Abstract
Purpose
Stereotactic radiosurgery (SRS) has been introduced for small-sized single and oligo-metastases in the brain. The aim of this study is to assess treatment outcome, efficacy, and prognostic variables associated with survival and intracranial recurrence.
Materials and Methods
This study retrospectively reviewed 123 targets in 64 patients with non-small cell lung cancer (NSCLC) treated with SRS between January 2006 and December 2012. Treatment responses were evaluated using magnetic resonance imaging. Overall survival (OS) and intracranial progression-free survival (IPFS) were determined.
Results
The median follow-up was 13.9 months. The median OS and IPFS were 14.1 and 8.9 months, respectively. Fifty-seven patients died during the follow-up period. The 5-year local control rate was achieved in 85% of 108 evaluated targets. The 1- and 2-year OS rates were 55% and 28%, respectively. On univariate analysis, primary disease control (p < 0.001), the Eastern Cooperative Oncology Group (ECOG) performance status (0-1 vs. 2; p = 0.002), recursive partitioning analysis class (1 vs. 2; p = 0.001), and age (<65 vs. ≥65 years; p = 0.036) were significant predictive factors for OS. Primary disease control (p = 0.041) and ECOG status (p = 0.017) were the significant prognostic factors for IPFS. Four patients experienced radiation necrosis.
Conclusion
SRS is a safe and effective local treatment for brain metastases in patients with NSCLC. Uncontrolled primary lung disease and ECOG status were significant predictors of OS and intracranial failure. SRS might be a tailored treatment option along with careful follow-up of the intracranial and primary lung disease status.
Keywords: Brain neoplasm, Neoplasm metastasis, Non-small-cell lung carcinoma, Radiosurgery, Prognosis
Introduction
Brain metastases are currently both common in clinical oncology and a critical problem, since they negatively affect patients' quality of life (QOL) as well as survival. In particular, 30% of patients with non-small cell lung cancer (NSCLC) will develop brain metastases [1,2]. However, improved systemic treatment modalities have led to prolonged disease courses in large numbers of patients and subsequently to an increased incidence of brain metastases.
For the last several decades, whole brain radiation therapy (WBRT) and surgery have been the main treatment options for brain metastases from NSCLC. Surgery is preferred in the case of the metastatic lesions that are single, located near the skull, relatively large in size, and inducing urgent neurologic sign [1,2,3]. With the exception of these surgical candidates, the treatment of choice for most patients, not only for multiple brain metastases, but also for single or oligo-metastases (generally, two to four metastases) in the brain, has been WBRT [3]. However, the risks of long-term neurotoxicity as well as multiple short-term adverse effects are controversial aspects of WBRT [4]. The long-term toxicity of the treatment could become a serious clinical problem as patients with NSCLC have increased survival periods.
In response to these clinical concerns, stereotactic radiosurgery (SRS) has recently emerged as an alternative treatment modality for selected patients with single metastases and oligo-metastases [5,6,7]. Generally, SRS has been used in conjunction with WBRT. However, some published articles have suggested that although WBRT can control micrometastases, it might not be necessary or effective in all patients [8,9]. Several studies have shown that SRS is very effective in tumor control, and it can prevent neurological defects, and permit a good QOL in lung cancer patients [10,11,12,13]. SRS is accepted as a standard treatment modality for single metastases and oligo-metastases. In addition, there are studies underway on the possibility of omitting WBRT after SRS [14].
Both local control and distant intracranial failure can be significant in relation to patients' long-term prognosis as well as their functional status and QOL. Therefore, the decision of which up-front radiotherapy (RT) technique to select would be particularly important. To allow the performance of tailored RT, more pooled data regarding clinical outcomes and toxicity, as well as the assessment of clinical prognostic variables, is required to more effectively characterize which patients are at a relatively high or low risk for intracranial recurrence.
This retrospective study was performed to review our clinical data on SRS delivered to NSCLC patients with brain metastases using the CyberKnife system (Accuray Inc., Sunnyvale, CA, USA) and to evaluate its efficacy as well as the prognostic variables associated with survival and intracranial recurrence.
Materials and Methods
1. Patients
Out institution performs brain SRS for the patients with the Eastern Cooperative Oncology Group (ECOG) 0, 1, and 2 and limited numbers of brain lesion less than 5. Between April 2006 and December 2012, a total of 123 targets brain metastases in 64 NSCLC patients were treated with SRS at Seoul St. Mary's Hospital. Among the 64 patients, 12 patients had previously received WBRT and were treated with SRS as salvage treatment for recurrent intracranial metastases. The details of the patients' characteristics were summarized in Table 1. One patient received treatment with SRS to 5 targets. The patient initially planned to receive SRS on 3 targets but additional two lesions were founded in planning contrast-enhanced magnetic resonance imaging (MRI) and we decide to enforce SRS treatment as initially planned. Outlines of the targets were demonstrated in Table 2. This study was approved by the Institutional Review Board of our institution for retrospective clinical research using the medical records of these 64 patients.
Table 1. Patients' characteristics (n = 64).
