Abstract
Detecting the gaze direction of others is critical for many social interactions. We explore factors that may make the perception of mutual gaze more difficult, including the degradation of the stimulus and simulated vision impairment. To what extent do these factors affect the complex assessment of mutual gaze? Using an interactive virtual head whose eye direction could be manipulated by the subject, we conducted two experiments to assess the effects of simulated vision impairments on mutual gaze. Healthy subjects had to demarcate the center and the edges of the cone of gaze, that is, the range of gaze directions accepted for mutual gaze. When vision was impaired by adding a semi-transparent white contrast reduction mask to the display (Exp. 1), judgments became more variable and more influenced by the head direction (indicative of a compensation strategy). When refractive blur was added (Exp. 1), the gaze cone shrank from 12.9° (no blur) to 11.3° (3-diopter len s), which cannot be explained by a low-level process but might reflect a tightening of the criterion for mutual gaze as a response to the increased uncertainty. However, the overall effects of the impairments were relatively modest. Elderly subjects (Exp. 2) produced more variability but did not differ qualitatively from the younger subjects. In the face of artificial vision impairments, compensation mechanisms and criterion changes allow us to perform better in mutual gaze perception than would be predicted by a simple extrapolation from losses in basic visual acuity and contrast sensitivity.
Keywords: gaze perception, eye contact, head orientation, gaze cone, central vision loss, cataracts
The cone of gaze
The gaze of others is an important signal that regulates social interactions (Kleinke, 1986). The structure of the human eye, with high contrast between the iris and the white sclera, is especially well suited to signal gaze direction to another person (Ando, 2002), and our visual system seems to be particularly capable of processing gaze-related features. Rather surprisingly, however, human observers accept a wide range of gaze directions to constitute mutual gaze, suggesting that the precision of the sensory system is not being fully exploited (Gibson & Pick, 1963). More precisely, the sum of gaze directions that constitute mutual gaze should be thought of as a cone centered on the inter-pupillary point. This gaze cone is about 10° wide. We have established a psychophysical method to measure the direction and width of the gaze cone (for details see Gamer & Hecht, 2007). In the centering task, in which the direction of the gaze cone is obtained, the subject is asked to adjust the eyes of the looker (i.e. the virtual head) via button presses on the key pad until the looker gazes straight (directly) toward the subject. On each trial, the initial gaze direction of the looker is clearly toward the left or the right. In the decentering task, which assesses the width of the gaze cone, the subject is asked to guide the looker‘s eyes either to the left or to the right until the virtual head is about to subjectively stop gazing at the subject. In the centering task, the direction of the gaze cone is expressed as the average gaze deviation from the subject's straight ahead (in degrees). The width of the gaze cone is expressed as the angular difference between the leftward and rightward boundary of the sector within which gaze directions are considered as looking at the subject. Note that the direction and width of the gaze cone do not depend on the eye direction alone, but also on other parameters as well, for instance, the direction of the virtual head with respect to the eyes (Gamer & Hecht, 2007).
Visual impairments
Thus far, the gaze cone has typically been assessed in young observers with normal visual acuity, and in good lighting conditions. We know that the gaze cones measured with the virtual head are similar – albeit somewhat larger - to those obtained with a human looker, and we know that extraneous factors, such as the number of onlookers also looking toward the subject, do affect the cone of gaze (Gamer, Hecht, Seipp & Hiller, 2011; Hecht, Weiland & Boyarskaya, 2011). Here we explore other extraneous factors that may make the perception of mutual gaze more difficult. Specifically, we conducted two experiments to evaluate the effects of stimulus degradations and simulated vision impairments on mutual gaze perception in younger and older normally-sighted observers.
At first sight, it might be thought that vision impairment should negatively affect the cone of gaze. However, although sufficient visual acuity and contrast sensitivity are a prerequisite for the detection of gaze direction, the latter involves higher-level visual processes and judgment. If all other parameters remain unchanged, then decrements in acuity should reduce performance. However, if the higher-level processes change in response to the impairment, then its effects will be much harder to predict. It is not clear to what extent the perceptual system might attempt to cope with the impairment. This could be done by compensation strategies such as increasing the effort dedicated to the task or by searching for other perceptual cues that are correlated with the missing information but still available despite the impairment. Next to effort and information substitution, it is also conceivable that the criterion used for detection of mutual gaze is changed. Thus, the resultant perception of gaze direction need not necessarily suffer with visual impairment; it may or may not be robust in the face of moderate stimulus degradation. We conducted two experiments to assess the effects of vision impairments.
Experiment 1: Simulated vision impairments in young observers
The first experiment tested the hypothesis that degrading the appearance of the virtual head would interfere with mutual gaze perception. Specifically, we predicted that as the level of degradation increased, mutual gaze performance would become more variable and that subjects would be more willing to accept a given gaze as directed toward them (i. e., the gaze cone width would increase). The visibility of the head was reduced in two ways: A virtual semi-transparent white contrast reduction mask was used to decrease the contrast and high spatial frequency content of the stimulus, as illustrated in Figure 1. The other method of reducing visibility was the introduction of dioptric blur, which reduced the visual acuity of the subjects, simulating the situation of people with myopia (short-sightedness) not wearing their glasses or contact lenses.
Figure 1.

The virtual head as seen without the semi-transparent mask (100% transparency, left panel) and with the virtual mask (15 % transparency setting, right panel). In this example, the head is rotated 8° to its left such that the nose points to the subject's right and the virtual eyes are clearly averted to the subject's left. A green letter and arrow indicated the task for each trial (in this case, decenter the eyes to the left).
Methods
The experiment was approved by the Ethics Committee of the Johannes Gutenberg-University Mainz in Germany, where the data were collected. Voluntary, written informed consent was obtained from all subjects after a full explanation of the study procedures.
Subjects
24 students volunteered for the experiment (11 men, 13 women; aged 19-36 years, M = 24.15, SD = 4.53). About half of them received partial course credit for their participation. Most of them were psychology students at the University of Mainz. All had normal or corrected-to-normal visual acuity and contrast sensitivity.
Apparatus and stimuli
A natural-looking human head was rendered using the 3D software Vizard (3.18) (2010) on a DELL Precision 380 computer. It was displayed on a 17-in. flat screen with a resolution of 1,280 × 1,024 pixels. The effective color depth (Graphics card nVIDIA Quadro FX3500) was 24 + 8 bits (8 per channel + 8 alpha). The virtual head and the eyes were intrinsically 3D. We had previously presented the head in stereoscopic 3D and found that the 2D viewing of this stimulus had little effect on the gaze cone (Gamer & Hecht, 2007). Thus, we used the 2D view here. The width of the virtual head was 16.5 cm and the height 25.8 cm with an interpupillary distance of 6.4 cm. This screen size of the virtual head (looker) approximately equaled the visual angle of an adult human head when viewed at 0.5 m. The eyes were rendered independently and could be rotated interactively by pressing two keys. Their movement was confined to the horizontal plane such that the line of sight was level with the subject's eye height. The vergence of the eyes of the virtual head was set such that they always converged at the subject's eye plane. That is, when looking straight ahead, the looker's eyes converged at the subject's interpupillary point. When looking to the side, the virtual eyes would converge on the subject's eye plane to the left or right. The subject was seated at 0.5 m from the screen. A height-adjustable chair combined with a chin rest ensured that the subject's eye height was 1 m at all times. The eye height of the virtual head had been adjusted to the same height by mounting the flat screen on a table.
