Introduction and aims
The indications for transfusion therapy in the neonatal period are based on specific knowledge of various aspects of this particular period of life, such as the dynamic interaction of the mother-placenta-foetus/neonate, the pathophysiological changes in the perinatal and neonatal periods and the profound haematological modfications that are characteristic of the first weeks of life. Furthermore, the physiological immaturity of various organs and systems can expose neonates, in particular those with a very low birth weight (VLBW) (Appendix I), to metabolic alterations following the transfusion of various blood components and the additives in them, and to infectious and immunological risks, such as Graft-versus-Host disease (GVHD).
This implies the need for close and continuous collaboration between paediatricians-neonatologists and transfusion medicine specialists in order to obtain “dedicated” blood components, both with regards to quality and quantity, able to meet the particular needs of the neonate, especially considering the now increased survival of extremely low birth weight (ELBW) babies.
ELBW and “critically ill” neonates are categories of patients with high transfusion needs, even though the number of transfusions given to premature neonates has progressively decreased over the last decade. It is, however, essential to establish appropriate transfusion criteria for these subjects.
The scientific contributions on transfusion medicine in the neonatal period derive predominantly from consensus of opinions rather than controlled studies and the lack of clear scientific evidence makes it difficult to formulate high-grade recommendations based on solid levels of evidence. Furthermore, it should be appreciated that neonatal transfusion medicine is, like all other scientific fields, a continuously evolving discipline. These Recommendations, which represent the opinions of the authors and include evidence-based data, when available, have been formulated to facilitate the implementation of uniform transfusion practices. They are not intended to provide absolute indications, but aim to be a “guide” which nevertheless guarantees individual healthcare professionals freedom of choice in the various different clinical situations.
This document deals with pre-transfusion tests, indications for the transfusion of blood components, characteristics of the blood components and methods of their administration for neonates. Details on the levels of evidence and strengths of the recommendations are provided in Appendix II.
This document does not consider the indications for the use of blood derivatives and some highly specialised, life-saving techniques used in particular emergencies, such as extracorporeal membrane oxygenation and cardiopulmonary bypass.
General criteria
Blood donors and blood components
The choice of donor may contribute to reduce the risk of transmission of infectious diseases; it is, therefore, recommended that only blood components obtained from repeat blood donors are used, as set out in current legislation in Italy1–4.
Leucodepletion
The use of leucodepleted blood components has the now undisputed advantages of:
- preventing non-haemolytic febrile reactions;
- reducing the risk of alloimmunisation;
- lowering the risk of transmission of cytomegalovirus (CMV) infection.
For this reason, all cellular blood components used in the neonatal period, except granulocytes, which cannot currently be considered a standard therapy outside clinical studies, must be leucodepleted (white blood cells <1×106/unit), preferably at the time of collection (pre-storage)5,6 (Level of evidence IV, Grade of recommendation C).
Prophylaxis of cytomegalovirus infection
The subjects at greatest risk of transfusion-transmitted infections are: the foetus, the neonate weighing ≤1,500 g at birth and/or born at a gestational age of ≤30 weeks (independently of maternal serology), neonates with congenital or acquired immunodeficiency and those who receive haematopoietic stem cells. It is, therefore, recommended that CMV-safe blood components are used in the following circumstances:
- intrauterine transfusion of red blood cells (RBC) and platelets;
- neonates weighing ≤1,500 g at birth and/or with a gestational age ≤30 weeks;
- neonates with congenital or acquired immunodeficiency;
- seronegative candidates for or recipients of allografts;
- pregnant women.
Blood components can be considered CMV-safe if they have been obtained from CMV-negative donors or contain <5×106 leucocytes/unit. Thus, leucodepleted blood components (white blood cells <1×106/unit) can be considered CMV-safe (Level of evidence IIb, Grade of recommendation B). However, neither donation from CMV-negative donors nor leucodepletion, nor indeed the combination of strategies, is able to completely eliminate the risk of transmission of CMV infection, because of the possible, occasional cases of viraemia in the initial stage of the infection7.
Fresh-frozen plasma (FFP) does not transmit CMV infection and can be administered without regard to the donor’s serological status. Passive acquisition of antibodies can cause false positive results, giving rise to a patient’s pseudo-seroconversion.
Prophylaxis of Graft-versus-Host disease
In order to prevent Graft-versus-Host disease, RBC and platelets (but not FFP) must be irradiated in the following circumstances8–10 (Level of evidence III, Grade of recommendation B):
- intrauterine transfusion of RBC and platelets;
- transfusion of RBC (including exchange transfusion [ET]) and platelets after intrauterine transfusion;
- transfusion of RBC and platelets in neonates weighing ≤1,500 g at birth and/or with a gestational age ≤30 weeks;
- donated blood from a first or second degree relative or human leucocyte antigen (HLA)-like relative, although donation from a relative must be an exceptional event, to be discouraged;
- neonates with congenital or acquired immunodeficiency;
- recipients of haematopoietic stem cells.
The blood components must be irradiated with a dose ranging between 25 and 50 Gray (2,500–5,000 rad). Units destined for transfusion to neonates must be chosen from those collected within the preceding 5 days. Once irradiated, the RBC must be transfused within 24 hours; if that is not possible, they must be washed with physiological saline in order to remove any excess of potassium and, possibly, in a closed circuit to limit the risk of bacterial contamination. Once washed, the RBC must be transfused as soon as possible and, in any case, not later than 24 hours after preparation. It is good transfusion practice to irradiate the blood component immediately prior to transfusion1.
Irradiation does not change the expiry data of platelet concentrates1.
In the case of transfusion of small volumes, it is good practice to irradiate only the fraction destined for transfusion, rather than the whole unit (Level of evidence IV, Grade of recommendation C).
The remaining sub-units should be irradiated within a maximum of 14 days of collection of the parent unit1.
In order to guarantee optimal transfusion support, the turnover of irradiated units should be rapid, reserving the freshest units irradiated the least time previously (preferably on the same day as transfusion) for neonates.
In the exceptional case of administration of granulocyte concentrates, these must always be irradiated and transfused as soon as possible, but in any case within 24 hours1 (Level of evidence III, Grade of recommendation B).
Pre-transfusion tests
Serological investigations
The initial tests should include the following.
-
Tests to perform on the mother (if a blood sample is available):
- determination of ABO/Rh phenotype;
- screen for irregular erythrocyte antibodies with an indirect antiglobulin test.
-
Tests to perform on the neonate:
- determination of ABO/Rh phenotype (to be confirmed on a second sample);
- a direct antiglobulin test and, if positive, elution and identification of the eluted antibody;
- screen for irregular erythrocyte antibodies.
However, it is good practice to search for and identify antibody specificities in maternal blood; thus, the use of these tests in the neonate should be limited to cases in which a sample of maternal blood is not available.
Pre-transfusion compatibility tests
It is recommended that maternal serum/plasma is used for the search for irregular erythrocyte antibodies and/or cross-matching at the first transfusion1; when maternal serum/plasma is not available, the pre-transfusion tests can be performed only on the neonate’s serum/plasma although, if the direct antiglobulin test is positive, it is preferable to use the eluate obtained from the RBC rather than the neonate’s serum/plasma11. In cases in which the direct antiglobulin test and/or search for irregular antibodies are positive, cross-matching must always be performed, through the indirect antiglobulin test, using the mother’s serum/plasma (at the first transfusion) and/or eluate of the neonate’s RBC and/or the neonate’s serum/plasma (Level of evidence IV, Grade of recommendation C).
If the maternal serum contains a clinically relevant antibody, the neonate must be transfused with red blood cells lacking the antigen to which the antibody is directed. This practice must be maintained until the antibody disappears from the neonate’s circulation12.
The cross-match is mandatory in the case of transfusions following a first one, even when the direct antiglobulin test and/or search for irregular antibodies was initially negative. In this case the neonate’s serum/plasma must be used.
