Abstract
The inferior colliculus (IC), the midbrain component of the auditory pathway, integrates virtually all inputs from the auditory brainstem. These are a mixture of excitatory and inhibitory ascending inputs, and the inhibitory transmitters include both gamma-aminobutyric acid (GABA) and glycine (GLY). Although the presence of these inhibitory inputs is well established, their relative location in the IC is not, and there is little information on the mouse. Here, we study the distribution of GAD67 and GLYT2 in axonal terminals to better understand the relative contributions of these inputs. Large-scale mosaic composite images of immunohistochemistry sections of rat and mice were used to isolate the signals related to the concentrations of these axonal terminals in the tissue, and the ratio of GLYT2/GAD67 in each pixel was calculated. GLYT2 was seen only in the central nucleus of IC (ICC), while GAD67 was seen throughout the IC. The map of the GAD67 and GLYT2 axonal distribution revealed a gradient that runs from ventrolateral to dorsomedial along the axis of the laminae of the ICC and perpendicular to the tonotopic axis. Although anatomically different, both the mouse and the rat had relatively more GAD67 dorsomedially in ICC and relatively more GLYT2 ventrolaterally. This organization of GABA and GLY inputs may be related to functional zones with different properties in ICC that are based, in part, on different sets of inhibitory inputs to each zone.
Keywords: GAD67, GLYT2, ratiometric analysis, auditory pathways, presynaptic terminals, AB_2278725, AB_90953
INTRODUCTION
The inferior colliculus (IC) is integral to the processing of auditory information, as virtually all information from the lower auditory brainstem must converge there before continuing to the forebrain (Oliver, 2005). These brainstem inputs are supplemented by the collaterals of local neurons (Oliver et al., 1991) and descending inputs from the auditory cortex (Saldana et al., 1996). In the central nucleus of IC (ICC), these inputs converge along the fibro-dendritic laminae that maintain the tonotopic organization of the system (Malmierca et al., 1993; Merzenich and Reid, 1974; Morest and Oliver, 1984; Oliver and Morest, 1984; Schreiner and Langner, 1997).
It has become evident that the segregation of inputs on the laminae may be an important basis of function in IC. The synaptic domain hypothesis suggests that there are functional zones on the fibro-dendritic laminae that receive different combinations of inputs (Oliver, 2005) and that auditory function results from the integration of a specific combination of inputs. A number of lines of evidence support this idea. There is segregation of inputs from the dorsal cochlear nucleus and lateral superior olive (Cant and Benson, 2006; Oliver et al., 1997). Inputs from medial and lateral superior olives can differ in their targets in IC (Loftus et al., 2004). More recently, physiological studies of ICC neurons that code interaural time differences showed that different response properties were related to specific patterns of brainstem input (Loftus et al., 2010). This attaches a special importance to the location of the neuron in ICC since it suggests that specific locations in IC receive specific synaptic inputs from the brainstem. Despite these advances, the visualization of functional zones in IC based on different sets of inputs has been difficult. Markers such as cytochrome oxidase and NADPH diaphorase are useful to distinguish ICC from the surrounding cortex (Cant and Benson, 2005; Loftus et al., 2008), and certain inputs appear more obvious in tract tracing studies such as those from the medial superior olive (Cant, 2013; Oliver et al., 2003). However, for physiological study of functional zones in ICC, it is necessary to be able visualize them as anatomical regions within ICC.
It may be possible to visualize synaptic domains in IC with probes for inhibitory neurotransmitters. All regions of IC contain inhibitory synapses (Roberts and Ribak, 1987a; b), and they comprise 40% of the total ascending inputs from the brainstem (Oliver, 2000; Saint Marie et al., 1989). These ascending inhibitory inputs can use either gamma-aminobutyric acid (GABA) or glycine (GLY) as neurotransmitters and come from different brainstem sources (e.g. Adams and Mugnaini, 1984; Glendenning et al., 1992; Saint Marie and Baker, 1990; Saint Marie et al., 1989). In addition, the IC contains GABAergic neurons (Merchan et al., 2005; Oliver et al., 1994), and these also may contribute to the GABAergic inputs of IC neurons. It seems likely that the localization of GABAergic and glycinergic synapses in IC may be related to the distribution of different brainstem inputs, and hence, functional zones. Nevertheless, the exact location of the GABAergic and the glycinergic synapses and their relationship to each other is unclear at different locations in IC.
Previous maps of GABAergic and glycinergic synapses have used receptor binding or immunocytochemistry for localization with differing results. The earliest maps of glycine receptor binding in rat or mouse failed to examine the IC (Frostholm and Rotter, 1985; Zarbin et al., 1981). Later GLY receptor binding experiments in IC suggested diffuse GLY distribution in the cat (Glendenning and Baker, 1988), a dorsal to ventral gradient in the gerbil (Sanes et al., 1987), and a ventrolateral to dorsomedial gradient in the big brown bat (Fubara et al., 1996). Immunocytochemical detection of GLY receptors in rat suggested a diffuse distribution in the ICC (Friauf et al., 1997). Binding of GABA-A receptors was throughout IC and densest in the dorsal cortex in several species (Fubara et al., 1996; Glendenning and Baker, 1988; Milbrandt et al., 1996). Due to technical limitations, these studies did not localize the GABA and GLY synapses in relationship to each other in the same section. Since most were surveys of the entire auditory system, they were focused on the major divisions of the IC and not whether the ICC contains different subdivisions or functional zones. Moreover, there is still little information on the localization of GABA and GLY in axonal terminal in the IC of the mouse, now a widely used model for auditory systems.
Here, we use immunocytochemistry to examine the location of GAD67, a synthetic protein for GABA, and GLYT2, a neuron-specific glycine reuptake transporter, in axon terminal fields in order to map the location of these in relationship to each other in both the rat and mouse. If they have different distributions within ICC, this may allow the visualization of synaptic domains. High-resolution dual channel mosaics of entire sections were image processed to determine the ratio of GAD67and GLYT2 at each point in the IC at multiple rostrocaudal levels. We find that the ICC in both species displays a consistent pattern of two separate regions with GLY dominant in the ventrolateral ICC and GABA dominant in the dorsomedial ICC.
