Abstract
Objective:
To characterize the clinical, EEG, and brain imaging findings in an adult case series of patients with de novo refractory status epilepticus (SE) occurring after a febrile illness.
Methods:
A retrospective study (2010–2013) was undertaken with the following inclusion criteria: (1) previously healthy adults with refractory SE; (2) seizure onset 0–21 days after a febrile illness; (3) lacking evidence of infectious agents in CSF; (4) no history of seizures (febrile or afebrile) or previous or concomitant neurologic disorder.
Results:
Among 155 refractory SE cases observed in the study period, 6 patients (17–35 years old) fulfilled the inclusion criteria. Confusion and stupor were the most common symptoms at disease onset, followed after a few days by acute repeated seizures that were uncountable in all but one. Seizures consisted of focal motor/myoclonic phenomena with subsequent generalization. Antiepileptic drugs failed in every patient to control seizures, with all participants requiring intensive care unit admission. Barbiturate coma with burst-suppression pattern was applied in 4 out of 6 patients for 5–14 days. One participant died in the acute phase. In each patient, we observed a reversible bilateral claustrum MRI hyperintensity on T2-weighted sequences, without restricted diffusion, time-related with SE. All patients had negative multiple neural antibodies testing. Four out of 5 surviving patients developed chronic epilepsy.
Conclusions:
This is a hypothesis-generating study of a preliminary nature supporting the role of the claustrum in postfebrile de novo SE; future prospective studies are needed to delineate the specificity of this condition, its pathogenesis, and the etiology.
In the last 2 decades, several authors described a series of syndromes characterized by the development of a difficult to treat status epilepticus (SE) in previously healthy children after a febrile illness.1–7 The condition is characterized by a refractory SE and followed by drug-resistant epilepsy, with often severe neuropsychiatric sequelae or death. These entities have been identified by different acronyms, but febrile infection-related epilepsy syndrome (FIRES) is the one that best underscores the main features of the disorder.8 Cases with a similar clinical picture have been described also in adults and in these case series the most frequently used definition is new-onset refractory SE (NORSE).9–13 Recently, it was pointed out that different terms probably have been used to describe the same condition.14,15 However, there is no consensus among investigators. Adult cases are more heterogeneous, some with clear similarities with FIRES cases (with only the age at onset as the main difference), others probably representing different conditions.
We describe a case series and a literature review of young adults fulfilling the definition of FIRES, all showing in the early acute phase of the disease a striking alteration of the claustrum on MRI, mainly bilaterally.
The claustrum function has remained obscure for decades. In 1996, its involvement in a case of de novo refractory status was reported for the first time.16 Only in recent years has the scientific community gained a strong interest in the function of the claustrum, mainly with respect to its role in sensory integration and consciousness.17–21
METHODS
Patients and investigations.
In a retrospective multicenter study (2010–Dec 2013), information including demographic data, clinical features, diagnostic findings, therapeutic interventions, and clinical outcomes of patients fulfilling the following inclusion criteria were acquired: (1) previously healthy adults (>16 years of age) with refractory SE (failed IV treatment with antiepileptic drugs, requiring general anesthesia)22; (2) onset of seizures 0–21 days after a febrile illness; and (3) lacking evidence of infectious agents in CSF. Exclusion criteria were a history of seizures (febrile or afebrile), as well as previous or concomitant neurologic disorder. Data were extracted from the participating centers, reviewing clinical charts, EEG (video-EEG) recordings, and MRI (when available). During the study period, 155 cases of refractory SE were observed. Among these, 6 fulfilled our inclusion criteria.
No family history for febrile seizures or epilepsy was reported. No participant had personal or familial history of an immunologic disorder.
All patients were examined for viral and bacterial infections. The following blood and CSF analyses were performed: viral tests including herpes simplex virus 1 and 2, varicella-zoster virus, human herpesvirus 6, cytomegalovirus, Epstein-Barr virus, rubella, parvovirus B19, enterovirus, and mumps. All virologic studies were performed using DNA PCR. The presence of oligoclonal immunoglobulin G bands was checked with isoelectrofocusing, performed with agarose gel support. Antinuclear antibodies, antiphospholipid antibodies, anti-DNA antibodies, anticardiolipin antibodies, anti–extractable nuclear antigen antibodies, and antithyroid antibodies were analyzed with immune-enzymatic tests and indirect immunofluorescent staining.
Thorax-abdomen CT was performed in all patients, showing no occult neoplasm. An extensive blood analysis and testing of classical onconeural antibodies (anti-GAD, anti-Yo, Ri, Hu, anti-Ma2) was negative in all participants.
Standard protocol approvals, registrations, and patient consents.
The scientific advisory boards of participating institutions approved the research protocol according to local regulations and consent was obtained from patients or their relatives. This research is reported following Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Literature review.
Searches for identification of studies were run in from 1990 to 2014 in MEDLINE and PubMed. Searches were limited from 1990 to the present day since studies carried out previously would necessarily have included participants without MRI. The search keywords were as follows: “AERRPS”; “FIRES”; “NORSE”; “status epilepticus” and “claustrum”; “epilepsy” and “claustrum”; “status epilepticus” and “neuroimaging.” For each citation considered, the abstract was read (when available). The bibliography of each of the retrieved articles was examined to identify relevant references that could have been missed by electronic search. Only peer-reviewed original articles providing images of the claustrum involvement were accepted for inclusion in the review.
