differences in the processing of alcoholic vs. non-alcoholic beverages using the individual t-contrast images from each subject for second level group analysis. Significant clusters of activation from the conjunction analysis and the group contrast analyses (see Tables 2-4) were extracted using the MarsBar toolbox (http://marsbar.sourceforge.net/) and used for correlation analyses between regional activation and alcohol-related behavior (i.e., craving, length of sobriety) (Müller-Oehring et al., 2013).
Table 2a.
Identification of regions of interest (ROIs). Conjunction analysis: Statistical significance (T-score) of brain regional activation to viewing emotional faces (sad, angry, happy) and pictures of alcoholic beverages for clusters activated by both groups (AUD=CTL) and their location in Montreal Neurological Institute (MNI) space (mm), peak-level threshold of pFWE-corrected<0.05.
| Region label | BA | Cluster size | T | Z | MNI coordinates | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Emotional faces | |||||||
| R. IOG, MOG, fusiform | 18,19 | 1448 | 10.38 | Inf | 44 | −74 | −6 |
| L. Globus pallidus | 493 | 7.15 | 6.23 | −26 | −14 | −4 | |
| L. Hippocampus | 27 | 6.79 | 5.98 | −26 | −24 | −8 | |
| L. Lingual, IOG, fusiform | 17,18 | 245 | 6.01 | 5.42 | −18 | −88 | −8 |
| R. Thalamus | 121 | 5.35 | 4.91 | 22 | −20 | 0 | |
| R. Striatum (putamen, caudate) | 107 | 5.16 | 4.76 | 22 | 2 | 20 | |
| L. Insula | 13 | 261 | 4.97 | 4.61 | −46 | 0 | 14 |
| R. Putamen, claustrum | 29 | 4.70 | 4.39 | 30 | 2 | 14 | |
| L. Parahippocampal | 28 | 22 | 4.60 | 4.31 | −16 | −10 | −12 |
|
| |||||||
| Alcoholic beverages | |||||||
| L. Hippocampus | 13 | 5.53 | 5.06 | −26 | −24 | −8 | |
| L. Lateral globus pallidus | 119 | 5.31 | 4.88 | −26 | −14 | −4 | |
| R. MOG, MTG, fusiform | 37 | 141 | 4.79 | 4.46 | 42 | −68 | 0 |
| L. Inferior OFG | 11,47 | 39 | 4.72 | 4.41 | −22 | 34 | −8 |
Abbreviations: CTL, controls; AUD, alcohol use disorder; BA, Brodman area; IOG, inferior occipital gyrus; MOG, middle occipital gyrus; MTG, middle temporal gyrus; OFG, orbitofrontal gyrus.
Table 4. Mesolimbic network analysis.
Group comparison of global efficiency and cost (pFDR-corrected<0.05; 2-sided) using graph network analysis of functional connectivity between network nodes for ‘facial emotion’ and ‘alcohol beverage’ conditions.
| Analysis Measure | Analysis Contrast | Region label | T | pFDR-corrected |
|---|---|---|---|---|
| Emotional faces | ||||
| Global efficiency | CTL>AUD | L. hippocampus | 3.25 | 0.038 |
| Cost | CTL>AUD | L. hippocampus | 3.91 | 0.008 |
|
| ||||
| Alcoholic beverages 1 | ||||
| Global efficiency | CTL>AUD | L. inferior OFG | 3.98 | 0.003 |
| AUD>CTL | L. pallidum | 2.70 | 0.028 | |
Abbreviations: CTL, controls; AUD, alcohol use disorder; L, left; OFG, orbitofrontal gyrus.
Network measures of global efficiency and cost of a node are the same for ‘small’ networks such as the ‘alcohol beverage’ network (see Table 2), i.e., the group difference for the average inverse shortest-path distance between the left pallidum and all other nodes in the network (global efficiency or ‘centrality’ of a node) was the same than for the proportion of nodes that are connected to left pallidum (cost of a node) (Anteraper et al., 2013).
For functional network analysis, the “conn” toolbox was used (Whitfield-Gabrieli and Nieto-Castanon, 2012), and first level ROI-to-ROI connectivity maps were derived via individual time series correlations of activity over 244 time points. The ROIs for the brain network analysis were derived from the activation maps shown in Table 2a (facial emotion or alcohol pictures). For these network ROIs, graph theory analysis was employed to evaluate group differences in the efficiency and costs of each network node of the facial emotion and alcohol pictures processing networks. Each subject-specific ROI-to-ROI connectivity matrix is thresholded based on connectivity values. Suprathreshold connectivity values define an adjacency matrix characterizing a graph with nodes associated with ROIs, and edges associated with the strength of functional connectivity among these ROIs (Whitfield-Gabrieli and Nieto-Castanon, 2012). For each node in a graph, cost is defined as the proportion of connected neighbors (Anteraper et al., 2013) and can be understood as network density or present connections to all possible connections within the network (Hosseini et al., 2012). Global efficiency is defined as the average inverse shortest path distance from node to all other nodes in the graph (Whitfield-Gabrieli and Nieto-Castanon, 2012) and can be understood as the centrality of a node within the graph (Anteraper et al., 2013). For network-level estimations of global efficiency and cost, a fixed percentile cost threshold (top 15% of ROI-to-ROI connectivity) was used to calculate connectivity (adjacency) matrix within the network ROIs (Table 2a), followed by a threshold of pFDR-corr < 0.05 (2-tailed) for second-level between-group comparisons of these graph theoretical measures following published procedures (Anteraper et al., 2013; Whitfield-Gabrieli and Nieto-Castanon, 2012).
