Abstract
Leinamycin (LNM, 1) is a novel antitumor antibiotic produced by Streptomyces atroolivaceus S-140 and features an unusual 1,3-dioxo-1,2-dithiolane moiety that is spiro-fused to a thiazole-containing 18-membered lactam ring. The 1,3-dioxo-1,2-dithiolane moiety of LNM is essential for its antitumor activity via an episulfonium ion-mediated DNA alkylation upon reductive activation in the presence of cellular thiols. We recently isolated leinamycin E1 (LNM E1, 2) from an engineered strain S. atroolivaceus SB3033, which lacks the 1,3-dioxo-1,2-dithiolane moiety. Here we report the chemical synthesis of 8,4′-dideshydroxy-LNM (5) from 2 and determination of the cytotoxicity of 5 against selected cancer cell lines in comparison with 1; 5 exhibits comparable activity as 1 with the EC50 values between 8.21 and 275 nM. This work reveals new insight into the structure-activity relationship of LNM and highlights the synergy between metabolic pathway engineering and medicinal chemistry for natural product drug discovery.
Keywords: antitumor antibiotic, DNA alkylation, leinamycin, metabolic pathway engineering, structure-activity relationship
Leinamycin (LNM, 1) is a novel antitumor antibiotic produced by Streptomyces atroolivaceus S-140.1 It features an unusual 1,3-dioxo-1,2-dithiolane moiety that is spiro-fused to a thiazole-containing 18-membered lactam ring, a molecular architecture that has not been found to date in any other natural products (Fig. 1). LNM shows potent antitumor activity in vitro and in vivo and is active against tumors that are resistant to clinically important anticancer drugs, such as cisplatin, doxorubicin, mitomycin, or cyclophosphamide.2, 3
Fig. 1.
LNM (1) and 8,4′-dideshydroxy LNM (5) as novel anticancer drug leads via an episulfonium ion-mediated alkylation of the N7 position of guanine residues in double-stranded DNA, in completion with the formation of an inactive epoxide intermediate.
The mode of action of 1 has been extensively investigated. As shown in Fig. 1, upon reductive activation in the presence of cellular thiols, 1 undergoes a sequential rearrangement to form an episulfonium ion intermediate, which can alkylate the N7 position of guanine residues in double-stranded DNA, ultimately causing DNA cleavage and cell death. The episulfonium ion also forms an equilibrium with an epoxide through the intramolecular attack by the C-8 hydroxyl group, resulting in a competition with the DNA attack at the C-6 position.4-11 While the Z,E-5-(thiazol-4-yl)-penta-2,4-dienone moiety of 1 play an important role in enhancing noncovalent binding of the activated LNM to double-stranded DNA, thereby increasing the overall yield of DNA alkylation,9, 11 it is the 1,3-dioxo-1,2-dithiolane moiety that imbues LNM its potent DNA alkylation activity.
Inspired by the novel mode of action of 1, a series of analogues have been prepared by chemical synthesis. Those analogues with the 1,3-dioxo-1,2-dithiolane moiety but lacking the macrolactam ring showed significantly decreased cytotoxicity or DNA cleavage activities than 1.12-14 In contrast, several analogues, prepared from 1 by direct modifications at the C-8 hydroxyl and/or C-9 keto groups, as well as at the 1,3-dioxo-1,2-dithiolane moiety, showed improved antitumor activity,3, 15-17 supporting the wisdom of discovering novel anticancer drugs based on the LNM scaffold.