RESULTS
Clinical data, interventions, and outcomes of the 6 patients are summarized in table 1.
Table 1.
Clinical data of the reported patients
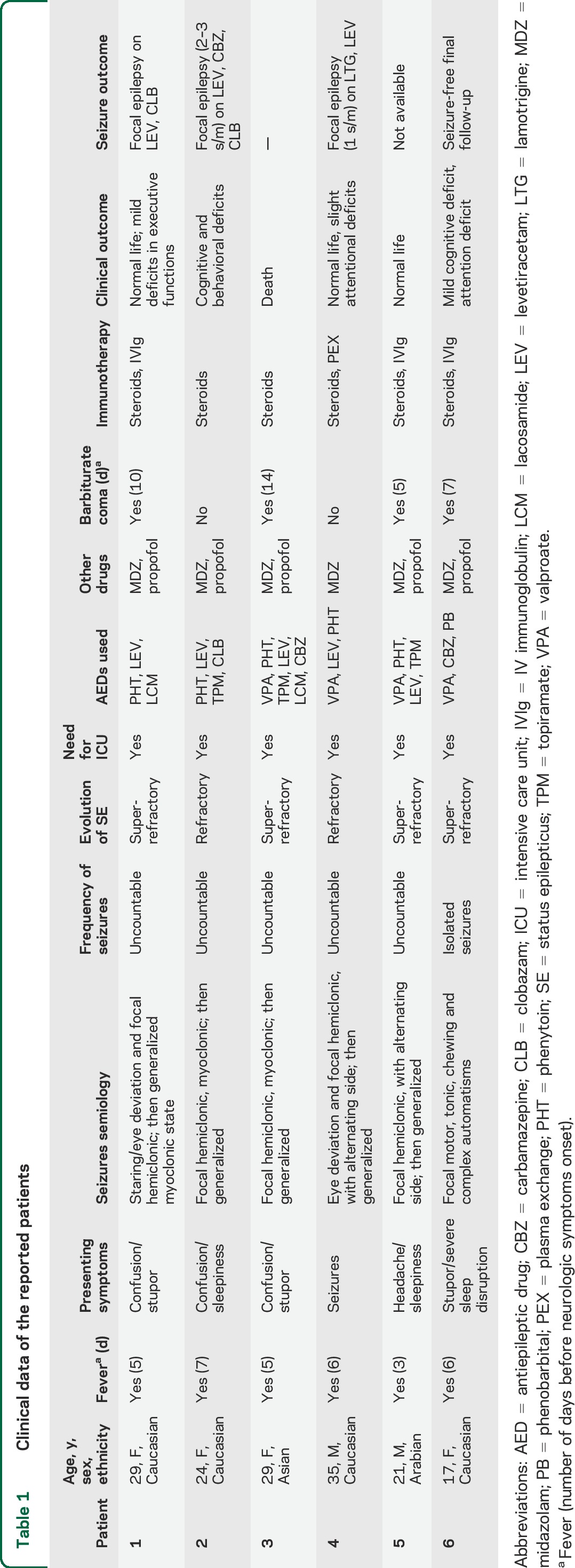
Age at onset was between 17 and 35 years; different ethnicities were represented. Fever, in the context of a flu-like condition, preceded the onset of neurologic symptoms in all patients. Confusion, stupor, sleepiness, and signs of consciousness alteration were the most common symptoms at disease onset, followed after a few days by acute repeated seizures that were uncountable in all but one patient. Seizures consisted in all participants of focal motor/myoclonic events with alternating side involvement and subsequent generalization. In patient 1, a dramatic generalized myoclonus was present through the entire acute phase of the disease. Antiepileptic drugs (AED) failed in every patient to control seizures, with all participants requiring intensive care unit (ICU) admission and treatment with midazolam and propofol. Barbiturate coma with burst-suppression pattern was applied in 4 out of 6 patients for 5–14 days. Corticosteroid IV treatment was used as adjunctive therapy in all patients during the acute early phase, while IV immunoglobulins (IVIg) or plasma exchange (PEX) were used in 4 patients. One patient did not survive the acute phase (patient 3: sepsis during ICU, staying 3 weeks after onset). One of the surviving patients was lost to follow-up since he returned to his home country (patient 5). Four patients are on AED therapy: 3 have active chronic focal epilepsy despite adequate drug regimens and 1 presented seizure clusters for months despite AED polytherapy before epilepsy control.
Brain imaging.
During the acute phase of the disorder, brain MRI showed bilateral claustrum involvement in all patients (figure 1): these positive imaging studies were acquired with a time lag of 3–10 days from SE onset (table 2). Notably, in all patients the first MRI study, undertaken in the few days preceding the development of seizures or in the first days (0–2 days) after the onset of refractory SE, was negative. Beyond high signal on fluid-attenuated inversion recovery/T2 and diffusion-weighted images (but with normal apparent diffusion coefficient [ADC]) (figure 2), no other sequences revealed claustrum abnormalities; no contrast enhancement was observed in any case. MRI studies undertaken after SE resolution no longer showed claustrum alterations (figure 2).