Simulated impairments
In the dioptric blur condition, subjects wore an optometrist's trial frame, into which lenses of different dioptric values (0, +1.5 and +3 diopters) were inserted. For long distance vision, the 1.5 and 3 diopter lenses would be expected to create about 1.5 and 3 diopters of myopia, respectively. However, at the 0.5 m test distance (where 2 diopters of accommodation is required for a clear image), it was expected that the 1.5 diopter lens would create only a subtle level of blur, while the 3 diopter lens would create more noticeable blur, equivalent to about 1 diopter of uncorrected myopia. In the mask condition, a virtual semi-transparent white mask was placed in front of the virtual head, producing transparency of 15%, which reduced stimulus contrast. The mask was applied to the entire image; it appeared as if fog had set in around the virtual head or the head were being viewed through ground glass (Figure 1). In order to have an independent measure of the effects of the dioptric blur and mask on visual acuity and contrast sensitivity, we recruited two groups (n = 6 and n = 9) of additional subjects from the same student population (average age 28 and 26 years, respectively). Visual acuity (VA) was measured using the Landolt C set of the Freiburg Acuity Test (FrACT, http://michaelbach.de/fract/html) (Bach, 1996; Bach, 2006) presented on a high-resolution monitor (52 cm × 29.4 cm, resolution 1920 × 1080, luminance 8.5 cd/m2) in a darkened room. Contrast sensitivity was measured with a custom test based on the Pelli-Robson chart (Pelli, Robson and Wilkins, 1988) with letters that subtended .5° by .5° of visual angle. The chart included 8 lines, with two groups of three letters on each line. The contrast of each triplet was reduced in steps by 0.15 log units, such that log contrast sensitivity varied from 0.00 to 2.25 (logCS Weber). Luminance of the white background was 85 cd/m² in the no-mask condition.
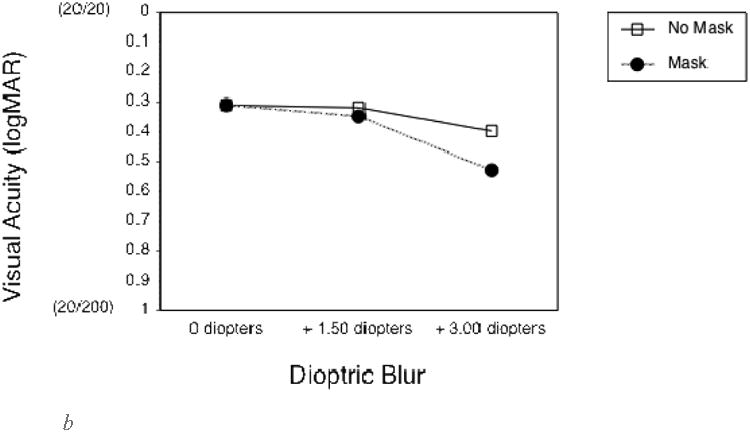
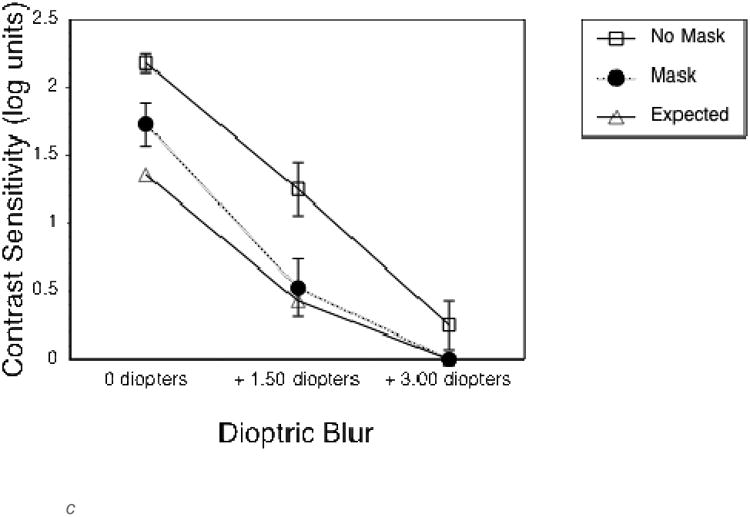
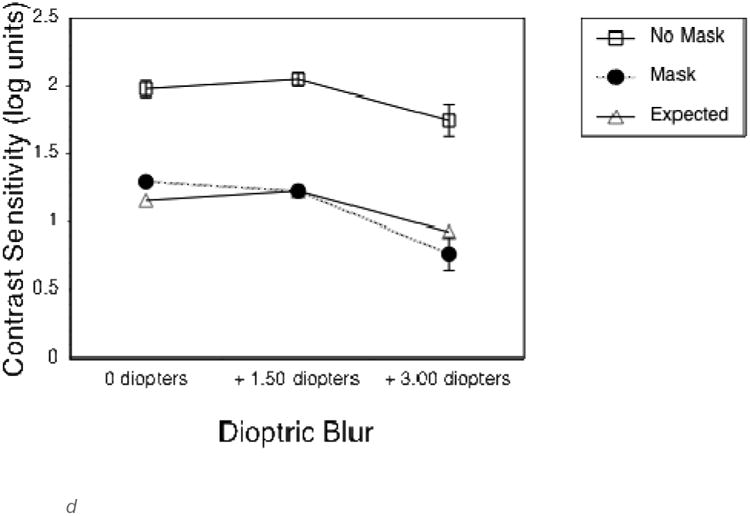
To quantify the effects of the optical blur and virtual mask on long distance vision, the first group of additional subjects completed VA and CS measures at a 4 m test distance. As expected, both the 1.5 and 3 diopter blur, and the mask, substantially reduced VA and CS (Figures 2a and 2c). VA measures were not limited by monitor resolution at this distance. The second group of additional subjects completed vision measures at the 0.5 m test distance, as used in the experimental gaze cone measurements. At this short distance, VA measures were limited by the smallest letter size that could be displayed, which was about 0.3 logMAR (the approximate resolution limit of the monitor). Therefore, VA without simulated impairments was limited to 0.3 logMAR (20/40) at 0.5 m (Figure 2b) compared to about -0.1 logMAR (20/15) at 4 m (Figure 2a). The 1.5 diopter blur had no effect on VA or CS at 0.5m (Figures 2b and d). By comparison, the 3 diopter blur reduced both VA and CS. The mask had relatively little effect on VA at 1.5 diopters of blur, but reduced VA at 3 diopters of blur (Figure 2b) and substantially reduced CS in all viewing conditions (Figure 2d).