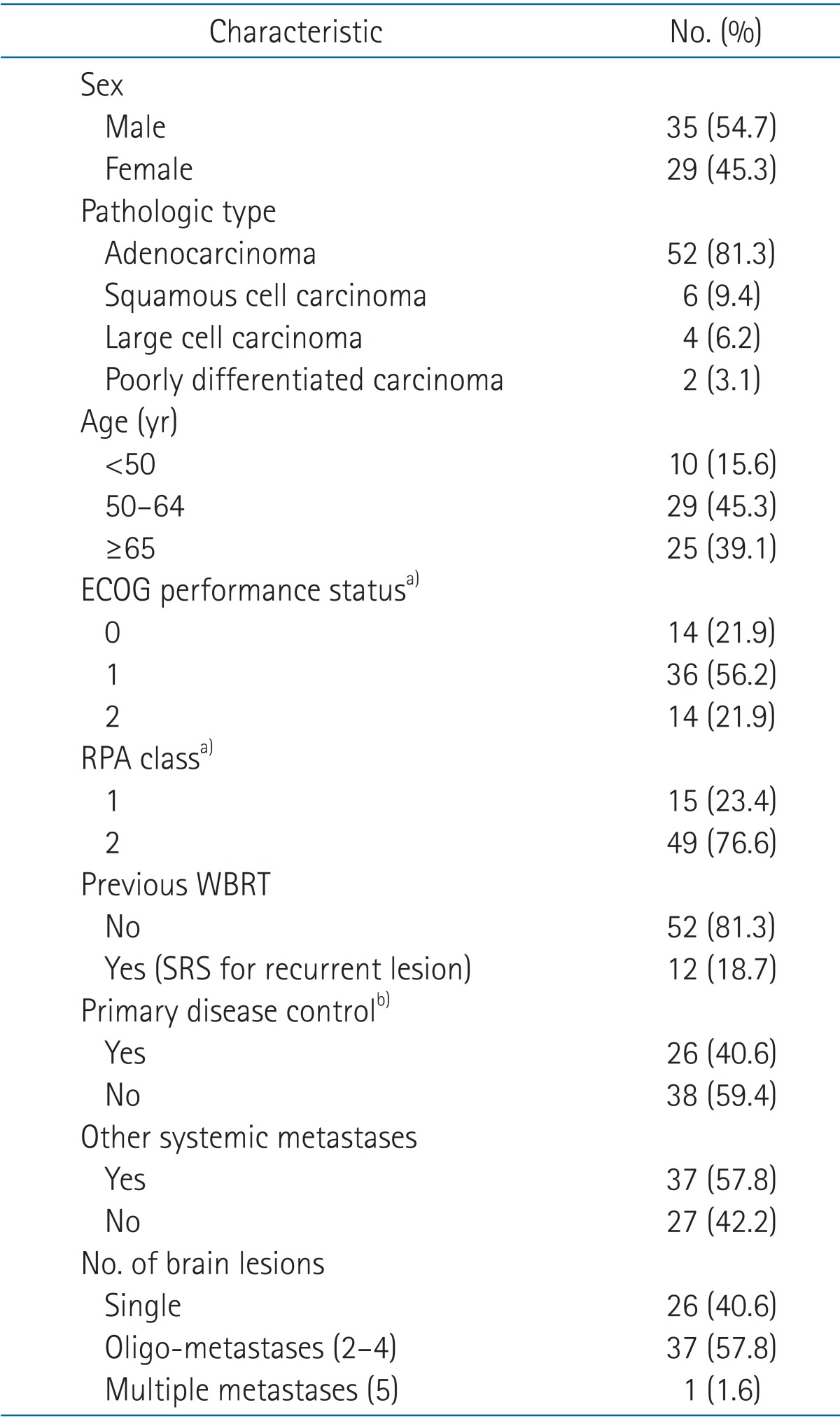
ECOG, Eastern Cooperative Oncology Group; RPA, recursive partitioning analysis; WBRT, whole brain radiation therapy; SRS, stereotactic radiosurgery.
a)Patients with ECOG status 3 and 4 and RPA class 3 were excluded in treatment. b)Disease control status of primary lung lesion.
Table 2. Stereotactic radiosurgery targets' distributions and dose schedule (n = 123).