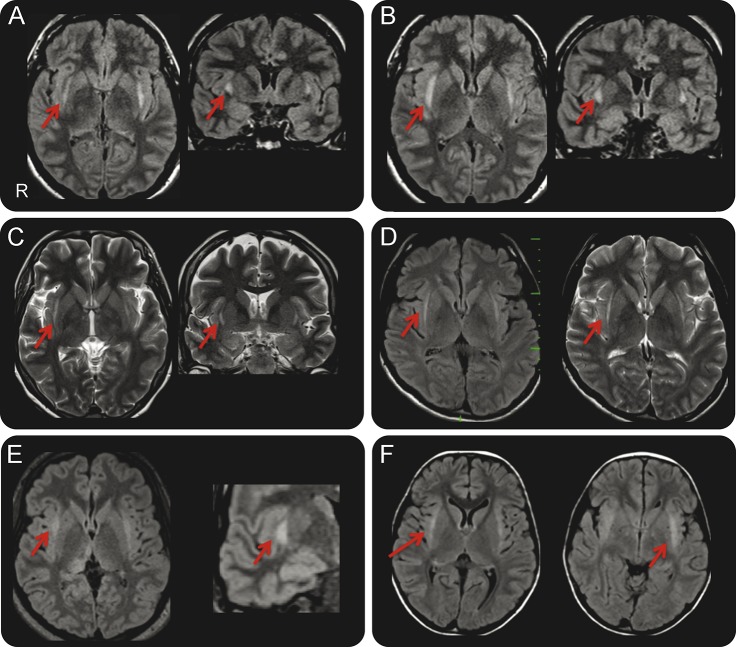
Figure 1. Claustrum sign.
(A, B) Axial and coronal fluid-attenuated inversion recovery (FLAIR) images show claustrum hyperintensity (red arrows) during the status epilepticus (SE) in patients 1 and 2, 10 and 4 days from onset, respectively. (C) Axial and coronal T2 images show claustrum hyperintensity (red arrows) during the SE in patient 3, 3 days from onset. (D) Axial FLAIR and axial T2 images show claustrum hyperintensity (red arrows) during the SE in patient 4, 9 days from onset. (E) Axial FLAIR images acquired during SE (left image) and coronal detail (right image) of claustrum hyperintensity in patient 5 (red arrows); note also the posterior thalamus hyperintensity (the patient also experienced visual seizures). (F) Axial FLAIR images show claustrum hyperintensity (red arrows) during the SE in patient 6, 7 days from onset.
Table 2.
Investigational data during the acute phase in the reported patients
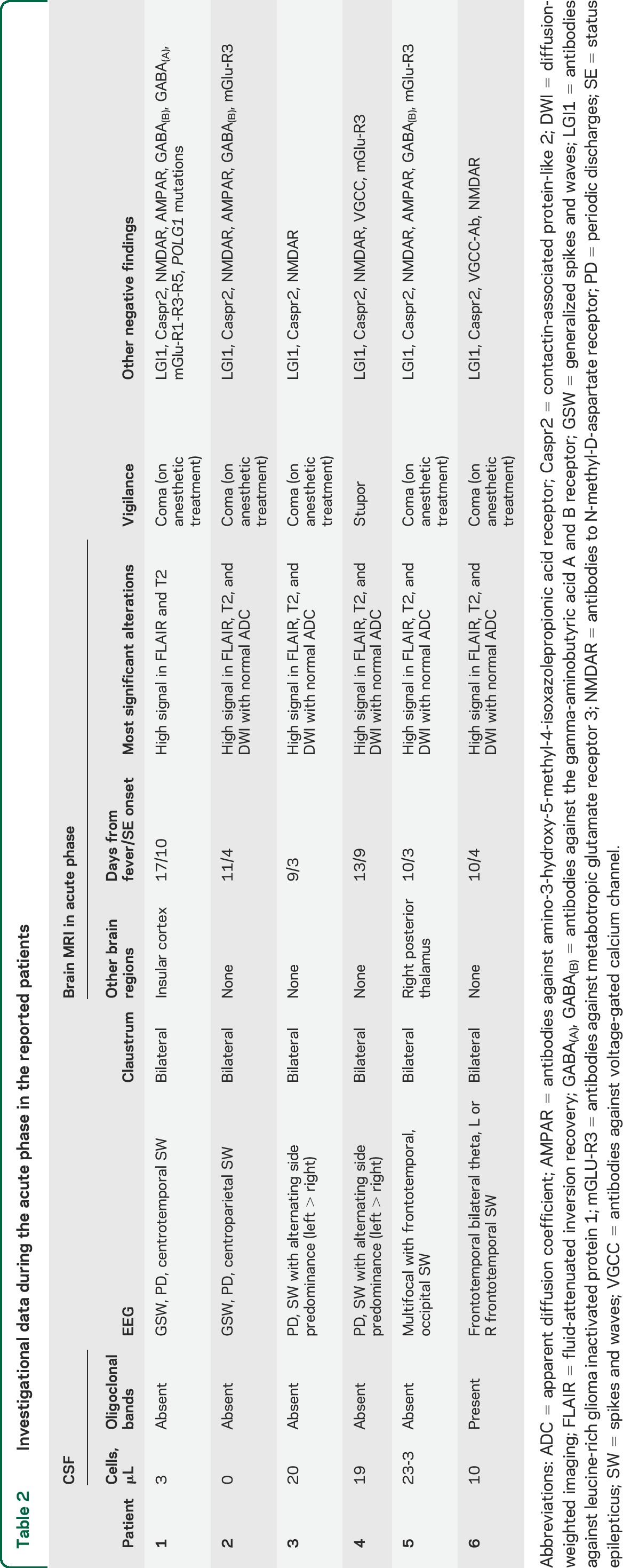
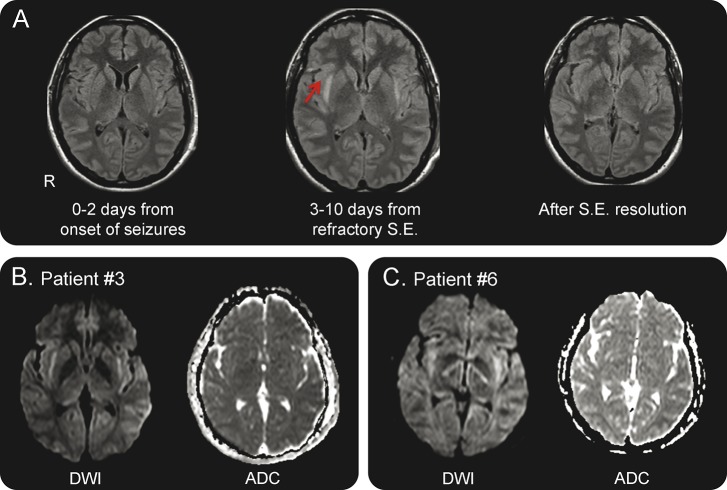
Figure 2. Typical temporal sequence of MRI alterations.
(A) Normal MRI (fluid-attenuated inversion recovery [FLAIR] sequences) before the fully developed status epilepticus (SE) (left); bilateral hyperintensity of the claustrum in FLAIR imaging during status epilepticus (central image); normal appearance of the claustrum after the resolution of the status (right image). Images refer to patient 2. (B, C) Diffusion-weighted imaging (DWI) (left) and apparent diffusion coefficient (ADC) (right) images of patients 3 and 6 show high signal in the region of the claustrum in DWI with normal ADC. The same pattern was observed in other patients. DWI sequences were not acquired in patient 1.
We did not observe signal alterations involving the medial temporal lobe, the splenium of corpus callosum, or other brain regions in any patient. MRI studies in the chronic phase were available for 3 patients and showed mild diffuse atrophy in one.
Notably, claustrum alterations were not observed in any other case of SE for which an MRI study was available during the acute phase (104 cases), considering any etiology.
Other investigational data.