In our effort to investigate LNM biosynthesis and manipulate the LNM biosynthetic machinery for LNM structural diversity,18-27 we recently engineered a recombinant strain S. atroolivaceus SB3033 that accumulated a LNM biosynthetic intermediate, LNM E1 (2). The structure of 2 contains a thiol moiety at the C-3 position and lacks the 1,3-dioxo-1,2-dithiolane moiety. Therefore, 2 exhibits no DNA alkylation activity under the thiol rich, reductive cellular environment. Interestingly, 2 can be oxidatively activated by cellular reactive oxygen species (ROS) to generate a similar episulfonium ion to alkylate DNA. This unprecedented mode of action by 2 to exert its cytotoxicity via oxidative activation complements that for 1 via reductive activation and could be exploited for anticancer drug discovery targeting cancer cells under high oxidative stress.27
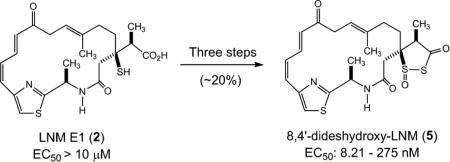
Here we report the chemical synthesis of 8,4′-dideshydroxy-LNM (5), containing the essential 1,3-dioxo-1,2-dithiolane moiety and macrolactam scaffold of 1 but lacking the C-8 and C-4’ hydroxyl groups, using 2 as the starting material. The cytotoxicity assay against selected cancer cell lines in comparison with 1 showed that 5 possessed comparable activity with the EC50 values between 8.21 and 275 nM (Table 1). This work reveals new insight into the structure-activity relationship of LNM and highlights the synergy between metabolic pathway engineering and medicinal chemistry for natural product drug discovery.
Table 1.
1H (700 MHz) and 13C (175 MHz) NMR data of 3-5 in CDCl3.a
| 3 |
4 |
5 |
||||
|---|---|---|---|---|---|---|
| positions | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) |
| 1 | 168.3, C | 167.7, C | 167.9, C | |||
| 2 | 46.3, CH2 | 3.01 d (13.4) | 42.3, CH2 | 2.95 d (14.0) | 36.9, CH2 | 3.16 d (16.0) |
| 2.79 d (13.4) | 2.77 d (14.0) | 2.75 d (16.0) | ||||
| 3 | 49.1, C | 62.1, C | 72.7, C | |||
| 4 | 34.8, CH2 | 2.25 m*, 1.73 m | 29.7, CH2 | 2.02 m, 1.58 m | 29.2, CH2 | 1.90 m, 1.80 m |
| 5 | 37.4, CH2 | 2.25 m* | 36.1, CH2 | 2.42 m, 2.19 m | 36.3, CH2 | 2.19 m, 2.08 m |
| 6 | 138.6, C | 138.5, C | 138.8, C | |||
| 7 | 119.5, CH | 5.83 dd (10.4, 4.9) | 119.8, CH | 5.79 br s | 119.2, CH | 5.76 br t (8.2) |
| 8 | 40.5, CH2 | 3.57 dd (13.2, 5.0) | 40.3, CH2 | 3.58 m* | 40.9, CH2 | 3.42 dd (13.7, 8.4) |
| 3.27 dd (13.2, 10.8) | 3.29 br t (12.3) | 3.23 dd (13.5, 8.0) | ||||
| 9 | 199.8, C | 199.8, C | 199.6, C | |||
| 10 | 132.2, CH | 6.09 d (16.3) | 132.1, CH | 6.11 d (16.2) | 132.5, CH | 6.02 d (15.8) |
| 11 | 142.9, CH | 8.90 dd (16.3, 11.3) | 142.8, CH | 8.86 dd (16.0, 11.4) | 142.1, CH | 8.63 dd (16.2, 11.3) |
| 12 | 128.6, CH | 6.41 t (11.4) | 128.4, CH | 6.40 t (11.2) | 129.2, CH | 6.39 t (11.3) |
| 13 | 127.7, CH | 6.63 d (11.4) | 128.0, CH | 6.62 d (11.2) | 127.9, CH | 6.63 d (11.3) |
| 13a | 153.8, C | 153.4, C | 153.8, C | |||
| 14 | 120.6, CH | 7.27 s | 121.0, CH | 7.28 s | 120.7, CH | 7.32 s |
| 15 | 169.7, C | 170.5, C | 171.0, C | |||
| 16 | 47.7, CH | 5.31 m | 47.7, CH | 5.36 m | 48.0, CH | 5.50 m |
| 17 | 22.9, CH3 | 1.62 d (6.5) | 23.2, CH3 | 1.63 d (6.6) | 20.7, CH3 | 1.77 d (6.7) |
| 18 | 15.9, CH3 | 1.81 s | 15.8, CH3 | 1.76 s | 15.6, CH3 | 1.64 s |
| 2′ | 193.4, C | |||||
| 3′ | 71.5, CH | 4.71 q (7.5) | 207.9, C | 202.2, C | ||
| 4′ | 10.7, CH3 | 1.28 d (7.5) | 55.1, CH | 3.55 m* | 51.2, CH | 3.37 q (7.0) |
| 5′ | 11.6, CH3 | 1.25 d (6.9) | 11.6, CH3 | 1.28 d (7.0) | ||
| NH | 6.42 d (6.6) | 6.56 d (6.1) | 6.23 d (6.7) | |||
Assignments were based on COSY, HMBC, and HSQC experiments.
