Abstract
Emotional stressors activate a stereotyped set of limbic forebrain cell groups implicated in constraining stress-induced hypothalamic-pituitary-adrenal (HPA) axis activation by inhibiting hypophysiotropic neurons in the paraventricular hypothalamic nucleus (PVH). We previously identified a circumscribed, anterior, part of the bed nuclei of the stria terminalis (aBST) that houses stress-sensitive, PVH-projecting, GABAergic neurons, as representing a site of convergence of stress-inhibitory influences originating from medial prefrontal and hippocampal cortices. Here we investigated whether exaggerated HPA axis responses associated with chronic variable stress (CVS; daily exposure to different stressors at unpredictable times over 14 d, followed by restraint stress on d 15), and diminished HPA output seen following repeated (14 d) restraint stress exposure, may be associated with differential engagement of the limbic modulatory network. Relative to acutely restrained rats, animals submitted to CVS showed the expected increase (sensitization) in HPA responses, and diminished levels of activation (Fos) of GABAergic neurons and glutamic acid decarboxylase (GAD) mRNA expression in aBST. By contrast, repeated restraint stress produced habituation in HPA responses, and maintained levels of activation of GABAergic neurons and increased GAD expression in aBST. aBST-projecting neurons in limbic sites implicated in HPA axis inhibition tended to show diminished activational responses in both repeated stress paradigms, with the exception of paraventricular thalamic nucleus, whose responsiveness was maintained in repeatedly restrained animals. The results are consistent with the view that differential engagement of HPA-inhibitory mechanisms in aBST may contribute to alterations in HPA axis responses to emotional stress in sensitization and habituation paradigms.
Keywords: chronic stress, bed nuclei of the stria terminalis, GABAergic neurons, HPA axis, medial prefrontal cortex, hippocampus, ventral subiculum, paraventricular nucleus, hypothalamus, AB_90738
INTRODUCTION
“Emotional” stress is a designation attached to one major subset of animal challenge paradigms that model fear, anxiety and social stress, such as are encountered in everyday life. Stressors of this type engage a seemingly stereotyped set of interconnected cell groups in the limbic forebrain, including aspects of the septum, amygdala, hippocampus and prefrontal cortex (Diorio et al., 1993; Cullinan et al., 1995; Cullinan et al., 1996; Li and Sawchenko, 1998; Dayas et al., 2001), each of which is implicated in modulating hormonal (hypothalamo-pituitary-adrenal, or HPA) responses to stress (Sapolsky et al., 1984; Kovacs and Makara, 1988; Diorio et al., 1993; Weinberg et al., 2010). None of these regions provides an appreciable innervation of neurosecretory neurons in the paraventricular hypothalamic nucleus (PVH) that comprise the final common path for stress-induced HPA axis activation. Instead, these influences appear to be mediated indirectly. We have recently identified a circumscribed, anterior, part of the bed nuclei of the stria terminalis (aBST) that houses stress-sensitive, PVH-projecting, inhibitory (GABAergic) neurons, and serves as a site of convergence of acute (restraint) stress-inhibitory influences imparted by prefrontal (i.e., prelimbic or PL) and hippocampal (ventral subiculum or vSUB) components of the limbic network, at least (Radley et al., 2009; Radley and Sawchenko, 2011).
This aBST region is thus positioned to serve as a clearinghouse that receives, integrates and distributes information for limbic regulation of the stress axis. We have now completed a study that probes the potential involvement of the aBST in adaptations to chronic stress. Secretory responses of the HPA axis tend to decline upon repeated exposure to the same (homotypic) stressor, but over-respond to a novel (heterotypic) challenge (Dallman et al., 1992; Dallman, 1993a; Bhatnagar and Dallman, 1998). These phenomena of habituation and facilitation, respectively, represent adaptations to chronic stress that are highly relevant to understanding and managing the negative health consequences of longer term stress exposure.
Chronic variable stress (CVS; a.k.a., chronic unpredictable stress, chronic mild stress, chronic intermittent stress) is a widely used facilitation paradigm that involves daily exposure of rodents to different stressors at unpredictable times daily typically over a 2-3 week period (Ottenweller et al., 1989; Willner, 1997; Grippo et al., 2003). The behavioral, physiological, and endocrine alterations that ensue following CVS exposure are similar to a number of the clinical features in stress-related psychiatric disorders (Willner, 1997). Here we address the question of whether limbic system modulation of PVH output via the aBST may be in position to participate in adaptations of HPA axis responses to repeated stress. We find that the dampening and enhancement of axis responses associated with habituation (repeated restraint) and facilitation (CVS) paradigms, respectively, have correlates in cellular activation/gene expression profiles in the aBST, as well as in a differential propensity to habituate among stress-sensitive cell groups in the limbic forebrain that project to aBST. The aBST is thus positioned to play a pivotal role in adaptations to repeated stress.
MATERIALS AND METHODS
Animals and treatments
Adult male Sprague-Dawley albino rats (275 - 325 g), maintained under standard laboratory conditions, were used in all experiments. All experimental protocols were approved by the Institutional Animal Care and Use Committees of the Salk Institute for Biological Studies and the University of Iowa. CVS involved daily exposure to either two brief or one sustained stressor over 14 d, in semi-randomized order, at unpredictable times of day. Brief stressors included elevated plus maze (5 min), shaker stress (30 min on an orbital shaker at 100 rpm), hypertonic saline injection (i.p.; 1 ml of 1.0 M saline), tail suspension (10 min), forced swim (10 min in RT water) and cold exposure (1 h at 5-7 °C). Sustained stressors included overnight (i.e., 18-24 h) exposure of rats to wet bedding, cage crowding (4 animals/cage), or isolation. Repeated restraint stress was performed in the morning (9:00) in plastic restrainers (Braintree Scientific, Braintree, MA) for 30 min each day over a 14-day period. Controls were handled comparably, but were not restrained. On day 15, animals were subjected to 30 min of restraint (acute restraint, CVS, repeated restraint), and remained in their home cages during restraint and until the prescribed time of perfusion for histology, 2 h after the termination of restraint. A separate group of handled, unstressed controls was included in these experiments to assess the effects of stress paradigms relative to baseline indices.
Retrograde labeling experiments
For retrograde labeling of afferent neurons in the aBST → PVH circuit, unilateral pressure injections of 2% Fluoro-Gold (FG; Fluorochrome LLC: (Schmued and Fallon, 1986) were made into either aBST or PVH in discrete volumes (30-60 nl for PVH: anteroposterior, −0.13 mm; mediolateral, +0.35-0.40 mm; dorsoventral, −7.20 mm from dura; 60-90 nl for aBST: anteroposterior, −0.10 mm; mediolateral, +1.20 mm; dorsoventral, −7.20 mm from dura). The quality of retrograde-labeling following deposits into aBST were verified by comparison with previous experiments employing iontophoretic injections into the same region (Radley et al., 2009). Upon analysis of tracer injection sites in separate series sections prepared for either immunoperoxidase or epifluorescence detection of FG, the most accurate localization of tracer deposits and size was achieved in fluorescence material. Thereafter, tracer placement and size were reconstructed under epifluorescence in separate series of sections (see below). For each regions in which retrograde labeling was analyzed in the present study, the number of retrogradely labeled neurons did not differ significantly as a function of treatment (stress) status (data not shown).
Histology and tissue processing
Rats were anesthetized with chloral hydrate (350 mg/kg, ip) and perfused via the ascending aorta with 100 ml 0.9% saline followed by 900 ml of ice-cold 4% paraformaldehyde in 0.1 M borate buffer, pH 9.5, at a flow rate of 55 ml/min. The brains were removed, postfixed for 3 h, and cryoprotected in 20% sucrose in 0.1 M phosphate buffer overnight at 4°C. Five one-in-five series of 30 μm-thick frozen coronal sections through the entire brain were cut and collected in cryoprotectant solution and stored at −20°C until processing.
Hybridization histochemistry
Techniques for probe synthesis, hybridization, and autoradiographic localization of mRNA signal were adapted from (Simmons et al., 1989). In situ hybridization was performed using 35S-labeled sense (control) and antisense cRNA probes labeled to similar specific activities encoding corticotropin-releasing factor (CRF) mRNA (1.2 kb; Dr. K. Mayo, Northwestern University), and the 67 kDa isoform of glutamic acid decarboxlyase, (GAD67, Dr. A. Tobin, University of California, Los Angeles)(Erlander et al., 1991). Sections were mounted onto poly-L-lysine-coated slides and dried under vacuum overnight. They were postfixed with 10% paraformaldehyde for 30 min at room temperature, digested with 10 μg/ml proteinase K for 15 minutes at 37°C, and acetylated for 10 min. Probes were labeled to specific activities of 1–3 109 dpm/μg and applied to the slides at concentrations of ~107 cpm/ml, overnight at 56°C in a solution containing 50% formamide, 0.3 M NaCl, 10 mM Tris, 1 mM EDTA, 0.05% tRNA, 10 mM dithiothreitol, 1× Denhardt’s solution, and 10% dextran sulfate, after which they were treated with 20 μg/ml of ribonuclease A for 30 minutes at 37°C and washed in 15 mM NaCl/1.5 mM sodium citrate with 50% formamide at 70°C. Slides were then dehydrated and exposed to X-ray films (Kodak Biomax MR, Eastman Kodak, Rochester, NY) for 18 hours. They were coated with Kodak NTB-2 liquid emulsion and exposed at 4°C for 10-14 days, as determined by the strength of signal on film. Slides were developed with Kodak D-19 and fixed with Kodak rapid fixer, and lightly counterstained with thionin.