EEG during the acute phase showed multifocal spikes and sharp waves over frontocentral regions in the majority, with periodic lateralized discharges (figure e-1 on the Neurology® Web site at Neurology.org). Seizures arose independently from the 2 hemispheres with secondary generalization in every patient.
All patients had negative multiple neural antibodies testing, including antibodies to NMDA receptor, leucine-rich glioma inactivated protein 1, and contactin-associated protein-like 2 (previously attributed to voltage-gated K channels) (table 2).23 One participant (patient 1) had additional negative testing for CSF and serum antibodies against γ-aminobutyric acid-A receptor (GABAA), responsible for the recently described acute autoimmune encephalopathy with refractory seizures/SE.24 This patient was also screened for mitochondrial disorders. The most common mutations in the POLG gene p.A467T, p.W748S, and p.G848S were tested and were negative25; muscle biopsy did not show specific alterations, and no respiratory chain defects were observed on muscle tissue specimens.
Literature review.
Overall, 16 cases were included in the review (14 studies, reported in table 3). The online supplementary references (e1–e53) report 53 studies on neuroimaging changes during SE, which did not show any case with claustrum involvement. The patient age range was 6–65 years (8 female). Fever, in the context of a flu-like condition, preceded the onset of neurologic symptoms in 15 out of 16. Drowsiness, confusion, and seizures were the presenting symptoms after a fever episode. In the acute phase, seizures configured a SE in all cases, with a predominance of focal motor and secondarily generalized seizures. In 7 cases, benzodiazepines and AED were successful in controlling SE, while in 8 patients the SE was considered refractory or super-refractory, with need for drug-induced coma.22 One patient died in SE.26 MRI studies showing bilateral claustrum alterations were acquired during the SE acute phase in stuporous/unconscious patients, with a time lag of 3–20 days from SE onset. MRI alterations in the acute phase consisted in every patient of hyperintense appearance of the claustrum on T2-weighted images. No case showed contrast enhancement. In 2 cases, ADC maps were reported, with no evidence of decreased diffusion, as in our cases.27,28 In 5 cases, medial temporal lobe hyperintensities were also present.26,27,29–31
Table 3.