Figure 2.
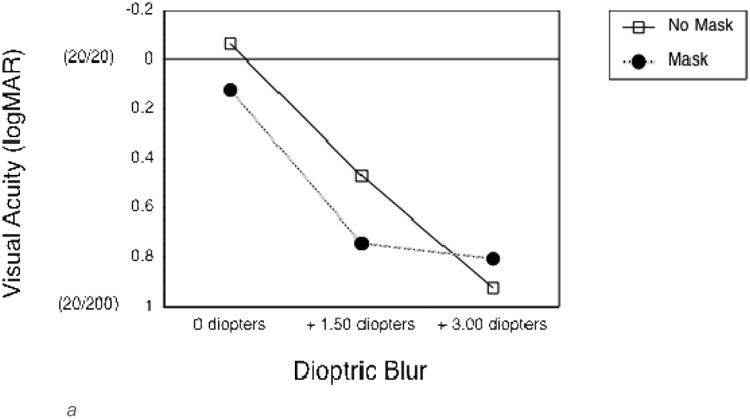
a. Landolt visual acuity thresholds without (empty squares) and with (black circles) the semi-transparent mask for each of the three levels of dioptric blur, measured at a viewing distance of 4 m. For reference, Snellen acuities are given in parentheses. Error bars represent standard error of the mean.
b. Landolt visual acuity thresholds without (empty squares) and with (black circles) the semi-transparent mask for each of the three levels of dioptric blur, measured at a viewing distance of .5 m. Note that, at this short viewing distance, visual acuity threshold measurements were limited by the smallest letter size that could be displayed (about 0.3 logMAR).
c. Letter contrast sensitivity without (empty squares) and with (black circles) the semi-transparent mask for each of the three levels of dioptric blur, measured at a viewing distance of 4 m. The expected values based on a contrast reduction of 15 % (decrement of .82 logCS) are indicated by the empty triangles. Error bars represent standard error of the mean.
d. Letter contrast sensitivity without (empty squares) and with (black circles) the semi-transparent mask for each of the three levels of dioptric blur, measured at a viewing distance of .5 m.
Design and procedure
Three factors were varied in a repeated measures design: Mask (two levels, transparency 100% and 15%), Dioptric blur (three levels: 0, 1.5 and 3.0 diopters) and Head orientation (three levels). The virtual head either pointed directly at the subject or was rotated around a vertical axis placed through a point between the eyes. In the latter case, head rotation was 8° to its right (yaw clockw ise), resulting in the nose pointing to the subject's left (Figure 1), or head rotation was 8° to the left (yaw counter-clockwise), resulting in the nose pointing to the subject's right. Note that the eyes were not always aligned with the head, such that head orientation was independent of gaze direction. Subjects sat in a chair adjusted to produce the desired eye height of 1 m. A letter and arrow on the screen indicated which one of the four adjustment tasks were to be completed (Figure 1). Centering was called for if the virtual eyes initially gazed at a direction between 7° and 9° to the left or -7° and -9°to the right of the subject. These values were randomly varied within this range to avoid a constant starting position and at the same time to leave sufficient room for adjustment. We instructed the subject to adjust the eyes of the virtual head such that it gazed directly at him or her. They rotated a trackball to move the gaze and were told that they could go back and forth, if needed, to home in on the setting that corresponded best to direct gaze. Decentering was called for whenever the virtual head initially gazed directly at the subject, who was instructed to rotate the head's eyes either to the left or to the right until they felt that the virtual head was about to stop gazing at the subject. The adjustment was accomplished with a maximal accuracy of 0.1° using the trackball. Subjects were encouraged to find the gaze direction that best specified the outer boundary of eye-contact. The order of all centering and decentering trials was randomly determined for each subject. They pressed a button when they were satisfied with their setting. No time limit was specified.
All factors were fully crossed and performed together with each of four instructions (centering from initial leftward/rightward gaze, decentering to the left and right). Thus, each subject performed a total of 72 trials (three levels of dioptric blur, two levels of mask, three head orientations, four instructions). The three dioptric blur and the two mask conditions were blocked and counterbalanced across subjects. The entire experiment lasted about 30 min.
Results
The direction of the gaze cone was computed as the average of all adjustments in the centering task expressed in degrees from the subject's straight ahead. The width of the gaze cone was the unsigned sum of the two matching decentering values (to the left and to the right) for any given level of head orientation, mask, and diopter value. Thus, the cone width amounted to the angular difference between the leftward and rightward boundary of the sector within which gaze directions were considered as looking at the subject.
Direction of the gaze cone
We conducted a 3 × 2 × 3 repeated measures analysis of variance (ANOVA) on the gaze cone's direction, with the factors head rotation (-8°, 0° or 8°), mask (transparency 100% o r 15%) and dioptric blur (0, 1.5 or 3 diopters). Degrees of freedom were Greenhouse-Geisser corrected to account for a lack of sphericity. We found a significant main effect for head rotation (see Figure 3): F(1.27, 29.17) = 186.13, p < .001, ηp2 = .89. When the head was rotated to its right, subjects adjusted the eyes to the right side of the actual center (by -2.4° on average). We describe the effect as the gaze being attracted by the observer. One might also want to think about the effect as the virtual head repulsing the perceived gaze direction1. When the head was rotated to the left (counterclockwise yaw), this effect was even larger. Subjects adjusted the eyes – which were actually looking directly at them - to 3.9° on the average, which is almost half the distance between the head rotation of 8° and the actual center at 0°. When the head orientation was centered directly toward the subject, subjects adjusted the eyes practically without bias to 0.6° on average. Neither mask nor dioptric blur produced a significant main effect. There was, however, a significant interaction between head rotation and mask (see Figure 4): F(1.95, 44.77) = 4.22, p = .022, ηp2 = .16. Adding the mask to the head increased the effect of head rotation.
Figure 3.