METHODS AND MATERIAL
Tissue Preparation
Three adult Long Evans rats (P56) were anaesthetized with a cocktail of Ketamine/Xylazine (40-80 mg/kg + 5-10 mg/kg, I.M.). Animals were perfused through the heart with 7.5 - 10 ml of phosphate buffered saline (PBS; 0.9% NaCl, 0.01 M phosphate buffer, pH 7.4) and 300ml of 4% paraformaldehyde in 0.1M phosphate buffer, pH 7.4. After dissection, the tissue was postfixed in 4% paraformaldehyde for 2 hours at 4° C. Three transgenic mice were used. Two were VGAT-CHR2-YFP-BAC mice [B6.Cg-Tg(Slc32a1-COP4*H134R/EYFP)8Gfng/J; #14548, Jackson Labs] on a C57BL/6J background that expressed channelrhodopsin (CHR2) and YFP under the promoter for the inhibitory amino acid transporter (VGAT). The third was a cross between a GAD67-GFP heterozygote on a Swiss Webster background and a VGAT-CHR2 mouse. Mice were anesthetized using Ketamine/Xylazine/Acepromazine (90-100 mg/kg+5-10mg/kg+3mg/kg) before the procedure began. The perfusion of the mice was similar to that above for the rat with the following exceptions: 0.1-1.0 ml of PBS, 0.1M phosphate buffer, or normal saline was injected as a washout before 20-30 ml of 4% buffered paraformaldehyde was perfused.
All experiments were approved by the Animal Care Committee at the University of Connecticut Health Center and were performed in accordance with the NIH “Guide for the Care and Use of Laboratory Animals” and institutional guidelines. All efforts were made to minimize the number of animals used and their suffering.
Immunohistochemistry
After cryoprotection overnight or longer in 30% sucrose, rat and mouse brains were cut at 40 µm and 30 µm, respectively, with a freezing microtome. Sections were collected and stored in PBS with 0.02% sodium azide. The sections were first washed for 30 minutes with PBS/0.02% sodium azide/1% normal goat serum/0.3% Triton-X. Next, they were transferred to the primary antibodies in PBS/0.02% sodium azide/1% normal goat serum/0.3% Triton-X where they remained overnight at room temperature.
The primary antibody for GAD-67 was mouse anti-GAD67 (Millipore, clone 1G10.2, MAB5406, lot #NG1839533, immunogen GAD67 protein, 67kDa molecular weight, AB_2278725, http://antibodyregistry.org/AB_2278725, JCN Database) used at 1:3000. Specificity has been shown previously (Ito et al., 2007; Parrish-Aungst et al., 2007)(Table 1). Specificity was previously confirmed by western blot and pre-absorption test. In the western blot, a single band around 67kDa was detected as the manufacturers had predicted. No signal was detected in rat brain sections that were pre-absorbed with recombinant rat GAD67 protein (180µg/ml)(Ito et al., 2007).
Table 1.
Primary Antibodies used
| Antigen | Description of Immunogen |
Source, Host Species, Cat#, Clone or Lot#, RRID |
Concentration Used |
|---|---|---|---|
| Glutamic acid decarboxylase 67 (GAD67) |
Recombination whole protein of mouse GAD67 |
Millipore, mouse monoclonal, Cat# MAB5406, Lot# NG1839533,RRID: AB_2278725 |
1:3,000 |
| Glycine transporter 2 (GLYT2) |
Recombination of whole protein of mouse GLYT2 |
Millipore, guinea pig polyclonal, Cat#AB1773, Lot#21080959,RRID:AB_90953 |
1:10,000 |
The primary antibody to GLYT2 was guinea pig polyclonal anti-GLYT2 (Millipore, AB1773, lot # 21080959, carboxy-terminus of cloned rat GLYT2 amino acids 780-799, AB_90953, http://antibodyregistry.org/AB_90953, JCN Database) used at 1:10,000. Specificity was shown previously (Table 1) by Western blot and pre-absorption of the antiserum; a single band of 98kDa was detected as specified by the manufacturer (Caminos et al., 2007; Toyoshima et al., 2009). Pre-absorption with the immunogenic peptide abolishes immunoreactivity in tissue sections from rat brain, in concordance with the manufacturer’s information. The Immunochemical signal matches the distribution of glycine transporter and synaptic endings of the rat central nervous system (Caminos et al., 2007).
After a wash in PBS for 10 minutes ×3, sections were incubated in the secondary antibody in PBS/0.02% sodium azide/1% normal goat serum/0.3% Triton-X for one hour at room temperature. The secondary antibodies were: AF568 goat anti-GP and AF647 goat anti-MS; both at a concentration of 1:200 (2.5µl in 500µl). Note, that these secondaries do not overlap with the wavelengths for visualization of GFP/YFP in transgenic mice. Finally, the sections were rinsed 10 minutes ×3 with PBS and left in fresh PBS at 4° C overnight. The next day, the sections were mounted and allowed to air-dry overnight. The following day, after a brief dip in PBS, the sections were soaked in 1mM solution of CuSO4 for an hour to remove auto-fluorescence, rinsed in PBS, and coverslipped using 2.5% 1,4-diazabicyclo[2.2.2]octane (DABCO) in glycerol/PBS, pH 7.4. The coverslips were sealed with nail polish.
Imaging and Image Processing
Mosaic images of the entire IC were captured on a Zeiss Axiovert 200M microscope using the MosaiX module of AxioVision Rel. 4.8 (Carl Zeiss Imaging Solutions) with a ×20/0.75 NA Planapo lens. Rat images usually consisted of 8 × 8 mosaics that were 10321 × 9517 pixels (3,700 × 3,300 µm). Mouse images were a 6 × 6 mosaic that measured 7216 × 5331 pixels (2,590 × 1,910 µm). Images were RGB format with separate color channels for GLYT2 and GAD67 immunofluorescence. Shading correction was applied to each fluorescent channel in order to ensure proper stitching and tiling. Stitching was used to align the edges of the individual images by comparing the shading and structures. Tiling converted the mosaics into a single image. Images were transferred to Adobe Photoshop CS6 (San Jose, CA) for processing. In order to remove the background for the individual fluorescent channels, a sample of the background was taken in a non-fluorescent region outside the IC, and the levels were adjusted so that only signal above the background would be visible. After the background was removed, the GABA and GLY channels were equalized by setting the mean value of their respective histograms to be equal to each other.
Ratio Data
After background removal and equalization, the individual GABA and GLY channels were transferred to Image-Pro Plus v6.1 (Media Cybernetics) in order to compare the data from individual pixels in each channel. Images were reduced by combining 5×5 pixel bins into a single pixel. This produced a matrix of illumination for each pixel in each channel for the XY coordinates of the IC. Each matrix was transferred to Excel 2010 (Microsoft) in order to convert the illumination data into a single matrix showing the proportion of glycine to GABA label, the ratio of illumination for each pixel. Thus,
Ratio = GLY / GABA
The ratio matrix was displayed in Origin 9.0 (OriginLab Corporation) as a layered mask where the layers were colored to create a heat map that represented the different ratios of GLY to GABA. Ratios less than 1.0 where GABA exceeded glycine were assigned a blue color, while ratios greater than 1.0 where GLY surpassed GABA were assigned a red color. These ratios were further subdivided into zones representing ratios of 0-0.009, 0.009-0.25, 0.25-0.5, 0.5-1.0, 1.0-2.0, 2.0-4.0, and 4.0-8.0.