RESULTS
Group similarities in mesocorticolimbic processing
1. Emotional faces picture processing
A conjunction analysis examined overlap in the activation pattern to emotional faces processing (pooled over valences: angry, sad, happy) in alcoholics and controls. Both groups activated the fusiform and inferior occipital gyri bilaterally, left hippocampus, left globus pallidus, left lingual gyrus, left insula, left parahippocampal gyrus, right middle occipital gyrus, right ventral posterolateral nucleus of the thalamus, right dorsal striatum (putamen and caudate), and right claustrum (pFWE-corrected<0.05) (Table 2a). Lower fusiform activity to emotional faces correlated moderately with longer sobriety in alcoholics (Rho=−.55, p=0.049).
Testing for emotion specificity of regional activations (Table 2a), repeated measures ANOVAs (SPSS 21) with activation to happy, angry, and sad faces as within-subjects factor revealed significant activation differences to emotional content for all ROIs except for the left hippocampal and parahippocampal ROIs (Table 2b), both genuine limbic regions (Nakano, 1998; Papez, 1937). Yet, the left hippocampal and parahippocampal ROIs showed significant more activation to emotional faces than to beverage pictures (alcohol and non-alcoholic beverages) (repeated measures ANOVAs with five conditions: for L. Hippocampus F(1,20)=28.95, p<0.0001; L. Parahippocampal gyrus F(1,20)=15.68, p<0.001).
Table 2b.
Emotion sensitivity of brain activation for identified regions of interest (Table 2a). Repeated measures ANOVAs (SPSS 21) testing regional activation differences to happy, angry, and sad faces. Significant emotion effects are in bold.
| Region label | Emotion ROIs | ||
|---|---|---|---|
| F (1,20) | p | Description of emotion effect | |
| Emotional faces | |||
| R. IOG, MOG, fusiform | 6.50 | 0.019 | Happy < Sad < Angry |
| L. Globus pallidus | 26.04 | 0.0001 | Happy < Angry < Sad |
| L. Hippocampus* | 2.57 | 0.13 | |
| L. Lingual, IOG, fusiform | 20.87 | 0.0001 | Happy = Sad < Angry |
| R. Thalamus | 46.37 | 0.0001 | Happy < Angry < Sad |
| R. Striatum (putamen, caudate) | 11.94 | 0.003 | Happy = Angry < Sad |
| L. Insula | 16.03 | 0.001 | Angry < Happy < Sad |
| R. Putamen, claustrum | 13.40 | 0.002 | Happy < Angry < Sad |
| L. Parahippocampal gyrus* | 1.97 | 0.18 | |
Significant effects were observed for emotional faces (happy, angry, and sad) vs. beverage pictures (alcohol and non-alcohol beverages): L. Hippocampus F(1,20)=28.95, p<0.0001; L. Parahippocampal gyrus F(1,20)=15.6, p<0.001).
2. Alcohol beverage picture processing
For alcoholic beverages, conjunction analysis showed that both groups activated left hippocampal, left pallidum, left inferior orbitofrontal gyrus, and right middle temporal gyrus extending towards middle occipital and fusiform gyri during alcohol picture processing (Table 2). Fusiform activation was greater for emotional faces than alcohol pictures (Figure 1) (pFWE-corrected<0.05). Within the AUD group, greater pallidum activity to alcoholic pictures correlated with longer sobriety (Rho=.70, p=0.013) and more craving (Rho=.71, p=0.048). In addition, lower orbitofrontal activity was correlated with longer sobriety in AUD (Rho=−.70, p=0.025).
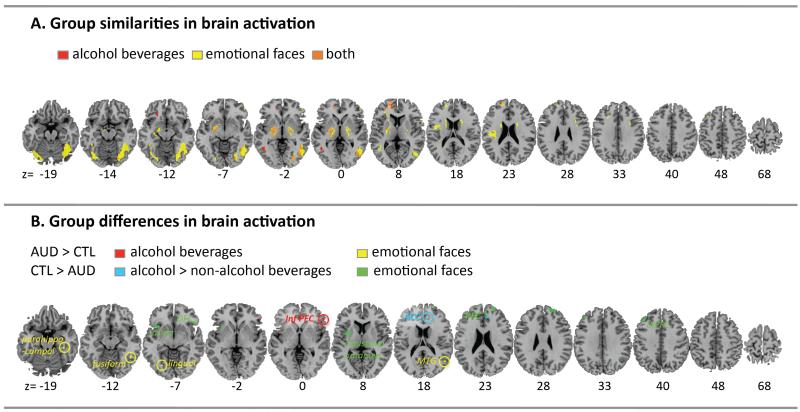
Figure 1. Brain activation elicited by expressions of facial emotion and alcohol beverage pictures.