Hormone assays
Separate groups of animals subjected to the stress regimens described above were implanted with indwelling jugular catheters 2 days prior to the first (i.e., acute restraint) or final stress episode (i.e., CVS/ repeated restraint) using previously described procedures (Ericsson et al., 1994). On the morning of day 15, blood samples (250 μl) were taken prior to restraint stress to estimate basal ACTH and corticosterone levels. Additional samples were collected at 0, 30, 60, 90, and 120 min after the termination of restraint. ACTH was measured using a two-site immunoradiometric assay obtained in kit form (DiaSorin, Stillwater, MN), with intra- and interassay coefficients of variation of 3 and 9%, respectively, and a sensitivity of 1.5 pg/ml. Plasma corticosterone was measured without extraction, using an antiserum raised in rabbits against a corticosterone-BSA conjugate, and 125I-corticosterone-BSA as tracer (MP Biomedicals, Solon, OH). The sensitivity of the assay was 0.8 μg/dl; intra- and interassay coefficients of variation were 5 and 10%, respectively.
Immunohistochemistry
Localization of Fos protein and other antigens was carried out on free-floating sections by using a avidin-biotin immunoperoxidase staining protocol (Sawchenko et al., 1990). Fos immunolocalization was performed using a primary antiserum raised against a fragment of rat Fos protein (residues 4-17, Radley et al., 2008). Endogenous peroxidase was neutralized by treating tissue for 10 minutes with 0.3% hydrogen peroxide, and sections were incubated with primary antiserum at 4°C for 48 hours in phosphate-buffered saline (PBS) containing 0.3% Triton X-100 and 3% blocking serum. The primary antiserum was localized using Vectastain Elite (Vector Laboratories, Burlingame, CA) reagents, and the reaction product was developed using a nickel-enhanced glucose oxidase method (Shu et al., 1988).
Characterization of neurons in aBST on the basis of projections to PVH, the GABAergic phenotype and stress-sensitivity involved concurrent labeling for (1) Fos- and FG-immunoreactivity, and (2) Fos protein and GAD 67 mRNA in separate series of sections. This approach was necessitated by our inability to efficiently localize all three markers in a single series (see results section, below). Dual immunolocalization of Fos and FG was carried out by sequentially localizing the antiserum against Fos using a nickel-enhanced diaminobenzidine method (black nuclear reaction product), as above, and then a FG antiserum (Chang et al., 1990), without nickel enhancement (brown cytoplasmic product). A combined immunoperoxidase and isotopic hybridization histochemical localization of Fos immunoreactivity and GAD67 mRNA, respectively, was carried out using a modification of protocols detailed previously (Chan et al., 1993). For these experiments, immunostaining was carried out first, and the two constituent methods were modified to allow efficient dual localization.
Antiserum characterization
The primary antibodies used in this study are listed in Table 1. Fos antiserum was raised in rabbits against an N-terminal synthetic fragment of rat Fos protein (residues 4-17; Radley et al., 2008). Western blotting of extracts from brains of stressed and unmanipulated rats displayed 52 kDa bands. Specificity was further evaluated by direct colabeling for c-fos mRNA over a range of challenge conditions, and specific staining in experimental and control tissue was abolished by preadsorbing the antiserum overnight at 4°C with 50 μM of the synthetic peptide immunogen.
Table 1.
Primary antibody list
| Name, RRID | Immunogen | Manufacturer, species, cat. no. | Concentrations used |
|---|---|---|---|
| anti-Fos | Rat Fos protein, residues 4-17 |
Dr. Paul Sawchenko, Salk Institute, rabbit polyclonal, N/A |
1: 10,000 – 1:20,000 |
| anti-Fluoro- Gold |
Fluoro-Gold-BSA conjugate |
Millipore, rabbit polyclonal, AB153 | 1: 10,000 |
Fluorogold antiserum was raised in rabbit against a fluorogold-BSA conjugate (Chang et al., 1990)(AB153; Millipore, Bedford, MA; RRID: AB_90738). Specificity was verified by detection of native fluorescence or immunoperodixase reaction product only in the brains of animals that received intracerebral injections of the fluorochrome.
Data analysis
Stereological methods were used to quantify the number of Fos-immunoreactive neurons. These analyses were performed using a computer-assisted morphometry system consisting of a photomicroscope equipped with an XYZ computer-controlled motorized stage, MicroFire camera (Optronics, Goleta, CA), Gateway microcomputer, and StereoInvestigator morphometry and stereology software (MBF Biosciences, Williston, VT). For each analysis, boundaries defining the regions of interest were drawn at 25X using an adjacent series of Nissl-stained sections. In regions identified as aBST-projecting (i.e., retrogradely labeled following FG injections in aBST), labeled cells were used as a guide to further aid the delineation of anatomical boundaries. Analyses of Fos immunoreactive cells were carried out on every fifth section, avoiding cells in the outermost plane of focus. Counts were then multiplied by five to estimate the total number of labeled neurons in the defined region of interest. Volume estimates from cross-sectional area measurements were obtained using the Cavalieri method to probe for possible treatment effects on PVH volume, but no reliable effects were observed. Fos- and GAD-labeled neurons were counted manually in every fifth section. In these analyses, the section thickness was too small relative to the average diameter of labeled neurons in to permit stereologic approaches, and estimates of cell numbers were obtained using the Abercrombie correction (Abercrombie, 1949).
Semiquantitative densitometric analysis of relative levels of CRF and GAD67 mRNA was performed on emulsion-coated slides using ImageJ software. The optical densities of hybridization signals were determined under darkfield illumination at 10X magnification. The hypophysyiotropic region of the PVH (i.e., dorsal medial parvicellular subdivision) was defined from Nissl staining pattern (Swanson and Kuypers, 1980) and aligned with corresponding darkfield images of hybridized sections by redirected sampling. aBST was defined from Nissl material using the parcellation scheme of Dong and colleagues (2001). Optical density readings, corrected for background, were taken from sections at regular, 150 μm, intervals and average values were determined through the extent of cell groups for each animal. Images from CRF and GAD mRNA densitometry were collected using a QImaging EXi Blue camera. Images collected from each analysis were exported first to Adobe PhotoShop (v. 8) for adjustments to optimize/balance contrast and brightness, and then to Canvas (v. 10) for assembly and labeling.
Statistics
Group data from the immunoperoxidase and hybridization histochemical experiments (N = 3-5 per group) were compared using a one-way analysis of variance for treatment status (control, acute restraint, CVS, repeated restraint) followed by post hoc pairwise comparisons using Tukey’s HSD. Group data from the hormone assays (N = 6-8 per group) were compared using a mixed design analysis of variance with one within- (time) and one between- (stress status) group variable, followed by individual pairwise comparisons as above. Data are expressed as the mean ± SEM.
RESULTS
HPA axis responses to differing repeated stress regimens
Previous studies have shown that 14 days’ exposure to CVS produces a robust enhancement of HPA axis responses to a novel challenge, relative to otherwise naïve controls subjected to the same insult (Ottenweller et al., 1989; Herman et al., 1995). As we have previously addressed the neural substrates for limbic forebrain modulation of HPA axis responses to acute restraint, the use of restraint as the novel challenge (on day 15) in a CVS paradigm allows for direct comparison with the acute stress situation. An additional group of animals subjected to 14 consecutive daily restraint sessions provided a contrast with the effects observed under CVS, as repeated exposure to the same (homotypic) challenge has been widely documented to produce response decrements (i.e., habituation) in HPA axis activation.
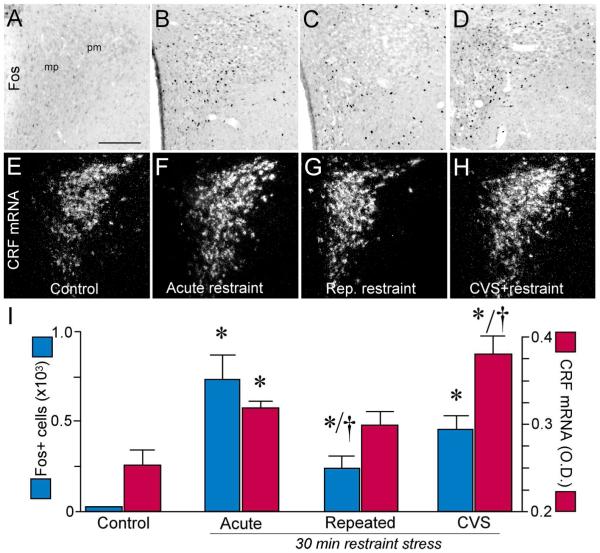
We initially surveyed two independent central indices of HPA axis activation at 2 hours after the termination of 30 min restraint in CVS, repeatedly restrained, and control (i.e., acute restraint and unstressed) groups (Fig. 1). This time point is one at which Fos protein levels are maximally elevated, and is sufficient for observing stress effects on CRF mRNA expression in PVH (Viau and Sawchenko, 2002). Main effects of stress were found on both endpoints (Fos: F3,15 = 10.7, p < 0.01; CRF mRNA: F3,15 = 18.4; p < 0.01). Each group exposed to restraint (acute, repeated, CVS) showed increases in the number of Fos-ir cells in the PVH as compared to the unstressed group (p < 0.01 for each; Fig. 1A-D, I). Whereas CVS produced increments in the number of Fos-activated neurons in PVH comparable to those of acutely restrained animals, repeatedly restrained animals’ response were blunted (by 67%; p < 0.05) as compared with the acute restraint group. CRF mRNA expression in PVH was reliably increased in the acute and CVS groups following 30 min restraint (by 28% and 52%, respectively), relative to unstressed controls (p < 0.05 for each; Fig. 1E-I). CVS-induced increments in relative levels of CRF message were significantly greater than those seen the acutely or repeatedly restrained animals (by 20% and 21%, respectively; p < 0.05); by contrast, the repeated restraint group failed to exhibit significant changes in CRF mRNA levels, as compared with unstressed controls (p = 0.5).