Precautions and considerations
In the neonatal period, as in every other period of life, all measures must be taken to avoid errors in identification of the units of blood components and of the recipient, exchanges of samples, and labelling errors.
ABO phenotype determination in neonates is based only on the identification of RBC antigens, because anti-A/-B isoagglutinins are absent.
Errors of blood group typing can derive, albeit rarely, from the weak expression of erythrocyte antigens on the neonate’s RBC or because of the presence of maternal antibodies capable of masking the corresponding antigens (RhD haemolytic disease of the foetus and neonate, HDFN)11.
Intrauterine foetal transfusion
In the last decade there has been a gradual decline in the use of this technique. A survey carried out by Italian Society of Transfusion Medicine and Immunohaematology in 2010 in about 60% of the Transfusion Services active in Italy showed that intrauterine foetal transfusion is now only practised rarely in centres specialised in Mother and Child Medicine13.
Plasmapheresis associated with the infusion of intravenous immunoglobulins (IVIG) seems to offer an effective, alternative antenatal treatment in cases of severe HDFN14,15. However, intrauterine foetal transfusion appears to be the most effective transfusion practice for a quick recovery from severe foetal anaemia16.
Intrauterine foetal transfusion with packed red cells is mainly indicated for correcting foetal anaemia secondary to the haemolytic action of alloantibodies against blood group antigens present on foetal erythrocytes (the antigens most frequently involved are: D, c, E, K, Fya, Jka). Other, less common indications are foetal anaemia following Parvovirus B19 infection, homozygous alpha-thalassaemia, “massive” foeto-maternal transfusion or foeto-foetal transfusion between twins11,17.
At present the indication for intrauterine foetal transfusion is provided non-invasively by the ultrasound evaluation of middle cerebral artery peak systolic velocity, which enables a moderate or high risk of anaemia to be determined in relation to gestational age18.
Intrauterine foetal transfusion can be performed in two ways: intravascular transfusion or intraperitoneal transfusion.
Intravascular transfusion is currently the technique of choice, in particular in foetuses with hydrops, in whom a survival of greater than 70% has been reported19.
Under ultrasound guidance a needle is passed transabdominally into the umbilical vein close to the site of entry of the cord in the placenta (cordocentesis). A small quantity of foetal blood is collected through this needle and used to evaluate the degree of anaemia, and then packed red cells are infused. The first intravascular transfusion can be performed from the 18th week of gestation, although the risk of foetal death decreases if the technique is used after the 20th week20.
The risk of foetal death within 48 h of the procedure is about 2%16.
With intraperitoneal transfusion red blood cells are infused directly through a needle placed in the peritoneal cavity of the foetus and reabsorbed slowly into the blood circulation through the subdiaphragmatic lymphatic vessels. This technique, which was widely used in the past, only allows gradual correction of the anaemia and, in the presence of ascites, the passage of transfused red cells can be altered or decreased. This procedure can, however, still be used in the case of failure of intravascular transfusion. Considering that an approximately one percentage point decrease in the haematocrit (Hct) can be expected daily, the procedure should be repeated at intervals of about 2–3 weeks until the time of the planned delivery.
In the case of very severe foetal anaemia, generally associated with foetal-placental hydrops, the post-transfusion Hct should not be more than four times higher than the initial Hct; in fact, a brusque increase in blood viscosity could compromise the cardiovascular system.
The delivery, to be performed by Caesarean section, is usually planned from the 34th week of gestational age, if the intrauterine transfusion has been successful, following induction of lung maturity. The use of intrauterine transfusion enables up to 80% of foetuses with severe HDFN to be treated successfully. Lower percentages are reported in cases of severe foetal-placental hydrops19.
Intravascular transfusion of platelet concentrates currently has few indications. It was recently used in a case of severe foetal thrombocytopenia associated with anaemia due to Parvovirus B19 infection21. This technique is no longer approved for the treatment of alloimmune foetal-neonatal thrombocytopenia because of the high risk of bleeding following cordocentesis and the good results obtained with non-invasive treatment, based on the use of IVIG and corticosteroids22.
The characteristics of the blood components and procedures for intrauterine foetal transfusion
Packed red cells
These must:
- be group O Rh (D) negative in the case of HDFN due to anti-D alloantibodies. RBC of the same blood group as the foetus can be used when this blood group is known and there is no ABO/Rh incompatibility with the mother;
- be compatible with maternal serum and, therefore, lacking the antigen against which the mother has produced alloantibodies: this is, of course, true for both HDFN due to anti-D and HDFN due to alloantibodies other than anti-RhD;
- be prepared within 5 days of collection;
- be leucodepleted/CMV-safe;
- be irradiated;
- have a Hct ~80%.
The volume to transfuse is calculated using the following formula:
The desired Hct is about 40–45%.
The transfusion is given at a rate of about 5 mL/min and even more slowly in the case of a hydropic foetus (2–3 mL/min)11.
Transfusions in the neonatal period
Exchange transfusion
Indications
The most frequent indication for exchange transfusion (ET) is marked hyperbilirubinaemia, since this technique quickly lower the levels of bilirubin considered responsible for neurological damage (kernicterus). The guidelines of the American Academy of Pediatrics supply indications on the levels of total bilirubin at which ET is advised in the case of lack of response to phototherapy. These levels depend primarily on post-natal age and the possible presence of numerous other risk factors such as HDFN, glucose-6-phosphate deficiency, prematurity, sepsis, acidosis, and hypoalbuminaemia23.
Other extremely rare indications are accumulation of endogenous toxic metabolites and drug overdoses. It was also reported recently that ET was an effective treatment in a case of neonatal haemochromatosis24,25.
The main indication for ET is, however, HDFN, even if recourse to this treatment is ever more uncommon because of the considerable decrease in the incidence of HDFN due to anti-D, the use of IVIG and the efficacy of modern phototherapy techniques26,27. The main aim of ET in HDFN is to remove the alloantibodies that are free in the serum or attached to RBC in order to simultaneously correct both the hyperbilirubinaemia and the anaemia resulting from antibody-mediated haemolysis.
"Early" exchange transfusion (within 9–12 h of birth) in haemolytic disease of the foetus and neonate
The criteria for early ET are based on the finding of:
- haemoglobin (Hb) values in the cord blood ≤8 g/dL;
- or levels of total bilirubin in cord blood >5.0–5.5 mg/dL and an increase in total bilirubin values ≥0.5–1.0 mg/dL/hour, despite the use of intensive phototherapy11.
In the days following birth, the indications for ET are based on the levels of total bilirubin, as recommended by the guidelines of the American Academy of Pediatrics23.
Methods of performance and recommendations
ET is performed using reconstituted whole blood with a “push-pull” technique, through a single vascular access, which is usually the umbilical vein. In exceptional cases, when this vein cannot be used, the ET can be performed with a technique involving two vascular accesses through which the reconstituted whole blood is contemporaneously removed and introduced. In these cases, two operators are necessary.
The volume exchanged each time should be about 5 mL/kg and the rate should not exceed 2–3 mL/kg/min, in order to avoid rapid fluctuations of intracranial pressure11 (Level of evidence IV, Grade of recommendation C).
Further details on the technique of ET can be found in other publication28.
The total volume of reconstituted whole blood (mL) to exchange (“double volume” exchange) is 160 mL/kg for neonates born at term and 200 mL/kg for those born prematurely.
With “double-volume” exchange, about 80–90% of the neonate’s RBC are removed and there is a reduction of approximately 50% of the pre-ET intravascular bilirubin level29. About 4 hours after the procedure there can be a rebound of about 60% of the total bilirubin level.
ET can be complicated by a series of side effects, such as thrombocytopenia, metabolic changes (hypocalcaemia, hyper- or hypo-glycaemia, hypernatraemia, hyperkalaemia) and thrombosis of the umbilical vein or lead to necrotising enterocolitis30. The platelet count must be controlled at the end of the exchange procedure because of a possible wash-out effect.