Top panel. A. Conjunction analysis showing the group overlap (AUD=CTL) of activations for the alcohol beverage pictures (red), emotional faces (yellow) and regions activated by both, alcohol-related and emotional face pictures (orange) using a peak threshold of pFWE-corrected<0.05. Bottom panel. B. Group differences in activations to alcohol beverage picture viewing for AUD > CTL (red) and the interaction between group (CTL>ALC) and beverage type (alcohol > no-alcohol) (blue). Group differences in activation to emotional faces for AUD > CTL (yellow) and for CTL > AUD (green) using a peak threshold of puncorrected<0.001. Abbreviations: Alcohol Use Disorder (AUD), age-matched controls (CTL).
Testing for alcohol specificity of regional activations (Table 2c) revealed significantly more activation to alcohol than nonalcoholic-beverage pictures for right middle temporal-occipital and fusiform gyri, a trend for the left hippocampus. Pallidum and inferior orbitofrontal ROIs showed significant activation differences only when comparing alcohol beverage pictures to emotional faces (repeated measures ANOVAs with five conditions: for L. Pallidum F(1,20)=33.85, p<0.0001; L. Inferior OFG F(1,20)=7.05, p=0.015). In addition, t-contrast analyses using SPM8 further showed that occipito-temporal and frontal mesocorticolimbic regions were sensitive to alcohol content (alcohol vs. non-alcohol) puncorrected<0.001) (Supplementary Table 1).
Table 2c.
Alcohol sensitivity of brain activation for identified regions of interest (Table 2a). Paired sample t-tests (SPSS 21) testing for regional activation specificity to alcohol > non-alcohol beverages; 1-tailed. Significant effects are in bold.
| Region label | Alcohol ROIs | ||
|---|---|---|---|
| t(20) | p | Description of effect | |
| Alcoholic beverages | |||
| L. Hippocampus | 1.69 | 0.05 | Alcohol > Non-alcohol |
| L. Lateral globus pallidus* | −0.79 | ns | |
| R. MOG, MTG, fusiform | 1.96 | 0.03 | Alcohol > Non-alcohol |
| L. Inferior OFG* | 0.03 | ns | |
Significant effects were observed for alcohol beverages vs. emotional faces (2-tailed): L. Lateral globus pallidus F(1,20)=33.85, p<0.0001; L. Inferior OFG F(1,20)=7.05, p=0.015).
Group differences in mesocorticolimbic processing
1. Emotional faces picture processing
Group contrast analysis revealed less activation in alcoholics than controls in frontal and subcortical brain regions including the left dorsolateral PFC (middle and superior gyri), left claustrum and putamen, left anterior insula, right anterior medial and superior PFC, and right inferior orbitofrontal gyrus (peak threshold at puncorrected<0.001) (Table 3). AUD patients further showed more brain activity than controls in occipito-temporal and premotor brain regions including in the right parahippocampal gyrus, right fusiform gyrus, right middle and superior temporal lobe, left premotor cortex and left lingual and fusiform gyrus.
Table 3.
Group contrast analysis: Statistical significance for group differences in brain regional responses to viewing emotional faces (sad, angry, happy) and pictures of alcohol beverages and their location in Montreal Neurological Institute (MNI) space (mm); peak-level threshold of puncorrected< 0.001.
| Region label | BA | Cluster size | T | Z | MNI coordinates | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Emotional faces | |||||||
| CTL>AUD | |||||||
| L. dorsolateral PFC, MFG, SFG | 9 | 67 | 4.79* | 4.46 | −38 | 38 | 38 |
| R. medial PFC | 9 | 39 | 4.31 | 4.07 | 6 | 48 | 24 |
| R. anterior PFC, MFG | 10 | 105 | 4.15 | 3.93 | 24 | 58 | 26 |
| L. Claustrum, Putamen | 52 | 3.94 | 3.75 | −30 | 0 | 8 | |
| R. SFG | 8 | 33 | 3.81 | 3.63 | 18 | 26 | 54 |
| R. Inferior OFG | 47 | 31 | 3.67 | 3.51 | 48 | 26 | −6 |
| L. Anterior insula | 13 | 69 | 3.57 | 3.42 | −34 | 14 | −4 |
| AUD>CTL | |||||||
| R. Parahippocampal | 36 | 18 | 3.62 | 3.46 | 40 | −32 | −16 |
| R. Fusiform | 37 | 11 | 3.60 | 3.45 | 46 | −52 | −12 |
| R. Temporal lobe, MTG, STG | 39 | 18 | 3.57 | 3.42 | 54 | −64 | 16 |
| L. Premotor cortex, SFG, MFG | 6 | 14 | 3.53 | 3.39 | −26 | −8 | 64 |
| L. Lingual, fusiform | 18 | 15 | 3.46 | 3.33 | −32 | −70 | −8 |
|
| |||||||
| Alcoholic beverages | |||||||
| AUD>CTL | |||||||
| R. Inferior PFC, triangularis | 47 | 11 | 3.46 | 3.33 | 54 | 32 | 0 |
|
| |||||||
| Alcoholic beverages > non-alcoholic beverages | |||||||
| CTL>AUD | |||||||
| R. Anterior cingulate cortex | 32 | 35 | 4.44 | 3.63 | 10 | 38 | 20 |
Abbreviations: BA, Brodman area; CTL, controls; AUD, alcohol use disorder; PFC, prefrontal cortex; MFG, middle frontal gyrus; SFG, superior frontal gyrus; OFG, orbitofrontal gyrus;
pFWE-corrected <0.05.
