Abstract
CD47, a self-recognition marker, plays an important role in both innate and adaptive immune response. To explore the potential role of CD47 in activation of autoreactive T and B cells and the production of autoantibodies in autoimmune disease, especially systemic lupus erythematosus (SLE), we have generated CD47 knockout Faslpr (CD47−/−–Faslpr) mice and examined histopathologic changes in the kidneys, cumulative survival rates, proteinuria, extent of splenomegaly and autoantibodies, serum chemistry and immunologic parameters. In comparison with Faslpr mice, CD47−/−–Faslpr mice exhibit a prolonged lifespan and delayed autoimmune nephritis including glomerular cell proliferation, basement membrane thickening, acute tubular atrophy and vacuolization. CD47−/−–Faslpr mice have lower levels of proteinuria, associated with reduced deposition of complement C3 and C1q, and IgG but not IgM in the glomeruli, compared to the age-matched Faslpr mice. Serum levels of antinuclear antibodies and anti-double-stranded DNA antibodies are significantly lower in CD47−/−–Faslpr mice than in Faslpr mice. CD47−/−–Faslpr mice also display less pronounced splenomegaly than Faslpr mice. The mechanistic studies further suggest that CD47 deficiency impairs the antigenic challenge-induced production of IgG but not IgM, and that this effect is associated with reduction of T follicular cells and impairment of germinal center development in lymphoid tissues. In conclusion, our results demonstrate that CD47 deficiency ameliorates lupus nephritis in Faslpr mice via suppression of IgG autoantibody production.
Keywords: CD47, autoimmunity, antibody production, T follicular cell
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the increased production of multiple autoantibodies and multi-organ damage. The kidneys are the most commonly affected organs in SLE and lupus nephritis is a frequent and potentially fatal complication [1]. Despite improvements in the prognosis over the past 30 years, lupus still progresses to end-stage renal disease within 10 years after initial diagnosis in 10–15% of patients [2]. The pathogenic cascade that mediates renal damage and kidney failure has been correlated with hyperactivities of T- and B-lymphocytes, production of autoantibodies, and formation of immune complexes, which deposit in kidney tissues causing subsequent inflammation. Given that many autoantibodies in SLE exhibit high affinity and somatic mutations [3, 4], the activation of auto-reactive B cells is probably preceded by the activation of auto-reactive T cells, which provide helper signals. Nucleosomes, ribonucleoprotein, and Sm-B are among the candidate immunogens for inducing pathogenic T cells and autoantibodies in SLE [5–7]. In addition to various autoantibodies, inflammation and proinflammatory cytokines, such as interleukin-1β (IL-1β), play key roles in renal damage in SLE [8–10].
CD47, a broadly expressed immunoglobulin superfamily transmembrane protein, influences immune responses at multiple levels and plays an important role in autoimmunity [11, 12]. Deficiency of CD47 in mice (CD47−/−) produces resistance to myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis (EAE), and this resistance is associated with reduction of T cells and antigen-presenting cells [13]. CD47−/− mice also display less severe bacteria- and LPS-induced lung inflammation [14], and dextran sulfate sodium-induced and trinitrobenzene sulfonic acid-induced colitis [15, 16]. Decreases in IL-17 induction resulting in low granulopoiesis and reduced PMN infiltration are attributable to the decreased tissue damage in colitis observed in CD47−/− mice [16]. A recent study further demonstrated that CD47−/− mice display increased incidence of autoimmune diabetes in a TCR transgenic mouse model [17]. Deficiency of CD47 on T cells has also been shown to promote Th1 phenotypic differentiation [18]. In addition to these studies of CD47 function by genetic depletion, antibody ligation of CD47 on leukocytes has suggested that CD47 regulates the transmigration of leukocytes, particularly neutrophils, across endothelial and epithelial monolayers [16, 19]. Ligation of CD47 by extracellular thrombospondin-1 is associated with down-regulation of IL-12 production by antigen-presenting cells [20], and inhibits human naive T cell differentiation to Th1 cells but not to Th2 cells [21]. Serving as a marker for self-recognition, CD47 interacting with its counter-receptor SIRPα expressed on myeloid cells initiates SIRPα-based inhibitory signaling that restrains myeloid leukocyte function [12]. CD47 also plays a role in dendritic cell (DC) recruitment into draining lymph nodes and the spleen, where T cell priming and immune responses are initiated [22]. However, although CD47 has been found to be involved in modulation of both innate and adaptive immune responses, its role in regulating the activation of autoreactive T and B cells and the production of autoantibodies in autoimmune disorders, in particular in SLE, remains unknown.
Mice deficient in the cell-surface Fas receptor (Faslpr) have long been used as a model for studying autoimmune diseases, especially lupus. Faslpr mice develop lupus nephritis, with high levels of autoantibodies and complement deposition in the glomeruli, increases in glomerular sclerosis and mesangial proliferation, thickening of the glomerular basement membrane, and proteinuria. In this study, we bred CD47−/− mice with Faslpr mice and produced animals deficient in both CD47 and Fas (CD47−/−–Faslpr).
Materials and Methods
Mice
Faslpr (B6.MRL-Faslpr/J) and CD47−/− (B6.129S7-Cd47tm1Fpl/J) mice and genetic background-matched wild-type (WT) C57BL/6J animals were purchased from the Jackson Laboratory and housed in a pathogen-free facility with free access to autoclaved water and food. To generate CD47−/−–Faslpr mice, Faslpr mice were cross-bred with CD47−/− mice, and the resulting heterozygous mice were further crossed to produce homozygous animals. PCR was performed to determine Faslpr (primers dGTAAATAATTGTGCTTCGTCAG, dTAGAAAGGTGCACGGGTGTG and dCAAATCTAGGCATTAACAGTG) and CD47−/− (dCTTGGGTGGAGAGGCTATTC, dAGGTGAGATGACAGGAGATC, dCACGTTTCAAAACAGGCAAA dCAAGCATAAATGAACAGTTGCAG) genotypes.
Statistical analysis
All images of immunofluorescence labeling, FACS and Western blots represent the results of at least three independent experiments. Data are represented as the mean ± SEM for three or more independent experiments. The log-rank test was used to determine differences in survival between groups. Differences were considered statistically significant if P<0.05, as analyzed using Student’s t-tests for paired samples and one-way ANOVA for groups (k) >2.