Figure 1.
Photomicrographs show representative examples of Fos immunoreactivity (A-D) and CRF mRNA (E-H) expression in PVH as a function of treatment status. mp, medial parvicellular subdivision; pm, posterior magnocellular subdivision. I: Mean + SEM number of Fos immunoperoxidase-labeled neurons in PVH (blue bars) and relative levels of CRF mRNA expression (red bars) in treatment groups. Both previously unstressed and CVS groups show significant increases in central measures of restraint-induced HPA activation following 30 min restraint stress. 14 d of CVS resulted in augmented CRF mRNA expression in PVH when exposed to this novel stressor, whereas animals exposed to daily restraint throughout this interval did not manifest increases in CRF mRNA relative to unstressed controls. Fos expression also showed significant decreases in the repeated restraint relative to acute stress group. *, p < 0.05, differs significantly from the unstressed control group; †, p < 0.05, differs significantly from the acute stress group. N = 4 control; N = 5 for acute, repeated and CVS groups. Scale bar: 150 μm (applies to all).
We next compared HPA secretory responses to restraint as a function of prior stress experience (acute, repeated, CVS; Fig. 2). For these experiments, indwelling jugular catheters were implanted on the afternoon of day 13 of CVS/ repeated restraint, after completion of stressor exposure for that day. Mixed design ANOVA of ACTH data (Fig. 2A), with time of blood sampling tested as a within-subjects factor, demonstrated main effects of treatment group (F2,19 = 5.2; p < 0.05), time of blood sampling (F5,19 = 22.8; p < 0.01), and a significant interaction between these variables (F10,19 = 5.6; p < 0.01). Both CVS-treated and acutely-stressed animals displayed significant increases in peak values of plasma ACTH relative to each group’s pre-stress levels (0 min vs. 30 min; p < 0.05 for each); CVS-treated animals also showed a more prolonged stress-induced rise in ACTH, which remained significantly elevated above baseline through the 60 min time point (p = 0.05). By contrast, repeatedly restrained animals failed to show any significant increase over basal (pre-stress) values of ACTH at any of the time points examined. Analysis of between-group differences at individual time points showed a significantly greater increase in peak plasma ACTH levels in CVS-treated animals as compared with both the acute and repeated restraint groups (by 3.8- and 2.6-fold, respectively; p = 0.05 for each).
Figure 2.
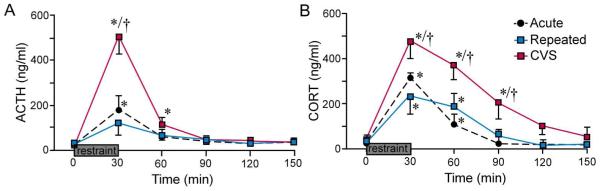
Effects of previous stress exposure on pituitary adrenal responses to 30 min restraint challenge. Mean ± SEM plasma ACTH and corticosterone (CORT) levels in control, repeated restraint, and CVS groups before (0 min) and at varying intervals after 30 min of exposure to restraint. A: Restraint significantly increased plasma levels of ACTH in previously unstressed (acute) animals (at 30 min), and 14 d CVS exposure significantly enhanced this effect. In contrast, plasma ACTH in animals previously subjected to daily restraint for the 14 consecutive days prior was not significantly elevated over pre-stress levels (p = 0.2). B: 30 min restraint exposure also significantly increased plasma CORT levels in all treatment groups (at 30 and 60 min. p < 0.05 for each), and resulted in a prolonged increase in CVS animals (p < 0.05 at 30, 60, and 90 min compared with 0 min). Notably, the repeated restraint group showed significantly lower peak values of CORT (at 30 min) as compared with acute stress and CVS groups, whereas CVS-treated animals displayed significantly elevated titers at 60 and 90 min time points as compared with other treatments. *, p < 0.05, differs significantly from basal (0 min) values within each group; †, p < 0.05, differs significantly from acutely and repeatedly restrained animals. N = 8 acute; N = 8 repeated; N = 6 CVS.
A similar pattern of results was seen in analysis of plasma corticosterone levels (Fig. 2B). Evident here were main effects of treatment group (F2,19 = 7.4; p < 0.01), time of blood sampling (F5,19 = 53.7; p < 0.01), and a significant interaction term (F10,19 = 3.6; p < 0.01). Within-group measures revealed reliable increases in plasma corticosterone immediately after the termination of restraint, and 30 min later, in all three groups (0 min vs. 30 min and 0 vs. 60 min, respectively; p < 0.05 for each); in only the CVS group were corticosterone response seen to be elevated beyond this, through the 90 min time point (p < 0.05). Although between-group comparisons failed to show a significant difference between peak levels of corticosterone in the CVS and acute restraint groups (p = 0.1), the response of the CVS animals was again more long-lasting, being significantly elevated through 90 min, vs. 60 for the others. Corticosterone titers of repeatedly restrained rats did not differ from those of acutely stressed animals at any time point examined (all p > 0.1). Collectively, these data are in line with previous reports that CVS and repeated restraint respectively result in sensitization and habituation of responses to restraint at each level of the HPA axis (Dallman, 1993a; Choi et al., 2008).
Differential engagement of aBST following CVS vs repeated restraint
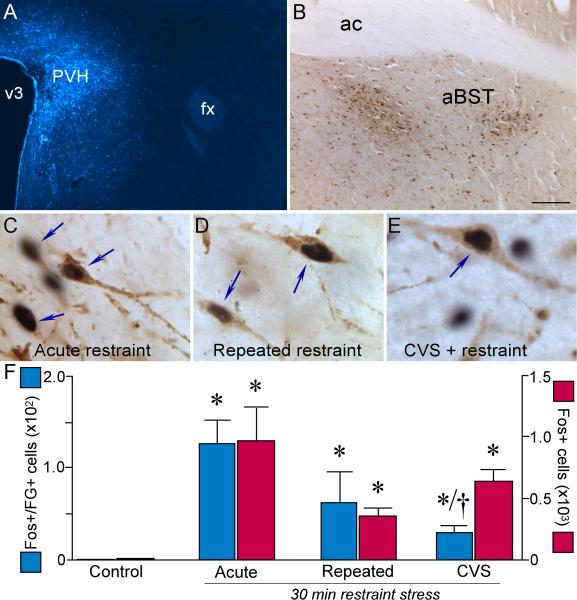
We have identified PVH-projecting, GABAergic neurons in aBST as providing a gateway for limbic forebrain influences to be exerted on HPA axis responses to acute restraint stress (Radley et al., 2009; Radley and Sawchenko, 2011). We now broach the possibility that such modulatory capabilities of aBST may also be involved in differential adaptations of axis responses to repeated stress, as are seen in CVS and/or repeated restraint paradigms. Retrograde tracer injections (FG) were placed in PVH, and neurons labeled in aBST were assayed for alterations in responsiveness (Fos induction) at 2 hr after a 30 min restraint exposure on the day following regimens of 14 d of repeated restraint, 14 d of CVS, or no prior stress exposure. The original intent was to couple tracing and Fos assays with markers of the GABAergic phenotype, but decrements in the sensitivity of one or more of the constituent methodologies in the triply labeled preparations made quantitative analyses unfeasible, so we were left to examine markers of interest singly or in pairs.
Tracer injections were aimed at the medial parvicellular part of the PVH (Fig. 3; 4A). Only animals bearing appropriately centered deposits, with lesser involvement of potentially confounding cell groups that adjoin the PVH, were included in the analysis. Whereas retrograde tracer placements were not devoid of any spread into aBST-projecting cell groups residing in close proximity to PVH (i.e., anterior hypothalamic nucleus or nucleus reuniens of the thalamus), no differences were noted in the distribution or density of retrograde labeling in aBST resulting from injections that may have differentially involved either of these two potential sources of contamination. In this material, retrograde labeling in the aBST was focused in the fusiform, dorsomedial, and subcommissural subnuclei defined by Dong and colleagues (2001)(Fig. 4B), in line with our prior characterization of GABAergic cell groups that issue projections to the hypophysiotropic zone of the PVH proper (Radley et al., 2009). Rats in each stress group displayed a robust Fos induction in aBST, including within the portion of neurons labeled concurrently for tracer, relative unstressed controls, which did not show constitutive Fos expression in this region (F3,11 = 8.0; p < 0.05; Fig. 4C-F). Fos induction in aBST of both repeatedly stressed groups tended to be reduced both globally and in identified PVH-projecting neurons, relative to acutely restrained controls, but the only statistically reliable effect was seen in retrogradely labeled neurons of the CVS group, which were reduced by 77% (p < 0.05).