2. Alcohol beverage picture processing
Group contrast analysis revealed greater activation in the right inferior PFC extending towards the pars triangularis in alcoholics than controls (peak threshold at puncorrected<0.001) (Table 3). Specificity of alcohol beverage processing in alcoholism was tested with the t-contrast ‘alcoholic vs. non-alcoholic beverages.’ AUD patients activated the ACC less than controls when processing alcoholic beverages relative to non-alcohol beverages (Table 3).
Mesocorticolimbic network connectivity analysis
We tested whether the groups showed different patterns of connectivity for the ‘alcohol’ and ‘emotion’ functional networks defined by the regions identified in the conjunction analyses of both groups (Table 2a). Graph theory analysis yielded measures of global efficiency and cost of a node within a network. For the ‘emotion’ network, the left hippocampus showed significantly lower global efficiency, i.e., less ‘centrality’ or long-range connectedness, in AUD than controls. A network-level analysis of cost revealed that connections of the left hippocampus were significantly weaker in AUD than controls, i.e., smaller proportion of the number of edges of left hippocampal connections relative to all possible connections within the network. In other words, the left hippocampus was less effectively connected during emotional face processing in AUD patients than in the controls. By contrast, graph analysis of the ‘alcohol’ network revealed higher global efficiency of the left pallidum node, but lower network efficiency of the inferior OFG node in AUD than CTL (Table 4) (for all analyses p<0.05, FDR-corrected for multiple comparisons). For inspection of global efficiency and cost for each network node and each group, see Supplementary Table 2.
DISCUSSION
This functional neuroimaging study identified similarities and differences in the neural response to emotional faces and to alcohol beverage pictures in abstinent AUD compared with controls.
Similarities in activation patterns
As hypothesized, AUD and controls engaged similar mesocorticolimbic nodes involving the fusiform area, left pallidum and hippocampal regions for both alcohol beverage pictures and emotional faces. These regions play a role in encoding facial emotion (Guyer et al., 2008; O’Daly et al., 2012) and also in alcohol cue processing (Dager et al., 2013). AUD and controls also showed similar stimulus-specific patterns with fusiform-hippocampal activation for facial emotion and pallidal-frontal activation for alcohol pictures. Occipital recruitment may indicate that the sensory analysis of visual information is comparable between groups and that differences in response to facial emotion may arise at higher cognitive processing levels. Thus, at a perceptual stage, processing of salient stimuli relies on similar brain regions in AUD and controls. Furthermore, the overlap of regions in the neurofunctional circuits for emotion and reward supports the idea that alcohol-related stimuli have inherent emotional/motivational quality, particularly in chronic alcoholism (Müller-Oehring et al., 2013).
Differences in activation patterns
In addition to the extensive overlap in activation patterns we also found significant group differences in the activation to emotional and alcohol-related stimuli. For alcohol beverage pictures, AUD activated the right inferior frontal gyrus (IFG) extending towards the pars triangularis more than controls. The IFG also plays a role in risk aversion (Christopoulos et al., 2009). Considering that the AUD patients in our study were abstinent, right IFG activation in response to viewing pictures of alcoholic beverages may be a neural correlate of ‘risk relative to gain’ evaluation of alcohol consumption.
For emotional face viewing, AUD activated a bilateral network less than controls, which was composed of the dorsolateral, medial and anterior PFC, left putamen and left anterior insula. These findings are consistent with literature reporting decreased prefrontal activation during emotional face processing in detoxified AUD patients compared with controls (O’Daly et al., 2012). More activation during emotional face viewing in AUD than controls was observed in more posterior occipito-temporal regions encompassing mainly the right temporal lobe, bilateral fusiform areas, and in a small cluster in the premotor cortex. Within the AUD group, those with less fusiform activity had longer durations of sobriety, which might indicate normalization of brain response along recovery (see also Charlet et al., 2014), considering that there was less fusiform response to facial emotion in controls than AUD. Together, these findings indicate that even during abstinence from alcohol neural processing differences exist in AUD that are marked by enhanced sensitivity to processing reward (alcohol-related pictures) and attenuated neural sensitivity to emotional faces that is modulated by sobriety length.