Line Density Analysis
To quantify the transition from a GABA-rich region to a GLY-rich region, we used a line density analysis in Origin. Five parallel lines, 136 pixels in length and spaced 10 pixels apart, were placed on the ratiometric map with a starting point in a GABA-rich area and the end point in a GLY-rich area. The ratio data for the pixels below the 5 lines were averaged and plotted to show the patterns of transition.
RESULTS
Overall distribution of GABA and GLY in the rat
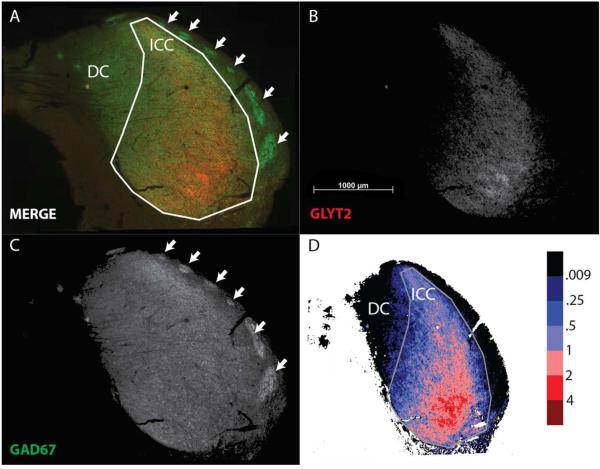
The large-scale mosaic images of GAD67 and GLYT2 immunoreactivity showed a heterogeneous distribution of GABAergic and glycinergic axon terminals within the IC. Figure 1A shows the merged, two-channel image from the IC of rat where the GLYT2 signal is red and the GAD67 signal is green. The individual channels are illustrated in Figures 1B and 1C, respectively, after the removal of the background and the equalization of the two channels.
Figure 1.
Rat immunohistochemistry and ratiometric data showing glycine-rich areas ventrolaterally in the ICC and GABA-rich areas dorsomedially. Images show the analysis performed. (A) Two-channel immunofluorescent images with the central nucleus (ICC) and dorsal cortex (DC) defined. Red, glycine (GLY) signal for the GLYT2 antibody. Green, GABA signal for GAD67 antibody. (B) The isolated GLY channel with the background removed and equalized with the GABA channel. (C) The isolated GABA channel with the background removed and equalized with the GLY channel. (D) Ratiometric analysis of these two channels and their relationship to the ICC and DC. The signal from the GLY channel was divided by the signal from the GABA channel in order to obtain a ratio of GLY level as compared to GABA. This ratio data was converted into a heat map in order to visually represent the ratios obtained. Ratios lower than 0.009 are black.
The GLY channel (Fig. 1B) was easily distinguished from the GABA since the GLYT2 signal was concentrated ventral and lateral with the heaviest signal in the ventrolateral quadrant of ICC. In the merged image, the red stained GLY is seen as a backbone traversing the lateral part of the ICC from ventral to dorsal. However, it was not restricted to that part only. The GLYT2 signal extended throughout the ICC in the rat (Fig. 1B) and had a tear-shaped contour that matched the outline of the ICC.
In the GABA channel, the GAD67 signal (Fig. 1C) occupied the ICC and spread through the whole IC. The most obvious GABA concentrations were seen in the lateral, outer perimeter of the section as the “GABA modules” (Chernock et al., 2004). “GABA modules” were seen as dense patches of GABA immunoreactivity in layer 2 of LC (Fig. 1A, 1C, arrows). Elsewhere in the GABA channel (Fig. 1C) there were hints of GABA concentration, but the IC had a homogenous distribution GABA terminals.
In order to directly compare the distribution of GABA and GLY terminal distributions to each other, we computed the ratio of the GLYT2 to GAD67 signals in each pixel (Fig. 1D). The resulting heat maps illustrates that GABA (<1.0, Fig. 1D, blue and black shades) was dominant in most of IC and ICC. GLY (>1.0, Fig. 1D, red shades) was dominant only in the ventral and lateral ICC. In the most ventral and lateral ICC, the proportion of GLYT2 to GAD67 signal often exceeded 2.0, while in the dorsomedial ICC it fell to < 0.5 and to near 0 in black shaded areas in the DC and LC.
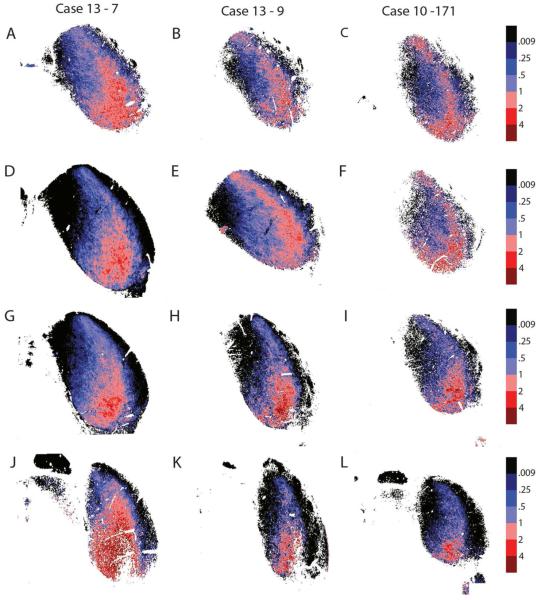
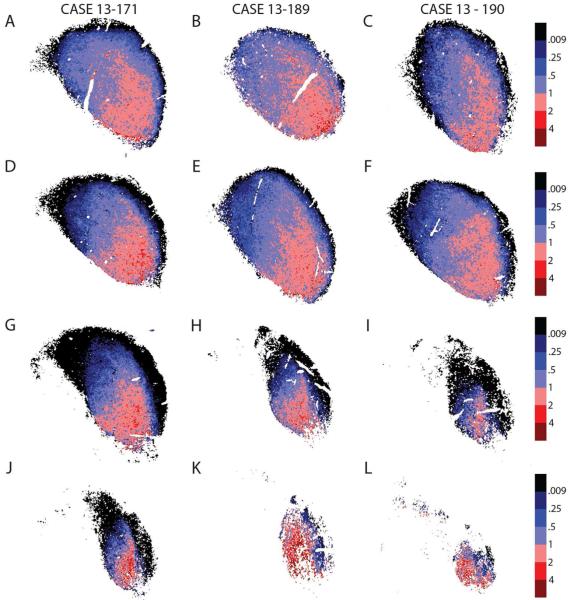
The GABA and GLY distribution are similarly distributed at different rostrocaudal levels of the ICC. In each of three rats, we sampled four sections spaced 440 µm ± 40 µm from each other (Fig. 2, GLYT2, red; GAD67, green). The segregation of neurochemical markers was slightly different as one moves from most caudal (Figure 2A-C) to the most rostral (Figure 2J-L), but the overall pattern was still represented in all sections. The most prominent GLYT2 signal was ventrolateral throughout, especially a very distinct GLY “Patch” (Figures 1, 2G-I), but it was disrupted rostrally where the lateral lemniscus entered the IC. Some moderate GLYT2 labeling was more prominent in caudal sections of the dorsal ICC.