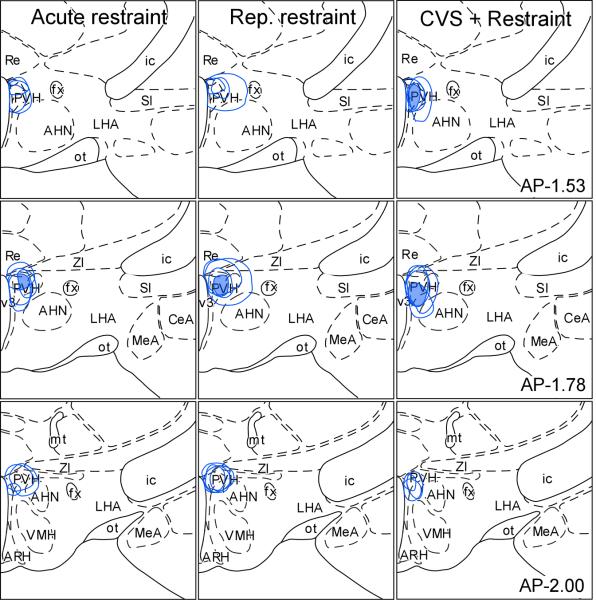
Figure 3.
Reconstructions of FG tracer injection placements in PVH in acute, repeated, and CVS groups (not shown are reconstructions in unstressed controls; N = 3-4/ group). The shaded regions in the diagrams indicate areas of overlap common to all tracer injections. Atlas plates are adapted from Swanson (1992); distance in millimeters relative to bregma is indicated. AHN, Anterior hypothalamic nucleus; ARH, arcuate nucleus hypothalamus; fx, fornix; ic, internal capsule; LHA, lateral hypothalamic nucleus; MeA, medial nucleus amygdala; ot, optic tract; Re, nucleus reunions; SI, substantia innominata; v3, third ventricle; VMH, ventromedial nucleus hypothalamus; ZI, zona incerta.
Figure 4.
A-B: Epifluorescence photomicrograph of an illustrating a FG tracer deposit centered within PVH following stereotaxic injection (A), and a brightfield image depicting the distribution of immunoperoxidase labeled FG neurons (brown reaction product) following a tracer injection in PVH (B). C-E: Higher magnification images show representative examples of dual immunoperoxidase staining of Fos and FG (arrows) as a function of treatment condition. Whereas each stress challenge led to the induction of Fos (black reaction product) activation within PVH-projecting neurons (brown), CVS-treated animals displayed an abrogated response. F: Histograms showing mean + SEM for Fos plus FG (blue bars) and Fos only (red bars). *, p < 0.05, differs significantly from unstressed controls; †, p < 0.05, differs significantly from the acute stress group. N = 3 control; N = 4 acute; N = 4 repeated, N = 4 CVS. Scale bar (in B): 150 μm, top row; 15 μm, middle row.
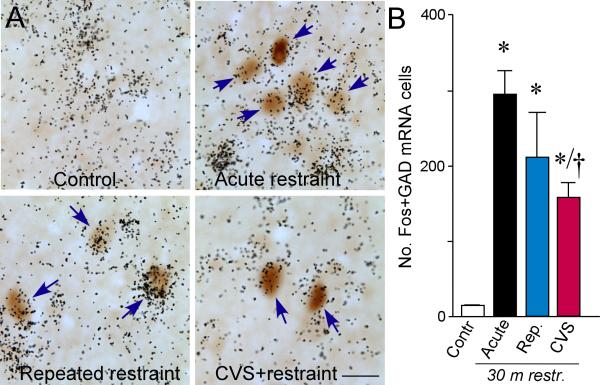
A compatible set of results was obtained in analyzing material in which immunolocalization of Fos protein was combined with hybridization histochemical detection of mRNA encoding for the 67 kDa isoform of glutamatic acid decarboxylase (GAD67; marker for GABAergic neurons) in aBST as a function of stress experience (Fig. 5). Animals in each of the stress groups displayed robust Fos induction in GAD67-expressing neurons as compared with unstressed animals (F3,12 = 8.2; p < 0.01). Of the two repeatedly stressed groups, however, only animals that were exposed to CVS showed a significant decrement (by 45%) in this measure relative to the acute restraint group (p < 0.05).
Figure 5.
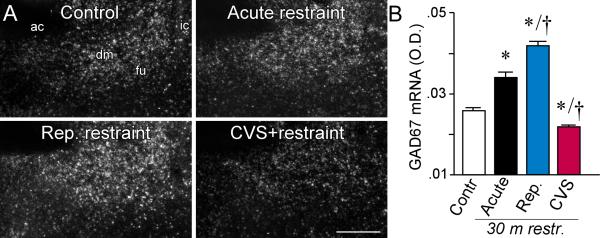
A: Photomicrographs show representative examples of concurrent labeling for Fos (brown) with GAD67 mRNA (black grains) in doubly-labeled cells (arrows) in aBST (notably within the dorsomedial and fusiform subdivisions). We have previously shown that these cell groups provide a source of GABAergic innervation of CRF-expressing neurons in PVH (Radley et al., 2009; Radley and Sawchenko, 2011). All animals subjected to 30 min of restraint stress showed increases in colabeled GAD+ and Fos+ cells in aBST as compared to unstressed controls, which were void of any Fos expression in this region. CVS resulted in significant decrements in doubly-labeled cells as compared with acutely stressed animals, consistent with the idea that the disinhibition of this pathway may underlie HPA axis sensitization under CVS conditions. B: Mean + SEM number of neurons co-labeled for Fos and GAD67 mRNA in aBST in treatment groups. *, p < 0.05, differs significantly from unstressed controls; †, p < 0.05, differs significantly from the acute stress group. N = 4 control, acute, and repeated groups; N = 5 CVS. Scale bar: 15 μm (applies to all).
In the preceding experiment, apparent differences were noted in the strength of GAD67 mRNA signal as a function of stress status. This was confirmed in a subsequent densitometric comparison of relative levels of this transcript across treatment conditions (F3,12 = 11.3; p < 0.01; Fig. 6). Compared to the acute restraint group, in which GAD67 mRNA measures were reliably elevated over those of unstressed controls, repeatedly restrained values were further enhanced, and CVS measures significantly reduced (p < 0.05 for each), the latter to the levels of basal controls. Together, these findings indicate that the aBST responds differently to an emotional stressor (restraint) in animals with differing repeated stress histories. In CVS-exposed animals, the aBST under-responds to a novel restraint stimulus, while rats with repeated restraint experience exhibit aBST reactivity at least on par with that seen in naïve rats.
Figure 6.
A: Representative darkfield photomicrographs show GAD 67 mRNA expression in aBST in all four treatment groups. ac, anterior commissure; dm dorsomedial subdivision; fu, fusiform subdivision; sc, subcommissural subdivision. B: Mean + SEM relative optical densities for GAD67 mRNA in aBST of treatment groups. Whereas acute stress elevated, repeated restraint produced further enhancements in, GAD expression in aBST. Conversely, GAD expression was significantly decreased following CVS compared to all other treatment groups. *, p < 0.05, differs significantly from unstressed controls; †, p < 0.05, differs significantly from the acute stress group. N = 4 control, acute, and repeated groups; N = 5 CVS. Scale bar: 150 μm (applies to all).
Repeated stress effects on aBST afferents from limbic forebrain
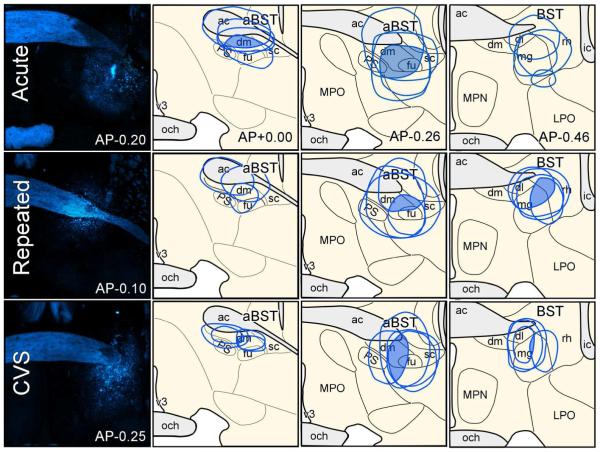
A network of interconnected cell groups in the limbic forebrain has been previously implicated in modulating HPA axis responses to acute emotional stressors (for reviews, see Van de Kar and Blair, 1999; Ulrich-Lai and Herman, 2009). An important feature of this modulation is that none of these regions have been shown to provide any appreciable innervation of PVH, suggesting a more complex, or indirect interaction with the stress axis. Our previous work shows that aBST GABAergic neurons form a disynaptic circuit interceding for stress-inhibitory influences of PL and vSUB on HPA activation (Radley et al., 2009; Radley and Sawchenko, 2011; Radley, 2012). These data raise the possibility that aBST may serve as a neural hub imparting inhibitory influences from other portions of the limbic forebrain (i.e., septum, PVT, amygdala), and importantly, that altered activation in these upstream cell groups may contribute to altered aBST GABAergic influences on PVH and corresponding HPA axis adaptations to repeated restraint and/or CVS. This was interrogated by placing discrete retrograde tracer (FG) injections in aBST, and examining for alterations in Fos induction in identified aBST-projecting neurons in cell groups implicated in HPA modulation, as a function of stress condition. In Figure 7, reconstructions of tracer deposits demonstrate areas of overlap common to all FG injections, and the maximal extent of diffusion of tracer deposits for each animal. Areas of overlap common to all placements judged to be appropriate for inclusion in the analysis encompassed aspects of the dorsomedial and fusiform subnuclei of BST just ventral to the anterior commissure. The maximal extent of diffusion involved portions of the parastrial nucleus (medially), the subcommissural and magnocellular nuclei (caudally) and/or the anterior commissure (dorsally) of the BST (see Dong et al., 2001, for pacellation/terminology). Although spread of the tracer to involve aspects of the anterior commissure represented a potential source of spurious retrograde labeling, we failed to detect such on either side of the brain in olfactory structures (e.g., olfactory bulb and tubercle, anterior olfactory nucleus) whose axons cross the midline via this fiber tract. This is consistent with the weight of evidence that under minimally invasive delivery conditions (pressure, iontophoresis), FG is at least relatively resistant to uptake and transport by axons-of-passage (Schmued and Fallon, 1986; Cullinan et al., 1993; Radley and Sawchenko, 2011).