Findings in reports describing claustrum damage in patients with new-onset status epilepticus
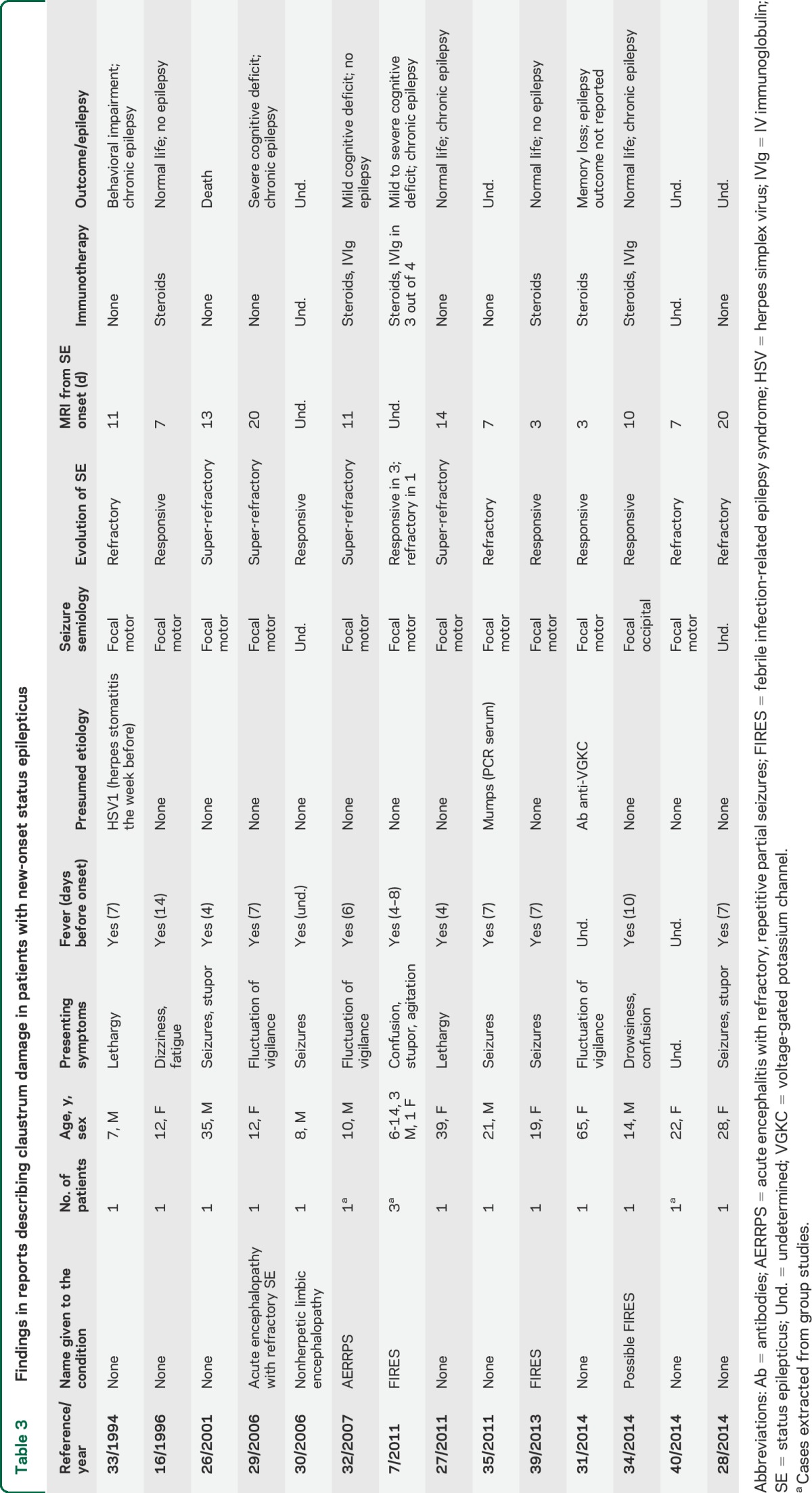
Follow-up MRIs were reported for 11 out of 16 patients showing normal findings or mild atrophy. In the MRI studies undertaken after SE resolution, the claustrum alterations were always reversed.
Clinical follow-up data were available for 10 patients. Eight out of 10 developed chronic focal epilepsy despite AED treatment.
The neuropathologic examination in the patient who died during SE showed marked astrocytic reaction in the Sommer sector and end folium of both hippocampi and in the claustrum bilaterally. No signs of encephalitis or global hypoxic-ischemic changes were observed.26
A presumed etiology was lacking in 13 out of 16 cases. Herpes simplex virus 1 encephalitis, mumps encephalitis, and voltage-gated potassium channel antibodies–related encephalitis were the presumed causes for 3 patients. In 7 patients (children or adolescents), the authors considered the condition as belonging to the acute encephalitis with refractory, repetitive partial seizures/FIRES spectrum.