RESULTS
Prolonged survival and mitigation of autoimmunity in CD47−/−–Faslpr mice
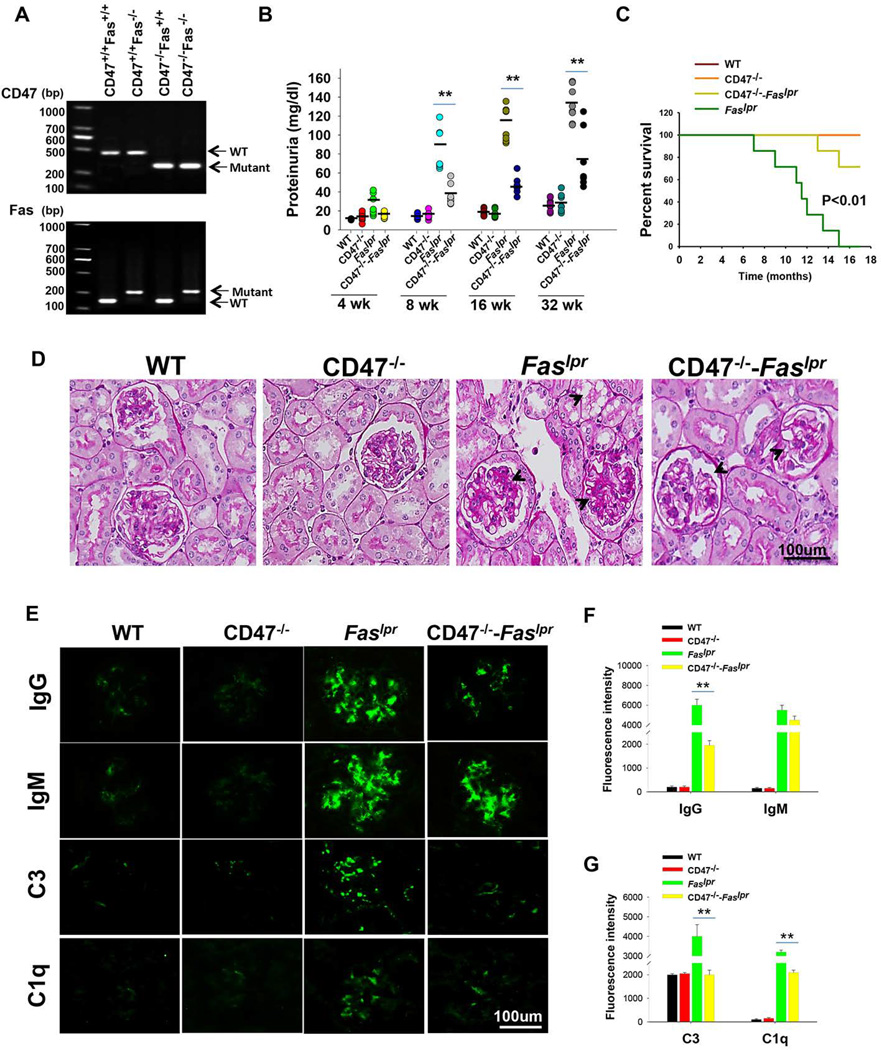
Systemic lupus often affects the kidney, resulting in proteinuria, glomerular inflammation and increased patient mortality. To determine if CD47 plays a role in the development of nephritis in lupus-prone Faslpr mice, we crossed CD47−/− mice with Faslpr mice and generated double-mutant CD47−/−–Faslpr mice (Figure 1A). We first compared lupus development in Faslpr mice with or without a CD47 knockout. In agreement with previous findings [23–25], Faslpr mice spontaneously developed lethal glomerular disease accompanied by various immunologic abnormalities. As shown in Figure 1B, at the age of 2 months, ~20% Faslpr mice presented pathological levels of proteinuria (total protein ≥100mg/dl). As mice further matured, the kidney disease condition aggravated and, by the age of 8 months, 100% of Faslpr mice displayed pathological levels of proteinuria. In contrast, all CD47−/−–Faslpr mice maintained proteinuria in the normal range (≤100mg/dl) at the 2-month age and only ~25% showed pathological proteinuria by 8 months. The finding that CD47−/−–Faslpr mice exhibited lower proteinuria than Faslpr mice suggests that absence of CD47 in Faslpr mice hindered nephritis progression. Indeed, all CD47−/−–Faslpr mice survived to the age of 12 months, while over 60% of Faslpr mice succumbed to fatal glomerulonephritis (Figure 1C).
Figure 1.
Prolonged survival and mitigation of autoimmunity in CD47−/−–Faslpr mice. A) Genotyping by PCR. B) Proteinuria. Starting from age of 4 weeks (WK), mice were monitored for proteinuria at various time points (n=7). C) Cumulative survival of WT, CD47−/−Faslpr and CD47−/−–Faslpr mice (n=7). The difference was significant (P<0.01, log-rank test). D) PAS staining of kidney sections at age of 8 months (n=7). E) Immune staining of deposition of IgG, IgM, C3 and C1q in the glomeruli at age of 8 months (n=7). F) Quantitative analysis of IgG and IgM level, using 12–15 images collected from three mice. G) Quantitative analysis of C3 and C1q deposition in glomeruli, using 12–15 images collected from three mice. **, P<0.01.
Histopathologic examination of kidneys from 8-month-old CD47−/−–Faslpr and Faslpr mice demonstrated that Faslpr mice displayed a pattern of glomerulonephritis typically found in systemic lupus, with increased glomerular hypercellularity, abnormal thickening of the glomerular basement membrane and mesangial cell proliferation (Figure 1D). The kidney disease in Faslpr mice was also associated with significant tubulointerstitial changes, including acute tubular injury and vacuolization (Figure 1D, arrow head). In contrast, glomerular lesions in CD47−/−–Faslpr mice of the same age were mild, associated with only a low degree of mesangial cell proliferation and only slight thickening of the basement membrane.
Excess of antoantibodies and their deposition in the kidneys play a key role in the pathogenesis of murine and human lupus nephritis [26, 27]. Not only antibodies, but also deposition of other immune complexes including complements in glomeruli are associated with kidney disease [28–31]. Labeling kidney tissue sections with antibodies against IgG, or IgM, or complement C3 or C1q revealed no such immune complexes in the glomeruli of WT or CD47−/− mice. In contrast, high intensities of antibody labeling for IgG, IgM, C3 and C1q, indicating significant deposition of these immune complexes, were observed in the glomeruli of Faslpr mice (Figure 1E–G). CD47−/−–Faslpr mice showed marked reduction of IgG, C3 and C1q deposits in the glomeruli compared to that in Faslpr mice. However, the levels of IgM deposition were only moderately reduced in CD47−/−–Faslpr mice.