Figure 2.
GABA and GLY at different rostrocaudal levels of the IC in three rats show a similar pattern. Transverse brain sections, 40µm thick, and are approximately 480 ± 40µm apart. The most caudal sections are seen in (A,B,C), while the most rostral sections are pictured in (J,K,L). Arrows, GABA modules; red, GLYT2; green, GAD67.
The same basic pattern was observed in all rostrocaudal sections in multiple animals (Fig. 3). The GLYT2 immunoreactivity was limited to the ICC, while the GAD67 immunoreactivity was throughout the IC. In the caudal half of IC (Fig. 3A-F), the GLY dominant region extended more dorsally and includes the low-frequency parts of ICC. In the rostral half of IC (Fig. 3G-L), the GLY-rich area was primarily ventral in ICC. In addition, as one moved from the most caudal to most rostral sections, there was a small migration of the GLY-rich area from lateral to medial. Seen through most sections, but specifically in the most rostral sections, there were areas where the heat map is discontinuous. In some sections, it was subdivided by the entering lateral lemniscus (Fig. 3J), or, in some cases, caused by missing data points or artifacts. However, in most examples the LC and lateral lemniscus remained lateral to the GLY dominant area.
Figure 3.
Ratiometric analysis of sections shown in Figure 2 showing GLY-rich areas ventrolaterally and GABA-rich areas dorsomedially across different rostrocaudal levels in the rat. Section order and orientation same as Fig. 2. The same basic pattern is seen on these heat maps as in Fig. 1; GLY-rich areas are ventro-lateral and blue GABA-rich areas found dorsomedial. Red = >1.0; blue = <1.0.
Laminar organization of GLYT2 and GAD67 labeling
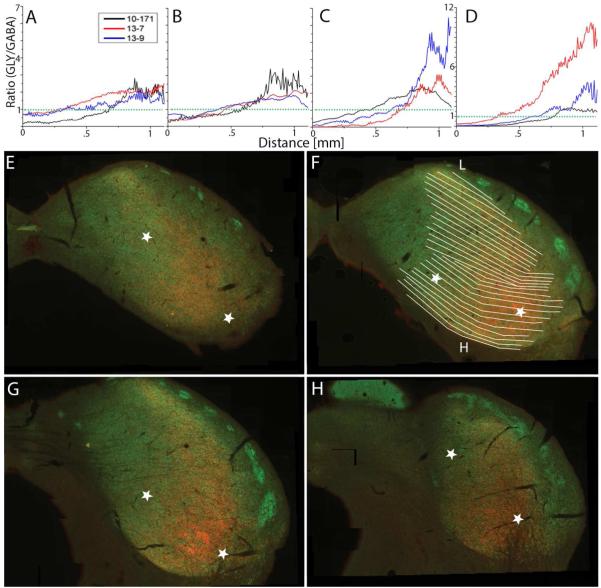
The fibro-dendritic laminae of ICC run from ventrolateral to dorsomedial. This is perpendicular to the axis for the tonotopic map, but it is parallel to the transition between GABA-rich and GLY-rich areas in ICC. To determine if these areas had a well-defined border along the laminae of ICC, we examined pixels in parallel to the direction of the laminae, ventrolateral to dorsomedial to determine the distance and rate at which the ratios of labeling shifted. The rate of change of GLYT2/GAD67 helped to identify whether the change was gradual or steep. The line graphs (Fig. 4A-D) show the mean ratios of the pixels under five parallel lines between the two white stars in each section. The lines, across all cases, are all of equal length starting in a high GABA-rich area and ending in a high GLY-rich area along the same fibrodendritic lamina; regardless of where the fibrodendritic lamina actually starts or ends. Only one case is depicted in Figure 4E-DH, but the line data for the same points in all three cases is presented in Figure 4A-D. The caudal to rostral sequence is the same as Figure 2. The line graphs show the data from the left star to the right star (GABA-rich to GLY-rich, respectively), and the dotted green line (Fig. 4A-D) represents the transition from GABA dominance to GLY dominance (ratio = 1.00). The right star is anchored at or near the lateral border of the GLY-rich area.
Figure 4.
Rate of ratiometric change parallel to layers in ICC within the same section. A-D, line histograms parallel to the lamina to show the changing ratio data between GABA-rich areas and GLY-rich areas for three rats shown in Figs. 2-3. Green dotted line represents GABA and GLY ratio = 1.00. E-H, shows location of the endpoints of lines used in A-D with the data reading from the left star to the right star (GABA-rich and GLY-rich, respectively). E-H shows images from one case, but the other cases were analyzed in similar areas. F has fibro-dendritic lamina defined with the tonotopic axis along the L and H corresponding to low and high frequency areas, respectively.
In all sections, there was a smooth transition from the GABA-rich area into the GLY-rich area over the first 0 - 0.5 mm, and the slope of the line does not change as it crosses over the 1.0 ratio line. However, in most sections there is a much steeper slope at about the 0.75 point of the lamina, and this indicates a more rapid increase in the proportion of the GLY signal. This is most prominent in the rostral sections (Fig. 4 C-D). At this point along the laminae, the transition is so steep it begins to approximate a stepwise transition, culminating in the high peaks of GLYT2 labeling. Note that this transition was so steep in Figure 4D, the graph is rescaled.