Figure 7.
Reconstructions of Fluoro-Gold (FG) tracer injection placements in aBST in acute, repeated, and CVS rats (top, middle, and bottom rows, respectively). Epifluorescence photomicrographs (left column) depict examples of FG tracer deposits, whereas the shaded regions in the diagrams indicate areas of overlap common to all tracer injections, and their approximate extent of diffusion into adjacent-lying structures. Not shown is a fourth group of unstressed controls were also included in these experiments. ac, anterior commissure; dl, dorsolateral subdivision of aBST; dm, dorsomedial subdivsion of aBST; fu, fusiform subdivision of aBST; ic, internal capsule; LPO, lateral preoptic area; mg, magnocellular subdivision of posterior BST (pBST); MPN, median preoptic nucleus; MPO, medial preoptic area; och, optic chiasm; PS, parastrial nucleus; rh, rhomboid subdivision of pBST; v3, third ventricle.
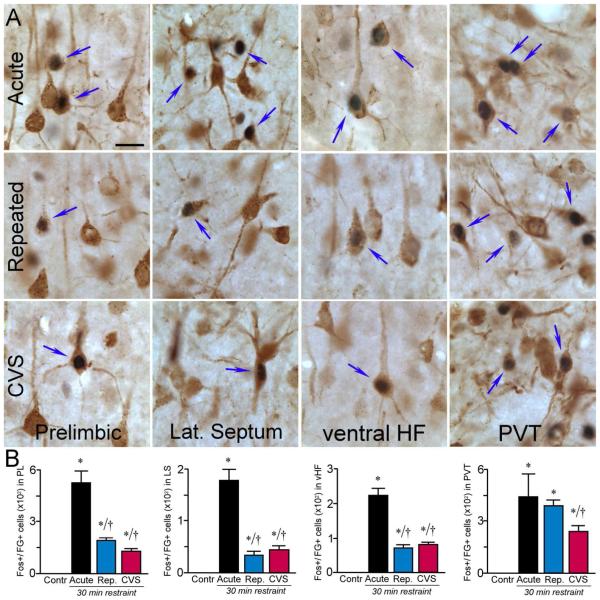
Tracer injections yielded moderate to dense retrograde labeling in cell groups implicated in the inhibitory control of the stress axis, including the lateral septum, PL, vSUB and the paraventricular nucleus of the thalamus (PVT). In unstressed control animals, these regions were conspicuously lacking in Fos-immunoreactivity, with the exception of PVT, which is known to display low-level constitutive Fos expression (Herdegen et al., 1995). Significant differences in activational responses to 30 min restraint as a function of stress experience were noted overall (Table 2), and aBST-projecting neurons (Fig. 8) in each of these regions (PL: F3,12 = 25.9; lateral septum: F3,12 = 7.0, p < 0.01; vSUB: F3,12 = 25.8; PVT: F3,12 = 13.9, all p < 0.01). Activational profiles in identified aBST afferent neurons in each cell group followed the general trend seen in both repeatedly stressed groups toward diminished numbers of neurons displaying restraint-induced Fos-ir, relative to acutely stressed rats (p < 0.05 for each; Fig. 8B), with one exception. In the PVT of animals subjected to repeated restraint, Fos responses to the final restraint session were maintained, that is, they did not differ significantly from those of the acutely stressed group (431 ± 25 and 448 ± 139, respectively; p = 0.5), contrasting with the significant reduction seen in CVS. Because of prior data implicating a distinct, posterior, region of the PVT in adaptations to repeated stress (Bhatnagar et al., 2002; Jaferi et al., 2003), we also analyzed data from the anterior and posterior PVT separately (anterior PVT: F3,12 = 14.3, p < 0.01; posterior PVT: F3,12 = 7.7, p < 0.01), finding that both responded similarly to the cell group as a whole.
Table 2.
Effect of different stress regimens on functional activation in select limbic forebrain regions
| No. Fos-labeled nuclei |
||||
|---|---|---|---|---|
| Cell Groups | Control | Acute Restr. | Repeated Restr. | CVS + Restr. |
| Prelimbic cortex | 255 ± 97 | 2,308 ± 242* | 801 ± 241*† | 1,976 ± 172* |
| Lateral septum | nil. | 105 ± 43* | 58 ± 13* | 83 ± 53* |
| Ventral hippocampal formation | nil. | 253 ± 36* | 235 ± 58* | 105 ± 24*† |
| Paraventricular thalamus (PVT) | 120 ± 11 | 1,183 ± 106* | 690 ± 134*† | 848 ± 47*† |
| PVT, anterior division | 58 ± 21 | 783 ± 103* | 398 ± 82*† | 533 ± 50*† |
| PVT, posterior division | 63 ± 23 | 400 ± 35* | 293 ± 55* | 315 ± 16* |
Values represent mean ± s.e.m. for counts made within each region. Statistical comparisons were made employing a one-way analysis of variance, followed by post-hoc pairwise comparisons using Tukey HSD.
p < 0.05 relative to the unstressed control group;
p < 0.05 relative to acutely stressed animals.
Figure 8.
A: Photomicrographs show representative examples of dual immunoperoxidase staining of FG and Fos (arrows) at high magnification in treatment groups (rows). This analysis reveals that many of the limbic forebrain regions (columns) previously shown to negatively regulate emotional stress-induced HPA activation are both aBST-projecting and functionally activated in response to acute stress. Notably, all of these regions underwent significant decreases in dual colocalization for Fos and FG following CVS. Many of these regions also showed similar decrements following repeated restraint stress, except for the posterior thalamic nucleus (PVT), which remained elevated. HF, hippocampal formation. B: Mean + SEM numbers of Fos+FG colabeling for each of the regions analyzed. *, p < 0.05, differs significantly from unstressed controls; †, p < 0.05, differs significantly from the acute stress group. N = 4 control, acute, and repeated groups; N = 5 CVS. Scale bar: 20 μm (applies to all).
Such differential propensities of aBST afferents to habituate to repeated restraint versus CVS (i.e., PVT versus PL, vSUB and lateral septum) could underlie the differential adaptations of aBST GABAergic neurons and the HPA axis seen under these two distinct repeated stress paradigms.
DISCUSSION
Here we addressed the question of how the activity of nodes in a limbic network implicated in modulating HPA axis responses to acute emotional stresses may be altered under differing repeated stress regimens, to determine whether and how that network may take part in mediating differential neuroendocrine adaptations to those regimens. Fourteen days’ exposure to CVS led to significant increases both in PVH and adrenocortical output in response to restraint on day 15 as compared with animals subjected acutely to the same challenge. Augmentation of HPA output in CVS animals was accompanied by a reduction of activational responses in PVH-projecting and GABAergic neurons in aBST, as well as decreases in overall GAD 67 mRNA expression in this region. By contrast, habituated HPA axis responses in the repeated restraint group were associated with an increased expression of GAD 67 mRNA and functional activation in PVH-projecting and GABAergic neurons in aBST. In view of evidence we have provided to support a role for aBST in integrating limbic modulatory influences on HPA axis responses to acute emotional stress (Radley et al., 2009; Radley and Sawchenko, 2011), these current findings justify consideration of an involvement of aBST in differential adaptations of the stress axis to repeated stress paradigms associated with sensitization (CVS) versus habituation (repeated restraint).
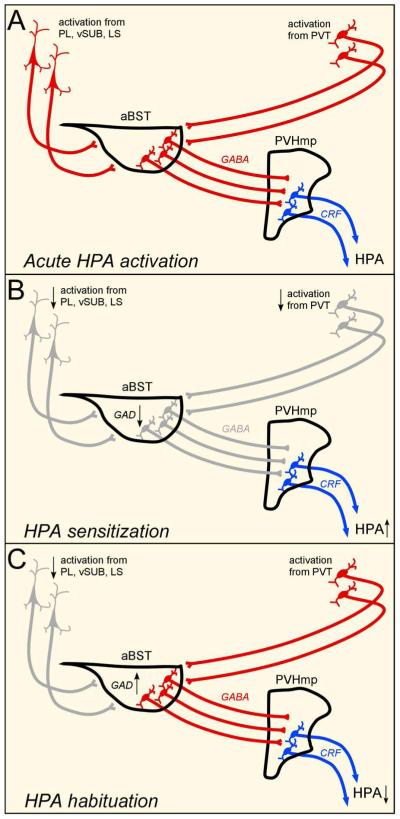
Further, in surveying key cell groups in an extended limbic forebrain circuitry that may underlie differential effects of the two repeated stress regimens, we found a pervasive tendency of identified aBST afferents from the septal region, vSUB, PL, and PVT to habituate under CVS conditions, which is consistent with a model of facilitation of axis responses by disinhibition (i.e., inhibiting an inhibitory relay in aBST). By contrast, we found that repeated restraint resulted in habituation of all but one of the aBST afferents listed above, namely the PVT, a cell group strongly implicated in adaptations to repeated stress (Bhatnagar and Dallman, 1998; Bhatnagar et al., 2002; Jaferi and Bhatnagar, 2006). Overall, our findings outline a network in which GABAergic neurons of the aBST may serve as a neural hub for stress-modulatory influences from the limbic forebrain, whereby inhibition or disinhibition of PVH-projecting components contributes to habituation or sensitization, respectively, of HPA axis adaptations to repeated stress (Fig. 9).