CD47 deficiency alleviates splenomegaly and reduces autoantibody production in Faslpr mice
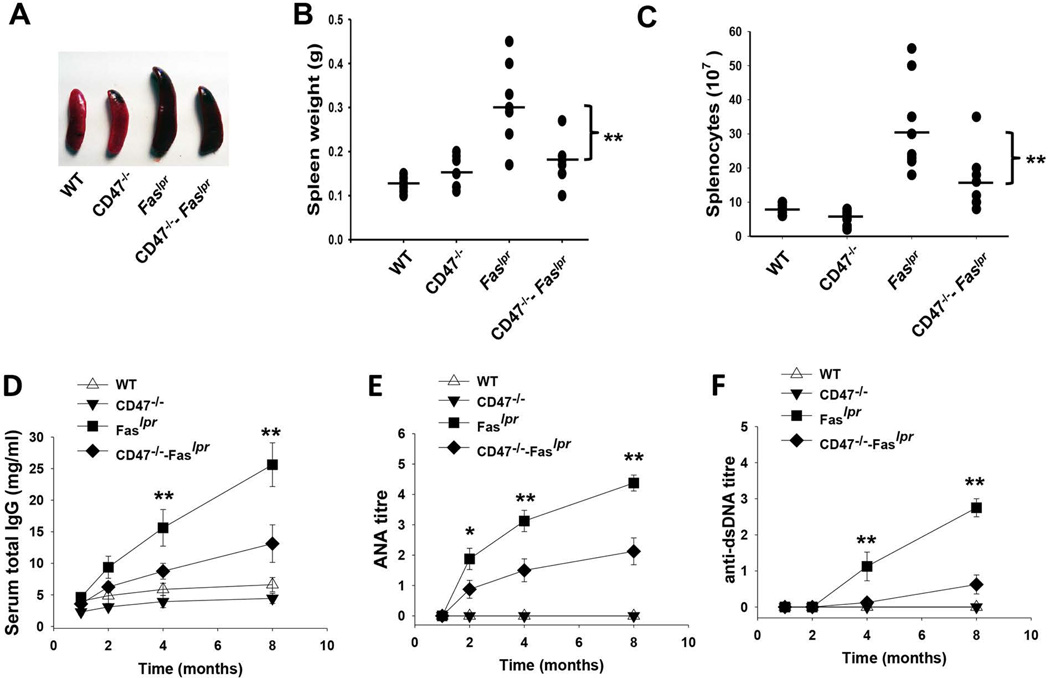
We found that deficiency of CD47 largely reversed the splenomegaly in Faslpr mice. Spleens from 8-month-old Faslpr mice were significantly enlarged compared to those from healthy WT mice (0.31±0.1 g vs. 0.13±0.03 g; Figure 2A–B). There was significantly less splenomegaly (0.19±0.08g) in 8-month-old CD47−/−–Faslpr mice than Faslpr mice without CD47 deficiency. The CD47 deficiency-associated reduction of splenomegaly in CD47−/−–Faslpr mice was also accompanied by a 2.7-fold reduction of splenocytes (1.2±0.3 × 108 cells) compared to Faslpr mice (3.2±1.2 × 108 cells) (Figure 2C).
Figure 2.
Ablation of CD47 mitigates splenomegaly and antibody deposition in CD47−/−–Faslpr mice. A–B) Photographs and weight comparison (n=7) of spleens from WT, CD47−/−, Faslpr and CD47−/−–Faslpr mice at 8 months. C) Splenocyte numbers in WT, Faslpr and CD47−/−–Faslpr mice (n=7). D–F) Kinetics of total serum IgG level (D), serum ANA level (E) and serum anti-dsDNA antibody level (F) in WT, CD47−/−, Faslpr and CD47−/−–Faslpr mice (n=7).
Mature Faslpr mice have high levels of autoreactive lymphocytes and excessive autoantibodies against double- or single-stranded DNA (dsDNA or ssDNA) autoantigens, and these correlate with lupus-associated symptoms [32, 33]. We next examined antinuclear antibodies (ANA) and anti-dsDNA IgG antibodies in the serum. Compared to WT mice and CD47−/− mice (in which IgG levels were low and were not specific towards ANA or dsDNA), Faslpr mice produced high levels of total IgG, ANA, and anti-dsDNA IgG after 2 months (Figure 2D–F). Both the total IgG and autoreactive IgGs increased with age. Although CD47−/−–Faslpr mice produced elevated IgG and autoreactive IgG, their levels as seen in ANA, and anti-dsDNA IgG were much lower compared to those in Faslpr mice. The reduction of autoreactive IgG in CD47−/−–Faslpr mice resulted in decreases in IgG deposition in the kidney glomeruli as shown in Figure 1E–F. Consistent with the tissue staining results shown in Figure 1, total IgM levels in CD47−/−–Faslpr mice were similarly elevated as those in Faslpr mice (data not shown).
CD47 deficiency causes impairment of IgG but not IgM production in mice following specific antigen challenge
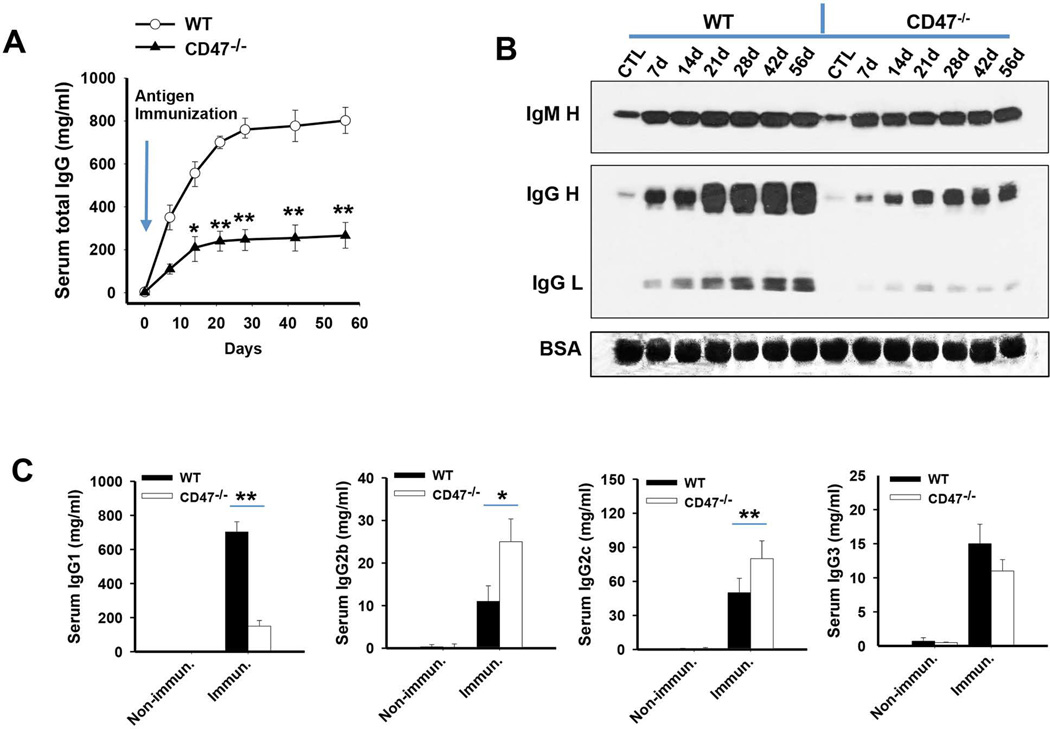
To explore how CD47 deficiency suppresses autoantibody production and attenuates the autoimmune condition in Faslpr mice, we explored the role of CD47 in modulating B cell activation and antibody production using CD47−/− mice. First, CD47−/− mice and WT mice were immunized with chicken ovalbumin or a GST fusion protein with complete Freund’s adjuvant, followed by boosters with the same protein with incomplete Freund’s adjuvant. WT mice but not CD47−/− mice produced high levels of IgG in response to antigen challenge; before challenge, serum IgG was 2.3 ± 0.6 mg/ml and 4.0 ± 1.6 mg/ml in CD47−/− and WT mice, respectively, while after four rounds of immunizations, levels were increased to 267.4 ± 86.5 mg/ml and 802.6 ± 85.7 mg/ml respectively (Figure 3A). Thus, compared to WT mice, CD47−/− mice exhibited impaired production of IgG after immunization. Western blots testing for serum IgG and IgM yielded similar results, CD47−/− mice manifesting significantly lower levels of IgG after immunization compared to WT mice, whereas IgM in CD47−/− mice was comparable to that in WT mice (Figure 3B). We further tested the isotypes of IgG following immunization. Since C57BL/6 mice were reported to produce IgG2c but not IgG2a, [34, 35], we detected IgG1, IgG2b, IgG2c and IgG3. On immunization, CD47−/− mice produced approximately 2-fold less IgG1, but higher levels of IgG2b and IgG2c, than WT mice (Figure 3C). These results agree with a previous report that CD47 ablation promotes a Th1-biased response, leading to IgG2 family isotypes (but not IgG1, which is related to Th2 response) [18]. No difference in IgG3 production between CD47−/− mice and WT mice was detected. Although IgG2 antibodies were elevated in CD47−/− mice, the reduction of IgG1, the predominant IgG, resulted in an overall IgG reduction in CD47−/− mice upon immunization.