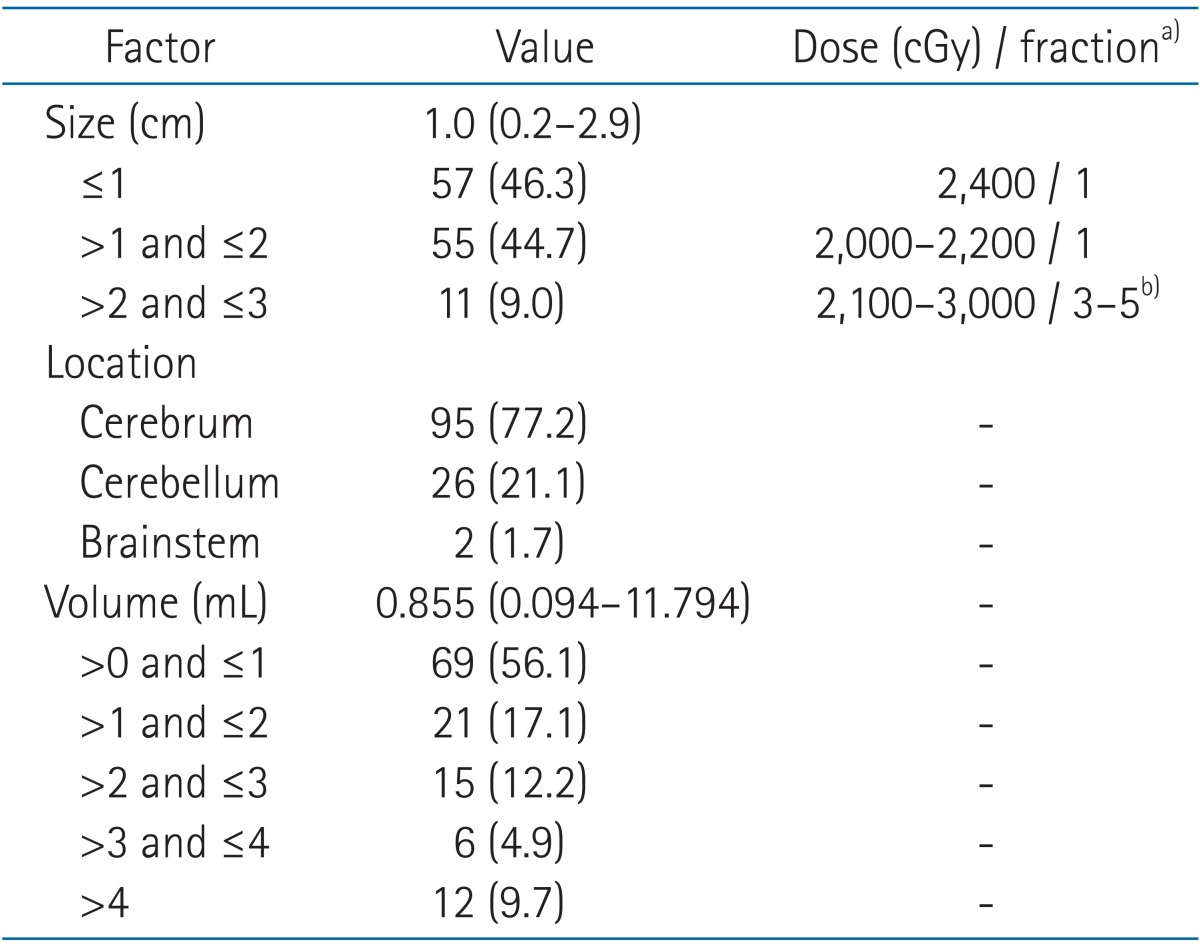
Values are presented as median (range) or number (%).
a)Prescription isodose line: median 80% (range, 70% to 92%). b)Patients with previous whole brain radiation therapy have followed this fractionated schedule regardless of target size.
2. Treatment planning, delivery, dose, and fractionation
All patients were immobilized with a tight thermoplastic stereotactic head mask in the supine position and underwent contrast-enhanced MRI and contrast-enhanced computed tomography (CT) with a slice thickness of 1 mm throughout the entire brain. The two sets of patient images were fused using the commercial software package CoreFusion (Seoul C&J Inc., Seoul, Korea).
The gross tumor volume (GTV) was determined as the evident, contrast-enhancing, gross disease on both images. We mainly delineated the GTV on the basis of CT images and incorporated additional information from fused MRI scans. We did not expand the GTV to form a clinical target volume or planning target volume from the GTV. Treatment planning was performed with the 'On Target' treatment planning system (Accuray Inc.). The GTV encompassed by a median 80% isodose line (range, 70% to 92%). Patients were treated with 6 MV photon beams using CyberKnife (Accuray Inc.). Our prescription guidelines for SRS were described in Table 2.
3. Follow-up
Follow-up was performed with clinical interviews, neurologic examinations, and MRI. The first follow-up MRI was routinely performed 1 to 3 months after SRS. Thereafter, follow-ups were performed every 3 to 6 months or sooner, if indicated. Response evaluations were based on the World Health Organization criteria.
Survival time was calculated from the date of diagnosis of brain metastases until death or the last follow-up in patients treated with SRS as initial treatment of brain metastases. In cases of salvage SRS, survival time was calculated from the date of the MRI on which recurrent brain metastases for SRS treatment was diagnosed. The cause of death was classified as lung cancer-related extracranial systemic progression which included primary lung disease progression, intracranial disease progression, other noncancerous conditions, and unknown causes.
The development of neurological defects was defined by a change in neurological status, including decreased strength, degraded ambulatory function, development of aphasia, altered vision or other sensation, or altered mental status. This assessment was based on a clinical interview with a clinician. We did not employ a written clinical questionnaire to evaluate neurocognitive function.
4. Statistics
All statistical analyses were performed using IBM SPSS ver. 22 (IBM, Armonk, NY, USA). The Kaplan-Meier method was employed to determine the actuarial survival rate or local recurrence rate at the SRS sites and the intracranial progression rate elsewhere in the brain. Prognostic factors for overall survival (OS) and intracranial progression-free survival (IPFS) were examined with the log-rank test. Multivariate prognostic factors were assessed with Cox regression analysis. A p-value of <0.05 (two-sided test) was considered significant.
Results
1. Treatment outcomes
The median follow-up duration was 13.9 months (range, 0.7 to 88.3 months) for the entire patient group. Response was evaluated in 108 of 123 targets 1 to 3 months after SRS. The other 15 targets from 9 patients were not evaluated because of early toxic death caused by other systemic treatment prior to follow-up imaging or systemic disease progression. We therefore included these 9 patients in the survival analysis because the cause of death was proven as systemic disease progression.
On the initial follow-up MRI, 43 targets (39.8%) showed a complete response, and another 40 targets (37.0%) showed a partial response. Thus, tumor response was achieved in 76.8% of the treated targets. Twenty-five targets (23.2%) showed stable disease. None of the targets showed progression.