Mesocorticolimbic networking in abstinent AUD
Graph network analysis revealed decreased mesocorticolimbic network efficiency for processing emotional faces in AUD compared with controls indicative of less functional integration of the hippocampal node for saliency of social-emotional stimuli in alcoholism. Diminished hippocampal network efficiency in AUD during facial emotion viewing might relate to difficulties in linking memories to emotional material. It has been proposed that the hippocampus acts as a gate between emotion and cognition (Alba-Ferrara et al., 2011), across different modalities (Alba-Ferrara et al., 2011; Baumgartner et al., 2006; Marinkovic et al., 2009). Marinkovic and colleagues (2009), for example, found weaker hippocampus activation in AUD patients compared to CTL when judging facial emotion, together with poorer performance in this task. Our data did not reveal less hippocampus activity in AUD per se, but a reduced number of hippocampal connection to its neighbors during facial emotion viewing. Thus, although the metabolic response within the hippocampus does not differ between groups (Table 3), the hippocampus was inefficiently integrated within the neural ‘facial emotion’ network in AUD. This finding adds to our understanding of the neurofunctional network mechanisms of face perception given the often-observed difficulties of chronic alcoholics in processing facial emotion, even during abstinence (Frigerio et al., 2002; e.g., Oscar-Berman et al., 1990; Philippot et al., 1999; e.g., Townshend and Duka, 2003).
For alcohol-cue processing, we had predicted increased mesocorticolimbic network efficiency in AUD due to the anticipated hedonic effects of alcohol consumption in chronic alcoholism. Indeed, in AUD relative to CTL, the left pallidum was more stongly connected (global efficiency) and had an increased number of left pallidal connections to its neighbors (increased nodal costs at the network level) (Anteraper et al., 2013) during alcoholic beverage viewing. Although the pallidum was not specifically sensitive to alcohol picture content (Table 2c), it is an integral part of the reward network (Haber, 2011). Also, pallidal activation correlated with longer sobriety and with craving. The ventral pallidum plays a causal role in “wanting” and “liking” (e.g., Castro and Berridge, 2014). For example, structural lesions as well as neurodisruptive stimulation of this node can cause cessation of alcohol and drug seeking and intake behaviors in dependent patients (for a review see Smith et al., 2009). In a meta-analysis including ten functional neuroimaging studies on alcohol-cue exposure, the pallidum was identified as one of the core brain region reliably linked to craving (Kuhn and Gallinat, 2011). Thus, our finding of increased pallidal activity in relation to more craving is consistent with the literature. In abstinent alcoholics, such relationship may mark an enhanced neural sensitivity to processing reward in the first year of drinking cessation and add to relapse risk. This finding is in agreement with the “deprivation-amplification” theory by which after intense psychotropic substances consumption there is enhanced sensitivity in the brain reward circuitry (Blum et al., 2009). Following this idea, the decreasing exposure to alcohol and avoidance of alcohol-related stimuli in our abstinent AUD might relate to an escalation in network excitability resulting in a rising sensitivity to alcohol cues. Neural sensitization, which represents an enduring neuroplastic change in the reward centers of the brain to maintain the enhanced behavior, occurs in alcohol addiction (Berridge and Robinson, 1998; Hyman et al., 2006; Vanderschuren and Pierce, 2010). Our data suggest that the pallidum node partakes in the sensitization of the ‘alcohol cue’ evoked mesolimbic network during abstinence, i.e., when the gradual disappearance of tolerance unmasks sensitization of neural circuits mediating incentive salience with potential implication for relapse risk (e.g.,Vanderschuren and Pierce, 2010).
Within this alcohol-cue exposure network, AUD further showed decreased left OFC global efficiency, i.e., long-range connectivity, compared with CTL. Here, lower OFC activation was related to longer sobriety. Both, the pallidum and the OFC are nodes in the mesocorticolimbic reward network, but appear to have different roles for the processing of alcohol cues in abstinent AUD. Whilst the pallidum has a role in facilitating/inhibiting an action leading to a reward (Tachibana and Hikosaka, 2012), the OFC seems to integrate multiple sources of information regarding the reward outcome, and to calculate how rewarding is a reward (Gallagher et al., 1999; Wallis, 2007) for decision-making (e.g., Dom et al., 2005; Kim et al., 2006; Tanabe et al., 2009). Along recovery, alcohol cues may trigger the OFC response to a lesser extent potentially reflecting changes in reward evaluation; yet, as the pallidum node is strongly connected within the reward network when processing alcohol cues, abstinent AUD patients might act on it and make disadvantageous decisions. Increased pallidal and decreased OFC nodal efficiency may signify mesocorticoclimbic network sensitization to alcohol cue exposure as part of the neuroadaptation process that occurs during early abstinence.
The present study has limitations. The sample size was small limiting the statistical power to detect all neural nodes potentially involved in processing of the experimental task conditions. Importantly, we presented FDR-corrected statistics to minimize possible type I errors. Another limitation is that although the preferred drug and most recent use was alcohol in the AUD sample, patients had also used other substances in the past (i.e., cocaine, marijuana, tobacco). It is possible that our finding on group differences in the mesocorticolimbic network response to emotion and alcohol cues are not entirely specific to alcohol addiction and applies to other addictive substances as well. Alcoholism today often occurs in concert with other addictions. Based on our previous findings that smoking can attenuate task-independent functional network connectivity (Müller-Oehring et al., 2014) weaker interregional connectivity strength in AUD than controls may be partially attributable to differences in smoking status between groups, thereby having the potential of curtailing brain functional reserve capability. As a cross-sectional study, our interpretations of brain activation patterns and strength with length of sobriety require verification with longitudinal testing.