Figure 3.
Absence of CD47 impairs mouse IgG but not IgM response following immunization. Six male WT or CD47−/− mice were immunized i.p. with peptide, followed by boosters on days 21, 35 and 49, with serum collection on day 7 after each immunization. A) Serum IgG measured by ELISA. B) Serum IgM and IgG levels asssessed by Western blotting C) Serum IgG1, IgG2b, IgG2c and IgG3 levels measured by ELISA. *, P<0.05; **, P<0.01.
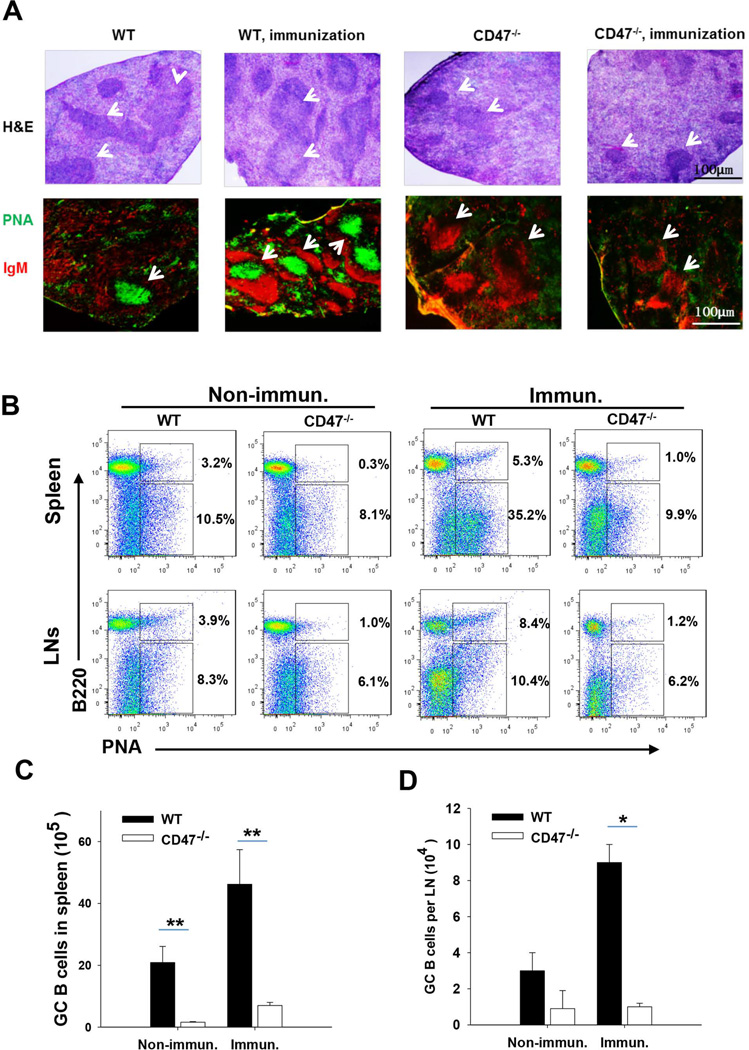
Given that germinal center (GC) development in lymphoid tissues is crucial for production of high affinity, class-switched antibodies, we asked if the failure of IgG production after immunization in CD47−/− mice is related to a defect of GCs. Spleens from WT and CD47−/− mice with and without peptide immunization were analyzed. As shown in Figure 4A, in the WT spleen, both H/E staining and staining with peanut agglutinin (PNA, green), a reagent specifically labeling GC, revealed development of GCs that appeared to be the lighter areas in the center of white pulp (H/E, arrows), and were wrapped by IgM+ lymphocytes (red). The GC structures were much increased in size and numbers after peptide immunization in WT mice, suggesting antigen-induced GC development. In contrast, the spleens of CD47−/− mice exhibited negligible GCs, both prior to and after peptide immunization. Spleens from CD47−/− mice displayed fewer and smaller white pulp areas, in which lymphocytes were densely packed and showed no lightly stained areas suggestive of GC structures (Figure 4A, H/E staining). There was nearly complete lack of PNA labeling, but positive labeling for anti-IgM, suggesting deficit of GC structures. Flow cytometry confirmed that CD47−/− mice were lacking GC-specific B cells (B220+PNAhi) in the spleen and LNs even after the peptide immunization (Figure 4B). These results were consistent with the serum detection and western blotting, both indicating that CD47−/− mice were deficient in the high affinity, class-switched antibodies, especially IgG, but not IgM. We also found that the total numbers of cells in the spleen, especially T and B lymphocytes, and in lymph nodes (LNs) were reduced (~ 25%) in CD47−/− mice compared to WT controls (Supplemental data, Table 1). Taken together, these results show that GC development in CD47−/− mice is severely impaired.
Figure 4.
Impairment of GC formation and reaction in immunized CD47−/− mice. A) Sections of spleen white pulp were stained with anti-IgM mAbs (red) and peanut agglutinin (PNA; green) to show GC architecture. B) Flow cytometry data displaying the frequency of GC B cells in spleen and lymph nodes in WT and CD47−/− mice (n=5). Cells were stained with FITC-PNA and PE-B220 mAb. C–D) The numbers of GC B cells in the spleen (C) and lymph nodes (D) from immunized WT and CD47−/− mice. *, P<0.05; **, P<0.01.