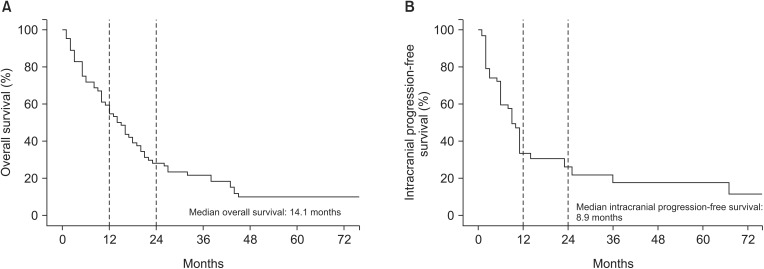
At the time of analysis, 57 patients (89.1%) were dead. The cause of death was classified into four categories: lung cancer-related systemic progression in 39 patients, intracranial progression in 8 patients, other noncancerous conditions in 3 patients, and unknown causes in 7 patients. The median OS for all patients was 14.1 months. The 1- and 2-year OS rates were 55% and 28%, respectively (Fig. 1A).
Fig. 1. Kaplan-Meier overall survival (A) and intracranial progression-free survival (B) curves (n = 64).
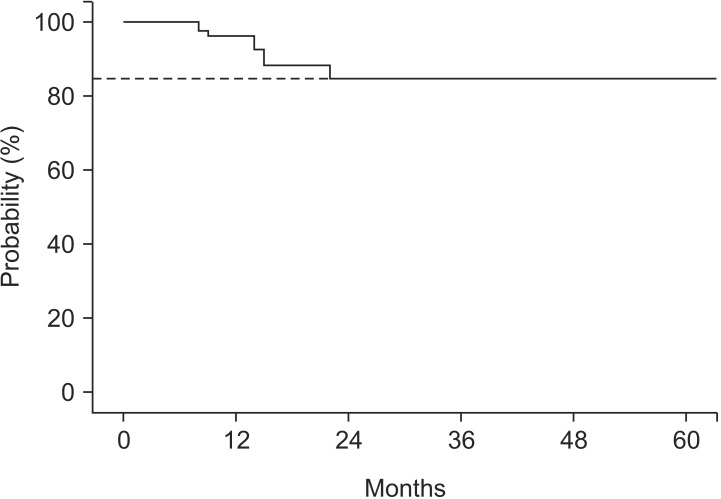
We assessed both local tumor recurrences in 108 evaluated treated sites and new brain metastases at other sites to determine IPFS in 64 patients. The median IPFS was 8.9 months (Fig. 1B). Local tumor recurrence was developed in 10 sites of 7 patients. The actuarial 5-year local control rate was 85% (Fig. 2). Among those 7 patients, one patient with single brain metastases and 6 patients with multiple brain metastases experienced local recurrence. Regarding the initial treatment responses of those 10 targets, 4 targets showed a complete response, 3 targets showed a partial response, and the other 3 targets demonstrated stable disease. However, new brain metastases at other sites occurred in 34 patients (53.1%); among them, 27 were in the group of initial SRS patients.
Fig. 2. Local control of evaluated stereotactic radiosurgery target (n = 108), 5-year local control rate was 85%.
2. Prognostic factors for OS
We performed a univariate analysis to identify predictive factors for OS (n = 64). On univariate analysis, whether or not the primary disease was controlled (31.5 months vs. 9.7 months; p < 0.001), ECOG performance status (0-1 vs. 2; 16.4 months vs. 4.9 months; p = 0.002), recursive partitioning analysis (RPA) class (1 vs. 2; 42.2 months vs. 11.2 months; p = 0.001), and age (<65 years vs. ≥65 years; 17.2 months vs. 7.3 months; p = 0.036) were significant predictive factors for OS. Table 3 presented the results of the univariate analysis. The other factors failed to achieve statistical significance. In the multivariate analysis, we excluded RPA class because this parameter encompasses other prognostic factors (age, ECOG performance status, and disease control of the primary lung lesion). Our results showed that primary disease control status (p < 0.001; hazard ratio [HR], 4.814; 95% confidence interval [CI], 10.413-2.226), ECOG performance status (p = 0.010; HR, 2.559; 95% CI, 5.254-1.246), and status of other systemic metastases (p = 0.006; HR, 2.419; 95% CI, 2.352-0.588) were significant prognostic factors for OS. The results are summarized in Table 4.
Table 3. Results of univariate analysis of OS and IPFS.