In summary, this study revealed similarities and differences in abstinent AUD and controls in the mesocorticolimbic system response to encoding reward and motivation signals for social and hedonic stimuli. The neurofunctional abnormalities in AUD were marked lower occipito-temporal sensitivity to emotional faces and enhanced striatal sensitivity to alcohol stimuli. Considering the role of the striatum in encoding reward, its activation enhancement with longer sobriety may reflect adaptive neural changes in the first year of drinking cessation. Neural sensitization of the mesocorticolimbic system during abstinence for encoding emotional salience and reward may potentially affect executive control ability and relapse propensity.
Supplementary Material
Acknowledgement
Support: AA018022, AA010723, AA023165, AA012388, AA017168
Footnotes
Conflict of Interest
Authors Lucia Alba-Ferrara, Eva M. Müller-Oehring, Edith V. Sullivan, Adolf Pfefferbaum, and Tilman Schulte declare that they have no conflict of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all participants for being included in the study.
References
- Adolphs R, Damasio H, Tranel D, Damasio A. Cortical systems for the recognition of emotion in facial expressions. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:7678–7687. doi: 10.1523/JNEUROSCI.16-23-07678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba-Ferrara L, Hausmann M, Mitchell RL, Weis S. The neural correlates of emotional prosody comprehension: disentangling simple from complex emotion. PLoS One. 2011;6:e28701. doi: 10.1371/journal.pone.0028701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anteraper SA, Whitfield-Gabrieli S, Keil B, Shannon S, Gabrieli JD, Triantafyllou C. Exploring functional connectivity networks with multichannel brain array coils. Brain Connect. 2013;3:302–315. doi: 10.1089/brain.2012.0113. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Lutz K, Schmidt CF, Jäncke L. The emotional power of music: how music enhances the feeling of affective pictures. Brain research. 2006;1075:151–164. doi: 10.1016/j.brainres.2005.12.065. [DOI] [PubMed] [Google Scholar]
- Becerra L, Breiter H, Wise R, Gonzalez R, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Berridge K, Robinson T. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain research Brain research reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Blum K, Chen TJ, Downs BW, Bowirrat A, Waite RL, Braverman ER, Madigan M, Oscar-Berman M, DiNubile N, Stice E, Giordano J, Morse S, Gold M. Neurogenetics of dopaminergic receptor supersensitivity in activation of brain reward circuitry and relapse: proposing “deprivation-amplification relapse therapy” (DART) Postgraduate medicine. 2009;121:176–196. doi: 10.3810/pgm.2009.11.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. Reinforcement expectancies and alcoholism treatment outcome after a one-year follow-up. Journal of studies on alcohol. 1985;46:304–308. doi: 10.15288/jsa.1985.46.304. [DOI] [PubMed] [Google Scholar]
- Carton JS, Kessler EA, Pape CL. Nonverbal decoding skills and relationship well-being in adults. Journal of Nonverbal Behavior. 1999;23:91–100. [Google Scholar]
- Castro D, Berridge K. Advances in the neurobiological bases for food‘liking‘versus’ wanting’. Physiology & behavior. 2014 doi: 10.1016/j.physbeh.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlet K, Schlagenhauf F, Richter A, Naundorf K, Dornhof L, Weinfurtner C, K√∂nig F, Walaszek B, Schubert F, M√°ller C. Neural activation during processing of aversive faces predicts treatment outcome in alcoholism. Addiction biology. 2014;19:439–451. doi: 10.1111/adb.12045. [DOI] [PubMed] [Google Scholar]
- Charlet K, Schlagenhauf F, Richter A, Naundorf K, Dornhof L, Weinfurtner CE, Konig F, Walaszek B, Schubert F, Muller CA, Gutwinski S, Seissinger A, Schmitz L, Walter H, Beck A, Gallinat J, Kiefer F, Heinz A. Neural activation during processing of aversive faces predicts treatment outcome in alcoholism. Addict Biol. 2013 doi: 10.1111/adb.12045. [DOI] [PubMed] [Google Scholar]
- Christopoulos GI, Tobler PN, Bossaerts P, Dolan RJ, Schultz W. Neural correlates of value, risk, and risk aversion contributing to decision making under risk. J Neurosci. 2009;29:12574–12583. doi: 10.1523/JNEUROSCI.2614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark U, Oscar-Berman M, Shagrin B, Pencina M. Alcoholism and judgments of affective stimuli. Neuropsychology. 2007;21:346–362. doi: 10.1037/0894-4105.21.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager AD, Anderson BM, Stevens MC, Pulido C, Rosen R, Jiantonio-Kelly RE, Sisante JF, Raskin SA, Tennen H, Austad CS, Wood RM, Fallahi CR, Pearlson GD. Influence of alcohol use and family history of alcoholism on neural response to alcohol cues in college drinkers. Alcohol Clin Exp Res. 2013;37(Suppl 1):E161–171. doi: 10.1111/j.1530-0277.2012.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickter CL, Forestell CA, Hammett PJ, Young CM. Relationship between alcohol dependence, escape drinking, and early neural attention to alcohol-related cues. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dom G, Sabbe B, Hulstijn W, van den Brink W. Substance use disorders and the orbitofrontal cortex: systematic review of behavioural decision-making and neuroimaging studies. The British journal of psychiatry: the journal of mental science. 2005;187:209–220. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- Flagel S, Clark J, Robinson T, Mayo L, Czuj A, Willuhn I, Akers C, Clinton S, Phillips P, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisy M-L, Kornreich C, Petiau C, Parez A, Hanak C, Verbanck P, Pelc I, Philippot P. Impaired emotional facial expression recognition in alcoholics: Are these deficits specific to emotional cues? Psychiatry research. 2007;150:33–41. doi: 10.1016/j.psychres.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Frigerio E, Burt D, Montagne B, Murray L, Perrett D. Facial affect perception in alcoholics. Psychiatry research. 2002;113:161–171. doi: 10.1016/s0165-1781(02)00244-5. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline J, Grasby P, Williams S, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Gallagher M, McMahan R, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman J, Ramchandani V, Crouss T, Hommer D. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37:467–477. doi: 10.1038/npp.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Fitzgerald DA, King AC, Phan KL. Alcohol attenuates amygdala-frontal connectivity during processing social signals in heavy social drinkers: a preliminary pharmaco-fMRI study. Psychopharmacology (Berl) 2013;229:141–154. doi: 10.1007/s00213-013-3090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Fromm SJ, Leibenluft E, Pine DS, Ernst M. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. Neuroanatomy of Reward: A View from the Ventral Striatum. In: Gottfried J, editor. Neurobiology of Sensation and Reward. CRC Press; Boca Raton (FL): 2011. [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grüsser S, Flor H, Braus D, Buchholz H, Gründer G. Correlation between dopamine D (2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. Social class and mental illness: Community study. John Wiley and Sons; New York: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SM, Hoeft F, Kesler SR. GAT: a graph-theoretical analysis toolbox for analyzing between-group differences in large-scale structural and functional brain networks. PloS one. 2012;7:e40709. doi: 10.1371/journal.pone.0040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben K, Wiers RW. Assessing implicit alcohol associations with the Implicit Association Test: fact or artifact? Addict Behav. 2006;31:1346–1362. doi: 10.1016/j.addbeh.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Hyman S, Malenka R, Nestler E. Neural mechanisms of addiction: the role of reward-related learning and memory. Annual review of neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O’Doherty J. Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. PLoS biology. 2006;4:e233. doi: 10.1371/journal.pbio.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Lee E, Ku J, Yoon KJ, Namkoong K, Jung YC. Disruption of orbitofronto striatal functional connectivity underlies maladaptive persistent behaviors in alcohol-dependent patients. Psychiatry Investig. 2013;10:266–272. doi: 10.4306/pi.2013.10.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love A, James D, Willner P. A comparison of two alcohol craving questionnaires. Addiction (Abingdon, England) 1998;93:1091–1102. doi: 10.1046/j.1360-0443.1998.937109113.x. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, O’Reilly CE, Howard JA, Sawyer K, Harris GJ. Alcoholism and dampened temporal limbic activation to emotional faces. Alcohol Clin Exp Res. 2009;33:1880–1892. doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Campanella S, Philippot P, Martin S, De Timary P. Face processing in chronic alcoholism: a specific deficit for emotional features. Alcoholism: Clinical and Experimental Research. 2008;32:600–606. doi: 10.1111/j.1530-0277.2007.00611.x. [DOI] [PubMed] [Google Scholar]
- Maurage P, Grynberg D, Noel X, Joassin F, Hanak C, Verbanck P, Luminet O, de Timary P, Campanella S, Philippot P. The “Reading the Mind in the Eyes” test as a new way to explore complex emotions decoding in alcohol dependence. Psychiatry Res. 2011;190:375–378. doi: 10.1016/j.psychres.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Montagne B, Kessels RP, Wester AJ, de Haan EH. Processing of emotional facial expressions in Korsakoff’s syndrome. Cortex. 2006;42:705–710. doi: 10.1016/s0010-9452(08)70408-8. [DOI] [PubMed] [Google Scholar]
- Müller-Oehring E, Jung Y, Pfefferbaum A, Sullivan E, Schulte T. The Resting Brain of Alcoholics. Cerebral cortex. 2014 doi: 10.1093/cercor/bhu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Oehring EM, Jung YC, Sullivan EV, Hawkes WC, Pfefferbaum A, Schulte T. Midbrain-driven emotion and reward processing in alcoholism. Neuropsychopharmacology. 2013;38:1844–1853. doi: 10.1038/npp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano I. The limbic system: An outline and brief history of its concept. Neuropathology. 1998;18:211–214. [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J-B. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Noel X, Van der Linden M, d’Acremont M, Bechara A, Dan B, Hanak C, Verbanck P. Alcohol cues increase cognitive impulsivity in individuals with alcoholism. Psychopharmacology (Berl) 2007;192:291–298. doi: 10.1007/s00213-006-0695-6. [DOI] [PubMed] [Google Scholar]