These signals overlapped with others.
The synthesis of 5 from 2, requiring the installment of the 1,3-dioxo-1,2-dithiolane moiety, was achieved by adopting a literature procedure with modifications.28 Thus, cyclization of the mercapto acid moiety of 2 was carried out in a solution of isobutyl chloroformate and trimethylamine, affording the corresponding thiolactone 3 as a slightly yellow oil. The thiolactone ring was then opened up by hydrogen sulfide to give rise to the mercapto thioic acid intermediate, which was subsequently converted into the dithiolane 4 under atmospheric oxygen through oxidative ring closure. Finally, m-CPBA was used to accomplish the S-oxidation of the 1,2-dithiolan-3-one moiety of 4 to produce the 1,3-dioxo-1,2-dithiolane moiety of 5 (Fig. 2 and Table 1).
Fig. 2.
Chemical synthesis of 8,4′-dideshydroxy LNM (5) from LNM E1 (2).
To evaluate the cytotoxicity of compounds 2-5 in comparison with 1, several cancer cell lines, including human malignant melanoma A375, human Burkitt's lymphoma Raji, human breast adenocarcinoma MCF7, human ductal breast epithelial T47D, human breast SKBR, and human Caucasian breast adenocarcinoma MDA-MB-231 cell lines, were selected. As reported previously,27 2 showed no significant cytotoxicity against all the cancer cell lines tested (EC50 > 10 μM). Compound 3 showed weak activities against MCF7, SKBR and MDA-MB-231 cell lines with EC50 values of 8.56, 7.37, and 1.95 μM, respectively. Compound 4 showed selective cytotoxicity against human Raji cell line with EC50 of 48.7 nM but no cytotoxicity against the other cell lines. It has been reported that the 3-oxo-1,2-dithiolane moiety in S-deoxyleinamycin may react with thiols to generate ROS causing DNA damage.29 Whether such a mechanism could be accounted for the observed cytotoxicity of 4 against Raji cell line, however, requires further investigation. As expected, 1 showed potent cytotoxicity against all the cell lines tested, with EC50 values between 4.69 and 52.3 nM. Compound 5 showed similar cytotoxicity as 1 against the A375 and Raji cell lines, with EC50 value of 12.5 and 8.21 nM, respectively. For the other four cell lines, 5 showed less potent cytotoxicity than 1 with about 5 to 13 fold higher EC50 values (Table 2).
Table 2.