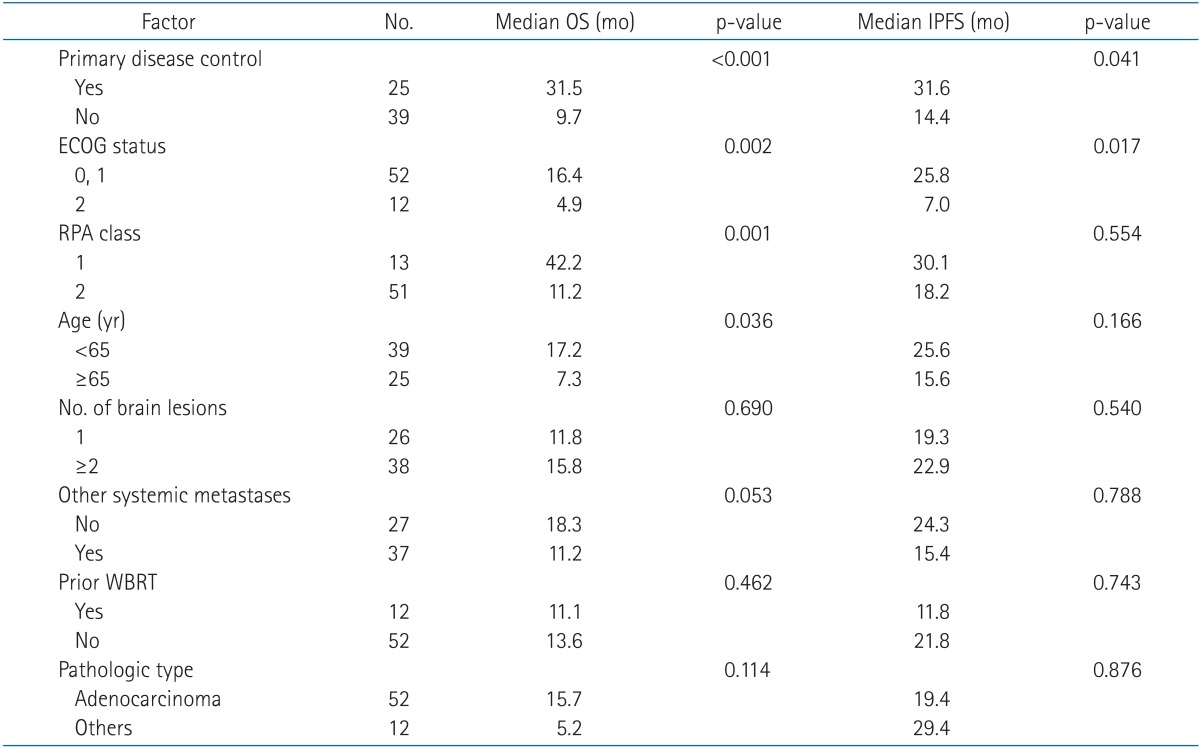
OS, overall survival; IPFS, intracranial progression-free survival; ECOG, Eastern Cooperative Oncology Group; RPA, recursive partitioning analysis; WBRT, whole brain radiation therapy.
Table 4. Multivariate analysis of overall survival and intracranial progression free survival.
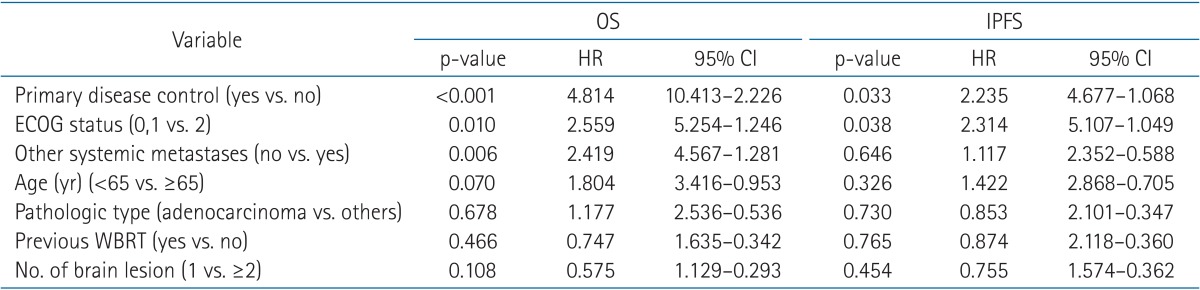
OS, overall survival; IPFS, intracranial progression-free survival; HR, hazard ratio; CI, confidential interval; ECOG, Eastern Cooperative Oncology Group; WBRT, whole brain radiation therapy.
3. Prognostic factors for IPFS
We also performed a univariate analysis to identify predictive factors for intracranial tumor recurrences, including local recurrences and new intracranial metastases (n = 64). Primary disease control (31.6 months vs. 14.4 months; p = 0.041) and ECOG performance status (0-1 vs. 2; 25.8 months vs. 7.0 months; p = 0.017) were statistically significant prognostic factors. Neither the number of sites of metastases nor a history of WBRT correlated with IPFS. Table 3 presented the results of the univariate analysis. On multivariate analysis, primary disease control (p = 0.033; HR, 2.235; 95% CI, 4.677-1.068) and ECOG performance status (p = 0.038; HR, 2.314; 95% CI, 5.107-1.049) were statistically significant prognostic factors for IPFS. The results were summarized in Table 4.
4. Treatment-induced toxicity
There were no cases of neurocognitive dysfunction in the initial-SRS treated patients during the follow-up period. Four patients presented with radiation necrosis that was diagnosed using perfusion/diffusion MRI and/or brain CT; only one of these patients underwent surgical resection and received a confirmed pathologic diagnosis of radiation necrosis. None of these 4 patients experienced permanent deterioration of neurologic function. The details of the radiation necrosis cases were summarized in Table 5.
Table 5. Summary of patients with radiation necrosis.