Figure 9.
Diagrams illustrating the proposed circuitry that may provide for modulation of HPA-inhibitory influences under repeated stress conditions. These data support activation in these pathways (highlighted in red) and changes in HPA output as a function of various stress regimens. Whereas GABAergic neurons in aBST impart restraining influences from the limbic forebrain on parvicellular PVH neurons that control HPA output during acute emotional stressors (A), dampened activational responses in this network (gray), and decreases in GAD expression in aBST, following CVS (B) are associated with HPA axis sensitization. By contrast, activation of aBST-projecting neurons from PVT, maintained levels of activation of GABAergic neurons, and increased GAD expression in aBST, may be associated with HPA axis habituation following repeated restraint stress (C). Whereas functional decrements in aBST and related circuits correspond well with HPA axis sensitization following CVS, they do not as easily explain the pattern of results observed under habituation.
Methodologial Considerations
Two technical-methodological issues that bear on the interpretation of the present findings warrant additional consideration. One has to do with our heavy reliance on Fos protein induction as an index of cellular activation under longer-term, complex stimulation conditions, when there is evidence to suggest that other factors, notably ΔFosB (e.g., Perrotti et al., 2004) may be more suitable makers of chronic activational effects. First, we would note that the stress models employed here are not chronic, in the sense of being continuously applied over a substantial time period (typically days), but rather involve intermittent exposure to discrete episodes. This is an important distinction, as we are not asking Fos to register the effect of a chronic treatment; its ability to inhibit its own expression for a period of a few hours after acute induction (Morgan and Curran, 1990; Brown and Sawchenko, 1997) makes it ill-equipped to do so. Instead, we are asking it to indicate how the response to discrete events varies with the amount and nature of prior stress experience, as attested by its capacity to be acutely induced in response to a range of signaling mechanisms. Perhaps the strongest argument we can offer for the relevance of Fos as a marker in this context is that its restraint-induced expression in the hypothalamus of animals with vastly differing stress histories (no stress, prior repeated restraint, prior repeated variable stress) varied in tandem with the effects of that final restraint episode on an independent cellular marker (CRF mRNA), and, critically, with stress hormone secretion, the PVH-dependent endpoints whose regulation we ultimately aimed to clarify.
We considered including immunohistochemical analyses of additional transcription factor markers, including ΔFosB, which shows a more gradual increase and persistent expression in some brain regions under repeated stress paradigms (Melia et al., 1994; Perrotti et al., 2004). However, the appeal of this candidate for examining long-term alterations in HPA-modulatory pathways in response to repeated challenges is offset by technical limitations. ΔFosB is a truncated form of FosB, and the two forms exhibit some degree of overlap in their temporal expression patterns, while spatial resolution is limited by the fact that immunohistochemical approaches are unable to distinguish the full-length proteins from splice variants (for discussion, see Perrotti et al., 2004). Furthermore, evidence from immunoblotting methods that can accurately differentiate Fos family proteins according to molecular weight, fail to reveal any frank differences in forebrain regional expression patterns of ΔFosB as a function of repeated restraint or CVS (Perrotti et al., 2004). While ΔFosB may well provide an index of enduring changes in cellular function as a result of experience, it is clearly not a useful marker for purposes of the present study. This was confirmed by surveying (Radley, unpublished) ΔFosB expression following repeated restraint and CVS exposure (i.e., omitting the restraint challenge on the final day of stress). Analysis using several different antibodies for ΔFosB reported in other published studies failed to demonstrate any convincing degree of regional or treatment specificity for this protein. Western blot experiments comparing extracts from forebrain regions of interest (e.g., prefrontal cortex) resulted in the recognition of at least 4 molecular weight bands that migrated at positions corresponding to FosB, ΔFosB, and several other unknown proteins, probably in the Fos family.
A second technical issue of concern in the present study has to do with the fact that group sizes in anatomically guided experiments (i.e., those involving subjects that received tracer injections) are small, which would limit confidence, particularly in negative (non-significant) comparisons. The limitations that this imposes may be offset, to a degree at least, by the fact that carrying out the study in anatomically identified neurons facilitates detection of effects in subpopulations of interest that might otherwise be diluted by considering complex cell groups as a whole. In addition, for the first two phases of the study, the use of multiple indices of the behavior of individual cell groups (e.g., Fos-ir, CRF mRNA, ACTH secretion for hypophysiotropic PVH neurons) bolsters confidence in convergent findings.
Concern over the interpretation of non-significant findings in studies with small Ns is more noteworthy in our final experiment, where we attach potential importance to a non-significant difference between the number of Fos-positive PVT neurons that project to the BST under acute versus repeated restraint conditions (see discussion below), which contrasted with highly reliable differences in this comparison in three other identified BST afferent populations. Calculating statistical power offers no help in this instance, as it is generally agreed among statisticians that post hoc power determinations are neither meaningful nor valid (see e.g., Hoenig and Heisey, 2001). As an alternative, we used an equivalence testing procedure, TOST (two one-sided tests; Walker and Nowacki, 2011), to test the joint null hypothesis that the difference between the mean counts of doubly labeled cells in the PVT from groups exposed to acute versus repeated restraint falls within a specified range of equivalence, which we defined as the average percent difference between the means from the other three cell groups examined (PL, septum, ventral HF) under these same stress conditions. The results supported equivalence of the acute-repeated comparison of PVT data, leading us to conclude (conservatively) that the PVT responded differently than the other three cell groups to acute versus repeated restraint.
Notwithstanding the considerations detailed above, the heavy reliance on Fos as an activation marker and the interpretation of negative outcomes of underpowered experiments remain valid concerns. It will remain for tests of the principal interpretations/hypotheses to emerge from the present study, which posit roles for the aBST and its inputs from limbic forebrain in adaptations of HPA axis responses to repeated stress, to judge the ultimate value of these approaches.
Relation to previous studies
The tendency for HPA responses to diminish with repeated exposure to the same (homotypic) stressor is a common (Mason, 1972; Keim and Sigg, 1976; Pollard et al., 1976), though not universal (Hennessy and Levine, 1977; Kant et al., 1983), finding. Habituation may be viewed as an adaptive phenomenon, which serves to protect against adverse catabolic and immunosuppressive effects of sustained elevations in circulating glucocorticoids. When evident, habituation is not generally attributable to an exhaustion of response capacity, since repeated exposure may sensitize, or facilitate, HPA secretory responses to a novel (heterotypic) insult (see e.g., Dallman et al., 1992; Dallman, 1993b). Decrements in immediate-early gene induction with repeated exposure to restraint or immobilization have been described in the PVH, as well as several extrahypothalamic cell groups, leading to the general belief that habituation is likely to be mediated by decreased neuronal activity in facilitatory afferents (Lachuer et al., 1994; Melia et al., 1994; Umemoto et al., 1994; Chen and Herbert, 1995b; cf. Campeau et al., 2002). Conversely, several laboratories have identified cell groups/circuitries whose responses to a novel stressor are enhanced in facilitation paradigms, as potential candidates for mediating increased HPA axis responses under such (sensitization) conditions (e.g., Bhatnagar and Dallman, 1998; Ma et al., 2008). One major inference to be drawn from the present findings is that the aBST is in a position to participate in mediating both kinds of adaptations.
The role of aBST in adaptations to repeated stress
Although several limbic forebrain sites are capable of inhibiting the HPA axis during acute emotional stress (Sapolsky et al., 1984; Kovacs and Makara, 1988; Herman et al., 1989; Diorio et al., 1993; Weinberg et al., 2010), none of these provides a substantial direct innervation of the PVH. Combined pathway tracing and immediate-early gene mapping studies have identified candidate cell groups that could serve as disynaptic relays to link forebrain regulators and the PVH, and suggests a complex network of higher-order structures interconnected with PVH in a parallel or multisynaptic manner (Cullinan et al., 1993; Roland and Sawchenko, 1993; Van de Kar and Blair, 1999; Ulrich-Lai and Herman, 2009). However, recent evidence lends support for at least two limbic cortical regions, PL and vSUB, that impart inhibitory influences over acute restraint-induced HPA output by converging on a discrete target, the aBST, that in turn inhibits the PVH (Radley et al., 2009; Radley and Sawchenko, 2011). Our observation of decreased functional activation throughout this network under HPA-sensitizing conditions highlights the need for studies to directly assess whether these perturbations drive increases in circulating glucocorticoids resulting from repeated stress exposure.