Cytotoxicity of 8,4′-dideshydroxy-LNM (5) against selected cancer cell lines in comparison with LNM (1)
| Cell lines | EC50 (nM) |
||||
|---|---|---|---|---|---|
| LNM (1) | LNM E1 (2) | 3 | 4 | 5 | |
| A375 | 4.69 | >10 × 103 | >10 × 103 | 12.5 | |
| Raji | 6.29 | >10 × 103 | 48.7 | 8.21 | |
| MCF7 | 42.4 | >10 × 103 | 8.56 × 103 | >10 × 103 | 275 |
| T47D | 15.2 | >10 × 103 | >10 × 103 | 196 | |
| SKBR | 52.3 | 7.37 × 103 | >10 × 103 | 251 | |
| MDA-MB-231 | 8.99 | 1.95 × 103 | >10 × 103 | 71.4 | |
The difference in cytotoxicity between 5 and 1 reveals new insights into the structure-activity relationship of LNM family of anticancer antibiotics. Thus, without the C-8 hydroxyl group, 5 would exclude the equilibrium between the active episulfonium ion intermediate and the inactive epoxide, thereby contributing to the overall improvement of the DNA alkylation activity (Fig. 1). LNM analogues with modifications at the C-8 hydroxyl group indeed have been prepared previously, displaying similar or improved antitumor activities than 1 in vitro and in vivo.3, 16, 17 However, the role of the C-4’ hydroxyl group in 1 has not been examined previously. The fact that 5, which lacks the C-4’ hydroxyl group, showed decreased activities than 1, would strongly suggest that the C-4’ hydroxyl group at the 1,3-dioxo-1,2-dithiolane moiety is very important for the DNA alkylation activity of the LNM family of antitumor drugs.
In conclusion, we synthesized a new LNM analogue 8,4’-dideshydroxy-LNM (5) from LNM E1 (2), which was produced by a recombinant strain S. atroolivaceus SB3033, engineered from S. atroolivaceus S-140, the wild-type producer of LNM (1). The synthesis involved a facile three-step installation of the 1,3-dioxo-1,2-dithiolane moiety, which is essential for the DNA alkylation activity of the LNM family of antitumor drugs. Compound 5 lacks both the C-8 and C-4’ hydroxyl groups, representing a novel LNM analogue that cannot be readily prepared from 1. Cytotoxicity comparison between 1 and 5 against the selected cancer cell lines revealed new insight into the structure-activity relationship of LNM and the importance of the C-4’ hydroxyl group in its activity. This work highlights the synergy between metabolic pathway engineering and medicinal chemistry for natural product drug discovery.
Supplementary Material
Acknowledgements
We thank Kyowa Hakko Kogyo Co. Ltd (Tokyo, Japan) for the wild-type S. atroolivaceus S-140 strain and the NMR Core facility at the Scripps Research Institute, Jupiter, Florida in obtaining the 1H and 13C NMR data. This work was supported in part by National Institutes of Health Grant CA106150.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Materials, methods, and detailed experimental procedures are provided in Supplementary data. Also included in Supplementary data are Fig. S1 of representative dose-response curves for cytotoxicity of 3-5 against selected cancer lines in comparison with 1 and Figs. S2-S13 of the 1H and 13C NMR spectra of 3-5. This material can found in the online version at http://dx.doi.org/10.1016/j.bmcl.xxx.
References
- 1.Hara M, Asano K, Kawamoto I, Takiguchi T, Katsumata S, Takahashi K, Nakano H. J. Antibiot. 1989;42:1768. doi: 10.7164/antibiotics.42.1768. [DOI] [PubMed] [Google Scholar]
- 2.Hara M, Saitoh Y, Nakano H. Biochemistry. 1990;29:5676. doi: 10.1021/bi00476a005. [DOI] [PubMed] [Google Scholar]
- 3.Ashizawa T, Kawashima K, Kanda Y, Gomi K, Okabe M, Ueda K, Tamaoki T. Anticancer Drugs. 1999;10:829. doi: 10.1097/00001813-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Gates K. Chem. Res. Toxicol. 2000;13:953. doi: 10.1021/tx000089m. [DOI] [PubMed] [Google Scholar]