Mouse
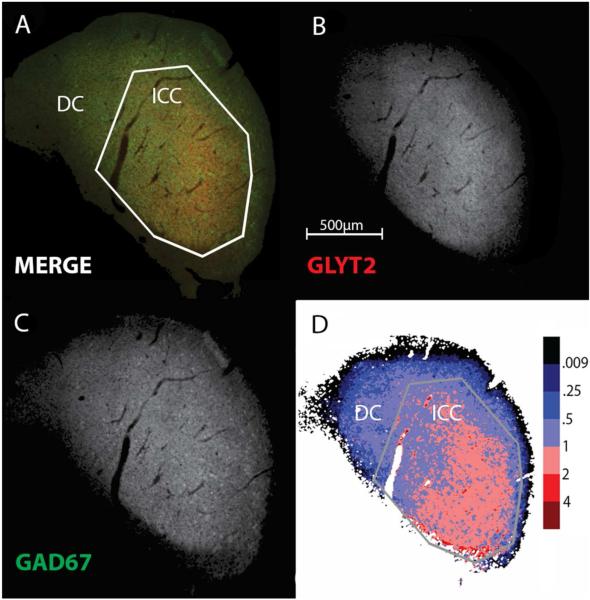
The difference between the rat and the mouse was minimal. The most prominent GLY-rich regions are ventral and lateral in ICC. In the rat, the ICC had a well-defined tear-shaped pattern that spans the whole ICC. In the mouse (Fig. 5), the ICC dorsally had a less prominent and less pointed low frequency region. Consequently, the ICC and the GLYT2 immuno-positive area were more rounded and less tear-shaped. In contrast to the rat, the mouse had fewer pixels with GLY/GABA ratios over 2.0 (Figs. 5D; 7) and virtually no quadruple or higher signal.
Figure 5.
GAD67 and GLYT2 immunohistochemistry in the mouse. The conventions are the same as in Fig. 1.
Figure 7.
Ratiometric analysis GLY/GABA for mouse sections in Fig. 6. with glycine rich areas ventrolaterally and GABA rich areas dorsomedially across different sections. Image is the mouse counterpart for Fig. 3 of rat. Less GLY-rich areas are seen dorsolaterally compared to the rat. Red = >1.0; blue = <1.0.
Over the rostro-caudal extent of the IC in the mouse (Figs. 6, 7), a larger proportion of ICC appeared to be devoted to the GLYT2 signal. The sections in mouse were an average of 180µm ± 30µm apart. The GLYT2-rich area was greater than half of the ICC in caudal sections (Figs. 6-7, A-C). However, the ICC in the rostral sections (Figs. 6-7, J-L) was small and there were discontinuities due to the fibers entering from the lateral lemniscus.
Figure 6.
GAD67 and GLYT2 immunohistochemistry in the mouse IC at four different rostrocaudal levels. Compared to Fig. 2 in the rat, the GLY pattern in mouse is more oval than tear-shaped. The most caudal sections are seen in (A,B,C), while the most rostral sections are pictured in (J,K,L) Arrows, GABA modules; red, GLYT2; green, GAD67.
The analysis of the GLY to GABA regions in parallel to the laminae in the mouse (Fig. 8) suggested similarities to the rat (Fig. 4). In caudal sections (Fig. 8A-B), the slope of the line of ratios did not change as it crossed 1.0, and it reached a plateau level at about the 0.75 point. However, in more rostral sections the slope was somewhat steeper, and in the most rostral sections it jumped up dramatically at the 0.5 point.
Figure 8.
Ratiometric change parallel to laminae across single sections in mouse. Rate of change is gradual up to the maximum at the ventrolateral region. The analysis is similar to that in the rat as shown in Figure 4. Green dashed line represents GABA and GLY ratio = 1.00.
DISCUSSION
The main findings show that GAD67-labeled input was dominant in the dorsomedial part of the ICC and GLYT2-labeled input was most prevalent in the ventrolateral part of the ICC. Thus, the GABA and GLY distribution does not follow the tonotopic map. Instead, the data suggest that the distribution of the neurotransmitters is ventrolateral to dorsomedial, perpendicular to the tonotopic map that defines the ICC.
The transition from GABA-dominant to GLY-dominant innervation along the laminae of ICC is not sharp in most cases (Figs. 4, 8). However, the level of GLY innervation increases rapidly in the most ventrolateral 25% of the ICC laminae in rat and maintains its highest level in that region in both rat and mouse. These maps suggest a segregation of inputs to ICC that is consistent with functional zones with different properties.
Technical Development and Artifacts
In order to study the distribution of two different types of axonal terminals within a brain section, we used a large mosaic of IC made up from smaller, higher magnification images that were stitched together. One artifact we encountered was created by the stitching effect of the mosaic. If there were small amounts of uneven illumination in the field of view, it led to slightly darker edges of an image. When these images were stitched together, it created a visible grid superimposed on the mosaic that compromised the analysis of the terminal density. To eliminate this artifact, shading correction was necessary for each channel. This resulted in mosaic images with smooth transitions between tiles of the mosaic, and there was no evidence of the mosaic artifact.
In the heat maps (Figures 3 and 7), there is some evidence of missing data points. These gaps occurred for several reasons. If GAD67 and GLYT2 were not co-localized to the same pixel, there cannot be an analysis of the signal in the two channels. In most cases, the lack of co-localization was due to the lack of GLY, as GABA is ubiquitous in the IC. When both GABA and GLY signal were missing, it was in very rostral sections where the fibers of the lateral lemniscus enter the IC (Figs. 3J-L and 7J-L) or within the blood vessels including small capillaries.
Since the heat map represents the ratio of GLYT2/GAD67, zero values created problems. When there was trace amounts or no GLY, a value at or near 0 is obtained. To represent these levels, the ratios from 0-0.009 were represented with black shading. These areas were predominantly in the dorsomedial part of the IC.
We chose to use ratios rather than the absolute values of the GABA and GLY immunofluorescence. To do this, the mean signals of GAD67 and GLYT2 in the entire ICC were normalized, so the ratio could be determined over the same range of values in each section from each case. The relative amount of GABA to GLY was easy to visualize, and this may be more physiologically relevant than the absolute amounts of immunolabeling. Ultimately, the ratios should be related to the relative amounts of synaptic current from GABA and GLY synapses on neurons in these regions of the ICC. The ratiometric data can predict relatively more GLY synaptic current in neurons in the GLY-rich zone than in the GABA-rich zone, and vice-versa. However, this does not mean that the amplitude of the GLY synaptic current is necessarily larger than the amplitude of the GABA synaptic current in IC neurons in the GLY-dominant zone.