Impairment of intrinsic CD4+CXCR5+ T follicular helper (Tfh) cell production in CD47−/− mice
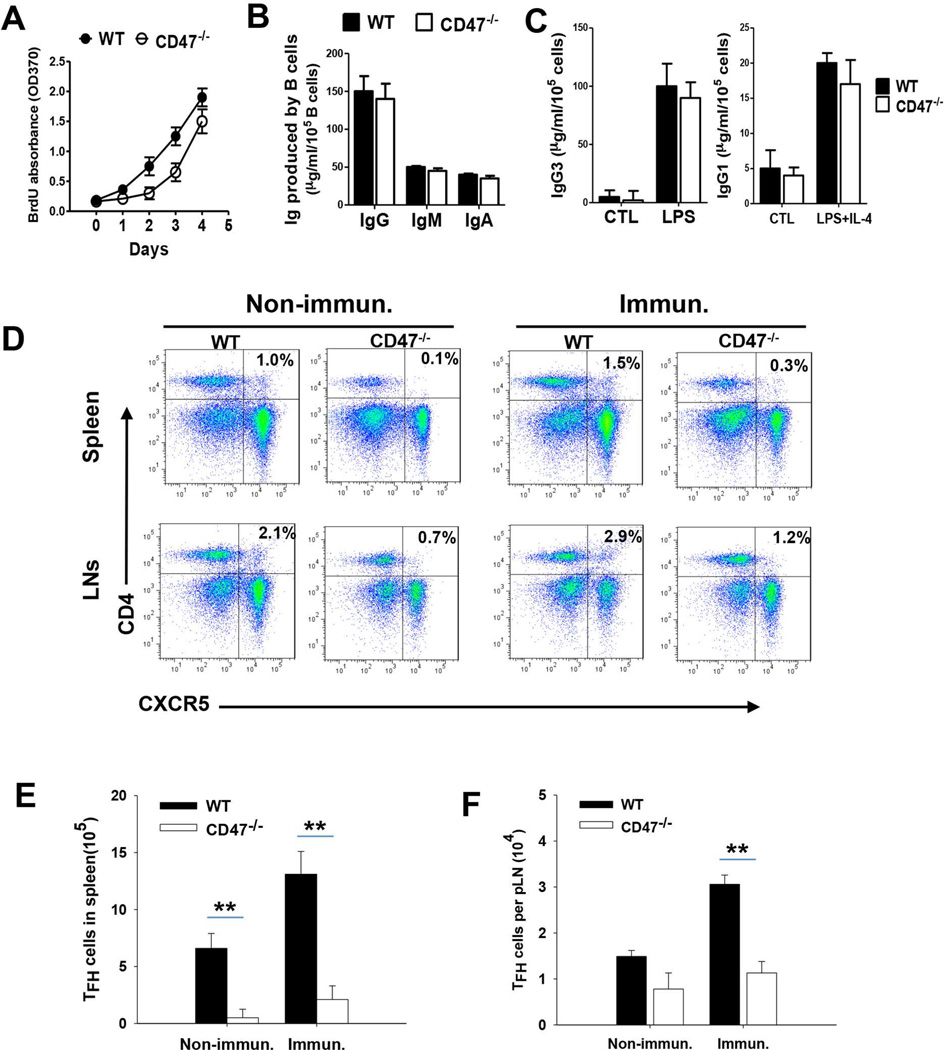
There are several possible explanations for the impaired GC development and low IgG but normal IgM response in CD47−/− mice. One is that CD47 deficiency directly affects B cell division and/or the capacity for immunoglobulin isotype switching upon antigen stimulation. Another possibility may be related to Tfh cells, the essential Th cells that support the antigen-induced GC development and B cells for Ig isotype switch [36–41]. To test if B cells had intrinsic defects secondary to CD47 deficiency, we harvested splenocytes from CD47−/− and WT mice and induced B cell proliferation ex vivo by LPS. As shown in Figure 5A, compared to splenic B cells from WT mice, CD47−/− B cells exhibited delayed onset of proliferation (day 1–3) upon LPS stimulation, indicated by the limited BrdU incorporation into B cells (labeled by PE-anti-B220). However, once the cell proliferation started, CD47−/− B cells showed fast proliferation and, by day 4, produced B cell numbers comparable to that of WT. Similar amounts of IgG, IgM and IgA were produced by CD47−/− and WT B cells (Figure 5B). To test if CD47−/− B cells were inefficient at antibody class switching, we further performed ummunoglobulin class switch recombination (CSR) assays and stimulated B cells with LPS and IL-4. Similar levels of IgG1 and IgG3 were detected from WT and CD47−/− B cells stimulated with LPS plus IL-4 (Figure 5C), suggesting that CD47−/− B cells have normal CSR activities.
Figure 5.
CD47 deficiency decreases the production of Tfh cells. Splenocytes from WT and CD47−/− mice were stimulated with LPS in the presence or absence of IL-4 for 4 days. A) Splenic B cell proliferation was assessed using BrdU assay in vitro after LPS stimulation. B) IgG, IgM and IgA in the culture medium of splenocytes stimulated with LPS were detected by ELISA (n=5). C) IgG1 and IgG3 antibodies were measured by ELISA (n=5). D–F) Male WT or CD47−/− mice (n = 4 to 6) were immunized i.p. with peptide in CFA/IFA, followed by splenocyte collection at day 7 after the fourth immunization. D) The frequency of follicular T cells in spleen and lymph nodes stained with APC-CD4 mAb and PE-CXCR5 mAb. E–F) The number of CD4+ CXCR5+ T follicular cells in spleen (E) and lymph nodes (F). **, P<0.01.
We then analyzed Tfh cells from WT and CD47−/− mice prior to and after peptide immunization. As shown in Figure 5 (D–F), double staining with anti-CD4 and anti-CXCR5 antibodies demonstrated markedly fewer Tfh cells (~60% decreases) in spleens and LNs from CD47−/− mice compared to those from WT mice. Also, although peptide immunization induced increases in total splenocytes and LN cells in both WT and CD47−/− mice, CD4+CXCR5+ Tfh cells remained low in CD47−/− mice (Figure 5E–F). As Tfh cells are essential for triggering GC formation, it could thus be expected that lower numbers of Tfh cells in spleens and LNs in CD47−/− mice would lead to a deficiency of GC development. These results therefore suggest that inability to induce Tfh cells is likely the mechanism for impaired GC development and IgG production in CD47−/− mice upon antigen challenge.