- 5.Asai A, Hara M, Katita S, Kanda Y, Yoshida M, Saito H, Saitoh Y. J. Am. Chem. Soc. 1996;118:6802. [Google Scholar]
- 6.Breydo L, Gates K. J. Org. Chem. 2002;67:9054. doi: 10.1021/jo020568l. [DOI] [PubMed] [Google Scholar]
- 7.Nooner T, Dutta S, Gates K. Chem. Res. Toxicol. 2004;17:942. doi: 10.1021/tx049964k. [DOI] [PubMed] [Google Scholar]
- 8.Viswesh V, Gates K, Sun D. Chem. Res. Toxicol. 2010;23:99. doi: 10.1021/tx900301r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fekry MI, Szekely J, Dutta S, Breydo L, Zang H, Gates KS. J. Am. Chem. Soc. 2011;133:17641. doi: 10.1021/ja2046149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viswesh V, Hays AM, Gates K, Sun D. Bioorg. Med. Chem. 2012;20:4413. doi: 10.1016/j.bmc.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sivaramakrishnan S, Breydo L, Sun D, Gates KS. Bioorg. Med. Chem. Lett. 2012;22:3791. doi: 10.1016/j.bmcl.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behroozi S, Kim W, Dannaldson J, Gates KS. Biochemistry. 1996;35:1768. doi: 10.1021/bi952257t. [DOI] [PubMed] [Google Scholar]
- 13.Szilagyi A, Fenyvesi F, Majercsik O, Pelyvas IF, Bacskay I, Feher P, Varadi J, Vecsernyes M, Herczegh P. J. Med. Chem. 2006;49:5626. doi: 10.1021/jm060471h. [DOI] [PubMed] [Google Scholar]
- 14.Keerthi K, Rajapakse A, Sun D, Gates KS. Bioorg. Med. Chem. 2013;21:235. doi: 10.1016/j.bmc.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanda Y, Ashizawa T, Saitoh Y, Saito H, Gomi K, Okabe M. Bioorg. Med. Chem. Lett. 1998;8:909. doi: 10.1016/s0960-894x(98)00133-4. [DOI] [PubMed] [Google Scholar]
- 16.Kanda Y, Ashizawa T, Kakita S, Takahashi Y, Kono M, Yoshida M, Saitoh Y, Okabe M. J. Med. Chem. 1999;42:1330. doi: 10.1021/jm9900366. [DOI] [PubMed] [Google Scholar]
- 17.Kanda Y, Ashizawa T, Kawashima K, Ikeda S, Tamaoki T. Bioorg. Med. Chem. Lett. 2003;13:455. doi: 10.1016/s0960-894x(02)00949-6. [DOI] [PubMed] [Google Scholar]
- 18.Cheng Y, Tang G, Shen B. J. Bacteriol. 2002;184:7013. doi: 10.1128/JB.184.24.7013-7024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng Y, Tang G, Shen B. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3149. doi: 10.1073/pnas.0537286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang G, Cheng Y, Shen B. Chem. Biol. 2004;11:33. doi: 10.1016/j.chembiol.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Tang G, Cheng Y, Shen BJ. Nat. Prod. 2006;69:387. doi: 10.1021/np050467t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang G, Cheng Y, Shen BJ. Biol. Chem. 2007;282:20273. doi: 10.1074/jbc.M702814200. [DOI] [PubMed] [Google Scholar]
- 24.Liu T, Huang Y, Shen B. J. Am. Chem. Soc. 2009;131:6900. doi: 10.1021/ja9012134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y, Huang S-X, Ju J, Tang G, Liu T, Shen B. Org. Lett. 2011;13:498. doi: 10.1021/ol102838y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohman JR, Bingman CA, Phillips GN, Shen B. Biochemistry. 2013;52:902. doi: 10.1021/bi301652y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang S-X, Yun B-S, Ma M, Basu HS, Church DR, Ingenhorst G, Huang Y, Yang D, Lohman JR, Tang G, Ju J, Liu T, Wilding G, Shen B. doi: 10.1073/pnas.1506761112. manuscript submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee AHF, Chan ASC, Li T. Tetrahedron. 2003;59:833. [Google Scholar]
- 29.Sivaramakrishnan S, Gates KS. Bioorg. Med. Chem. Lett. 2008;18:3076. doi: 10.1016/j.bmcl.2007.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