Furthermore, depending on the amount of calcium in the blood, it may be necessary to administer calcium gluconate (Level of evidence III, Grade of recommendation B). Despite the risks associated with this procedure, the reported mortality rate is <0.6%, although it may be higher in preterm babies and in those with severe pathologies11.
Characteristics of the reconstituted whole blood
The whole blood is obtained from reconstituting red cell concentrates with fresh-frozen plasma. The reconstitution procedure must only be performed in Transfusion Services, using appropriate formulae to obtain the desired Hct.
Characteristics of the red blood cells:
- the same blood group or ABO/Rh compatible with the neonate and the maternal plasma. In particular:
Rh(D) negative in cases of HDFN Rh(D);
O phenotype in cases of ABO incompatibility neonatal haemolytic disease;
- lacking the antigens against which any irregular antibodies are directed (HDFN due to anti-c, anti-K, etc.) identified in the mother’s or neonate’s serum/plasma;
- fresh (collected within the preceding 5 days). In cases in which fresh blood (collected within the preceding 5 days) is not available, products that have been stored for longer can be used, provided they are compatible with irradiation which, by law, must be performed within 14 days of donation, with an additional washing procedure to remove storage residues;
- cleaned of any additive or preservative, before reconstitution;
- leucodepleted, CMV-safe;
- heated to 37 °C, if specific equipment is available.
Characteristics of the plasma:
- safe FFP is used (quarantined or inactivated);
- AB phenotype.
The final product must:
- have a Hct between 0.50 and 0.60;
- be irradiated;
- be transfused within 24 hours from the preparation.
The product has the same metabolic and haemostatic characteristics as fresh, whole blood but lacks platelets.
"Partial" exchange transfusion
This is used in cases of severe anaemia at birth associated with congestive heart failure (HDFN with hydrops; chronic foeto-maternal or foeto-foetal post-haemorrhagic anaemia), in order to correct the anaemia without increasing blood volume.
The product used for partial ET is packed red cells with a Hct of about 0.70. The amount is determined from the following formula:
Partial ET is more commonly used in the treatment of polycythemia or hyperviscosity syndrome. This develops when the Hct of a venous sample is >0.65–0.70 and is characterised by symptoms such as tachypnoea, hypotonia, tremor, seizures, cardio-circulatory compromise and renal failure. In such cases it is advisable to perform partial ET in order to lower the Hct to about 0.50. There are no clinical data demonstrating either a short-term or long-term benefit of partial ET used as a treatment for polycythemia (Hct>65%), when performed in neonates in a stable clinical condition or with few symptoms31. A potential disadvantage of partial ET is the possibility of side effects, including a higher risk of necrotising enterocolitis. Data concerning long-term neurological development are vitiated by the inadequate follow-up and, consequently, indications on the benefits and risks cannot be formulated.
The Hct is corrected by using crystalloid solutions instead of plasma or albumin, which were widely used in the past (Level of evidence Ib; Grade of recommendation A)32.
The volume of crystalloid solution to exchange is calculated using the following formula:
Recent studies evaluating the long-term outcome of neonates with symptomatic polycythemia undergoing partial ET have not found clear benefits with regards to neurocognitive development33.
Transfusion of packed red cells
The use of packed red cells in the anaemia of very low birthweight babies
For various reasons VLBW babies have lower Hct and Hb levels at birth and in the first weeks of life than babies born at term (Table I)34. This particular haematological profile, called anaemia of prematurity, can further worsen the clinical course of a premature baby, which is often complicated by cardiorespiratory, metabolic and haemorrhagic disorders.
Table I.
Concentration of haemoglobin (g/dL) in prematurely born babies in the first 16 weeks of life.
| Age (weeks) | Weight at birth | |
|---|---|---|
|
| ||
| 1,000–1,500 g | 1,501–2,000 g | |
| 2 | 16.3 (11.7–18.4) | 16.8 (11.8–19.6) |
| 4 | 10.9 (8.7–15.2) | 11.5 (8.2–15.0) |
| 8 | 8.8 (7.1–11.5) | 9.4 (8.0–11.4) |
| 12 | 9.8 (8.9–11.2) | 10.2 (9.3–11.8) |
| 16z | 11.3 (9.1–13.1) | 11.3 (9.1–13.1) |
From Lundstrom U et al. 197734.
Thus, VLBW and, in particular, ELBW, babies form a class of neonates more frequently administered transfusion therapy and, precisely because of the extreme immaturity of their various organs and systems, may be predisposed to more side effects of the blood transfusion35.
Advances in the last 20 years in Transfusion Medicine have led to drastic reductions in infectious risks and adverse events related to blood transfusion35. Furthermore, the particular attention given to this vulnerable category of patients has led to further improvements, including a decrease in side effects and, above all, a reduction in the number of donors to which each neonate is exposed36,37.
Studies carried out since the early 1990s aimed at evaluating the efficacy of recombinant human erythropoietin (rHuEPO) in anaemia of prematurity have contributed to both the production and application of specific transfusion protocols, promoting better use of blood transfusions in neonatal intensive care units. It has been demonstrated that transfusing according to pre-established criteria limits both the number of neonates given a transfusion and the number of donors to which each neonate is exposed38–40. For this reason it is recommended that individual neonatal intensive care units adopt transfusion protocols “dedicated” to this particular category of neonates (Level of evidence Ib, Grade of recommendation A).
The ever more restrictive transfusion practices adopted in recent years have led to the need to evaluate both the short-term and long-term outcomes of reduced transfusion regimes41. Most studies are concordant in not showing statistically significant differences in immediate outcomes, such as mortality, or long-term ones (auditory, visual or psychocognitive deficits) in neonates managed with a restricted transfusion regime compared to neonates managed according to more “liberal” criteria42,43. Other studies which have evaluated outcomes in school age have even found better neurocognitive development in neonates managed with the restricted transfusion regime, compared to those who received more blood transfusions44.
The possible association between blood transfusions and adverse events, such as intraventricular haemorrhage and necrotising enterocolitis, frequently reported in recent years45–48, justifies the current tendency to adopt ever lower Hct (or Hb) thresholds for blood transfusions49.
The transfusion criteria used for VLBW babies are based more on consensus of opinions of “experts” than on scientific evidence. In any case, the diagnostic means that we have make it currently difficult, if not impossible, to formulate motivated indications (for example, reduced tissue oxygenation) on the need to give a blood transfusion. In the first few weeks of life, VLBW neonates generally have cardio-respiratory disorders and may have to undergo major surgical interventions, meaning that they need transfusions to maintain the levels of Hb>12g/dL. In contrast, a less aggressive transfusion approach is recommended in neonates in a stable clinical condition, particularly during the phase of recovery of body growth. The finding of an absolute reticulocyte count >75–100×103/mL is indicative of a rapid increase in Hb values, so, in the presence of a stable clinical condition, the decision to transfuse can be deferred50.
The indications listed in Table II are given with the purpose of providing “threshold” values of Hb drawn from the most recent studies, leaving large decisional power to each professional on the appropriate choice to make faced with different, specific clinical situations42.
Table II.
Indications for transfusion of packed red blood cells in VLBW neonates according to haemoglobin levels (g/dL)*.
| Age (days) | Type of sample | Neonates receiving respiratory aid** | Neonates not receiving respiratory aid |
|---|---|---|---|
| 1–7 | Skin prick | ≤11.5 | ≤10.0 |
| Central | ≤10.4 | ≤9.0 | |
|
| |||
| 8–14 | Skin prick | ≤10.0 | ≤8.5 |
| Central | ≤9.0 | ≤7.7 | |
|
| |||
| ≥15 | Skin prick | ≤8.5 | ≤7.5 |
| Central | ≤7.7 | ≤6.8 | |
Modified from Kirpalani et al. 200642.