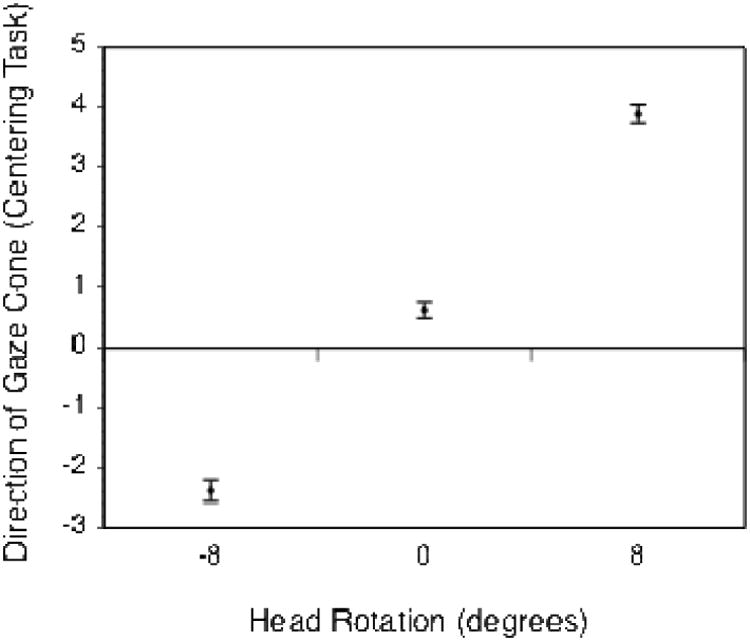
Mean eye adjustment error (in degrees) for the direction of the gaze cone as a function of head rotation. Negative head rotation values indicate a rotation to the right (clockwise yaw of the virtual head), positive values a rotation to the left. A value of 0° means that the head was centered. Error bars represent standard error of the mean.
Figure 4.
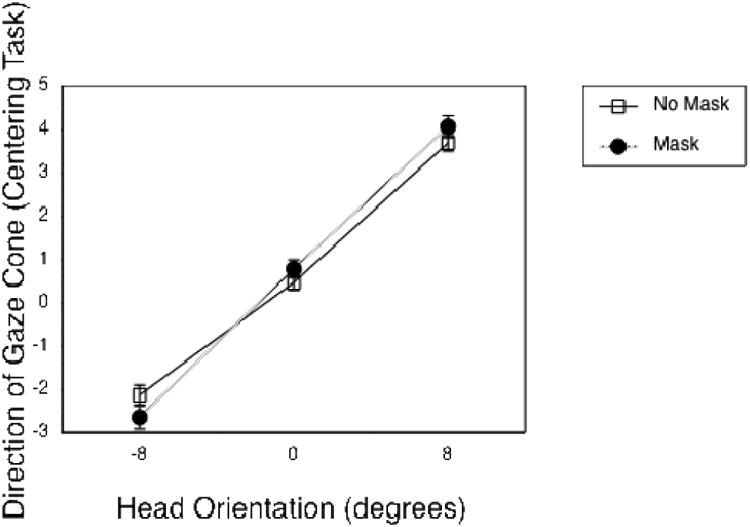
Mean eye adjustment for the direction of the gaze cone as a function of head rotation, grouped by the factor mask. Error bars represent standard error of the mean.
A separate ANOVA was conducted on the standard deviations of the gaze centering data. Trials with the mask were associated with greater variability in the gaze direction judgments, M = 2.89° without mask vs. M = 3.29° with mask; F(1.00, 23.00) = 1.99, p = .009.
Width of the gaze cone
We conducted a 3 × 2 × 3 rmANOVA on the gaze cone's width, using the same factors as before. We found a small, but significant main effect for dioptric blur value (see Figure 5): F(1.96, 45.08) = 5.18, p = .01, ηp2 = .18. The higher the level of dioptric blur, the smaller the width of the gaze cone (12.9° at 0 diopters, 11.7° for 1.5 diopters, 11.3° for 3 diopters). The main effect for mask was not significant (F(1.00, 23.00) = 0.64, p = .43, ηp2 = .03) neither was the interaction between mask and blur (F(1.51, 34.82) = 2.46, p = .11, ηp2 = .1). Head orientation did not significantly affect the width of the gaze cone.
Figure 5.
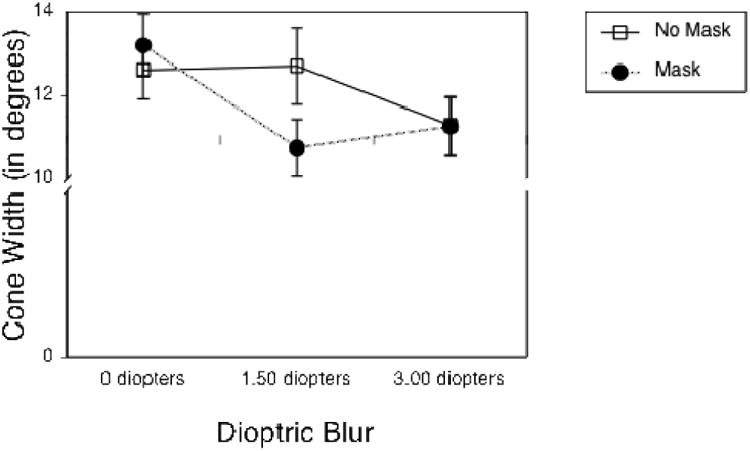
Mean eye adjustment for the width of the gaze cone as a function of the three levels of dioptric blur, grouped by the factor mask. Error bars represent standard error of the mean.
Discussion
Inducing dioptric blur did not affect the judgment of the direction of gaze. It did, however, affect the width of the gaze cone. The cone became narrower as the level of blur increased. This effect might be counter-intuitive as the reduction in visual acuity created by the blurring lenses should increase uncertainty. Subjects seemed to have tightened their criterion for mutual gaze in the face of a less crisp image.
The degradation caused by the virtual mask increased the attractor effect of the head. This can be taken as a strategy shift in reaction to the poorer stimulus quality, which is very intuitive. Head orientation is factored into the judgments to a larger extent as the high spatial frequency information of the looker's eyes is being reduced. Thus, coarser information replaces the fine-grained information that is no longer available. The virtual mask also made the gaze direction judgments more variable, which reflects the higher uncertainty. It is remarkable, however, that on average, the effects were very small indeed. Gaze direction was not seriously affected by the impairments brought about by degrading the stimulus and the observer's acuity.
Subjects in Experiment 1 were young (19-36 years) normally-sighted subjects. In Experiment 2 we extended our investigation to include both young and older normally-sighted subjects. We also extended our experimental paradigm to include measurements of mutual gaze in the vertical plane, as gaze direction in the real world is judged not only when people look from the left or right, but also when looking upward or downward (depending on height differences between the looker and the subject).
Experiment 2: Simulated impairment in younger and older observers
In Experiment 2 we used a diffusing filter commonly applied to simulate loss of visual acuity and contrast sensitivity, as might occur in patients with moderate cataract, an age-related ocular condition that impairs the ability to recognize faces and facial expressions (Elliott, Patla & Bullimore, 1997). Based on the findings from Experiment 1, we tested the hypothesis that this simulated vision impairment would increase variability in performance on the gaze perception task and would reduce the gaze cone width. We also investigated the effects of age on mutual gaze perception and predicted that the effects of the simulated cataract would be greater in older than younger subjects.
Methods
This experiment was approved by the institutional review board at Schepens Eye Research Institute where the data were collected. Voluntary, written informed consent was obtained from all subjects after a full explanation of the study procedures.