DISCUSSION
We report clinical, EEG, and brain imaging findings of a homogeneous group of 6 adult patients who developed a new-onset refractory or super-refractory SE starting a few days after a febrile illness and showing a striking claustrum alteration, mainly bilaterally. We ascertained through an extensive literature review that at least 16 other cases have been described from 1996 to the present. Some of these cases were observed in children and were attributed to FIRES.7,32 Claustrum involvement in febrile-related new-onset SE is probably more frequent than has been reported. Indeed, even for the cases included in the review, several of the imaging findings were not identified as claustrum alterations, but rather as peri-insular or external capsule lesions.27,33,34 For these reasons, multicentric studies are needed to evaluate the actual claustrum involvement in both children and adults with febrile-related new-onset SE.
Our observations are important for several reasons. First, this imaging sign seems to be associated with a very aggressive form of SE, with focal motor seizures and myoclonus, often requiring ICU admission and anesthetic drugs. Indeed, considering our patient group and the previously observed cases, refractory or super-refractory SE cases were observed in 14 out of 22 patients (64%), and chronic epilepsy was reported in 12 out of 14 cases for which follow-up was available/possible (85%). Second, no definite etiology was ascertained, with the exception of 3 patients.31,33,35 Considering the striking homogeneity of the observed cases, it seems highly probable that an unknown but specific etiology accounts for febrile-related SE with claustrum hyperintensity. From this point of view, the literature review and the reported cases allow us to affirm that claustrum involvement was not observed in the context of refractory SE secondary to acute structural brain damage or to other specific SE etiologies. Importantly, no other case with claustrum involvement was observed in our cohort of SE cases observed during the 4-year study period across the different participating centers (104 cases with MRI peri-SE, post-SE). Moreover, we screened 56 publications on peri-ictal imaging changes in SE and only 3 cases were identified, notably all fulfilling our inclusion criteria. It seems therefore unlikely that claustrum lesions represent a consequence of refractory SE per se.
This retrospective uncontrolled study prevents us from making inferences about analysis of outcomes in relation to treatments. However, immunomodulatory treatment could have been relevant to outcomes.12,13 Notably, in the reported 6 patients, the 4 who received early IVIg/PEX (beyond IV steroids) had a good outcome also concerning cognitive aspects, while the 2 patients who did not receive such treatment died or had severe cognitive deficits. This observation, together with the reversibility of the claustrum lesions, the preceding febrile illness, and the absence of any detectable infectious agent, supports a role of inflammation-mediated mechanisms as relevant in the determination of SE and encephalopathy in the presented cases.15 The neuropathologic findings in one previously reported patient who died during SE showing evidence of only reactive gliosis without signs of encephalitis support this hypothesis.26
Our findings underscore the key role of claustrum dysfunction in the pathophysiology of this form of refractory or super-refractory SE. Indeed, the time course of the MRI alterations suggests that the SE was strictly time-related to claustrum dysfunction/damage. Of course, it is not possible to affirm if claustrum damage is the initial event leading to ictogenesis and SE, or if conversely it is the consequence of this peculiar form of febrile-related SE. Fever, seizures, or both could be relevant to induce claustrum alterations. However, we suggest that claustrum could have a relevant role in the maintenance of this very aggressive form of refractory SE.
Experimental evidence in animal models indicates that some subcortical structures and circuits act as critical modulators of seizure propagation and maintenance.36 In recent years, the concept that during focal seizure activity specific cortical-subcortical circuits can be critical in sustaining epileptic activity and ictogenesis has been supported by functional brain imaging studies.37 Notably, however, few observations pointed attention to the claustrum within this framework, even in animal models.38 This can be explained by the paucity of clinical data about claustrum involvement in epilepsy and by the unknown functions of this thin layer of gray matter. Recent advances in neuroanatomy and physiology indicate this subcortical region as a highly relevant hub center in neural synchronization of several (and distant) cortical areas, and, as a consequence, a structure involved in integration of conscious perception.17–21 Considering these physiologic properties of the claustrum, this structure could also be a key region in promoting the propagation and synchronization of abnormal epileptic activity from several cortical regions. To this point, 2 properties of claustral neurons are relevant. First, claustro-cortical fibers connect the claustrum with several cortical areas, including the prefrontal, pre-postcentral, posterior parietal, orbitofrontal, and medial temporal cortex.20,21 Second, the claustrum is constituted by densely packed and tightly interconnected GABAergic interneurons.17,18 Therefore, the claustrum can potentially bind together and modulate neural activities (epileptic activity in our case) from and to widespread cortical areas.
Interestingly, a recent EEG/fMRI study in patients with focal drug-resistant epilepsies found a common brain region with increased hemodynamic responses in relation to interictal epileptiform discharges, regardless of the localization of interictal and ictal activity.37 This region was close to the frontal piriform cortex, and its Talairach coordinates suggest that it corresponds to the claustrum. Notably, GABAA receptor binding (measured by flumazenil PET) was reduced in patients with more frequent seizures.
Even though this report is a hypothesis-generating study of preliminary nature, our findings suggest that the claustrum could be an attractive new target for epilepsy and SE therapy that deserves to be explored in future studies. Future prospective studies are needed to delineate the specificity of this imaging biomarker, its pathogenetic role in refractory SE, and the etiology of the condition.