Previous Studies Suggested a Tonotopic Gradient
Previous studies of the distribution of GABA or GLY presynaptic axons and terminals suggested a dorsal to ventral gradient in the IC of rat. Merchan et al (Merchan et al., 2005) specifically studied the axosomatic terminals made by GLY and GABA axons on neurons in ICC with high-resolution light microscopy on semi-thin sections. A higher density of GLY puncta were seen on cell bodies of glutamatergic neurons in the high frequency ICC than in the low frequency ICC. In the rostrocaudal dimension of ICC, the GABA axosomatic puncta were higher in density rostrally on GABAergic neurons. There was no difference in the distribution of GABA axosomatic puncta in the frequency dimension. However, consistent with the present study, the density of GLY axosomatic puncta was higher on GABA and glutamatergic neurons in the ventrolateral ICC laminae than in the dorsomedial parts, and the reverse was seen for GABA axosomatic puncta on glutamatergic neurons. Also consistent with the present findings, the density of axosomatic GLY puncta was higher in the ICC than in LC or DC.
Some of the previous studies of receptor distribution also suggested spatial differences in localization although they could not compare GLY and GABA-A receptor localization directly on the same sections for technical reasons. Caspary and co-workers used autoradiography methods to localize GABA-A and GABA-B receptors in the auditory system as a part of their study of aging. The GABA-A receptor binding was significantly higher in the dorsal cortex than in the ICC and lateral cortex (Milbrandt et al., 1996). A similar pattern was seen for GABA-BR1 and GABA-BR2 receptors with immunohistochemical methods (Jamal et al., 2012). In a similar study of GLY receptors in the IC of the gerbil (Sanes et al., 1987), their distribution was described as being at the highest concentrations in the ventral, high frequency region of the IC. Only a study of both GABA-A, GABA-B, and GLY receptor binding in the big brown bat shows a pattern similar to that seen here in the rat (Fubara et al., 1996). There was a gradient of receptor binding in IC with the highest binding of the GABA_A probe dorsomedially and the highest binding of the GLYR probe ventrolaterally.
It is unclear why the results of the receptor binding studied have varied so dramatically with only one showing a gradient of binding within ICC. There may be species differences. On the other hand, the spatial location of GABA and GLY receptors may not vary dramatically in the rodent, despite our findings that there is a gradient of axonal terminal labeling for the presynaptic elements of GABA and GLY synapses.
Functional Significance
The location of GABA dominant zone in the dorsomedial ICC, and a GLY dominant zone in the ventrolateral IC suggests that there is a segregation of inputs to ICC. This is consistent with the synaptic domain hypothesis of organization for the ICC (Loftus et al., 2010; Oliver, 2005). The most prominent GLY inputs are from the ipsilateral lateral superior olive (LSO) and ventral nucleus of the lateral lemniscus (VNLL) (Glendenning et al., 1992; Saint Marie and Baker, 1990; Saint Marie et al., 1989). It is well established in cat and gerbil that the afferents from LSO terminate ventral and lateral in IC and occupy a ventrolateral position on the ICC laminae (Cant and Benson, 2006; Oliver et al., 1997). Glycinergic VNLL inputs may terminate more widely (Kelly et al., 1998), but are evidently confined to the ICC. In contrast, the most prominent GABA inputs to IC are from the dorsal nucleus of the lateral lemniscus (DNLL) and superior periolivary nucleus (SPON) (Saldana et al., 2009; Shneiderman et al., 1993; Shneiderman and Oliver, 1989; Zhang et al., 1998). The DNLL is most likely to terminate more dorsomedially on the laminae of ICC (Shneiderman et al., 1988), while the SPON may terminate more widely. In addition to the afferent inputs, GABAergic neurons in IC project to the contralateral IC via the commissure (Hernandez et al., 2006). GABA synapses from local axonal collaterals of ipsilateral GABAergic neurons are theoretically possible, but, their existence has not been shown.
If the GABA-rich and GLY-rich zones correspond to functional areas with different inputs in ICC, the visualization of these areas makes it possible, for the first time, to investigate the functional properties of different portions of the laminae in the ICC. Visualization of GABA and GLY zones in ICC with immunofluorescence in fixed tissue after electrophysiological recording is compatible with a wide variety of in vivo and in vitro experiments that may shed light on how the ventrolateral GLY-rich area differs in function from the dorsomedial GABA-prevalent area.
A number of properties may differ for these two zones of ICC. For example, as suggested above, the glycine synaptic currents may be stronger in the ventrolateral ICC than elsewhere and might display different properties. GABA and GLY kinetics often differ on the same postsynaptic neuron and may be related to the sources of these inhibitory inputs (Donato and Nistri, 2000; Dumoulin et al., 2001; Kuo et al., 2009). The dorsomedial and ventrolateral ICC may process binaural information differently since they may receive binaural inputs from different sources (Loftus et al., 2010; Oliver et al., 1997; Shneiderman et al., 1988). Other properties also may differ in the dorsomedial to ventrolateral axis, perpendicular to the tonotopic axis. Maps of periodicity and temporal rate have been demonstrated in the cat and the rhesus monkey (Baumann et al., 2011; Schreiner and Langner, 1988), and these may be related to the GABA and GLY regions identified in the present report.
Understanding the functional zones of the IC may facilitate the development of implantable prosthetic devices for use in the IC. Early studies in animals suggest that stimulation of different parts of a lamina in ICC leads to different responses in auditory cortex (Lim and Anderson, 2006; 2007). To use these devices effectively in human subjects (Calixto et al., 2012; Lim et al., 2009), it may be necessary to account for regions of ICC with different properties and stimulate them differentially.