Previous reports have demonstrated increases in GAD mRNA expression and functional activation of GAD-expressing neurons afferent to PVH following acute stress exposure (Bowers et al., 1998; Bali et al., 2005). In agreement with these studies, we observed increases in GAD67 mRNA expression in aBST following acute restraint that were paralleled by enhanced functional activation of PVH-projecting GABAergic neurons, and corresponding decreases in both of these indices following CVS exposure. Increased GAD67 expression has been associated with enhanced inhibition, and disrupted GAD67 expression is associated with impaired inhibitory mechanisms (Asada et al., 1997; Lewis et al., 2005; Kobori and Dash, 2006). These findings are consistent with the idea that activation of GABAergic afferents to PVH restrain HPA activation during acute stress, and that following CVS, dampened activation/ GAD expression is associated with a diminished inhibitory capacity. We also observed increased GAD67 mRNA expression in aBST during HPA axis habituation, lending further support to the idea of a functional link between alterations in GAD expression in PVH-projecting inhibitory cell groups and the differential modulation of HPA output (Bowers et al., 1998; Bali et al., 2005). However, this general interpretation may be complicated by the fact that repeatedly restrained animals failed to display reliable enhancement of Fos-immunoreactivity in PVH-projecting GABAergic neurons in aBST (Fig. 4, 5), leaving questions as to whether changes in the inhibitory capacity of proximate mediators of the stress axis may be sufficient to impart activational influences from upstream regions, such as PVT.
The BST is a limbic forebrain structure that is intimately associated with aspects of the amygdala, and projects in turn to hypothalamic and brainstem target areas that mediate many autonomic and behavioral responses to aversive or threatening stimuli. While earlier behavioral studies tended to treat BST as an homogeneous entity, recent work has defined cytoarchitectonic subdivisions of BST that are associated with distinct types of adaptive behavioral and autonomic responses (Jennings et al., 2013; Kim et al., 2013). BST influences on HPA responses may be differentiated in the rostrocaudal dimension, and more recent evidence suggests that the anterior aspect, alone, may harbor co-mingled excitatory and inhibitory controls over the stress axis (Dunn, 1987; Cecchi et al., 2002; Choi et al., 2007; Radley et al., 2009). On one hand, non-selective lesions to aBST mildly attenuate, while, selective ablation of GABAergic cell groups in this region augment, HPA axis responses to a single acute restraint episode (Choi et al., 2007; Radley et al., 2009). Evidence also supports a role for a distinction in aBST modulation of the stress axis as a function of stressor duration. Excitotoxin lesions of aBST were found to further enhance HPA activation to a novel restraint challenge following CVS exposure (Choi et al., 2008), which suggests a restraining influence on the stress axis even under conditions of HPA axis sensitization. Our finding that the CVS + restraint group showed decreased functional activation in both PVH-projecting and GABAergic neurons in aBST is consistent with the possibility that this cell group generally constrains HPA activation following prolonged stress exposure.
Extended circuitry
The most thorough and systematic analysis of the CNS substrates that might underpin adaptations to repeated stress was initiated by Bhatnagar and Dallman (1998) and developed by Bhatnagar and colleagues (Bhatnagar et al., 2000; Bhatnagar et al., 2002; Jaferi and Bhatnagar, 2006; Grissom and Bhatnagar, 2009). The initial studies identified the posterior paraventricular nucleus of the thalamus (pPVT) at the core of an interconnected series of cell groups, including the lateral parabrachial and several amygdaloid nuclei that are known to project to the PVH, and PVH itself, that displayed increased Fos staining in a facilitation model (i.e., 30 min restraint on day 7, following 6 daily cold stress exposures). Lesions of pPVT enhanced ACTH secretory responses to restraint in previously cold-stressed, but not naïve, rats. The subsequent demonstration that pPVT ablation disrupted habituation of HPA axis activity under conditions of repeated restraint stress, again without affecting responses to acute restraint, has fostered a general championing of the pPVT as a pivotal structure in “stress memory,” that is, in effecting alterations in axis output as a consequence of prior stress experience.
Aspects of the present findings are compatible with this model. For one, the differential propensity for a relevant (aBST-projecting) PVT subpopulation to habituate under repeated stress paradigms, in which restraint is presented as a final homotypic versus heterotypic challenge, is consistent with a unique role for this thalamic cell group in adaptations to chronic stress. Second, the prior finding that lesions of pPVT increase HPA secretory output in a facilitation paradigm (Bhatnagar et al., 1998), is indicative of an inhibitory role in HPA control, in line with our identification of a GABAergic population in the aBST as providing a gateway for convergent limbic forebrain influences on the central limb of the axis (Radley and Sawchenko, 2011).
In addition to issuing prominent inputs to aBST, PVT is interconnected with other upstream components of the limbic-PVH inhibitory network, including the ventral subiculum, amygdala, and, most prominently, mPFC (Moga et al., 1995; Heidbreder and Groenewegen, 2003; Li and Kirouac, 2012). This raises the possibility that PVT may serve as a primary interface between pathways conveying stress-related information to the limbic forebrain (Bubser and Deutch, 1999; Otake et al., 2002), and may indicate a more prominent role for this thalamic region in modulating adaptive responses to prolonged challenges by interceding for higher-order cognitive processing systems such as mPFC. Some progress has been made describing the synaptic mechanisms underlying habituation of the HPA axis following repeated stress (e.g., Levy and Tasker, 2012), although their relationship to the upstream neural substrates that influence the PVH have yet to be sorted out. Activation of forebrain arginine vasopressin 1A receptors and endocannibinoids have been implicated as mediators of HPA axis adaptations following repeated stress exposure (Chen and Herbert, 1995a; Hill et al., 2010; Gray et al., 2014), and further experimental work is be needed to evaluate whether and how such factors may map onto the network outlined here.
Acknowledgements
We thank Carlos Arias, Soon Lee, and Joan Vaughan for technical assistance. This work was supported by National Institutes of Health Grants MH-095972 (J.J.R.) and DK-26741 (P.E.S.). P.E.S. is a senior investigation with the Clayton Medical Research Foundation.
Footnotes
Author contributions: J.J.R. designed research; J.J.R. performed research; J.J.R. analyzed data; J.J.R. and P.E.S. wrote the paper.
The authors declare no conflicts of interest.
REFERENCES
- Abercrombie M. Estimation of nuclear populations from microtome populations sections. Anatomical Record. 1949;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(12):6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali B, Erdelyi F, Szabo G, Kovacs KJ. Visualization of stress-responsive inhibitory circuits in the GAD65-eGFP transgenic mice. Neuroscience letters. 2005;380(1-2):60–65. doi: 10.1016/j.neulet.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84(4):1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman MF, Roderick RE, Basbaum AI, Taylor BK. The effects of prior chronic stress on cardiovascular responses to acute restraint and formalin injection. Brain research. 1998;797(2):313–320. doi: 10.1016/s0006-8993(98)00382-5. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. Journal of neuroendocrinology. 2002;14(5):403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Viau V, Chu A, Soriano L, Meijer OC, Dallman MF. A cholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamic-pituitary-adrenal function. J Neurosci. 2000;20(14):5564–5573. doi: 10.1523/JNEUROSCI.20-14-05564.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers G, Cullinan WE, Herman JP. Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18(15):5938–5947. doi: 10.1523/JNEUROSCI.18-15-05938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ER, Sawchenko PE. Hypophysiotropic CRF neurons display a sustained immediate-early gene response to chronic stress but not to adrenalectomy. Journal of neuroendocrinology. 1997;9(4):307–316. doi: 10.1046/j.1365-2826.1997.00586.x. [DOI] [PubMed] [Google Scholar]
- Bubser M, Deutch AY. Stress induces Fos expression in neurons of the thalamic paraventricular nucleus that innervate limbic forebrain sites. Synapse. 1999;32(1):13–22. doi: 10.1002/(SICI)1098-2396(199904)32:1<13::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Campeau S, Dolan D, Akil H, Watson SJ. c-fos mRNA induction in acute and chronic audiogenic stress: possible role of the orbitofrontal cortex in habituation. Stress. 2002;5(2):121–130. doi: 10.1080/10253890290027895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Morilak DA. Modulatory effects of norepinephrine, acting on alpha 1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology. 2002;43(7):1139–1147. doi: 10.1016/s0028-3908(02)00292-7. [DOI] [PubMed] [Google Scholar]
- Chan RK, Brown ER, Ericsson A, Kovacs KJ, Sawchenko PE. A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13(12):5126–5138. doi: 10.1523/JNEUROSCI.13-12-05126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HT, Kuo H, Whittaker JA, Cooper NG. Light and electron microscopic analysis of projection neurons retrogradely labeled with Fluoro-Gold: notes on the application of antibodies to Fluoro-Gold. J Neurosci Methods. 1990;35(1):31–37. doi: 10.1016/0165-0270(90)90091-s. [DOI] [PubMed] [Google Scholar]
- Chen X, Herbert J. Alterations in sensitivity to intracerebral vasopressin and the effects of a V1a receptor antagonist on cellular, autonomic and endocrine responses to repeated stress. Neuroscience. 1995a;64(3):687–697. doi: 10.1016/0306-4522(94)00413-y. [DOI] [PubMed] [Google Scholar]
- Chen X, Herbert J. Regional changes in c-fos expression in the basal forebrain and brainstem during adaptation to repeated stress: correlations with cardiovascular, hypothermic and endocrine responses. Neuroscience. 1995b;64(3):675–685. doi: 10.1016/0306-4522(94)00532-a. [DOI] [PubMed] [Google Scholar]