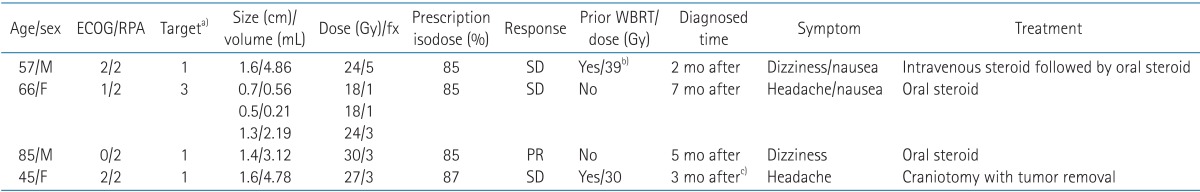
ECOG, Eastern Cooperative Oncology Group; RPA, recursive partitioning analysis; WBRT, whole brain radiation therapy; fx, fraction; SD, stable disease; PR, partial remission.
a)All lesions were located in cerebrum. b)30 Gy WBRT followed by 9 Gy boost radiation therapy on metastatic mass. c)Patient underwent surgical removal for symptom relief and pathology confirm.
Discussion and Conclusion
In the current study, we investigated patients with brain metastases from NSCLC. Although this study was limited by its nature as a single institutional retrospective study with a short follow-up period, we found that patients who underwent SRS demonstrated excellent local control and survival compared with traditional WBRT. According to an analysis of randomized clinical trials by the Radiation Therapy Oncology Group (RTOG), the median survival time of patients with brain metastases after WBRT was 4 to 6 months [13]. In the current study, the median survival of all patients was 14.1 months; moreover, only 8 patients died due to intracranial lesions. When SRS was used as a salvage approach, patients (n = 12; median OS, 11.1 months) showed a trend toward longer survival compared with the reported survival rates of lung cancer patients with brain disease progression [15,16]. However, it is difficult to determine whether the improved treatment results can be attributed solely to the advantages of SRS, so that we didn't perform direct comparison study between SRS group and WBRT group.
According to our results, univariate and multivariate analyses showed that primary disease control is a statistically significant predictor of OS and IPFS. Similar to the findings of our study, primary disease control was found to be the most important factor for clinical outcome in several previous studies, showing a strong correlation with OS as well as with intracranial progression [10,17,18]. Kress et al. [19] also found that primary lung disease control was the most powerful prognostic factor in a study comparing SRS to other treatment modalities in the control of brain disease. Patients with NSCLC live longer than predicted because of advance in systemic chemotherapy. During their prolonged survival, uncontrolled systemic disease continuously increases the risk of other intracranial failures. These findings are very important for predicting long-term outcomes and tailoring plans for patient surveillance. Prospective studies are needed to optimize patient selection for up-front SRS, and to refine follow-up schedules with the goal of minimizing the impact of intracranial failure on patients' QOL.
Our study also demonstrated that ECOG performance status is a statistically significant predictor of both OS and IPFS. The RTOG provides an RPA classification method that includes the Karnofsky performance status (KPS) score, age, status of extracranial metastases, and primary disease control. This RPA classification was derived from and validated by the results of several clinical trials [20,21,22]. In our study, the RPA classification showed a statistically significant correlation with OS and was adequate in predicting the prognosis of NSCLC patients treated with SRS.
The number of brain lesions is also an important point in brain SRS. Previously, WBRT was considered for brain metastases, regardless of the number of sites. Recently, SRS was initially adopted to increase local control in cases with a few metastases. Kondziolka et al. [23] reported that the combination of WBRT and SRS improved local control without treatment side effects. RTOG 9508 [13,24] was a phase III multi-institute randomized study that compared WBRT with WBRT plus SRS boost. The study determined that patients with one surgically unresectable metastases benefited from an SRS boost and that patients with 2 to 3 metastases can consider SRS boost as a treatment option. After the RTOG 9508 report, SRS became a treatment option for oligo-metastases in the brain.
Meanwhile, Sperduto et al. [24] found, in a secondary analysis of RTOG 9508, that patients with poor graded prognostic assessment (GPA) scores receive no clinical benefit from SRS. The GPA score is composed of age, KPS score, presence of extracranial metastases, and number of brain metastases, and poorer the prognostic factors, lower are the scores. Patients with GPA scores of 3.5 and 4.0 show a survival benefit with SRS, while SRS does not significantly affect survival in patients with GPA scores from 0.0 to 3.0. This result emphasizes that the patient's status including the presence of systemic metastases, performance status and number of brain metastases should be judiciously considered before performing SRS.
Some studies have investigated the possibility of omitting WBRT after SRS in suitable patient groups, and the debates are ongoing. To avoid the acute and chronic morbidity of WBRT, this modality may be reserved as a salvage treatment in cases of intracranial relapse. Aoyama et al. [25] failed to find survival differences in patients with 1-4 metastases who were treated with SRS alone or SRS plus WBRT. Similarly, the European Organization for Research and Treatment of Cancer (EORTC) 22952-26001 trial [14,26] also found that the addition of WBRT to SRS did not affect survival, although it did increase the risk of delayed radiation necrosis. In addition, a randomized study of 58 patients conducted by Chang et al. [27] was stopped due to a high probability (96%) of decreased learning and memory function in the WBRT plus SRS group.
In fact, the probability of new brain metastases at other sites was higher in patients treated with SRS alone. However, the lack of a survival benefit and the controversy regarding WBRT-induced neurocognitive dysfunction might become logical evidence for the omission of WBRT when SRS is administered to patients with single metastases or oligo-metastases (2-4 lesions). WBRT may be omitted in patients with a good performance status with otherwise stable systemic disease and a limited number of brain metastases if serial MRI is performed during follow-up. In the current study, 53.1% of patients developed new brain metastases in the initial SRS treated patients those who did not receive prior WBRT. However, their median survival duration of 13.6 months was not inferior compared to the traditional outcome of WBRT.
