Abstract
Background
Pulmonary infections remain more common in HIV-infected (HIV+) compared to uninfected individuals. The increase in chronic lung diseases among aging HIV+ individuals may contribute to this persistent risk. We sought to determine whether chronic obstructive pulmonary disease (COPD) is an independent risk factor for different pulmonary infections requiring hospitalization among HIV+ patients.
Methods
We analyzed data from 41,993 HIV+ Veterans in the nationwide Veterans Aging Cohort Study Virtual Cohort (VACS-VC) from 1996–2009. Using ICD-9 codes, we identified baseline comorbid conditions, including COPD, and incident community-acquired pneumonia (CAP), pulmonary tuberculosis (TB) and Pneumocystis jirovecii pneumonia (PCP) requiring hospitalization within two years after baseline. We used multivariable Poisson regression to determine incidence rate ratios (IRR) associated with COPD for each type of pulmonary infection, adjusting for comorbidities, CD4+ cell count, HIV viral load, smoking status, substance use, vaccinations and calendar year at baseline.
Results
Unadjusted incidence rates of CAP, TB and PCP requiring hospitalization were significantly higher among persons with COPD compared to those without COPD (CAP: 53.9 vs. 19.4 per 1,000 person-years; TB: 8.7 vs. 2.8; PCP: 15.5 vs. 9.2; p ≤0.001). In multivariable Poisson regression models, COPD was independently associated with increased risk of CAP, TB and PCP (IRR 1.94, 95% CI 1.64–2.30; IRR 2.60, 95% CI 1.70–3.97; and IRR 1.48, 95% CI 1.10–2.01, respectively).
Conclusions
COPD is an independent risk factor for CAP, TB and PCP requiring hospitalization among HIV+ individuals. As the HIV+ population ages, the growing burden of COPD may confer substantial risk for pulmonary infections.
Keywords: COPD, pulmonary infection, pneumonia, HIV, comorbidities
INTRODUCTION
The widespread use of antiretroviral therapy (ART) has resulted in a decline in many infectious complications of HIV. Chronic medical comorbidities, including non-infectious pulmonary, cardiovascular, renal and liver diseases, are increasingly recognized amongst aging HIV-infected (HIV+) persons.1–6 Pulmonary infection, however, persists as an important contributor to morbidity and mortality, with over 10% of hospitalizations among those with HIV attributed to community-acquired pneumonia (CAP), pulmonary tuberculosis (TB) and Pneumocystis jirovecii pneumonia (PCP) in the contemporary ART era.7 Despite a decreased incidence of CAP requiring hospitalization, CAP represents the most frequent admission diagnosis for HIV+ patients.8 Established factors, including low CD4+ cell counts, HIV treatment interruption, PCP prophylaxis, prior pneumonia, cigarette smoking, injection drug use and pneumococcal vaccination continue to play important roles in the risk for pulmonary infections among HIV+ individuals.9–14
With prolonged survival of HIV+ patients on ART, a growing burden of chronic comorbidities may affect the incidence of pulmonary infections requiring hospitalization. Chronic obstructive pulmonary disease (COPD) is one of the most common non-infectious pulmonary complications observed among HIV+ patients,15–17 and occurs more frequently in this patient population than among uninfected individuals.4,18 While COPD is strongly associated with increased CAP risk in uninfected patients,19–23 no studies have focused on the role of COPD in the risk for CAP and other pulmonary infections amongst HIV+ patients. Identifying HIV+ populations at highest risk for pulmonary infection is important for the development of novel preventative and therapeutic strategies.
In this study, we hypothesized that COPD is an independent risk factor for pulmonary infections requiring hospitalization among HIV+ patients, adjusting for other comorbidities and risk factors, such as low CD4+ cell count, detectable HIV viral load, smoking and substance use. We assessed whether the risk associated with COPD differed for CAP, TB or PCP requiring hospitalization. Additionally, as HIV management and characteristics of the HIV+ population have evolved over the course of the HIV epidemic, we examined trends in the association between COPD and risk for CAP, TB or PCP hospitalization over time between 1996 and 2012, dividing our cohort into three time periods, with the first corresponding to the beginning of the combination ART era and the last to more contemporaneous management.