Supplementary Material
ACKNOWLEDGMENTS
SUPPORT: NIH grant R01 DC00189 to DLO
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest
REFERENCES
- Adams JC, Mugnaini E. Dorsal nucleus of the lateral lemniscus: A nucleus of GABAergic projection neurons. Brain Res Bull. 1984;13:585–590. doi: 10.1016/0361-9230(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Baumann S, Griffiths TD, Sun L, Petkov CI, Thiele A, Rees A. Orthogonal representation of sound dimensions in the primate midbrain. Nature neuroscience. 2011;14(4):423–425. doi: 10.1038/nn.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto R, Lenarz M, Neuheiser A, Scheper V, Lenarz T, Lim HH. Coactivation of different neurons within an isofrequency lamina of the inferior colliculus elicits enhanced auditory cortical activation. J Neurophysiol. 2012;108(4):1199–1210. doi: 10.1152/jn.00111.2012. [DOI] [PubMed] [Google Scholar]
- Caminos E, Garcia-Pino E, Martinez-Galan JR, Juiz JM. The potassium channel KCNQ5/Kv7.5 is localized in synaptic endings of auditory brainstem nuclei of the rat. J Comp Neurol. 2007;505(4):363–378. doi: 10.1002/cne.21497. [DOI] [PubMed] [Google Scholar]
- Cant NB. Patterns of convergence in the central nucleus of the inferior colliculus of the Mongolian gerbil: organization of inputs from the superior olivary complex in the low frequency representation. Front Neural Circuits. 2013;7:29. doi: 10.3389/fncir.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant NB, Benson CG. An atlas of the inferior colliculus of the gerbil in three dimensions. Hearing research. 2005;206(1-2):12–27. doi: 10.1016/j.heares.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Organization of the inferior colliculus of the gerbil (Meriones unguiculatus): differences in distribution of projections from the cochlear nuclei and the superior olivary complex. J Comp Neurol. 2006;495(5):511–528. doi: 10.1002/cne.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernock ML, Larue DT, Winer JA. A periodic network of neurochemical modules in the inferior colliculus. Hearing research. 2004;188(1-2):12–20. doi: 10.1016/S0378-5955(03)00340-X. [DOI] [PubMed] [Google Scholar]
- Donato R, Nistri A. Relative contribution by GABA or glycine to Cl(−)-mediated synaptic transmission on rat hypoglossal motoneurons in vitro. Journal of neurophysiology. 2000;84(6):2715–2724. doi: 10.1152/jn.2000.84.6.2715. [DOI] [PubMed] [Google Scholar]
- Dumoulin A, Triller A, Dieudonne S. IPSC kinetics at identified GABAergic and mixed GABAergic and glycinergic synapses onto cerebellar Golgi cells. J Neurosci. 2001;21(16):6045–6057. doi: 10.1523/JNEUROSCI.21-16-06045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friauf E, Hammerschmidt B, Kirsch J. Development of adult-type inhibitory glycine receptors in the central auditory system of rats. J Comp Neurol. 1997;385(1):117–134. doi: 10.1002/(sici)1096-9861(19970818)385:1<117::aid-cne7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Frostholm A, Rotter A. Glycine receptor distribution in mouse CNS: autoradiographic localization of [3H]strychnine binding sites. Brain Res Bull. 1985;15(5):473–486. doi: 10.1016/0361-9230(85)90038-3. [DOI] [PubMed] [Google Scholar]
- Fubara BM, Casseday JH, Covey E, Schwartz-Bloom RD. Distribution of GABAA, GABAB, and glycine receptors in the central auditory system of the big brown bat, Eptesicus fuscus. J Comp Neurol. 1996;369(1):83–92. doi: 10.1002/(SICI)1096-9861(19960520)369:1<83::AID-CNE6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Glendenning KK, Baker BN. Neuroanatomical distribution of receptors for three potential inhibitory neurotransmitters in the brainstem auditory nuclei of the cat. The Journal of comparative neurology. 1988;275(2):288–308. doi: 10.1002/cne.902750210. [DOI] [PubMed] [Google Scholar]
- Glendenning KK, Baker BN, Hutson KA, Masterton RB. Acoustic chiasm V: inhibition and excitation in the ipsilateral and contralateral projections of LSO. J Comp Neurol. 1992;319(1):100–122. doi: 10.1002/cne.903190110. [DOI] [PubMed] [Google Scholar]
- Hernandez O, Rees A, Malmierca MS. A GABAergic component in the commissure of the inferior colliculus in rat. Neuroreport. 2006;17(15):1611–1614. doi: 10.1097/01.wnr.0000236857.70715.be. [DOI] [PubMed] [Google Scholar]
- Ito T, Hioki H, Nakamura K, Tanaka Y, Nakade H, Kaneko T, Iino S, Nojyo Y. Gamma-aminobutyric acid-containing sympathetic preganglionic neurons in rat thoracic spinal cord send their axons to the superior cervical ganglion. J Comp Neurol. 2007;502(1):113–125. doi: 10.1002/cne.21309. [DOI] [PubMed] [Google Scholar]
- Jamal L, Khan AN, Butt S, Patel CR, Zhang H. The level and distribution of the GABA(B)R1 and GABA(B)R2 receptor subunits in the rat's inferior colliculus. Front Neural Circuits. 2012;6:92. doi: 10.3389/fncir.2012.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SP, Bradley LA, Trussell LO. Heterogeneous kinetics and pharmacology of synaptic inhibition in the chick auditory brainstem. J Neurosci. 2009;29(30):9625–9634. doi: 10.1523/JNEUROSCI.0103-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HH, Anderson DJ. Auditory cortical responses to electrical stimulation of the inferior colliculus: implications for an auditory midbrain implant. Journal of neurophysiology. 2006;96(3):975–988. doi: 10.1152/jn.01112.2005. [DOI] [PubMed] [Google Scholar]
- Lim HH, Anderson DJ. Spatially distinct functional output regions within the central nucleus of the inferior colliculus: implications for an auditory midbrain implant. J Neurosci. 2007;27(32):8733–8743. doi: 10.1523/JNEUROSCI.5127-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HH, Lenarz M, Lenarz T. Auditory midbrain implant: a review. Trends Amplif. 2009;13(3):149–180. doi: 10.1177/1084713809348372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus WC, Bishop DC, Oliver DL. Differential patterns of inputs create functional zones in central nucleus of inferior colliculus. J Neurosci. 2010;30(40):13396–13408. doi: 10.1523/JNEUROSCI.0338-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus WC, Bishop DC, Saint Marie RL, Oliver DL. Organization of binaural excitatory and inhibitory inputs to the inferior colliculus from the superior olive. J Comp Neurol. 2004;472(3):330–344. doi: 10.1002/cne.20070. [DOI] [PubMed] [Google Scholar]
- Loftus WC, Malmierca MS, Bishop DC, Oliver DL. The cytoarchitecture of the inferior colliculus revisited: a common organization of the lateral cortex in rat and cat. Neuroscience. 2008;154(1):196–205. doi: 10.1016/j.neuroscience.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca MS, Blackstad TW, Osen KK, Karagulle T, Molowny RL. The central nucleus of the inferior colliculus in rat: a Golgi and computer reconstruction study of neuronal and laminar structure. J Comp Neurol. 1993;333(1):1–27. doi: 10.1002/cne.903330102. [DOI] [PubMed] [Google Scholar]