- Choi DC, Evanson NK, Furay AR, Ulrich-Lai YM, Ostrander MM, Herman JP. The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress. Endocrinology. 2008;149(2):818–826. doi: 10.1210/en.2007-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(8):2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE, Helmreich DL, Watson SJ. Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. The Journal of comparative neurology. 1996;368(1):88–99. doi: 10.1002/(SICI)1096-9861(19960422)368:1<88::AID-CNE6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64(2):477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. The Journal of comparative neurology. 1993;332(1):1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Adaptation of the hypothalamic-pituitary adrenal axis to chronic stress. Trends in Endorcrinology and Metabolism. 1993a;4:62–69. doi: 10.1016/s1043-2760(05)80017-7. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Stress update Adaptation of the hypothalamic-pituitary-adrenal axis to chronic stress. Trends in endocrinology and metabolism: TEM. 1993b;4(2):62–69. doi: 10.1016/s1043-2760(05)80017-7. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker CD, Strack AM, Cascio CS. Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. Journal of neuroendocrinology. 1992;4(5):517–526. doi: 10.1111/j.1365-2826.1992.tb00200.x. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. The European journal of neuroscience. 2001;14(7):1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13(9):3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. The Journal of comparative neurology. 2001;436(4):430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dunn JD. Plasma corticosterone responses to electrical stimulation of the bed nucleus of the stria terminalis. Brain research. 1987;407(2):327–331. doi: 10.1016/0006-8993(87)91111-5. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14(2):897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7(1):91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Gray M, Innala L, Viau V. Central vasopressin V1A receptor blockade alters patterns of cellular activation and prevents glucocorticoid habituation to repeated restraint stress exposure. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2014:1–11. doi: 10.1017/S1461145714000935. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav. 2003;78(4-5):703–710. doi: 10.1016/s0031-9384(03)00050-7. [DOI] [PubMed] [Google Scholar]
- Grissom N, Bhatnagar S. Habituation to repeated stress: get used to it. Neurobiology of learning and memory. 2009;92(2):215–224. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neuroscience and biobehavioral reviews. 2003;27(6):555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Levine S. Effects of various habituation procedures on pituitary-adrenal responsiveness in the mouse. Physiology & behavior. 1977;18(5):799–802. doi: 10.1016/0031-9384(77)90186-x. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Kovary K, Buhl A, Bravo R, Zimmermann M, Gass P. Basal expression of the inducible transcription factors c-Jun, JunB, JunD, c-Fos, FosB, and Krox-24 in the adult rat brain. J Comp Neurol. 1995;354(1):39–56. doi: 10.1002/cne.903540105. [DOI] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61(2):180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Herman JP, Schafer MK, Young EA, Thompson R, Douglass J, Akil H, Watson SJ. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1989;9(9):3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT, Gray JM, Hillard CJ, Gorzalka BB, Viau V. Endogenous cannabinoid signaling is essential for stress adaptation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(20):9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenig JM, Heisey DM. The abuse of power: The pervasive fallacy of power calculations for data analysis. The American Statistician. 2001;55:1–6. [Google Scholar]
- Jaferi A, Bhatnagar S. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology. 2006;147(10):4917–4930. doi: 10.1210/en.2005-1393. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Nowak N, Bhatnagar S. Negative feedback functions in chronically stressed rats: role of the posterior paraventricular thalamus. Physiology & behavior. 2003;78(3):365–373. doi: 10.1016/s0031-9384(03)00014-3. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496(7444):224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant GJ, Bunnell BN, Mougey EH, Pennington LL, Meyerhoff JL. Effects of repeated stress on pituitary cyclic AMP, and plasma prolactin, corticosterone and growth hormone in male rats. Pharmacology, biochemistry, and behavior. 1983;18(6):967–971. doi: 10.1016/s0091-3057(83)80022-7. [DOI] [PubMed] [Google Scholar]
- Keim KL, Sigg EB. Physiological and biochemical concomitants of restraint stress in rats. Pharmacology, biochemistry, and behavior. 1976;4(3):289–297. doi: 10.1016/0091-3057(76)90244-6. [DOI] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, Malenka RC, Warden MR, Neve R, Tye KM, Deisseroth K. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496(7444):219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori N, Dash PK. Reversal of brain injury-induced prefrontal glutamic acid decarboxylase expression and working memory deficits by D1 receptor antagonism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(16):4236–4246. doi: 10.1523/JNEUROSCI.4687-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KJ, Makara GB. Corticosterone and dexamethasone act at different brain sites to inhibit adrenalectomy-induced adrenocorticotropin hypersecretion. Brain research. 1988;474(2):205–210. doi: 10.1016/0006-8993(88)90435-0. [DOI] [PubMed] [Google Scholar]
- Lachuer J, Delton I, Buda M, Tappaz M. The habituation of brainstem catecholaminergic groups to chronic daily restraint stress is stress specific like that of the hypothalamo-pituitary-adrenal axis. Brain research. 1994;638(1-2):196–202. doi: 10.1016/0006-8993(94)90650-5. [DOI] [PubMed] [Google Scholar]
- Levy BH, Tasker JG. Synaptic regulation of the hypothalamic-pituitary-adrenal axis and its modulation by glucocorticoids and stress. Frontiers in cellular neuroscience. 2012;6:24. doi: 10.3389/fncel.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature reviews Neuroscience. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Li HY, Sawchenko PE. Hypothalamic effector neurons and extended circuitries activated in "neurogenic" stress: a comparison of footshock effects exerted acutely, chronically, and in animals with controlled glucocorticoid levels. The Journal of comparative neurology. 1998;393(2):244–266. [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain structure & function. 2012;217(2):257–273. doi: 10.1007/s00429-011-0360-7. [DOI] [PubMed] [Google Scholar]
- Ma S, Mifflin SW, Cunningham JT, Morilak DA. Chronic intermittent hypoxia sensitizes acute hypothalamic-pituitary-adrenal stress reactivity and Fos induction in the rat locus coeruleus in response to subsequent immobilization stress. Neuroscience. 2008;154(4):1639–1647. doi: 10.1016/j.neuroscience.2008.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JW. Corticosteroid response to chair restraint in the monkey. The American journal of physiology. 1972;222(5):1291–1294. doi: 10.1152/ajplegacy.1972.222.5.1291. [DOI] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14(10):5929–5938. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. The Journal of comparative neurology. 1995;359(2):221–238. doi: 10.1002/cne.903590204. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Inducible proto-oncogenes of the nervous system: their contribution to transcription factors and neuroplasticity. Progress in brain research. 1990;86:287–294. doi: 10.1016/s0079-6123(08)63185-4. [DOI] [PubMed] [Google Scholar]
- Otake K, Kin K, Nakamura Y. Fos expression in afferents to the rat midline thalamus following immobilization stress. Neurosci Res. 2002;43(3):269–282. doi: 10.1016/s0168-0102(02)00042-1. [DOI] [PubMed] [Google Scholar]
- Ottenweller JE, Natelson BH, Pitman DL, Drastal SD. Adrenocortical and behavioral responses to repeated stressors: toward an animal model of chronic stress and stress-related mental illness. Biol Psychiatry. 1989;26(8):829–841. doi: 10.1016/0006-3223(89)90123-6. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(47):10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard I, Bassett JR, Cairncross KD. Plasma glucocorticoid elevation and ultrastructural changes in the adenohypophysis of the male rat following prolonged exposure to stress. Neuroendocrinology. 1976;21(4):312–330. doi: 10.1159/000122539. [DOI] [PubMed] [Google Scholar]
- Radley JJ. Toward a limbic cortical inhibitory network: implications for hypothalamic-pituitary-adrenal responses following chronic stress. Frontiers in behavioral neuroscience. 2012;6:7. doi: 10.3389/fnbeh.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(22):7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sawchenko PE. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(26):9683–9695. doi: 10.1523/JNEUROSCI.6040-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Williams B, Sawchenko PE. Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(22):5806–5816. doi: 10.1523/JNEUROSCI.0552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1993;332(1):123–143. doi: 10.1002/cne.903320109. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci U S A. 1984;81(19):6174–6177. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Cunningham ETJ, Mortrud MT, Pfeiffer SW, Gerfen CR. Phaseolus vulgaris-leucoagglutanin (PHA-L) anterograde axonal transport technique. Methods Neurosci. 1990;3:247–260. [Google Scholar]
- Schmued LC, Fallon JH. Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986;377(1):147–154. doi: 10.1016/0006-8993(86)91199-6. [DOI] [PubMed] [Google Scholar]
- Shu SY, Ju G, Fan LZ. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988;85(2):169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- Simmons DM, Swanson LW, Arriza JL. A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radiolabeled single-stranded RNA probes. J Histotechnol. 1989;12:169–181. [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194(3):555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto S, Kawai Y, Senba E. Differential regulation of IEGs in the rat PVH in single and repeated stress models. Neuroreport. 1994;6(1):201–204. doi: 10.1097/00001756-199412300-00051. [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Blair ML. Forebrain pathways mediating stress-induced hormone secretion. Front Neuroendocrinol. 1999;20(1):1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- Viau V, Sawchenko PE. Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress: rapid publication. The Journal of comparative neurology. 2002;445(4):293–307. doi: 10.1002/cne.10178. [DOI] [PubMed] [Google Scholar]
- Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. Journal of general internal medicine. 2011;26(2):192–196. doi: 10.1007/s11606-010-1513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MS, Johnson DC, Bhatt AP, Spencer RL. Medial prefrontal cortex activity can disrupt the expression of stress response habituation. Neuroscience. 2010;168(3):744–756. doi: 10.1016/j.neuroscience.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134(4):319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Amsterdam; Elsevier: 1992. [Google Scholar]