These recommendations are not valid in the case of major surgery, sepsis, shock, haemorrhage or symptoms suggestive of anaemia(tachycardia, tachypnoea).
Includes assisted ventilation, continuous positive-pressure ventilation, and administration of free-flowing oxygen.
Use of packed red cells in post-haemorrhagic and haemolytic anaemias and anaemia due to reduced or altered red blood cell production
Anaemia present at birth and in the first week of life
In cases of severe anaemia (Hb<8g/dL) with hypovolaemic shock (loss of blood volume >20%) following bleeding from a placenta previa, abruptio placentae, ruptured cord, etc., the intravascular volume must be restored quickly and the anaemia corrected in the ways reported in Table III (Level of evidence IV, Grade of recommendation C).
Table III.
Use of packed red cells at birth in the case of acute post-haemorrhagic anaemia with hypovolaemic shock.
| Correction of hypovolaemia: urgent transfusion (20 mL/kg) of physiological saline, a “volume expander” or reconstituted blood (if available). | |
|
Correction of anaemia: (unless reconstituted blood has been used to correct the hypovolaemia, restore the haematocrit [Hct] to about 0.35 without giving more than 20 mL/kg) transfuse packed red cells (PRC) according to the following formula:
|
In cases of anaemia that develop during the first week of life, for which the Hb values are moderately lower than the reference values for post-natal age (Table IV)51 and the neonate is clinically stable, it is justifiable to wait for recovery of erythropoietic activity (evaluated from the reticulocyte count). Transfusion therapy is, however, necessary in the presence of severe cardiorespiratory difficult or surgery to maintain the Hct >0.35.
Table IV.
Red blood cell parameters in neonates born at term in the first 6 months of life.
| Age | Hb g/dL | Hct % | RBC 1012/L | MCV fL | MCH pg | MCHC g/dL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Mean | −2 SD | Mean | −2 SD | Mean | −2 SD | Mean | −2 SD | Mean | −2 SD | Mean | −2 SD | |
| Cord | 16.5 | 13.5 | 51 | 42 | 4.7 | 3.9 | 108 | 98 | 34 | 31 | 33 | 30 |
| 1–3 days | 18.5 | 14.5 | 56 | 45 | 5.3 | 4.0 | 108 | 95 | 34 | 31 | 33 | 29 |
| 1 week | 17.5 | 13.5 | 54 | 42 | 5.1 | 3.9 | 107 | 88 | 34 | 28 | 33 | 28 |
| 2 weeks | 16.5 | 12.5 | 51 | 39 | 4.9 | 3.6 | 105 | 86 | 34 | 28 | 33 | 28 |
| 1 month | 14.0 | 10.0 | 43 | 31 | 4.2 | 3.0 | 104 | 85 | 34 | 28 | 33 | 29 |
| 2 months | 11.5 | 9.0 | 35 | 28 | 3.8 | 2.7 | 96 | 77 | 30 | 26 | 33 | 29 |
| 3–6 months | 11.5 | 9.5 | 35 | 29 | 3.8 | 3.1 | 91 | 74 | 30 | 25 | 33 | 30 |
Modified from Dallman PR et al. 197751.
Hb: haemoglobin; Hct: haematocrit; RBC: red blood cells; MCV: mean cell volume; MCH: mean corpuscular haemoglobin; MCHC: mean cell haemoglobin concentration; SD: standard deviation.
Late-onset neonatal anaemias (after the first week of life)
When evaluating these forms of anaemia it is essential to consider the reference ranges for Hb (or Hct) in the post-natal period (Table IV)51 and the presence of any symptoms suggesting inadequate tissue oxygenation, such as apathy, difficulty in suckling, poor growth, tachycardia, and tachypnoea. It is also very important to evaluate the degree of reticulocyte response, in that a reticulocyte count >100×103/μL is an indicator of effective bone marrow compensation. Neonates who undergo ET in the first week of life can tolerate even very low levels of Hb (~6–7 g/dL) in the following weeks because of the high proportion of HbA and consequent increased release of oxygen to tissues.
Characteristics of packed red blood cells
It is considered good practice to give VLBW babies small aliquots (pedi-packs), obtained via a connecting device, of a single unit of packed red cells. The unit should be stored for the same neonate, when it is predicted that more than one transfusion will be necessary within a certain period of time, in order to reduce exposure of the neonate to different donors (Level of evidence III, Grade of recommendation B).
The packed red cells must:
- be the same ABO/Rh group or a group compatible with the neonate and the mother’s serum/plasma (Tables Va and Vb);
- lack the antigens against which any irregular antibodies found in the maternal or neonatal serum/plasma are directed; thus they must be negative in a cross-match test with maternal or neonatal serum/plasma;
- have a final haematocrit of about 0.70;
- be leucodepleted/CMV-safe;
- be irradiated, if indicated;
- be used within 14 days of collection if irradiation is necessary and preferably transfused immediately after irradiation.
In premature babies the volume of packed red cells to administer varies from 10 to 20 mL/kg or can be calculated using the following formula:
It is not necessary to warm small quantities of red cell concentrate before its administration; the transfusion must be completed within 3–4 hours11.
Transfusion of fresh-frozen plasma
Proof of the efficacy of FFP in neonates is extremely limited52. The use of this blood component in sepsis or as a volume expander in the neonate with hypotension is not considered appropriate53. Furthermore, the administration of FFP as a strategy to prevent intracranial haemorrhage has not been shown to be beneficial and is not, therefore, indicated54 (Level of evidence Ib, Grade of recommendation A).
The administration of FFP is, however, recommended for bleeding associated with coagulopathy (Tables VI and VII). It should be noted that the longer clotting times in the neonate than in the adult do not correlate with an increased risk of bleeding55–59. This is all the more the case in premature neonates; thus, isolated changes in clotting tests, in the absence of bleeding, are not indications for the transfusion of FFP (Table VII).
Table VI.
Indications for the transfusion of fresh-frozen plasma.
|
Table VII.
Definition of coagulopathy in premature neonates and neonates born at term, at birth (A) and in the post-natal period (B), and recommended interventions with level of evidence and strenght of recommendation.
| (A) At birth | |||
|---|---|---|---|
|
| |||
| Category | Fibrinogen | PT | PTT |
| Lower limit | Upper limit | Upper limit | |
| Neonate <28 weeks (1) | <71 mg/dL | >21 sec. | >64 sec. |
| Neonate 28–34 weeks (1) | <87 mg/dL | >21 sec. | >57 sec. |
| Neonate 30–36 weeks (2) | <150 mg/dL | >16 sec. | >79 sec. |
| Neonate at term (3) | <167 mg/dL | >16 sec. | >55 sec. |
|
| |||
| Recommended intervention | |||
|
| |||
| In neonate without bleeding | Observation (IIb/B) | Observation (IIb/B) | Observation (IIb/B) |
| In neonate with bleeding or to undergo an invasive procedure | Cryoprecipitate* | FFP | FFP |
| 5–10 mL/kg (IV/C) | 15–20 mL/kg (III/B) | 15–20 mL/kg (III/B) | |
|
| |||
| (B) Post-natal period | |||
|
| |||
| Category | Fibrinogen | PT | PTT |
| Lower limit | Upper limit | Upper limit | |
|
| |||
| Neonate 30–36 weeks (2) - Post-natal age | |||
| Day 5 | <160 mg/dL | >15 sec. | >74 sec. |
| Day 30 | <150 mg/dL | >14 sec. | >62 sec. |
| Day 90 | <150 mg/dL | >15 sec. | >51 sec. |
| Neonate at term (3) - Post-natal age | |||
| Day 5 | <162 mg/dL | >15 sec. | >60 sec. |
| Day 30 | <162 mg/dL | >14 sec. | >55 sec. |
| Day 90 | <150 mg/dL | >14 sec. | >50 sec. |
|
| |||
| Recommended intervention | |||
|
| |||
| Neonate without bleeding | Observation (IIb/B) | Observation (IIb/B) | Observation (IIb/B) |
| Neonate with bleeding or to undergo an invasive procedure | Cryoprecipitate* | FFP | FFP |
| 5–10 mL/kg (IV/C) | 15–20 mL/kg (III/B) | 15–20 mL/kg (III/B) | |
Reference ranges drawn from:
Christensen RD et al. 201455;
Andrew M et al. 198856;
Andrew M et al. 199257.