Supplementary Material
GLOSSARY
- ADC
apparent diffusion coefficient
- AED
antiepileptic drug
- FIRES
febrile infection-related epilepsy syndrome
- ICU
intensive care unit
- IVIg
IV immunoglobulin
- NORSE
new-onset refractory status epilepticus
- PEX
plasma exchange
- SE
status epilepticus
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Stefano Meletti: design and conceptualization of the study, interpretation of the data, drafting and revising the manuscript. Jana Slonkova, Iva Mareckova, Nicola Specchio: data collection, interpretation of the data, drafting and revising the manuscript. Giulia Monti, Giada Giovannini, Nicola Pietrafusa: data collection, drafting and revising the manuscript. Petr Hon, Vaclav Marcian, Petr Krupa, Dagmar Berankova, Annalisa Chiari, Michal Bar: analysis and interpretation of data, revising the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
S. Meletti received research grant support from the Ministry of Health (MOH), from the non-profit organization CarisMo Foundation, and has received personal compensation as a scientific advisory board member for UCB and EISAI. J. Slonkova, I. Mareckova, G. Monti, N. Specchio, P. Hon, G. Giovannini, V. Marcian, A. Chiari, P. Krupa, N. Pietrafusa, D. Berankova, and M. Bar report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Baxter P, Clarke A, Cross H, et al. Idiopathic catastrophic epileptic encephalopathy presenting with acute onset intractable status. Seizure 2003;12:379–387. [DOI] [PubMed] [Google Scholar]
- 2.Kramer U, Shorer Z, Ben-Zeev B, Lerman-Sagie T, Goldberg-Stern H, Lahat E. Severe refractory status epilepticus owing to presumed encephalitis. J Child Neurol 2005;20:184–187. [DOI] [PubMed] [Google Scholar]
- 3.Mikaeloff Y, Jambaqué I, Hertz-Pannier L, et al. Devastating epileptic encephalopathy in school-aged children (DESC): a pseudo encephalitis. Epilepsy Res 2006;69:67–79. [DOI] [PubMed] [Google Scholar]
- 4.Awaya Y, Fukuyama Y, Hayashi K, Osawa M. Acute non-herpetic encephalitis with severe refractory status epilepticus: its overwhelming ictogenicity, epileptogenicity, long-term prognosis and review of the literature [in Japanese]. No To Hattatsu 2007;39:138–144. [PubMed] [Google Scholar]
- 5.Sakuma H, Awaya Y, Shiomi M, et al. Acute encephalitis with refractory, repetitive partial seizures (AERRPS): a peculiar form of childhood encephalitis. Acta Neurol Scand 2010;12:251–256. [DOI] [PubMed] [Google Scholar]
- 6.van Baalen A, Häusler M, Boor R, et al. Febrile infection-related epilepsy syndrome (FIRES): a nonencephalitic encephalopathy in childhood. Epilepsia 2010;51:1323–1328. [DOI] [PubMed] [Google Scholar]
- 7.Specchio N, Fusco L, Claps D, et al. Childhood refractory focal epilepsy following acute febrile encephalopathy. Eur J Neurol 2011;18:952–961. [DOI] [PubMed] [Google Scholar]
- 8.Kramer U, Chi CS, Lin KL, et al. Febrile infection-related epilepsy syndrome (FIRES): pathogenesis, treatment, and outcome: a multicenter study on 77 children. Epilepsia 2011;52:1956–1965. [DOI] [PubMed] [Google Scholar]
- 9.Van Lierde I, Van Paesschen W, Dupont P, Maes A, Sciot R. De novo cryptogenic refractory multifocal febrile status epilepticus in the young adult: a review of six cases. Acta Neurol Belg 2003;103:88–94. [PubMed] [Google Scholar]
- 10.Wilder-Smith EP, Lim EC, Teoh HL, et al. The NORSE (new-onset refractory status epilepticus) syndrome: defining a disease entity. Ann Acad Med Singapore 2005;34:417–420. [PubMed] [Google Scholar]
- 11.Costello DJ, Kilbride RD, Cole AJ. Cryptogenic new onset refractory status epilepticus (NORSE) in adults-infectious or not? J Neurol Sci 2009;277:26–31. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Saldivar C, Maganti RK. Plasma exchange in cryptogenic new onset refractory status epilepticus. Seizure 2013;22:70–73. [DOI] [PubMed] [Google Scholar]
- 13.Gall CR, Jumma O, Mohanraj R. Five cases of new onset refractory status epilepticus (NORSE) syndrome: outcomes with early immunotherapy. Seizure 2013;22:217–220. [DOI] [PubMed] [Google Scholar]
- 14.Ismail FY, Kossoff EH. AERRPS, DESC, NORSE, FIRES: multi-labeling or distinct epileptic entities? Epilepsia 2011;52:e185–e189. [DOI] [PubMed] [Google Scholar]
- 15.Nabbout R, Vezzani A, Dulac O, Chiron C. Acute encephalopathy with inflammation-mediated status epilepticus. Lancet Neurol 2011;10:99–108. [DOI] [PubMed] [Google Scholar]
- 16.Sperner J, Sander B, Lau S, Krude H, Scheffner D. Severe transitory encephalopathy with reversible lesions of the claustrum. Pediatr Radiol 1996;26:769–771. [DOI] [PubMed] [Google Scholar]