Anti-CD47 antibody impairs IgG production in WT mice but exacerbates glomerulonephritis in Faslpr mice
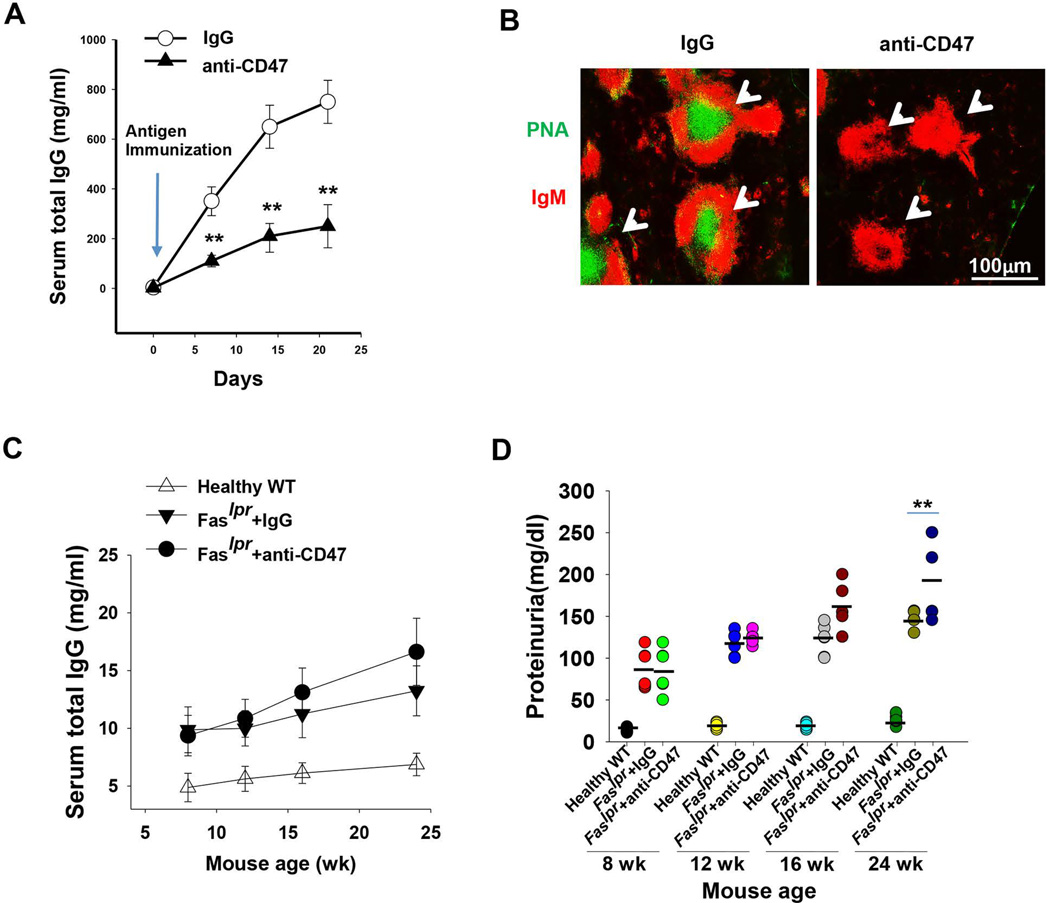
Based on the finding that CD47 deficiency ameliorates lupus nephritis in Faslpr mice by suppressing IgG autoantibody production, we next tested whether inhibition of CD47 in WT mice by an inhibitory anti-CD47 antibody had a similar effect on antibody production. In these experiments, we administrated healthy WT mice with a murine-specific anti-CD47 antibody (mIAP301), or a control IgG (100 µg, i.p. on alternate days) seven days before and after immunization (1 x). Lower IgG levels were detected in mice administered with anti-CD47 antibody compared to mice administered with control, non-specific IgG (Figure 6A). Mice that received anti-CD47 antibody also displayed an impaired germinal center development induced by antigen immunization compared to those receiving control IgG (Figure 6B). However, administration of the same anti-CD47 antibody into 8- and 16-week old Faslp mice, which were displaying lupus symptoms, failed to reverse the abnormally increased antibody production and proteinuria (Figure 6, C–D). Instead, and to our surprise, the anti-CD47 antibody administration resulted in further increased serum IgG levels and worse proteinuria (Figure 6, C–D), suggesting aggravated kidney damage and exacerbated lupus.
Figure 6.
Blocking of CD47 by anti-CD47 antibody impaired IgG production in peptide/CFA-immunized WT mice but aggravated glomerulonephritis in Faslpr mice. In each experiment, six male WT or CD47−/− mice were treated with IgG control or anti-CD47 antibodies followed by peptide in CFA/IFA immunization. A) Serum IgG levels measured by ELISA every 7 days after immunization. B) Impairment of GC formation and reaction in immunized mice treated with anti-CD47 antibody on day 7. The sections of mouse spleen white pulp were stained with anti-IgM mAbs (red) and peanut agglutinin (PNA; green) to show GC architecture. C) Kinetics of total serum IgG levels in mice treated with IgG control or anti-CD47 antibodies after 8 weeks (wk). D) Proteinuria levels assayed by BCA methods. The results in panel A–D were presented as mean ± SE from five independent experiments. **, P<0.01.
DISCUSSION
Autoantibody production is one of the hallmarks of systemic lupus erythematosus. Although many factors, such as failure of B cell and T cell tolerance, increased or abnormal presentation of autoantigens, and defects in cell death and apoptotic clearance [42] have been identified to be involved in the overproduction of antoantibodies, the underlying molecular mechanisms remain unknown. In this study, we demonstrate that CD47 plays an important role in the production of high-affinity antibodies, and that CD47-knockout in Faslpr mice reduces autoantibodies and protects mice against autoimmune lupus.
It has been well documented that GCs, the essential sites for B lymphocyte activation, immunoglobulin isotype class switch, and B cell proliferation, are crucial for producing mature immune responses, including high affinity IgG antibodies, plasma cells and memory B cells [41]. We have found that mice with CD47 deficiency display a severe defect in heavy chain class switch to IgG during humoral immune response, and that this defect is associated with incapability to induce GC formation upon antigen immunization. However, CD47 deficiency does not affect B cell production of IgM, nor does it directly impair B cell response and proliferation. B cell proliferation and production of various antibodies, including class-switched high affinity IgGs, can be successfully induced upon ex vivo stimulation with LPS and IL-4. We found that reduction of Tfh cells is likely to be the reason for impairment of GC and the reduction of IgG in CD47−/− mice. Tfh cells are a specialized subset of CD4+ T cells that through secretion of cytokines and cell surface engagement, promote B cells to produce affinity-matured antibodies [43]. Tfh cell number and Tfh activity directly correlate with GC formation [41]. However, in CD47−/− mice, Tfh cell levels in the spleen and LNs were low and could not be elevated even with peptide immunization. Van et al.[22] reported that the lack of CD47 curtails the migration of DC from the peripheral tissue to central lymphoid organs, and as a result reduces antigen-specific T-cells under steady state and inflammatory conditions. The same poor DC function in CD47−/− mice may also account for the decrease in Tfh cells and the subsequent failure of GC formation.