Product not easily standardised and subject to donor- and manual preparation-dependent variation, with a very wide range of fibrinogen concentrations. Fibrinogen is currently available as a plasma-derivative, but there is insufficient experience with its use in the neonatal period.
PT: prothrombin time; PTT: partial thromboplastin time; FFP: fresh-frozen plasma.
FFP can be used in the treatment of congenital deficiencies of single clotting factors for which the relative blood derivative is not available (Table VI).
In the cases in which FFP is advised (Table VI), it should be transfused at a dose of about 15–20 mL/kg (Level of evidence IV, Grade of recommendation C).
Characteristics of fresh-frozen plasma
It is recommended that the Transfusion Service divides a single unit of plasma, possibly produced by apheresis, into several fractions of suitable volume, prior to freezing, to be reserved for an individual neonate.
The FFP must be:
- ABO compatible or AB phenotype;
- “safe”, that is, quarantined or subjected to pathogen inactivation.
Transfusion of platelet concentrates
Thrombocytopenia is common in premature neonates (occurring in up to 73% of neonates weighing <1,000 g and up to 85–90% in neonates weighing <750 g) and is associated with a risk of severe intraventricular haemorrhage60.
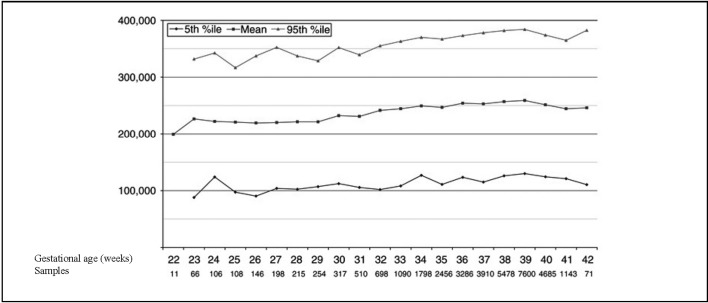
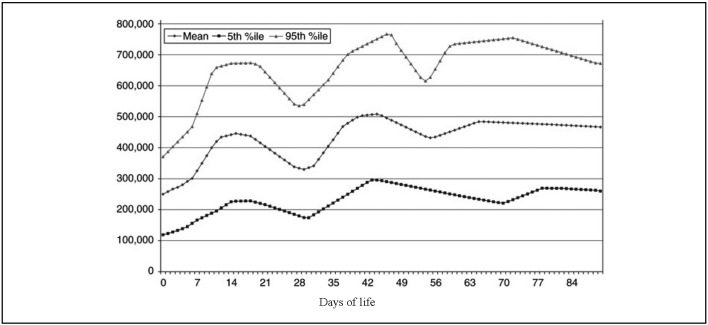
The incidence of thrombocytopenia varies according to the definition used; new reference ranges were proposed recently, taking into consideration the effect of gestational age at birth and post-natal age on the platelet count (Figure 1 and Figure 2)61.
Figure 1.
Reference range of platelet counts at birth in neonates with a gestational age between 22 and 42 weeks.
Modified from Wiedmeier SE et al., 200961.
Figure 2.
Reference ranges of platelet counts in the first 90 days of life in neonates born at a gestational age of 22 to 42 weeks.
Modified from Wiedmeier SE et al., 200961.
In the light of these new reference ranges, it has been suggested that the classification of thrombocytopenia into mild, moderate and severe should be abandoned, except to assign a preliminary risk to the thrombocytopenia because platelet counts of 5,000/μL and 45,000/μL cause very different clinical problems, despite both being included in the same category of “severe thrombocytopenia”.
The administration of platelets in the case of moderate thrombocytopenia (50,000–100,000/μL) does not, in any case, seem to reduce the severity of bleeding62.
In the absence of randomised, controlled clinical studies, the indications for transfusing platelets in this category of children is based on clinical experience63,64.
In healthy neonates born at term, the risk of bleeding is low if the platelet count is kept above 20,000–30,000/μL. Higher levels are recommended for premature neonates, particularly in the first few days of life, a period in which the risk of intraventricular haemorrhage is high, or when a concomitant coagulopathy is present. It is, therefore, recommended that the platelet count is kept above 50,000/μL in premature neonates (weight <1,000 g; gestational age <28 weeks) in the first week of life, in critically ill neonates (with sepsis or fluctuating blood pressure) or in the case of invasive procedures. In neonates who are bleeding, the platelet count should be maintained above 100,000/μL (Level of evidence IV, Grade of recommendation C).
In order to determine the risk of bleeding, the thrombocytopenia should be evaluated taking into consideration the mean platelet volume, the haematocrit, gestational age, post-natal age, use of drugs, stability of blood pressure, the presence of other comorbid conditions such as patent ductus arteriosus, sepsis and pulmonary hypertension65,66.
Table VIII summarises the indications for platelet transfusion while Table IX summarises the practical aspects of these transfusions.
Table VIII.
Indications for the transfusion of platelet concentrates.
|
Table IX.
Transfusion of platelet concentrates: practical aspects.
|
Characteristics of the platelet concentrates
There is no evidence that the increase in platelet count is greater when 15–20 mL/kg is transfused compared to 10 mL/kg67; the rate of infusion should be 5–10 mL/kg/h (Level of evidence III, Grade of recommendation B).
The increase in platelet count can be measured from 10 minutes to 3 hours after transfusion68.
The platelets must be:
- of an identical or compatible ABO phenotype;
- human platelet antigen (HPA)-compatible in the case of alloimmune thrombocytopenia;
- leucodepleted/CMV-safe;
- irradiated, if indicated.
In the case of alloimmune thrombocytopenia, compatible platelets must be searched for as soon as possible. The product must have the following characteristics:
- lack human platelet antigens (HPA) against which the mother has produced specific antibodies: in the absence of donors typed for the main HPA, maternal platelets can be used; these should be obtained by apheresis, washed to remove the plasma containing the antibodies, and irradiated;
- in the absence of HPA-compatible platelets, IVIG should be administered together with platelet concentrates from “random” donors69.
Granulocyte concentrates
The transfusion of granulocyte concentrates has been proposed in the past for neonates with severe neutropenia who have severe sepsis resistant to antibiotic treatment. The data available so far do not, however, seem to justify such a practice and so, at present, there are no precise indications in this regard.
A meta-analysis performed in the early 2000s showed that there were no statistically significant differences in morbidity or mortality between neonates treated with granulocyte concentrates or those managed with “standard” treatment70.
Thus, considering the potential serious side effects (transmission of infections), it is current practice to use recombinant granulocyte growth factors (recombinant granulocyte colony-stimulating factor, recombinant granulocyte-monocyte colony-stimulating factor) (Level of evidence IV, Grade of recommendation C).
Granulocyte concentrates must be ABO/Rh-compatible with the neonate and must be irradiated before being transfused. Consult specific instructions for information on the dose and methods of administering recombinant granulocyte growth factors.
Particular conditions
Tactivation
The main red blood cell membrane glycoproteins, glycophorins A, B and C, contain oligosaccharides (tetrasaccharides) conjugated with sialic acid. If the molecules of sialic acid are removed, an antigen named T is exposed. This phenomenon is called T activation.