- 17.Crick FC, Koch C. What is the function of the claustrum? Philos Trans R Soc Lond B Biol Sci 2005;360:1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smythies J, Edelstein L, Ramachandran V. Hypotheses relating to the function of the claustrum. Front Integr Neurosci 2012;6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koubeissi MZ, Bartolomei F, Beltagy A, Picard F. Electrical stimulation of a small brain area reversibly disrupt consciousness. Epilepsy Behav 2014;37:32–35. [DOI] [PubMed] [Google Scholar]
- 20.Milardi D, Bramanti P, Milazzo C, et al. Cortical and subcortical connections of the human claustrum revealed in vivo by constrained spherical deconvolution tractography. Cereb Cortex 2015;25:406–414. [DOI] [PubMed] [Google Scholar]
- 21.Torgerson CM, Irimia A, Goh SY, Van Horn JD. The DTI connectivity of the human claustrum. Hum Brain Mapp 2015;36:827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shorvon S, Ferlisi M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain 2011;134:2802–2818. [DOI] [PubMed] [Google Scholar]
- 23.Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007;61:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petit-Pedrol M, Armangue T, Peng X, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol 2014;13:276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uusimaa J, Gowda V, McShane A, et al. Prospective study of POLG mutations presenting in children with intractable epilepsy: prevalence and clinical features. Epilepsia 2013;54:1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nixon J, Bateman D, Moss T. An MRI and neuropathological study of a case of fatal status epilepticus. Seizure 2001;10:588–591. [DOI] [PubMed] [Google Scholar]
- 27.Gujjar A, Jacob PC, Al-Asmi A, Ramachandran N, Obaidi A, Jain R. Reversible MRI changes in prolonged status epilepticus: a case report. Int J Neurosci 2011;121:341–345. [DOI] [PubMed] [Google Scholar]
- 28.Hwang KJ, Park KC, Yoon SS, Ahn TB. Unusual lesion in the bilateral external capsule following status epilepticus: a case report. J Epilepsy Res 2014;4:88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiihara T, Kato M, Ichiyama T, et al. Acute encephalopathy with refractory status epilepticus: bilateral mesial temporal and claustral lesions, associated with a peripheral marker of oxidative DNA damage. J Neurol Sci 2006;250:159–161. [DOI] [PubMed] [Google Scholar]
- 30.Ishida H, Hattori H, Takaura N, et al. A child with non-herpetic acute limbic encephalitis affecting the claustrum and hippocampus [in Japanese]. No To Hattatsu 2006;38:443–447. [PubMed] [Google Scholar]
- 31.Hiraga A, Watanabe O, Kamitsukasa I, Kuwabara S. Voltage-gated potassium channel antibody-associated encephalitis with claustrum lesions. Intern Med 2014;53:2263–2264. [DOI] [PubMed] [Google Scholar]
- 32.Saito Y, Maegaki Y, Okamoto R, et al. Acute encephalitis with refractory, repetitive partial seizures: case reports of this unusual post-encephalitic epilepsy. Brain Dev 2007;29:147–156. [DOI] [PubMed] [Google Scholar]
- 33.Kimura S, Nezu A, Osaka H, Saito K. Symmetrical external capsule lesions in a patient with herpes simplex encephalitis. Neuropediatrics 1994;25:162–164. [DOI] [PubMed] [Google Scholar]
- 34.Mumoli L, Labate A, Palamara G, Sturniolo M, Gambardella A. Reversible symmetrical external capsule hyperintensity as an early finding of autoimmune encephalitis. Neurol Sci 2014;35:1147–1149. [DOI] [PubMed] [Google Scholar]
- 35.Ishii K, Tsuji H, Tamaoka A. Mumps virus encephalitis with symmetric claustrum lesions. AJNR Am J Neuroradiol 2011;32:E139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gale K. Subcortical structures and pathways involved in convulsive seizure generation. J Clin Neurophysiol 1992;9:264–277. [DOI] [PubMed] [Google Scholar]
- 37.Laufs H, Richardson MP, Salek-Haddadi A, et al. Converging PET and fMRI evidence for a common area involved in human focal epilepsies. Neurology 2011;77:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wada JA, Tsuchimochi H. Role of the claustrum in convulsive evolution of visual afferent and partial nonconvulsive seizure in primates. Epilepsia 1997;38:897–906. [DOI] [PubMed] [Google Scholar]
- 39.Serrano-Castro PJ, Quiroga-Subirana P, Payán-Ortiz M, Fernandez-Perez J. The expanding spectrum of febrile infection-related epilepsy syndrome (FIRES). Seizure 2013;22:153–155. [DOI] [PubMed] [Google Scholar]
- 40.Cartagena AM, Young GB, Lee DH, Mirsattari SM. Reversible and irreversible cranial MRI findings associated with status epilepticus. Epilepsy Behav 2014;33:24–30.24614522 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.