Whereas CD47 knockout in Faslpr mice leads to reduction of IgG autoantibodies and mitigation of lupus nephritis, anti-CD47 antibody administration to Faslpr mice aggravates glomerulonephritis. The discrepancy may imply that the anti-CD47 antibody, through breaking the CD47-SIRPα interaction, activates macrophage inflammatory function and phagocytosis of self-cells in kidney tissues. Given that macrophages play an essential role in kidney inflammation and renal failure [45, 46], enhancing macrophage inflammatory function and phagocytosis may directly increase renal tissue damage and exacerbate nephritis in Faslpr mice.
Recently, CD47 interaction with its counter-receptor SIRPα has been proposed to function in autoimmune diseases other than lupus. CD47−/− mice were protected from colitis [15], and the underlying mechanism may be that CD47 is required to promote the migration of a pathogenic SIRPα positive dendritic cell subset that drives Th17-biased response. The resistance to colitis of CD47−/− mice was confirmed by our previous study [16]; however, our results suggest that this is due to attenuated granulopoiesis and paucity of PMN infiltration. Employing CD47−/− mice and monoclonal anti-CD47 antibody, Han et al. [13] reported a Janus-like opposing effect of CD47 on experimental autoimmune encephalomyelitis (EAE). They showed that CD47−/− mice are refractory to EAE after immunization with myelin antigen. However, blocking CD47 with a monoclonal antibody at the peak of paralysis worsened the EAE and enhanced immune activation in the peripheral immune system. As macrophage phagocytosis through Fc within the active MS lesion was increased by anti-CD47 antibody, which likely disrupted the CD47-SIRPα interaction, increasing macrophage phagocytic activity may contribute to the aggravation of EAE in the presence of anti-CD47. This finding, together with our observations, suggest that anti-CD47 diminishes IgG production in peptide-immunized WT mice, but exacerbates glomerulonephritis in Faslpr mice through different mechanisms. In contrast to the above findings that CD47−/− mice are refractory to autoimmune diseases following immunization, Dugas et al.[17] found that CD47 deficiency provoked the onset of autoimmune diabetes via reduction in the number of regulatory (Treg)-like CD4−CD8− T cells. Taken together, these studies suggest that the immune regulation mediated by CD47 signaling in autoimmune inflammatory disease is complicated, and that CD47 may have different functional roles depending on the cell type, the tissue location and the disease stage.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Jill Leslie Littrell (Georgia State University, Atlanta, GA) for critical reading and constructive discussion of the manuscript. This work was supported, in part, by National Institutes of Health Grant (AI106839) and a Research Scholar Grant from the American Cancer Society (YL), National Basic Research Program of China (973 Program, 2012CB517603 and 2011CB504803, KZ), and the program of China Scholarships Council (No. 201406190123, LS)
Footnotes
Author Contribution Statement
YL initiated the study, and together with KZ designed and supervised the entire project. LS and ZB carried out the animal experiments and they received help, especially for lupus analyses, from CZ (Caihong Zeng) and ZL (Zhihong Liu), both being doctors specialized in autoimmune kidney diseases. Celia X-J Chen did in vitro B cell studies. Ya-Nan Guo and Zhiyuan Lv contributed to animal breeding, genotyping, tissue sectioning and staining, ect. All authors were involved in paper writing and had approved the submitted version.
CONFLICT OF INTEREST
All authors promise that there is no conflict interest to disclose.
REFERENCES
- 1.Cameron JS. Lupus nephritis. J Am Soc Nephrol. 1999;10:413–424. doi: 10.1681/ASN.V102413. [DOI] [PubMed] [Google Scholar]
- 2.Faurschou M, Dreyer L, Kamper AL, et al. Long-term mortality and renal outcome in a cohort of 100 patients with lupus nephritis. Arthritis Care Res (Hoboken) 2010;62:873–880. doi: 10.1002/acr.20116. [DOI] [PubMed] [Google Scholar]
- 3.William J, Euler C, Christensen S, et al. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 4.Wellmann U, Letz M, Herrmann M, et al. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci U S A. 2005;102:9258–9263. doi: 10.1073/pnas.0500132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohan C, Adams S, Stanik V, et al. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993;177:1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monneaux F, Hoebeke J, Sordet C, et al. Selective modulation of CD4+ T cells from lupus patients by a promiscuous, protective peptide analog. J Immunol. 2005;175:5839–5847. doi: 10.4049/jimmunol.175.9.5839. [DOI] [PubMed] [Google Scholar]
- 7.Talken BL, Holyst MM, Lee DR, et al. T cell receptor beta-chain third complementarity-determining region gene usage is highly restricted among Sm-B autoantigen-specific human T cell clones derived from patients with connective tissue disease. Arthritis Rheum. 1999;42:703–709. doi: 10.1002/1529-0131(199904)42:4<703::AID-ANR13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Munoz LE, Janko C, Grossmayer GE, et al. Remnants of secondarily necrotic cells fuel inflammation in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1733–1742. doi: 10.1002/art.24535. [DOI] [PubMed] [Google Scholar]
- 9.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan DC, Yui MA, Wuthrich RP, et al. Tumor necrosis factor and IL-1 in New Zealand Black/White mice. Enhanced gene expression and acceleration of renal injury. J Immunol. 1989;143:3470–3475. [PubMed] [Google Scholar]
- 11.van den Berg TK, van der Schoot CE. Innate immune 'self' recognition: a role for CD47-SIRPalpha interactions in hematopoietic stem cell transplantation. Trends Immunol. 2008;29:203–206. doi: 10.1016/j.it.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014;32:25–50. doi: 10.1146/annurev-immunol-032713-120142. [DOI] [PubMed] [Google Scholar]
- 13.Han MH, Lundgren DH, Jaiswal S, et al. Janus-like opposing roles of CD47 in autoimmune brain inflammation in humans and mice. J Exp Med. 2012;209:1325–1334. doi: 10.1084/jem.20101974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su X, Johansen M, Looney MR, et al. CD47 deficiency protects mice from lipopolysaccharide-induced acute lung injury and Escherichia coli pneumonia. J Immunol. 2008;180:6947–6953. doi: 10.4049/jimmunol.180.10.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortin G, Raymond M, Van VQ, et al. A role for CD47 in the development of experimental colitis mediated by SIRPalpha+CD103- dendritic cells. J Exp Med. 2009;206:1995–2011. doi: 10.1084/jem.20082805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bian Z, Guo Y, Luo Y, et al. CD47 deficiency does not impede polymorphonuclear neutrophil transmigration but attenuates granulopoiesis at the postacute stage of colitis. J Immunol. 2013;190:411–417. doi: 10.4049/jimmunol.1201963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dugas V, Beauchamp C, Chabot-Roy G, et al. Implication of the CD47 pathway in autoimmune diabetes. J Autoimmun. 2010;35:23–32. doi: 10.1016/j.jaut.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Bouguermouh S, Van VQ, Martel J, et al. CD47 expression on T cell is a self-control negative regulator of type 1 immune response. J Immunol. 2008;180:8073–8082. doi: 10.4049/jimmunol.180.12.8073. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Merlin D, Burst SL, et al. The role of CD47 in neutrophil transmigration - Increased rate of migration correlates with increased cell surface expression of CD47. Journal of Biological Chemistry. 2001;276:40156–40166. doi: 10.1074/jbc.M104138200. [DOI] [PubMed] [Google Scholar]