RBC exposing this antigen can be polyagglutinated by anti-T IgM antibodies, which are naturally and constantly present in the plasma of adults71. These naturally occurring antibodies seem to be produced by exposure to intestinal bacterial flora containing structures antigenically similar to the red cell “crypto-antigens”.
The T antigen on RBC can be activated when the erythrocytes come into contact with some enzymes (neuroaminidases) produced by aerobic and anaerobic bacteria (Clostridium spp.), able to remove the sialic acid residues. This phenomenon has been described in Gram-negative neonatal sepsis and in particular during necrotising enterocolitis72.
The passive transfusion of anti-T antibodies with FFP, packed red cells and/or platelet concentrates in T-activated subjects can induce a haemolytic transfusion reaction of variable severity. This phenomenon must be suspected in neonates when the expected post-transfusion increase in Hb is not achieved (unexpected increase in transfusion requirements) and in the presence of a haemolytic transfusion reaction with haemoglobinuria due to intravascular haemolysis.
All patients with necrotising enterocolitis and/or systemic infection who develop haemolysis must be investigated to determine the cause of the haemolysis, taking into consideration the possibility of T activation73. The treatment includes the use of carefully washed cellular blood components and industrially pathogen-inactivated plasma74.
Each Centre should establish a protocol for the diagnosis and treatment of this situation (Level of evidence IV, Grade of recommendation C).
Table Va.
Choice of ABO group of blood components to administer to a neonate ABO-compatible with the mother.
| ABO blood group of the neonate | ABO blood group that can be transfused | |||
|---|---|---|---|---|
|
| ||||
| Red blood cells | Platelets | Plasma | ||
| O | First choice | O | O | O |
| Second choice | - | AB or A or B | AB or A or B | |
|
| ||||
| A | First choice | A | A | A |
| Second choice | O | AB | AB | |
|
| ||||
| B | First choice | B | B | B |
| Second choice | O | AB | AB | |
|
| ||||
| AB | First choice | AB | AB | AB |
| Second choice | O or A or B | Plasma-free A or B | - | |
Table Vb.
Choice of ABO group of blood components to administer to a neonate ABO-incompatible with the mother.
| ABO blood group of the neonate | ABO blood group of the mother | ABO group that can be transfused | ||
|---|---|---|---|---|
|
| ||||
| Red blood cells | Platelets | Plasma | ||
| O | A or B | O | O | O or AB or A or B |
|
| ||||
| A | O or B | O | Plasma-free O | A or AB |
|
| ||||
| B | O or A | O | Plasma-free O | B or AB |
|
| ||||
| AB | O | O | Plasma-free O | AB |
| A | O or A | Plasma-free A or O | ||
| B | O or B | Plasma-free B or O | ||
Appendix I.
Definitions
Neonate: baby ≤28 days of life
LBW: low birthweight neonate, <2,500 g
VLBW: very low birthweight neonate, <1,500 g
ELBW: extremely low birthweight neonate, <1,000 g
Appendix II.
The definitions of the levels of evidence and grades of recommendation used in these guidelines are those from the US Agency for Health Care Policy and Research.
Levels of evidence
| Ia | Evidence obtained from meta-analysis of randomised, controlled clinical studies (RCT). |
| Ib | Evidence obtained from at least one RCT. |
| IIa | Evidence obtained from at least one well-designed, not randomised, controlled trial. |
| IIb | Evidence obtained from at least one other well-designed type of study. |
| III | Evidence obtained from well-designed descriptive studies, such as case-controlled studies, cohort studies and case studies. |
| IV | Evidence obtained from reports of expert commissions or opinions and/or clinical experience of authoritative persons. |
Strength of the recommendations
A (Levels of evidence Ia, Ib)
Requires at least one RCT as part of a set of literature of overall good quality and consistency which suggests specific recommendations.
B (Levels of evidence IIa, IIb, III)
Requires availability of well-conducted clinical studies, but not RCT, on the object of the recommendation.
C (Level of evidence IV)
Requires evidence obtained from reports of expert commissions or opinions and/or clinical experience of authoritative persons. Indicates the lack of good quality,
References
- 1.Decree of the Italian Ministry of Health, March 3, 2005: [Caratteristiche e modalità per la donazione di sangue e di emocomponenti]. Gazzetta Ufficiale della Repubblica Italiana, Serie Generale, n. 85 of April 13, 2005. [In Italian].
- 2.Decree of the Italian Ministry of Health, March 3, 2005: [Protocolli per l’accertamento della idoneità del donatore di sangue e di emocomponenti]. Gazzetta Ufficiale della Repubblica Italiana, Serie Generale, n. 85 of April 13, 2005. [In Italian].
- 3.Italian Legislative Decree, December 20, 2007, n. 261. Revisione del DL 19 agosto 2005, n. 191, recante attuazione della direttiva 2002/98/CE che stabilisce norme di qualità e di sicurezza per la raccolta, il controllo, la lavorazione, la conservazione e la distribuzione del sangue umano e dei suoi componenti. Gazzetta Ufficiale della Repubblica Italiana, Serie Generale, n. 19 of January 23, 2008. [In Italian].
- 4.Italian Law n. 219 of October 21, 2005: [Nuova disciplina delle attività trasfusionali e della produzione nazionale degli emoderivati]. Gazzetta Ufficiale della Repubblica Italiana, Serie Generale, n. 251 of October 27, 2005. [In Italian].
- 5.Josephson CD, Castillejo MI, Caliendo AM, et al. Prevention of transfusion-transmitted cytomegalovirus in low-birth weight infants (≤1500 g) using cytomegalovirus-seronegative and leukoreduced transfusions. Transfus Med Rev. 2011;25:125–32. doi: 10.1016/j.tmrv.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Council of Europe. Recommendation No R (95) 15 on the preparation, use and quality assurance of blood components. 17th ed. Strasbourg: Council of Europe Publishing; 2013. Guide to Preparation, Use and Quality Assurance of Blood Components. [Google Scholar]
- 7.Ziemann M, Hennig H. Prevention of transfusion-transmitted cytomegalovirus infections: which is the optimal strategy? Transfus Med Hemother. 2014;41:40–4. doi: 10.1159/000357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roback J, Grossman B, Harris T, Hillyer CD. Technical Manual. 17th ed. Bethesda, MD: American Association of Blood Banks; 2011. pp. 645–760. [Google Scholar]
- 9.Dwyre DM, Holland PV. Transfusion-associated graft-versus-host disease. Vox Sang. 2008;95:85–93. doi: 10.1111/j.1423-0410.2008.01073.x. [DOI] [PubMed] [Google Scholar]
- 10.British Committee for Standards in Haematology. Blood Transfusion Task Force. Guidelines on the use of irradiated blood components. Br J Haematol. 2011;152:35–51. doi: 10.1111/j.1365-2141.2010.08444.x. [DOI] [PubMed] [Google Scholar]
- 11.Fasano RM, Luban NLC. Transfusion practices. In: De Alarcon PA, Werner EJ, Christensen RD, editors. Neonatal Hematology, Pathogenesis, Diagnosis and Management of Hematologic Problems. 2nd ed. Cambridge University Press; 2013. pp. 303–27. [Google Scholar]
- 12.Price TH. Standards for Blood Banks and Transfusion Services. 25th ed. Bethesda, MD: American Association of Blood Banks; 2008. pp. 7–41. [Google Scholar]