Subjects
32 normally-sighted subjects participated (14 men, 18 women; aged 23-87 years, M = 55.66, SD = 20.51). All had normal or corrected-to-normal visual acuity (M = 0.04 logMAR, SD = 0.08) and contrast sensitivity (M = 1.88 log units, SD = 0.14).
Design and Procedure
The study consisted of one session and took approximately 1 to 2 hours including vision measures, and two blocks of the gaze cone task. One block was performed with the subject's normal or corrected-to-normal vision with the spectacles that they normally used in social situations when interacting with people at about a 1 m distance. The other block was performed while wearing lightweight goggles with an 0.3 opacity Bangerter diffusing filter (Fresnel Prism & Lens Co., Eden Prairie, MN) in front of each eye (and over the habitual spectacles, where needed). These diffusing filters reduced both visual acuity and contrast sensitivity (see results below) (McCulloch et al., 2011; Bowers and Reid, 1997; Pérez, Archer, & Artal, 2010). The 0.3 opacity was selected to provide a simulation of moderate cataract (Wood and Carberry, 2006). The order of blocks was counterbalanced.
Vision measures
Vision measures were conducted binocularly. Visual acuity (VA) measurements were taken at a 1 m test distance (the distance at which the gaze cone measurements were performed) using the ‘Tumbling E’ option of the Freiburg Acuity Test (FrACT, http://michaelbach.de/fract/html) program on a NEC MultiSync LCD 2090UXi high resolution monitor (1600 × 1200 at 85 Hz) with average luminance of 280 cd/m2 controlled by a Cyperpower PC (Bach, 1996; Bach, 2006). Contrast sensitivity was assessed at a 450 mm testing distance using a custom computerized program, equivalent to the Pelli-Robson chart, for letters subtending 2.5° visual angle.
Gaze cone task
Subjects were seated at 1 m from a computer monitor displaying the same 2D virtual head as in Experiment 1 (but sized appropriately for the longer 1 m viewing distance). A chin-rest was used to align the subject to the eye-level of the virtual head target and maintain the 1 m testing distance. All subjects were presented with a virtual head that was either aligned with the subject or turned away by 8° to the left, or to the right for measurements of the horizontal gaze cone, and turned away 8° up or down for measurements of the vertical gaze cone. As in Experiment 1, the subjects received instruction to perform one of four tasks: centering from an initial gaze to the left/right or up/down, and decentering of the eyes of the virtual head toward the left/right or up/down. Subjects used the computer mouse to interactively adjust the eyes. There were a total of 24 trials for the centering and 24 trials for the decentering task, randomly interleaved.
Results
The data of one subject was removed because the vertical and horizontal gaze cone size exceeded four standard deviations of the remaining sample suggesting that this individual failed to understand the instructions. To look at the effect of age on our measures, we conducted a median split (Mdn = 59 years) of the subjects into two Age Groups: an older group (M = 71 years, SD = 9, n = 16) and a younger group (M = 36 years, SD = 12, n = 15). An alpha level of .05 was used for all statistical tests.
Vision measures
Visual acuity (VA) and contrast sensitivity (CS) were subjected to a 2 × 2 repeated measures analysis of variance (ANOVA) having Vision (no filters vs. diffusing filters) and Age Group (older vs. younger) as factors. As expected, we found that the diffusing filters significantly reduced VA (F(1, 29) = 198.82, ηp2 = 0.87, p < 0.001) and CS (F(1, 28) = 57.07, ηp2 = 0.67, p < 0.001) (Figure 6). In addition, there was a significant main effect for age group for both VA (F(1, 29) = 5.16, ηp2 = 0.15, p = 0.03) and CS (F(1, 28) = 7.80, ηp2 = 0.22, p = 0.009), with better VA and CS in the younger than the older group. Finally, there was a marginally significant interaction between Vision and Age Group for both VA (F(1, 29) = 3.91, ηp2 = 0.13, p = 0.048) and CS (F(1, 28) = 5.10, ηp2 = 0.15, p = 0.032). Specifically, the diffusing filters had a greater negative effect on the VA and CS of the older group than the younger group (see Figure 6).
Figure 6.
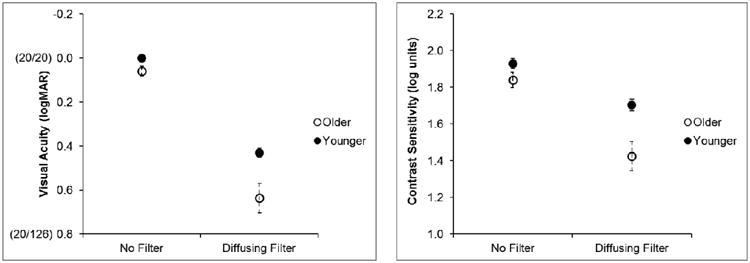
Mean visual acuity (left panel) and contrast sensitivity (right panel) of the subjec in the older and younger groups without and with the diffusing filters. Values in parenthes on the y-axis on the left panel are Snellen acuities. Error bars represent standard errors of the mean.
Horizontal direction of the gaze cone
We conducted a 2 × 2 × 3 repeated measures ANOVA on the horizontal direction of the gaze cone. The factors were Age Group (older vs. younger), Vision (no filters vs. diffusing filters), and Head Rotation (-8°, 0° or 8°). As in Experiment 1, we found a significa nt main effect for Head Rotation: F(2, 56) = 64.44, ηp2 = 0.70, p < 0.001. As can be seen in Figure 7, the perceived horizontal direction of the gaze cone shifted towards the direction to which the virtual head was oriented (attractor effect of head orientation). This effect was robust for both the younger and older observers. The analysis also revealed a significant main effect for Vision (F(1, 28) = 6.52, ηp2 = 0.19, p = 0.02); the overall head orientation effect was less with than without the filter. In addition, there was a Vision × Age Group interaction effect approaching significance: F(1, 28) = 3.95, ηp2 = 0.12, p = 0.057. Specifically, the attractor effect of head orientation was similar with and without the filter for the younger group, but decreased with the filter for the older group (Figure 7). There were no other significant main effects or interactions.
Figure 7.
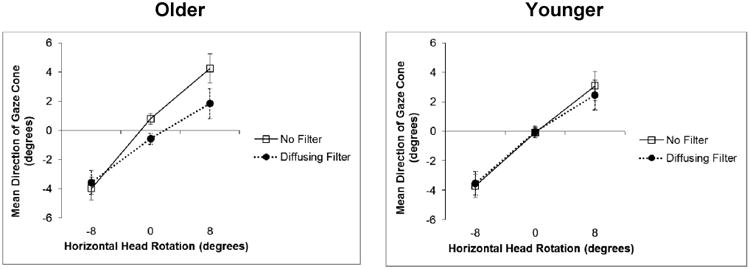
Mean eye adjustments for the direction of the gaze cone, by the older (left panel) and younger (right panel) age groups, as a function of the horizontal head rotations without and with the diffusing filters. Negative values indicate a rotation to the right, positive values a rotation to the left. A value of 0° means that the head was centered. Error bars represent standard error of the mean.