Furthermore, the development of novel chemotherapeutic agents for lung cancer has resulted in improvements in survival. Targeted agents in particular, such as epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs), compared to other cytotoxic chemotherapy agents, show superior pharmacokinetics in penetrating through the bloodbrain barrier. Despite the fact that few prospective data are available on the efficacy of EGFR TKIs in this setting, several authors have recently reported a growing number of cases showing partial or complete responses in brains treated with these agents. Park et al. [28] treated NSCLC patients with brain metastases and EGFR exon 19 or 21 mutations using EGFR TKIs. Out of 28 patients, 23 patients showed a partial response with EGFR TKIs only. If these drugs can adequately affect tumor cells in the brain, it would reduce the incidence of other intracranial recurrences. Unfortunately, we have no date on some of our patients have regarding chemotherapy or mutation studies for additional evaluation. Further studies will be needed to determine whether EGFR TKIs can replace or permit the omission of WBRT in selected NSCLC patients harboring EGFR mutations.
Our study only included patients with NSCLC. There are few similar studies focusing specifically on NSCLC brain metastases. Kim et al. [29] investigated the treatment of 77 NSCLC patients with brain metastases using linear accelerator based SRS. Seventy-one patients also underwent WBRT. The authors reported that the overall median survival was 10 months and the extent of systemic disease was a significant factor affecting survival. Those findings were similar to our results. Sheehan et al. [30] investigated Gamma Knife surgery (GKS) for the treatment of brain metastases in NSCLC patients. The median survival of 273 patients was 15 months from the diagnosis of brain metastases, which was similar to our data. In this study, GKS resulted in a crude local control rate of 84%, and a higher KPS score, female sex, adenocarcinoma, and a long time interval from diagnosis of lung cancer to metastases to the brain were significant prognostic factors; active systemic disease was a poor prognostic factor, similar to the results of our study.
The side effects of SRS are usually limited but can occasionally be serious. There are few radiation-related acute side effects of SRS. The treatment may cause mild fatigue and occasionally, temporary loss of a patch of hair. The risk of symptomatic radiation injury is usually below 5%, depending on factors such as the treatment volume, radiation dose, pathology, interval since prior SRS, dose distribution conformity, and previous radiation history [18,31]. In a prior study conducted by our institute [32], a history of diabetes mellitus, hypertension, cerebral infarction and chemotherapy history were also found to increase the risk of brain necrosis. Radiation injury must be differentiated from tumor recurrence, which presents with similar MRI findings. Such findings are focally increased enhancements, accompanied by perilesional edema. The efficacies of various modalities such as MRI spectrography, perfusion/diffusion MRI/CT, or positron emission tomography with fluorodeoxyglucose or methionine have been reported. However, it is sometimes difficult to distinguish between tumor recurrence and radiation injury, due to the occurrence of mixed lesions that contain both tumor recurrences and radiation injuries.
In our study, 4 patients were diagnosed with radiation necrosis. The diagnosis was based on imaging in 3 patients, and one patient underwent surgical resection, with the findings pathologically proven as necrosis. We obtained enhanced perfusion MRI/CT images of suspicious lesions, and the imaging findings suggested that the lesions might be radiation necrosis rather than tumor recurrence. The details of the 4 radiation necrosis cases are summarized in Table 5. Fortunately, no patients died or experienced chronic irreversible neurocognitive dysfunction in response to SRS, and the adverse treatment effects were controlled by oral steroids and surgical resection.
We acknowledge that this study has several limitations; particularly the inclusion of patients who had a previous WBRT and the patient with 5 treatment targets in the brain. Additionally, there was poor pathological information regarding the mutation status of EGFR and anaplastic lymphoma kinase, as mutation studies were adopted in routine clinical practice after 2010. Targeted agents for these mutations lead to tremendous benefits in disease control, OS, and intracranial disease control.