- 13.Bennardello F, Curciarello G. Survey on the prevention and incidence of haemolytic disease of the newborn in Italy. Blood Transfus. 2013;11:518–27. doi: 10.2450/2013.0179-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novak DJ, Tyler LN, Reddy RL, Barsoom MJ. Plasmapheresis and intravenous immunoglobulin for the treatment of D alloimmunization in pregnancy. J Clin Apher. 2008;23:183–5. doi: 10.1002/jca.20180. [DOI] [PubMed] [Google Scholar]
- 15.Bellone M, Boctor FN. Therapeutic plasma exchange and intravenous immunoglobulin as primary therapy for D alloimmunization in pregnancy precludes the need for intrauterine transfusion. Transfusion. 2014;54:2118–21. doi: 10.1111/trf.12633. [DOI] [PubMed] [Google Scholar]
- 16.Birchenall KA, Illanes SE, Denbow M. Neonatal outcomes of pregnancies affected by haemolytic disease of the foetus and newborn and managed with intrauterine transfusion: a service evaluation. Blood Transfus. 2013;11:548–52. doi: 10.2450/2013.0288-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genova L, Slaghekke F, Klumper FJ, et al. Management of twin anemia- polycythemia sequence using intrauterine blood transfusion for the donor and partial exchange transfusion for the recipient. Fetal Diagn Ther. 2013;34:121–6. doi: 10.1159/000346413. [DOI] [PubMed] [Google Scholar]
- 18.Mari G for the Collaborative Group For Doppler Assessment of the Blood Velocity in Anemic Fetuses. Non-invasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. N Engl J Med. 2000;342:9–14. doi: 10.1056/NEJM200001063420102. [DOI] [PubMed] [Google Scholar]
- 19.Altunyurt S, Okyay E, Saatli B, et al. Neonatal outcomes of foetuses receiving intrauterine transfusion for severe hydrops complicated by Rhesus hemolytic disease. Int J Gynaecol Obstet. 2012;117:153–6. doi: 10.1016/j.ijgo.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Lindemburg ITM, van Kamp IL, van Zwet EW, et al. Increased perinatal loss after intrauterine transfusion for alloimmune anemia before 20 weeks of gestation. BJOG. 2013;120:847–52. doi: 10.1111/1471-0528.12063. [DOI] [PubMed] [Google Scholar]
- 21.Argoti PS, Bebbington M, Moise KJ. Serial intrauterine transfusions for a hydropic fetus with severe anemia and thrombocytopenia caused by Parvovirus: lessons learned. AJP Rep. 2013;3:75–8. doi: 10.1055/s-0033-1341576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson JA, McFarland JG, Curtis BR, Aster RH. Neonatal alloimmune thrombocytopenia: pathogenesis, diagnosis and management. Br J Haematol. 2013;161:3–14. doi: 10.1111/bjh.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.AAP Subcommittee on hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316. doi: 10.1542/peds.114.1.297. [DOI] [PubMed] [Google Scholar]
- 24.Pugni L, Riva E, Pietrasanta C, et al. Severe hypertriglyceridemia in a newborn with monogenic lipoprotein lipase deficiency: an unconventional therapeutic approch with Exchange Transfusion. JIMD Rep. 2014;13:59–64. doi: 10.1007/8904_2013_272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babor F, Hadzik B, Stannigel H, et al. Successful management of neonatal hemochromatosis by exchange transfusion and immunoglobulin: a case report. J Perinatol. 2013;33:83–5. doi: 10.1038/jp.2012.9. [DOI] [PubMed] [Google Scholar]
- 26.Kumar P, Chawla D, Deorari A. Light-emitting diode phototherapy for unconjugated hyperbilirubinaemia in neonates. Cochrane Database Syst Rew. 2011;7:CD007969. doi: 10.1002/14651858.CD007969.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chitty HE, Ziegler N, Savoia H, et al. Neonatal exchange transfusion in the 21st century: a single hospital study. J Paediatr Child Health. 2013;49:825–32. doi: 10.1111/jpc.12290. [DOI] [PubMed] [Google Scholar]
- 28.Ramasethu J. Exchange transfusion. In: Macdonald MG, Ramasethu J, Rais-Bahrami K, editors. Atlas of Procedures in Neonatology. 5th ed. Vol. 2013. Philadelphia: Lippincott & Co; 2012. pp. 315–22. [Google Scholar]
- 29.Thayyil S, Milligan DW. Single versus double volume exchange transfusion in jaundiced newborn infants. Cochrane Database System Rev. 2006;18:CD004592. doi: 10.1002/14651858.CD004592.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Smiths-Winti Jens VE, Rath ME, van Zwet EW, et al. Neonatal morbidity after exchange transfusion for red cell alloimmune hemolytic disease. Neonatology. 2013;103:141–7. doi: 10.1159/000343261. [DOI] [PubMed] [Google Scholar]
- 31.Ozek E, Soll R, Schimmel MS. Partial exchange transfusion to prevent neurodevelopmental disability in infants with polycythemia. Cochrane Database Syst Rev. 2010;1:CD005089. doi: 10.1002/14651858.CD005089.pub2. [DOI] [PubMed] [Google Scholar]
- 32.de Waal KA, Baerts W, Offringa M. Systematic review of the optimal fluid for dilutional exchange transfusion in neonatal polycythaemia. Arch Dis Child Fetal Neonatal Ed. 2006;91:F7–10. doi: 10.1136/adc.2004.063925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarkar S, Rosenkratz TS. Neonatal polycythemia and hyperviscosity. Semin Fetal Neonatal Med. 2008;13:248–55. doi: 10.1016/j.siny.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Lundstrom U, Siimes MA, Dallman PR. At what age does iron supplementation become necessary in low birth weight infants? J Pediatr. 1977;91:882–6. doi: 10.1016/s0022-3476(77)80881-0. [DOI] [PubMed] [Google Scholar]
- 35.Bolton-Maggs PHB, Cohen H. Serious hazards of transfusion (SHOT) haemovigilance and progress is improving transfusion safety. Br J Haematol. 2013;163:303–14. doi: 10.1111/bjh.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bifano EM, Curran TR. Minimizing donor blood exposure in the neonatal intensive care unit. Current trend and future prospects. Clin Perinatol. 1995;22:657–69. [PubMed] [Google Scholar]
- 37.Pupella S, Girelli G, Casadei AM, et al. Protocollo operativo per la terapia trasfusionale del neonato: risultati preliminari. La Trasf del Sangue. 1999;44:298–303. [Google Scholar]
- 38.Miyashiro AM, dos Santos N, Guinsburg R, et al. Strictred blood cell transfusion guideline reduces the need for transfusions in very-low-birth weight infants in the first 4 weeks of life: a multicentre trial. Vox Sang. 2005;88:107–13. doi: 10.1111/j.1423-0410.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- 39.Motta M, Testa M, Tripodi G, Radicioni M. Changes in neonatal transfusion practice after dissemination of neonatal recommendations. Pediatrics. 2010;125:e810–7. doi: 10.1542/peds.2009-0502. [DOI] [PubMed] [Google Scholar]
- 40.Baer VL, Henry E, Lambert DK, et al. Implementing a program to improve compliance with neonatal intensive care unit transfusion guidelines was accompanied by a reduction in transfusion rate: a pre-post analysis within a multihospital health care system. Transfusion. 2011;51:264–9. doi: 10.1111/j.1537-2995.2010.02823.x. [DOI] [PubMed] [Google Scholar]
- 41.Kasat K, Hendrics-Munoz H, Mally PV. Neonatal red blood cell transfusion: searching for better guidelines. Blood Transf. 2011;9:86–94. doi: 10.2450/2010.0031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirpalani H, Whyte RK, Andersen C, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized controlled trial of restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301–7. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 43.Whyte RK, Kirpalani H, Asztalos EV, et al. Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics. 2009;123:207–13. doi: 10.1542/peds.2008-0338. [DOI] [PubMed] [Google Scholar]