The same analysis was performed on the variability (i. e., standard deviation) of the horizontal direction of the gaze cone. Although there was no significant main effect of Vision (no filters vs. diffusing filters), the ANOVA yielded a main effect for Head Rotation (F(2, 56) = 5.85, ηp2 = 0.17, p = 0.005) such that the variability was greater when the virtual head was rotated right than centered or left. In addition, the main effect for Age Group was significant (F(1, 28) = 9.93, ηp2 = 0.26, p = 0.004), indicating that the older age group had more variability in their perceived direction of the gaze cone than the younger group. However, there was an interaction between Head Rotation and Age Group (F(2, 56) = 4.04, ηp2 = 0.13, p = 0.02); thus, the significant difference between the two groups was due to the centered and right head rotations (Figure 8).
Figure 8.
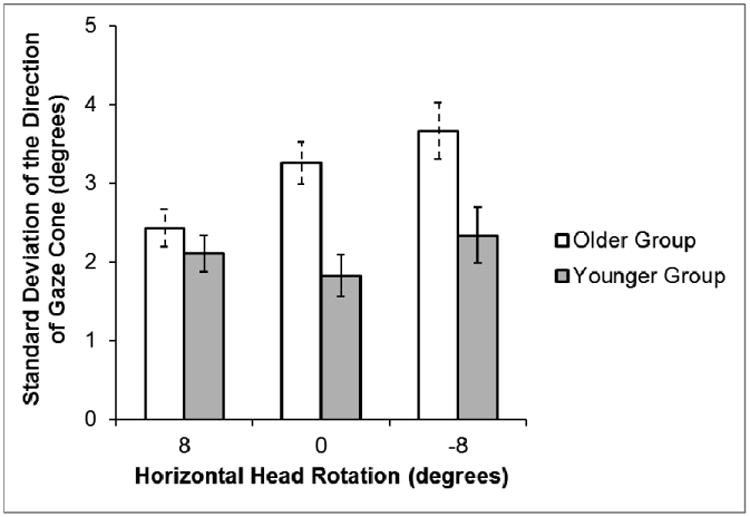
Mean of the standard deviations (i. e., variability) of the average eye adjustment for the direction of the gaze cone as a function of horizontal head rotations for the subjects in the older group and younger group. Error bars represent standard error of the mean.
Vertical direction of the gaze cone
The vertical direction of the gaze cone data was analyzed with the same 2 × 2 × 3 ANOVA as the horizontal data. The orientation of the virtual head had a significant effect on the perceived vertical direction of gaze: F(2, 56) = 60.64, ηp2= 0.68, p < 0.001. As in the horizontal direction, the subjects' perception of the direction of gaze shifted towards the vertical rotation of the virtual head (Figure 9). The analysis also showed a significant main effect for age group (F(1, 28) = 86.82, ηp2 = 0.28, p = 0.003), indicating that the older group tended to judge the direction of mutual gaze to be more upward than the younger group, regardless of the orientation of the virtual head (Figure 9). There were no other significant main effects (no effect of the filters), and no significant interactions.
Figure 9.
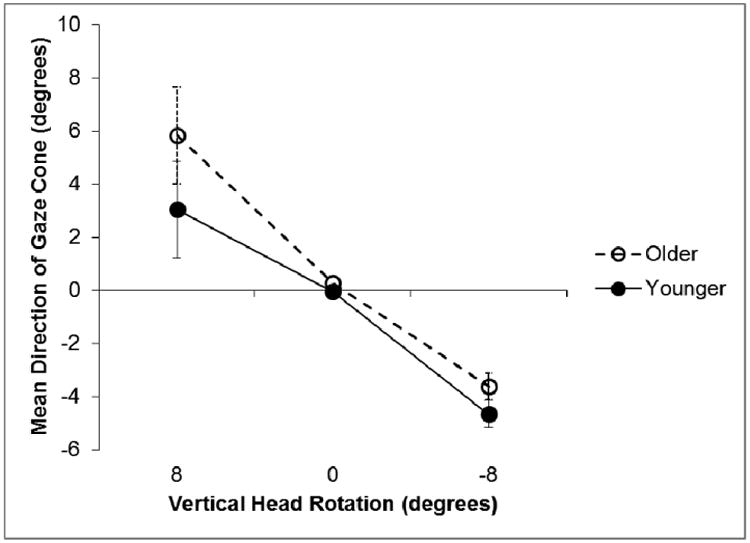
Mean eye adjustment for the direction of the gaze cone as a function of the vertical head rotations. Negative values indicate a downward rotation, positive values an upward rotation. A value of 0° means that the head was centered. Error bars represent standard error of the mean.
The same analysis performed on the variability (i. e., standard deviation) of the vertical direction of the gaze cone yielded only a main effect for Age Group: F(1, 28) = 8.56, ηp2 = 0.23, p = 0.007. In the vertical direction, the older group had more variability in their judgment of gaze (M = 3.32°, SD = 1.62) than the younger group (M = 2.06°, SD = 1.62).
Horizontal width of gaze cone
A 2 × 2 × 2 repeated measures ANOVA using Age Group (older vs. younger), Vision (no filters vs. diffusing filters), and Head Orientation (centered vs. angled) as factors was conducted on the horizontal width of the gaze cone. Replicating Experiment 1, we found the main effect of vision to be significant (F(1, 28) = 4.32, ηp2 = 0.13, p = 0.047), indicating that the horizontal gaze cone width was smaller when the subjects wore the filters than when they did not (see Figure 10). There was also a significant main effect for head orientation (F(1, 28) = 9.50, ηp2 = 0.25, p = 0.005), such that the gaze cone was wider when the virtual head was centered than when it was rotated to the right or the left (see Figure 10). There were no other significant main effects or interactions.
Figure 10.
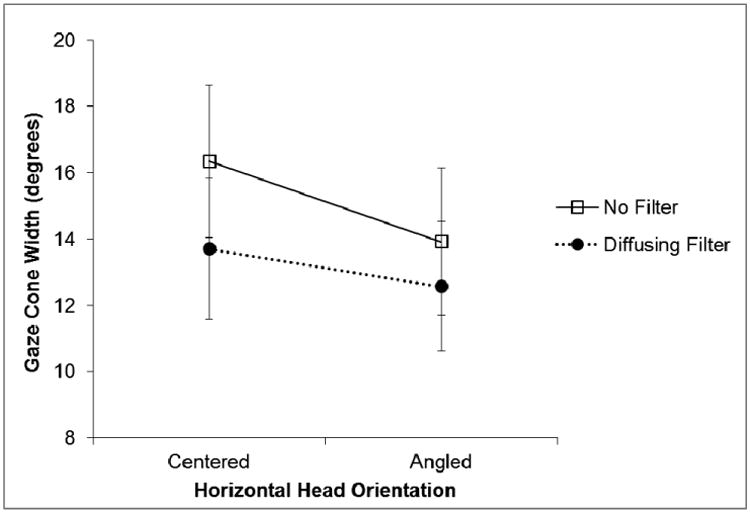
Mean eye adjustments for the width of the gaze cone with and without diffusing filters when the virtual head was centered compared to the virtual head angled right or left. Error bars represent standard error of the mean.