- 20.Armant M, Avice MN, Hermann P, et al. CD47 ligation selectively downregulates human interleukin 12 production. J Exp Med. 1999;190:1175–1182. doi: 10.1084/jem.190.8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avice MN, Rubio M, Sergerie M, et al. CD47 ligation selectively inhibits the development of human naive T cells into Th1 effectors. Journal of Immunology. 2000;165:4624–4631. doi: 10.4049/jimmunol.165.8.4624. [DOI] [PubMed] [Google Scholar]
- 22.Van VQ, Lesage S, Bouguermouh S, et al. Expression of the self-marker CD47 on dendritic cells governs their trafficking to secondary lymphoid organs. EMBO J. 2006;25:5560–5568. doi: 10.1038/sj.emboj.7601415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mixter PF, Russell JQ, Durie FH, et al. Decreased CD4-CD8- TCR-alpha beta + cells in lpr/lpr mice lacking beta 2-microglobulin. J Immunol. 1995;154:2063–2074. [PubMed] [Google Scholar]
- 24.Thacker SG, Duquaine D, Park J, et al. Lupus-prone New Zealand Black/New Zealand White F1 mice display endothelial dysfunction and abnormal phenotype and function of endothelial progenitor cells. Lupus. 2010;19:288–299. doi: 10.1177/0961203309353773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lech M, Weidenbusch M, Kulkarni OP, et al. IRF4 deficiency abrogates lupus nephritis despite enhancing systemic cytokine production. J Am Soc Nephrol. 2011;22:1443–1452. doi: 10.1681/ASN.2010121260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz N, Goilav B, Putterman C. The pathogenesis, diagnosis and treatment of lupus nephritis. Curr Opin Rheumatol. 2014;26:502–509. doi: 10.1097/BOR.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterner RM, Hartono SP, Grande JP. The Pathogenesis of Lupus Nephritis. J Clin Cell Immunol. 2014;5 doi: 10.4172/2155-9899.1000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.French LE, Tschopp J, Schifferli JA. Clusterin in renal tissue: preferential localization with the terminal complement complex and immunoglobulin deposits in glomeruli. Clin Exp Immunol. 1992;88:389–393. doi: 10.1111/j.1365-2249.1992.tb06459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bao L, Haas M, Kraus DM, et al. Administration of a soluble recombinant complement C3 inhibitor protects against renal disease in MRL/lpr mice. J Am Soc Nephrol. 2003;14:670–679. doi: 10.1097/01.asn.0000051597.27127.a1. [DOI] [PubMed] [Google Scholar]
- 30.Tan Y, Song D, Wu LH, et al. Serum levels and renal deposition of C1q complement component and its antibodies reflect disease activity of lupus nephritis. BMC Nephrol. 2013;14:63. doi: 10.1186/1471-2369-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trouw LA, Groeneveld TW, Seelen MA, et al. Anti-C1q autoantibodies deposit in glomeruli but are only pathogenic in combination with glomerular C1q-containing immune complexes. J Clin Invest. 2004;114:679–688. doi: 10.1172/JCI21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao Z, Duncan GS, Seagal J, et al. Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity. 2008;29:615–627. doi: 10.1016/j.immuni.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 34.Morgado MG, Cam P, Gris-Liebe C, et al. Further evidence that BALB/c and C57BL/6 gamma 2a genes originate from two distinct isotypes. EMBO J. 1989;8:3245–3251. doi: 10.1002/j.1460-2075.1989.tb08484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Methods. 1998;212:187–192. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 36.Liu YJ, Johnson GD, Gordon J, et al. Germinal centres in T-cell-dependent antibody responses. Immunology Today. 1992;13:17–21. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- 37.McHeyzer-Williams LJ, Malherbe LP, McHeyzer-Williams MG. Helper T cell-regulated B cell immunity. Curr Top Microbiol Immunol. 2006;311:59–83. doi: 10.1007/3-540-32636-7_3. [DOI] [PubMed] [Google Scholar]
- 38.Arnold CN, Campbell DJ, Lipp M, et al. The germinal center response is impaired in the absence of T cell-expressed CXCR5. European Journal of Immunology. 2007;37:100–109. doi: 10.1002/eji.200636486. [DOI] [PubMed] [Google Scholar]
- 39.McHeyzer-Williams M, Okitsu S, Wang N, et al. Molecular programming of B cell memory. Nat Rev Immunol. 2012;12:24–34. doi: 10.1038/nri3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bayry J, Hermine O, Webster DA, et al. Common variable immunodeficiency: the immune system in chaos. Trends Mol Med. 2005;11:370–376. doi: 10.1016/j.molmed.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 41.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 42.Waldman M, Madaio MP. Pathogenic autoantibodies in lupus nephritis. Lupus. 2005;14:19–24. doi: 10.1191/0961203305lu2054oa. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Li L, Jung J, et al. The distinct roles of T cell-derived cytokines and a novel follicular dendritic cell-signaling molecule 8D6 in germinal center-B cell differentiation. J Immunol. 2001;167:49–56. doi: 10.4049/jimmunol.167.1.49. [DOI] [PubMed] [Google Scholar]
- 44.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Kuroiwa T, Lee EG. Cellular interactions in the pathogenesis of lupus nephritis: the role of T cells and macrophages in the amplification of the inflammatory process in the kidney. Lupus. 1998;7:597–603. doi: 10.1191/096120398678920712. [DOI] [PubMed] [Google Scholar]
- 46.Carvalho-Pinto CE, Garcia MI, Mellado M, et al. Autocrine production of IFN-gamma by macrophages controls their recruitment to kidney and the development of glomerulonephritis in MRL/lpr mice. J Immunol. 2002;169:1058–1067. doi: 10.4049/jimmunol.169.2.1058. [DOI] [PubMed] [Google Scholar]
- 47.Li X, Pabla N, Wei Q, et al. PKC-delta promotes renal tubular cell apoptosis associated with proteinuria. J Am Soc Nephrol. 2010;21:1115–1124. doi: 10.1681/ASN.2009070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonzalez-Buitrago JM, Gonzalez C. Present and future of the autoimmunity laboratory. Clin Chim Acta. 2006;365:50–57. doi: 10.1016/j.cca.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 49.Heinke J, Kerber M, Rahner S, et al. Bone morphogenetic protein modulator BMPER is highly expressed in malignant tumors and controls invasive cell behavior. Oncogene. 2012;31:2919–2930. doi: 10.1038/onc.2011.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.