- 44.McCoy TE, Conrad AL, Richman LC, et al. Neurocognitive profiles of preterm infants randomly assigned to lower or higher hematocrit thresholds for transfusion. Child Neuropsychol. 2011;17:347–67. doi: 10.1080/09297049.2010.544647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christensen RD. Association between “early” red blood transfusion and severe intraventricular hemorrhage, and between “late” red blood cell transfusion and necrotizing enterocolitis. Semin Perinatol. 2012;36:283–9. doi: 10.1053/j.semperi.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Paul DA, Mackley A, Novitsky A, et al. Increase odds of necrotizing enterocolitis after transfusion of red blood cell in premature infants. Pediatrics. 2011;127:635–41. doi: 10.1542/peds.2010-3178. [DOI] [PubMed] [Google Scholar]
- 47.Demirel G, Celik IH, Aksoy HT, et al. Transfusion-associated necrotizing enterocolitis in very low birth weight premature infants. Transfus Med. 2012;22:332–7. doi: 10.1111/j.1365-3148.2012.01170.x. [DOI] [PubMed] [Google Scholar]
- 48.Sing R, Visintainer PF, Frantz ID, et al. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J Perinatol. 2011;31:176–82. doi: 10.1038/jp.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chirico G, Beccagutti F, Sorini A, et al. Red blood cell transfusion in preterm infants: restrictive versus liberal policy. J Matern Fetal Neonatal Med. 2011;24(Suppl 1):20–2. doi: 10.3109/14767058.2011.607566. [DOI] [PubMed] [Google Scholar]
- 50.Widness JA. Treatment and prevention of neonatal anemia. Neoreviews. 2008;9:e526–33. doi: 10.1542/neo.9-11-e526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dallman PR. Blood and blood-forming tissues. In: Rudolph A, editor. Pediatrics. 16th ed. New York: Appleton-Century-Crofts; 1977. p. 1111. [Google Scholar]
- 52.Yang L, Stanworth S, Hopewell S, et al. Is fresh-frozen plasma clinically effective? An update of a systematic review of randomized controlled trials. Transfusion. 2012;52:1673–86. doi: 10.1111/j.1537-2995.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 53.Osborn DA, Evans N. Early volume expansion for prevention of morbidity and mortality in very preterm infants. Cochrane Database Syst Rev. 2004;2:CD002055. doi: 10.1002/14651858.CD002055.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.A randomized trial comparing the effect of prophylactic intravenous fresh frozen plasma, gelatin or glucose on early mortality and morbidity in preterm babies. The Northern Neonatal Nursing Initiative [NNNI] Trial Group. Eur J Pediatr. 1996;155:580–8. doi: 10.1007/BF01957909. [DOI] [PubMed] [Google Scholar]
- 55.Christensen RD, Baer VL, Lambert DK, et al. Reference intervals for common coagulation tests of preterm infants. Transfusion. 2014;54:627–32. doi: 10.1111/trf.12322. [DOI] [PubMed] [Google Scholar]
- 56.Andrew M, Paes B, Milner R, et al. Development of the human coagulation system in the healthy premature infant. Blood. 1988;72:1651–7. [PubMed] [Google Scholar]
- 57.Andrew M, Vegh P, Johnston M, et al. Maturation of the hemostatic system during childhood. Blood. 1992;80:1998–2005. [PubMed] [Google Scholar]
- 58.Tripodi A, Ramenghi LA, Chantarangkul V, et al. Normal thrombin generation in neonates in spite of prolonged conventional coagulation tests. Haematologica. 2008;93:1256–9. doi: 10.3324/haematol.12566. [DOI] [PubMed] [Google Scholar]
- 59.Motta M, Del Vecchio A, Perrone B, et al. Fresh frozen plasma use in the NICU: a prospective, observational, multicentred study. Arch Dis Child Fetal Neonatal Ed. 2014;99:F303–8. doi: 10.1136/archdischild-2013-304747. [DOI] [PubMed] [Google Scholar]
- 60.Christensen RD, Henry E, Wiedmeier SE, et al. Thrombocytopenia among extremely low birth weight neonates: data from a multi hospital healthcare system. J Perinatol. 2006;26:348–53. doi: 10.1038/sj.jp.7211509. [DOI] [PubMed] [Google Scholar]
- 61.Wiedmeier SE, Henry E, Sola-Visner MC, Christensen RD. Platelet reference ranges for neonates, defined using data from over 47,000 patients in a multihospital healthcare system. J Perinatol. 2009;29:130–6. doi: 10.1038/jp.2008.141. [DOI] [PubMed] [Google Scholar]
- 62.Andrew M, Vegh P, Caco C, et al. Randomised controlled trial of platelet transfusion in thrombocytopenic premature infants. J Pediatr. 1993;123:285–91. doi: 10.1016/s0022-3476(05)81705-6. [DOI] [PubMed] [Google Scholar]
- 63.Del Vecchio A, Sola MC, Theriaque DW, et al. Platelet transfusions in the neonatal intensive care unit: factors predicting which patients will require multiple transfusions. Transfusion. 2001;41:803–8. doi: 10.1046/j.1537-2995.2001.41060803.x. [DOI] [PubMed] [Google Scholar]
- 64.Roberts I, Murray NA. Neonatal thrombocytopenia: causes and management. Arch Dis Child Fetal Neonatal Ed. 2003;88:F359–64. doi: 10.1136/fn.88.5.F359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Del Vecchio A, Motta M, Radicioni M, Christensen RD. A consistent approach to platelet transfusion in the NICU. J Matern Fetal Neonatal Med. 2012;25:93–6. doi: 10.3109/14767058.2012.716985. [DOI] [PubMed] [Google Scholar]
- 66.Baer VL, Lambert DK, Henry E, et al. Do platelet transfusions in the NICU adversely affect survival? Analysis of 1600 thrombocytopenic neonates in a multi hospital healthcare system. J Perinatol. 2007;27:790–6. doi: 10.1038/sj.jp.7211833. [DOI] [PubMed] [Google Scholar]
- 67.Kline A, Mackley A, Taylor SM, et al. Thrombopoietin following transfusion of platelets in preterm neonates. Platelets. 2008;19:428–31. doi: 10.1080/09537100802220476. [DOI] [PubMed] [Google Scholar]
- 68.Murray NA, Howarth LJ, MaCloy MP, et al. Platelet transfusion in the management of severe thrombocytopenia in neonatal intensive care unit patients. Transfus Med. 2002;12:35–41. doi: 10.1046/j.1365-3148.2002.00343.x. [DOI] [PubMed] [Google Scholar]
- 69.Bakchoul T, Bassler D, Heckmann M, et al. Management of infants born with severe neonatal alloimmune thrombocytopenia: the role of platelet transfusions and intravenous immunoglobulin. Transfusion. 2014;54:640–5. doi: 10.1111/trf.12336. [DOI] [PubMed] [Google Scholar]
- 70.Mohan P, Brocklehurst P. Granulocyte transfusions for neonates with confirmed or suspected sepsis and neutropenia. Cochrane Database Syst Rev. 2003;4:CD003956. doi: 10.1002/14651858.CD003956. [DOI] [PubMed] [Google Scholar]
- 71.Ramasethu J, Luban NL. T activation. Br J Haematol. 2001;112:259–63. doi: 10.1046/j.1365-2141.2001.02301.x. [DOI] [PubMed] [Google Scholar]
- 72.Osborn DA, Lui K, Pussell P, et al. T and Tk antigen activation in necrotizing enterocolitis: manifestations, severity of illness, and effectiveness of testing. Arch Dis Child Fetal Neonatal Ed. 1999;80:F192–7. doi: 10.1136/fn.80.3.f192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eder AF, Manno CS. Does red cell T activation matter? Br J Haematol. 2001;114:25–30. doi: 10.1046/j.1365-2141.2001.02886.x. [DOI] [PubMed] [Google Scholar]
- 74.Boralessa H, Modi N, Cockburn H, et al. RBC T activation and hemolysis in a neonatal intensive care population: implications for transfusion practice. Transfusion. 2002;42:1428–34. doi: 10.1046/j.1537-2995.2002.00237.x. [DOI] [PubMed] [Google Scholar]