Vertical width of gaze cone
The same 2 × 2 × 2 repeated measures ANOVA performed on the horizontal gaze cone width data was used to analyze the vertical gaze cone width. As with the horizontal gaze cone, the mean width of the vertical gaze cone was significantly wider (F(1,28) = 24.50, ηp2 = 0.47, p < 0.001) when the virtual head was centered (M = 12.44°, SD = 6.51) than when it was rotated up or down (M = 10.11°, SD = 6.58). There were no other significant main effects or interactions.
Discussion
Simulation of cataracts via Bangerter diffusing filters, which reduced visual acuity and contrast sensitivity, affected the judgment of the direction and width of the horizontal gaze cone. As in Experiment 1, the width of the horizontal gaze cone was narrower when the subjects' vision was reduced. However, unlike the virtual mask condition in Experiment 1, the attractor effect of the head on perceived gaze direction did not increase with the filters. This difference may be because the Bangerter diffusing filters did not degrade vision as much as the virtual semi-transparent white mask.
We extended the paradigm in Experiment 2 to include trials performed in the vertical (up and down) plane in addition to the horizontal (right and left) plane. Unlike the horizontal gaze cone, the vertical gaze cone parameters were not affected by the simulated vision impairment. However, we found that the orientation of the virtual head attracted the mean gaze direction toward the direction the head was rotated, regardless of whether the head rotation was horizontal or vertical.
In Experiment 2, we widened our population sample (23-87 years) to investigate the effects of aging on the gaze cone parameters. We found that the gaze cone width was unaffected by the age of the subject. The direction of mutual gaze, on the other hand, significantly differed between the younger and older subjects. The younger subjects were more consistent (i. e., had less variability) in their judgment of horizontal and vertical gaze direction. Furthermore, in the vertical direction, the younger group tended to judge the mean direction of gaze to be lower than the older group.
General Discussion
Under optimal viewing conditions, we take a large range of gaze directions to constitute mutual gaze. This range of tolerance, the cone of gaze, is typically about 10° when using a human looker, and it can be considerably larger when using a virtual head whose facial expression is changed to express anger, or when the subject is anxious (Harbort, Witthöft, Spiegel, Nick, & Hecht, 2013). But what happens when stimulus uncertainty is increased by suboptimal viewing conditions such as low light levels, refractive blur or the onset of age-related ocular disease? Does the large tolerance range in the normal cone of gaze have the advantage of allowing for maintenance of perceptual and communicative skills in the face of stimulus degradation or visual impairment?
To address these questions, we conducted two experiments to investigate how the perceived center of gaze and the perceived cone of gaze would change when visual acuity and contrast sensitivity were reduced. The results suggest that perceived gaze direction tends to be very robust. The width of the cone of gaze, however, was more readily affected by simulated visual impairment. Interestingly, the increase of uncertainty that accompanied the blur, did not lead to a more relaxed criterion for mutual gaze, but rather to a tightened criterion; in both experiments, the width of the horizontal cone of gaze was reduced as uncertainty increased. Thus, subjects behaved as if they required a given degree of certainty to decide for mutual gaze to be established.
It should be noted that, in direct contrast to our findings, Mareschal, Calder, Dadds & Clifford (2013) found gaze discrimination thresholds to be higher when noise was added to the eye region of synthetic face stimuli. This finding suggests that gaze is more likely to be taken as directed at the observer whenever uncertainty is introduced. Within our paradigm, such a bias should result in a wider gaze cone with increasing uncertainty. We propose that this difference in the results may be attributed to the differing methodologies. Whereas our subjects made direct interactive assessments of mutual gaze, somewhat similar to deliberate gaze judgments in everyday situations, their subjects had to make mutual gaze judgments of briefly (400 ms) presented pictures of faces by means of a forced choice task. It is possible that we did not replicate Mareschal et al.'s (2013) results because they blurred only the eyes of their stimuli, whereas we manipulated the entire image. However, it is more likely that the method of assessment is the critical factor underlying the difference.
In addition to altering the criterion for accepting mutual gaze, the results of Experiment 1 suggest that subjects may change their strategy for judging gaze direction when access to visual information is limited. In both experiments, the direction of the head attracted the central direction of the gaze cone. However, when a virtual semi-transparent mask degraded the stimulus (Experiment 1) this effect became stronger. Thus, it appears that when eye position became more difficult to discern, subjects adopted a strategy of judging mutual gaze that relied more heavily on head orientation than eye position. This makes sense as head position is carried by lower spatial frequencies and would, therefore, be less affected by the stimulus degradation. However, neither the dioptric blur (Experiment 1) nor the Bangerter diffusing filters (Experiment 2) increased the effect of the head rotation on the central direction of the gaze cone, possibly because these simulations degraded the visibility of the virtual eyes to a lesser extent than did the virtual mask.
In Experiment 2, we investigated the effects of age, as well as vision impairment, on mutual gaze perception. Overall, the gaze judgments of the older group were fairly similar to those of the younger group. The main difference was greater variability in the judgments of gaze direction in the older group, but there was little effect of age on the perceived width of the gaze cone. Contrary to our prediction that older subjects would be more strongly affected by the simulated impairments than the younger subjects, there was only one interaction between vision (no filter or diffusing filter) and age group (young or old) that approached significance. This may seem surprising given that the filters reduced visual acuity and contrast sensitivity to a greater extent in the older than the younger subjects. However, one interpretation of these findings is that mutual gaze perception is relatively robust in the face of increasing age and mild-to-moderate levels of vision impairment.
In this study, we used simulations of vision impairments that might be experienced by normally-sighted people when wearing an incorrect spectacle prescription or by people with central vision loss due to cataracts (or any other condition that reduces visual acuity and contrast sensitivity). We previously found that patients with central vision loss that included a central scotoma (a blind area in central vision) had significantly more variable gaze direction judgments than age-matched normally-sighted subjects (Sheldon, Quint, Hecht & Bowers, 2014). Our present simulations produced similar results in healthy subjects. This is compatible with findings by Geringswald, Baumgartner, and Pollmann (2012) who simulated the effects of a central scotoma in healthy observers using a gaze-contingent stimulus presentation. In a complex visual search task involving contextual cueing, the artificial scotoma reduced the facilitation effect of stimulus repetition. Thus, even complex visual processes that leave room for compensation were negatively affected by the scotoma. Surprisingly, apart from the increased variability, the central vision loss affected our patients to a remarkably small extent when judging mutual gaze (Sheldon et al., 2014). In the current study, the healthy subjects appeared to compensate for the impairment by tightening their criterion for mutual gaze when confronted with dioptric blur or a diffusing filter, which could point to a process by which patients adapt to their visual impairment and are able to achieve close to normal performance with regard to higher-order visual perception although their acuity is compromised.