- O’Daly OG, Trick L, Scaife J, Marshall J, Ball D, Phillips ML, Williams SS, Stephens DN, Duka T. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology. 2012;37:2267–2276. doi: 10.1038/npp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin B, Dzemidzic M, Tran S, Soeurt C, Albrecht D, Yoder K, Kareken D. Beer flavor provokes striatal dopamine release in male drinkers: mediation by family history of alcoholism. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013;38:1617–1624. doi: 10.1038/npp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Hancock M, Mildworf B, Hutner N, Weber D. Emotional perception and memory in alcoholism and aging. Alcoholism, clinical and experimental research. 1990;14:383–393. doi: 10.1111/j.1530-0277.1990.tb00491.x. [DOI] [PubMed] [Google Scholar]
- Papez J. A proposed mechanism of emotion. Archives of Neurology & Psychiatry. 1937;38:211–214. [Google Scholar]
- Philippot P, Kornreich C, Blairy S, Baert I, Den Dulk A, Le Bon O, Streel E, Hess U, Pelc I, Verbanck P. Alcoholics’ deficits in the decoding of emotional facial expression. Alcoholism, clinical and experimental research. 1999;23:1031–1038. [PubMed] [Google Scholar]
- Rather BC, Sherman MF. Relationship between alcohol expectancies and length of abstinence among Alcoholics Anonymous members. Addict Behav. 1989;14:531–536. doi: 10.1016/0306-4603(89)90073-7. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Recovery of short-term memory and psychomotor speed but not postural stability with long-term sobriety in alcoholic women. Neuropsychology. 2004;18:589–597. doi: 10.1037/0894-4105.18.3.589. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Nolte-Zenker B, Patzer J, Bauer M, Schmidt LG, Heinz A. Psychopathological correlates of reduced dopamine receptor sensitivity in depression, schizophrenia, and opiate and alcohol dependence. Pharmacopsychiatry. 2001;34:66–72. doi: 10.1055/s-2001-15184. [DOI] [PubMed] [Google Scholar]
- Singleton E, Tiffany S, Henningfield J. The multidimensional aspects of craving for alcohol. Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health; Baltimore, MD: 1994. Unpublished research. [Google Scholar]
- Smith E, Weinberg A, Moran T, Hajcak G. Electrocortical responses to NIMSTIM facial expressions of emotion. Int J Psychophysiol. 2013;88:17–25. doi: 10.1016/j.ijpsycho.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res. 2009;196:155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcke K, Holst RJ, Brink W, Veltman DJ, Goudriaan AE. Physiological and Endocrine Reactions to Psychosocial Stress in Alcohol Use Disorders: Duration of Abstinence Matters. Clinical and Experimental Research; Alcoholism: 2013. [DOI] [PubMed] [Google Scholar]
- Tachibana Y, Hikosaka O. The primate ventral pallidum encodes expected reward value and regulates motor action. Neuron. 2012;76:826–837. doi: 10.1016/j.neuron.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Tregellas J, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biological psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townshend J, Duka T. Mixed emotions: alcoholics’ impairments in the recognition of specific emotional facial expressions. Neuropsychologia. 2003;41:773–782. doi: 10.1016/s0028-3932(02)00284-1. [DOI] [PubMed] [Google Scholar]
- Vanderschuren L, Pierce R. Sensitization processes in drug addiction. Current topics in behavioral neurosciences. 2010;3:179–195. doi: 10.1007/7854_2009_21. [DOI] [PubMed] [Google Scholar]
- Volkow N, Li T. Drug addiction: the neurobiology of behaviour gone awry. Nature reviews Neuroscience. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Wichert S, Rabinstein J, Bühler M, Klein O, Ende G, Hermann D, Mann K. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction (Abingdon, England) 2010;105:1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Vollstädt- Klein S, Loeber S, Richter A, Kirsch M, Bach P, von der Goltz C, Hermann D, Mann K, Kiefer F. Validating incentive salience with functional magnetic resonance imaging: association between mesolimbic cue reactivity and attentional bias in alcohol- dependent patients. Addict Biol. 2012;17:807–816. doi: 10.1111/j.1369-1600.2011.00352.x. [DOI] [PubMed] [Google Scholar]
- Volman SF, Lammel S, Margolis EB, Kim Y, Richard JM, Roitman MF, Lobo MK. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:17569–17576. doi: 10.1523/JNEUROSCI.3250-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Yang H, Devous MD, Briggs RW, Spence JS, Xiao H, Kreyling N, Adinoff B. Altered Neural Processing of Threat in Alcohol-Dependent Men. Alcohol Clin Exp Res. 2013;37:2029–2038. doi: 10.1111/acer.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.