Despite limitations mentioned above, these retrospective data demonstrated that SRS could be a safe and effective option for front-line treatment and for salvage treatment after WBRT for single metastases and oligo-metastases of the brain from NSCLC. Disease control of the primary lung lesion, ECOG performance status, age, and other systemic (extracranial) metastases were correlated with OS. Primary disease control and ECOG performance status were significant predictors of IPFS. These results signify that uncontrolled primary disease continuously increases the risk of other intracranial failures. Therefore, SRS combined with or without WBRT might be a tailored treatment option along with careful follow-up of intracranial and systemic disease status.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Peacock KH, Lesser GJ. Current therapeutic approaches in patients with brain metastases. Curr Treat Options Oncol. 2006;7:479–489. doi: 10.1007/s11864-006-0023-8. [DOI] [PubMed] [Google Scholar]
- 2.Cairncross JG, Kim JH, Posner JB. Radiation therapy for brain metastases. Ann Neurol. 1980;7:529–541. doi: 10.1002/ana.410070606. [DOI] [PubMed] [Google Scholar]
- 3.Diener-West M, Dobbins TW, Phillips TL, Nelson DF. Identification of an optimal subgroup for treatment evaluation of patients with brain metastases using RTOG study 7916. Int J Radiat Oncol Biol Phys. 1989;16:669–673. doi: 10.1016/0360-3016(89)90483-5. [DOI] [PubMed] [Google Scholar]
- 4.Kazda T, Jancalek R, Pospisil P, et al. Why and how to spare the hippocampus during brain radiotherapy: the developing role of hippocampal avoidance in cranial radiotherapy. Radiat Oncol. 2014;9:139. doi: 10.1186/1748-717X-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 6.Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys. 1994;29:711–717. doi: 10.1016/0360-3016(94)90558-4. [DOI] [PubMed] [Google Scholar]
- 7.Mehta MP, Tsao MN, Whelan TJ, et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2005;63:37–46. doi: 10.1016/j.ijrobp.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 8.Lester-Coll NH, Dosoretz AP, Yu JB. Decision analysis of stereotactic radiation surgery versus stereotactic radiation surgery and whole-brain radiation therapy for 1 to 3 brain metastases. Int J Radiat Oncol Biol Phys. 2014;89:563–568. doi: 10.1016/j.ijrobp.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25:1260–1266. doi: 10.1200/JCO.2006.09.2536. [DOI] [PubMed] [Google Scholar]
- 10.Wegner RE, Olson AC, Kondziolka D, Niranjan A, Lundsford LD, Flickinger JC. Stereotactic radiosurgery for patients with brain metastases from small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81:e21–e27. doi: 10.1016/j.ijrobp.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Serizawa T, Ono J, Iichi T, et al. Gamma knife radiosurgery for metastatic brain tumors from lung cancer: a comparison between small cell and non-small cell carcinoma. J Neurosurg. 2002;97:484–488. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 12.Chang EL, Wefel JS, Maor MH, et al. A pilot study of neurocognitive function in patients with one to three new brain metastases initially treated with stereotactic radiosurgery alone. Neurosurgery. 2007;60:277–283. doi: 10.1227/01.NEU.0000249272.64439.B1. [DOI] [PubMed] [Google Scholar]
- 13.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 14.Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31:65–72. doi: 10.1200/JCO.2011.41.0639. [DOI] [PubMed] [Google Scholar]
- 15.Caballero JA, Sneed PK, Lamborn KR, et al. Prognostic factors for survival in patients treated with stereotactic radiosurgery for recurrent brain metastases after prior whole brain radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:303–309. doi: 10.1016/j.ijrobp.2011.06.1987. [DOI] [PubMed] [Google Scholar]
- 16.Yomo S, Hayashi M. The efficacy and limitations of stereotactic radiosurgery as a salvage treatment after failed whole brain radiotherapy for brain metastases. J Neurooncol. 2013;113:459–465. doi: 10.1007/s11060-013-1138-y. [DOI] [PubMed] [Google Scholar]
- 17.Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118:2486–2493. doi: 10.1002/cncr.26515. [DOI] [PubMed] [Google Scholar]
- 18.Shehata MK, Young B, Reid B, et al. Stereotatic radiosurgery of 468 brain metastases < or =2 cm: implications for SRS dose and whole brain radiation therapy. Int J Radiat Oncol Biol Phys. 2004;59:87–93. doi: 10.1016/j.ijrobp.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Kress MA, Oermann E, Ewend MG, Hoffman RB, Chaudhry H, Collins B. Stereotactic radiosurgery for single brain metastases from non-small cell lung cancer: progression of extracranial disease correlates with distant intracranial failure. Radiat Oncol. 2013;8:64. doi: 10.1186/1748-717X-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaspar LE, Scott C, Murray K, Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001–1006. doi: 10.1016/s0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]
- 21.Kepka L, Cieslak E, Bujko K, Fijuth J, Wierzchowski M. Results of the whole-brain radiotherapy for patients with brain metastases from lung cancer: the RTOG RPA intra-classes analysis. Acta Oncol. 2005;44:389–398. doi: 10.1080/02841860510029699. [DOI] [PubMed] [Google Scholar]
- 22.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 23.Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427–434. doi: 10.1016/s0360-3016(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 24.Sperduto PW, Shanley R, Luo X, et al. Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole-brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1-3 brain metastases; poststratified by the graded prognostic assessment (GPA) Int J Radiat Oncol Biol Phys. 2014;90:526–531. doi: 10.1016/j.ijrobp.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoyama H, Shirato H, Onimaru R, et al. Hypofractionated stereotactic radiotherapy alone without whole-brain irradiation for patients with solitary and oligo brain metastasis using noninvasive fixation of the skull. Int J Radiat Oncol Biol Phys. 2003;56:793–800. doi: 10.1016/s0360-3016(03)00014-2. [DOI] [PubMed] [Google Scholar]
- 26.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 28.Park SJ, Kim HT, Lee DH, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012;77:556–560. doi: 10.1016/j.lungcan.2012.05.092. [DOI] [PubMed] [Google Scholar]
- 29.Kim YS, Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for patients with nonsmall cell lung carcinoma metastatic to the brain. Cancer. 1997;80:2075–2083. doi: 10.1002/(sici)1097-0142(19971201)80:11<2075::aid-cncr6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 30.Sheehan JP, Sun MH, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for non-small cell lung carcinoma metastatic to the brain: long-term outcomes and prognostic factors influencing patient survival time and local tumor control. J Neurosurg. 2002;97:1276–1281. doi: 10.3171/jns.2002.97.6.1276. [DOI] [PubMed] [Google Scholar]
- 31.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 32.Lee DS, Yu M, Jang HS, et al. Radiation-induced brain injury: retrospective analysis of twelve pathologically proven cases. Radiat Oncol J. 2011;29:147–155. doi: 10.3857/roj.2011.29.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]