Simulations offer the advantage of being able to conduct within-subjects analyses of the effects of different levels of impairment, which can only be studied between groups when real vision impairment is involved. However, simulations do not reproduce all of the characteristics of real vision impairment and, importantly, the responses of normally-sighted observers, who are exposed to simulations for only a short period of time, might not use the same compensatory strategies that develop as patients adapt to their vision impairment over a longer period of time.
In summary, it cannot be taken for granted that the effects of aging and visual impairment on the judgment of gaze direction and mutual gaze amount to a straightforward loss in performance. Our results suggest that compensation mechanisms and criterion changes take place which allow us to perform better than would be predicted by a simple extrapolation from losses in basic acuity and contrast sensitivity.
Significance.
We demonstrate that decrements in the available visual information do not necessarily translate into reduced perceptual performance in a gaze perception task. We used dioptric blur, a virtual semi-transparent white contrast reduction mask and diffusing filters to simulate loss of acuity and contrast sensitivity. Subjects were able to compensate for these impairments in a more complex visual task. Insights into the relation between lower-level visual parameters and gaze perception are gained.
Acknowledgments
Agnes Münch programmed the gaze cone task. Supported in part by NIH grant T35EY007149 and the Alice J. Adler Fellowship of the Schepens Eye Research Institute
Footnotes
Note that the eccentricity of the eye within the head (eye socket) appears to determine the subjective gaze direction. Thus, as the pupil of the virtual head has to approach the rightmost edge of the eye-socket (as seen by the subject) in order to be directed at the subject, the gaze looks more rightward than it actually is. This could be thought of as the virtual head repulsing the gaze away from its midline (direction of the nose). For a nice elaboration of the eccentricity account see Todorovic (2009).
References
- Ando S. Luminance-induced shift in the apparent direction of gaze. Perception. 2002;31:657–674. doi: 10.1068/P3332. [DOI] [PubMed] [Google Scholar]
- Bach M. The Freiburg Visual Acuity Test-Automatic Measurement of Visual Acuity. Optometry & Vision Science. 1996;73(1):49–53. doi: 10.1097/00006324-199601000-00008. [DOI] [PubMed] [Google Scholar]
- Bach M. The Freiburg Visual Acuity Test-Variability unchanged by post-hoc re-analysis. Graefe's Archive for Clinical and Experimental Ophthalmology. 2006;245(7):965–971. doi: 10.1007/s00417-006-0474-4. [DOI] [PubMed] [Google Scholar]
- Bowers AR, Reid VM. Eye movements and reading with simulated visual impairment. Ophthalmic and Physiological Optics. 1997;17:392–402. [PubMed] [Google Scholar]
- Elliott DB, Patla A, Bullimore MA. Improvements in clinical and functional vision and perceived visual disability after first and second eye cataract surgery. British Journal of Ophthalmology. 1997;80:799–804. doi: 10.1136/bjo.81.10.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M, Hecht H. Are You Looking at Me? Measuring the Cone of Gaze. Journal of Experimental Psychology. 2007;33(3):705–715. doi: 10.1037/0096-1523.33.3.705. [DOI] [PubMed] [Google Scholar]
- Gamer M, Hecht H, Seipp N, Hiller W. Who is looking at me? The cone of gaze widens in social phobia. Cognition and Emotion. 2011;25(4):756–764. doi: 10.1037/0096-1523.33.3.705. [DOI] [PubMed] [Google Scholar]
- Geringswald F, Baumgartner F, Pollmann S. Simulated loss of foveal vision eliminates visual search advantage in repeated displays. Frontiers in Human Neuroscience. 2012;6:134. doi: 10.3389/fnhum.2012.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JJ, Pick AD. Perception of Another Person's Looking Behavior. The American Journal of Psychology. 1963;76(3):386–394. doi: 10.2307/1419779. [DOI] [PubMed] [Google Scholar]
- Harbort M, Witthöft M, Spiegel J, Nick K, Hecht H. The widening of the gaze cone in patients with social anxiety disorder and its normalization after CBT. Behaviour Research and Therapy. 2013;51:359–367. doi: 10.1016/j.brat.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Hecht H, Weiland R, Boyarskaya E. The cone of gaze. IEEE Human System Interactions (HSI), 4th International Conference. 2011:378–385. doi: 10.1109/HSI.2011.5937396. [DOI] [Google Scholar]
- Kleinke CL. Gaze and eye-contact: a research review. Psychological Bulletin. 1986;100:78–100. doi: 10.1037//0033-2909.100.1.78. [DOI] [PubMed] [Google Scholar]
- Mareschal I, Calder AJ, Dadds MR, Clifford CWG. Gaze categorization under uncertainty: Psychophysics and modeling. Journal of Vision. 2013a;13(5):18, 1–10. doi: 10.1167/13.5.18. [DOI] [PubMed] [Google Scholar]
- Mareschal I, Calder AJ, Clifford CWG. Humans have an expectation that gaze is directed toward them. Current Biology. 2013;23(8):717–721. doi: 10.1016/j.cub.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch DL, Loffler G, Colquhoun K, Bruce N, Dutton GN, Bach M. The effects of visual degradation on face discrimination. Ophthalmic and Physiological Optics. 2011;31:240–248. doi: 10.1111/j.1475-1313.2011.00828.x. [DOI] [PubMed] [Google Scholar]
- Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clinical Vision Science. 1988;2:187–199. [Google Scholar]
- Pérez GM, Archer SM, Artal P. Optical Characterization of Bangerter Foils. Investigative Ophthalmology & Visual Science. 2010;51(1):609–613. doi: 10.1167/iovs.09-3726. [DOI] [PubMed] [Google Scholar]
- Sheldon S, Quint J, Hecht H, Bowers AR. The effect of central vision loss on perception of mutual gaze. Optometry and Vision Science. 2014;91(8):1000–1011. doi: 10.1097/OPX.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorović D. The effect of face eccentricity on the perception of gaze direction. Perception. 2009;38(1):109–132. doi: 10.1068/p5930. [DOI] [PubMed] [Google Scholar]
- Wood JM, Carberry TP. Bilateral cataract surgery and driving performance. British Journal of Ophthalmology. 2006;90:1277–1280. doi: 10.1136/bjo.2006.096057. [DOI] [PMC free article] [PubMed] [Google Scholar]