- Merchan M, Aguilar LA, Lopez-Poveda EA, Malmierca MS. The inferior colliculus of the rat: quantitative immunocytochemical study of GABA and glycine. Neuroscience. 2005;136(3):907–925. doi: 10.1016/j.neuroscience.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Reid MD. Representation of the cochlea within the inferior colliculus of the cat. Brain Res. 1974;77(3):397–415. doi: 10.1016/0006-8993(74)90630-1. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Albin RL, Turgeon SM, Caspary DM. GABAA receptor binding in the aging rat inferior colliculus. Neuroscience. 1996;73(2):449–458. doi: 10.1016/0306-4522(96)00050-4. [DOI] [PubMed] [Google Scholar]
- Morest DK, Oliver DL. The neuronal architecture of the inferior colliculus in the cat: defining the functional anatomy of the auditory midbrain. J Comp Neurol. 1984;222(2):209–236. doi: 10.1002/cne.902220206. [DOI] [PubMed] [Google Scholar]
- Oliver DL. Ascending efferent projections of the superior olivary complex. Microsc Res Tech. 2000;51(4):355–363. doi: 10.1002/1097-0029(20001115)51:4<355::AID-JEMT5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Oliver DL. Neuronal organization in the inferior colliculus. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. Springer; New York: 2005. pp. 69–114. Chapter 2. [Google Scholar]
- Oliver DL, Beckius GE, Bishop DC, Kuwada S. Simultaneous anterograde labeling of axonal layers from lateral superior olive and dorsal cochlear nucleus in the inferior colliculus of cat. J Comp Neurol. 1997;382(2):215–229. doi: 10.1002/(sici)1096-9861(19970602)382:2<215::aid-cne6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Beckius GE, Bishop DC, Loftus WC, Batra R. Topography of interaural temporal disparity coding in projections of medial superior olive to inferior colliculus. J Neurosci. 2003;23(19):7438–7449. doi: 10.1523/JNEUROSCI.23-19-07438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver DL, Kuwada S, Yin TC, Haberly LB, Henkel CK. Dendritic and axonal morphology of HRP-injected neurons in the inferior colliculus of the cat. J Comp Neurol. 1991;303(1):75–100. doi: 10.1002/cne.903030108. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Morest DK. The central nucleus of the inferior colliculus in the cat. J Comp Neurol. 1984;222(2):237–264. doi: 10.1002/cne.902220207. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Winer JA, Beckius GE, Saint Marie RL. Morphology of GABAergic neurons in the inferior colliculus of the cat. J Comp Neurol. 1994;340(1):27–42. doi: 10.1002/cne.903400104. [DOI] [PubMed] [Google Scholar]
- Parrish-Aungst S, Shipley MT, Erdelyi F, Szabo G, Puche AC. Quantitative analysis of neuronal diversity in the mouse olfactory bulb. The Journal of comparative neurology. 2007;501(6):825–836. doi: 10.1002/cne.21205. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Ribak CE. An electron microscopic study of GABAergic neurons and terminals in the central nucleus of the inferior colliculus of the rat. Journal of neurocytology. 1987a;16(3):333–345. doi: 10.1007/BF01611345. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Ribak CE. GABAergic neurons and axon terminals in the brainstem auditory nuclei of the gerbil. J Comp Neurol. 1987b;258(2):267–280. doi: 10.1002/cne.902580207. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Baker RA. Neurotransmitter-specific uptake and retrograde transport of [3H]glycine from the inferior colliculus by ipsilateral projections of the superior olivary complex and nuclei of the lateral lemniscus. Brain Res. 1990;524(2):244–253. doi: 10.1016/0006-8993(90)90698-b. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Ostapoff EM, Morest DK, Wenthold RJ. Glycine-immunoreactive projection of the cat lateral superior olive: possible role in midbrain ear dominance. J Comp Neurol. 1989;279(3):382–396. doi: 10.1002/cne.902790305. [DOI] [PubMed] [Google Scholar]
- Saldana E, Aparicio MA, Fuentes-Santamaria V, Berrebi AS. Connections of the superior paraolivary nucleus of the rat: projections to the inferior colliculus. Neuroscience. 2009;163(1):372–387. doi: 10.1016/j.neuroscience.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldana E, Feliciano M, Mugnaini E. Distribution of descending projections from primary auditory neocortex to inferior colliculus mimics the topography of intracollicular projections. J Comp Neurol. 1996;371(1):15–40. doi: 10.1002/(SICI)1096-9861(19960715)371:1<15::AID-CNE2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Geary WA, Wooten GF, Rubel EW. Quantitative distribution of the glycine receptor in the auditory brain stem of the gerbil. J Neurosci. 1987;7(11):3793–3802. doi: 10.1523/JNEUROSCI.07-11-03793.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner CE, Langner G. Periodicity coding in the inferior colliculus of the cat. II. Topographical organization. Journal of neurophysiology. 1988;60(6):1823–1840. doi: 10.1152/jn.1988.60.6.1823. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Langner G. Laminar fine structure of frequency organization in auditory midbrain. Nature. 1997;388(6640):383–386. doi: 10.1038/41106. [DOI] [PubMed] [Google Scholar]
- Shneiderman A, Chase MB, Rockwood JM, Benson CG, Potashner SJ. Evidence for a GABAergic projection from the dorsal nucleus of the lateral lemniscus to the inferior colliculus. Journal of neurochemistry. 1993;60:72–82. doi: 10.1111/j.1471-4159.1993.tb05824.x. [DOI] [PubMed] [Google Scholar]
- Shneiderman A, Oliver DL. EM autoradiographic study of the projections from the dorsal nucleus of the lateral lemniscus: a possible source of inhibitory inputs to the inferior colliculus. J Comp Neurol. 1989;286(1):28–47. doi: 10.1002/cne.902860103. [DOI] [PubMed] [Google Scholar]
- Shneiderman A, Oliver DL, Henkel CK. Connections of the dorsal nucleus of the lateral lemniscus: an inhibitory parallel pathway in the ascending auditory system? J Comp Neurol. 1988;276(2):188–208. doi: 10.1002/cne.902760204. [DOI] [PubMed] [Google Scholar]
- Toyoshima M, Sakurai K, Shimazaki K, Takeda Y, Nakamoto M, Serizawa S, Shimoda Y, Watanabe K. Preferential localization of neural cell recognition molecule NB-2 in developing glutamatergic neurons in the rat auditory brainstem. J Comp Neurol. 2009;513(4):349–362. doi: 10.1002/cne.21972. [DOI] [PubMed] [Google Scholar]
- Zarbin MA, Wamsley JK, Kuhar MJ. Glycine receptor: light microscopic autoradiographic localization with [3H]strychnine. J Neurosci. 1981;1(5):532–547. doi: 10.1523/JNEUROSCI.01-05-00532.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX, Li L, Kelly JB, Wu SH. GABAergic projections from the lateral lemniscus to the inferior colliculus of the rat. Hear Res. 1998;117(1-2):1–12. doi: 10.1016/s0378-5955(97)00202-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.