METHODS
Cohort and study design
We analyzed administrative and electronic health record (EHR) data from 41,993 HIV+ persons in the nationwide, longitudinal Veterans Aging Cohort Study Virtual Cohort (VACS-VC). HIV+ patients in VACS-VC are identified at the first occurrence of International Classification of Diseases, Ninth Revision (ICD-9) codes for HIV infection within Veterans Affairs (VA) administrative data, using a validated algorithm.24 VACS-VC is approved by the institutional review boards of participating institutions and has been previously described.24 In the current analyses, we included HIV+ Veterans whose first HIV ICD-9 code (defined as the baseline date) in the VA healthcare system fell between January 1996 and December 2009. A baseline window was defined as 12 months prior to and six months after the baseline date, and all individuals were followed for up to two years after the baseline window to determine the incidence of first CAP, TB or PCP requiring hospitalization at VA facilities, with the last follow-up date falling in June 2012. Given aging of the HIV+ population and changes in HIV care over time, we also evaluated differences in clinical characteristics and pulmonary infection rates over time, dividing individuals using their baseline date into three calendar-time periods of approximately equal time intervals (1996–2000, 2001–2005, 2006–2009).
Baseline characteristics and comorbidities
All data were abstracted from electronic VA databases. Age, sex and race/ethnicity were retrieved from VA administrative databases. Never, former and current smoking status was derived from VA Health Factors data.25 Alcohol and drug related diagnoses were identified using ICD-9 codes.26 Influenza and pneumococcal vaccinations within one and five year(s) prior to the baseline date, respectively, were obtained from VA immunization files, ICD-9 codes and Current Procedural Terminology codes. The CD4+ cell counts (cells/μL) and HIV viral load (copies/mL) closest to the baseline date and preceding hospitalization (within 1 year and up to 30 days prior) were obtained from VA laboratory data from the Decision Support System files. ART and PCP prophylaxis prescriptions were obtained from Pharmacy Benefits Management data.
Baseline COPD (primary exposure) was defined as one inpatient or two outpatient ICD-9 codes during the baseline window of 12 months prior to and six months after the baseline date. This method of one inpatient or two outpatient ICD-9 code identification of COPD and other comorbidities has been previously validated against chart review and improves the accuracy of ICD-9 codes.25,27 Comorbidities considered prevalent at baseline were defined using the same approach. We included those that have been associated with pneumonia in uninfected populations, namely congestive heart failure (CHF)22,23,28 and other cardiovascular diseases,29 chronic renal and liver disease,30,31 diabetes mellitus,23,32 malignancy30 and as well as hepatitis C infection (HCV). Prior pneumonia diagnoses were also defined by ICD-9 codes. A complete listing of ICD-9 codes for each diagnosis is available on www.vacohort.org.
Incident CAP, TB and PCP requiring hospitalization
The primary outcomes for this study were incident CAP, TB and PCP requiring hospitalization based on inpatient ICD-9 codes. We focused on pulmonary infections that required hospitalization and did not include outpatient events in our analysis; outpatient CAP has accounted for only 2% of CAP events in another HIV+ cohort.33 Although ICD-9 codes for TB could include pulmonary, extrapulmonary and primary TB, the majority of the codes represented pulmonary TB. For simplicity, we refer to the three conditions as pulmonary infections, particularly as the primary route of infection for TB is through the lungs. Microbiologic data were not available from the EHR for these analyses. Individuals were followed for two years beginning at six months after the baseline date, so that diagnoses of COPD preceded diagnoses of CAP, TB or PCP.
Statistical analysis
Baseline characteristics and comorbidities were compared across the three calendar-time periods, using one-way analysis of variance for continuous variables and the chi-square test for categorical variables. Score tests assessed trends across time periods. We calculated incidence rates for CAP, TB and PCP requiring hospitalization using time to the first event and person-years at risk during two years of follow-up. Incidence rates are reported per 1,000 person-years.
Univariate Poisson regression models were used to determine unadjusted incidence rate ratios (IRRs) for risk of CAP, TB and PCP requiring hospitalization associated with baseline diagnosis of COPD. Separate multivariable Poisson regression models were used to calculate IRRs to determine the independent association of COPD with risk for CAP, TB or PCP hospitalization. Multivariable models were generated adjusting for covariates added incrementally en bloc: first with age, race/ethnicity, smoking status, alcohol/drug related diagnoses, and calendar-time periods; then comorbidities; baseline CD4+ cell count <200 cells/μL, HIV viral load >500 copies/mL; and vaccination status. The same variables were included in all three multivariable models and were selected a priori given potential pathogenic mechanisms or epidemiologic data linking them with risk for different types of pulmonary infections.22,23,29–31,33 We did not include prior pneumonia in these models because we were unable to determine the temporal relationship between prior pneumonia events and development of COPD.
As smoking status was missing for 31% of persons from the earliest calendar-time period, we included “missing” as a smoking status category. As a sensitivity analysis, we generated multivariable models restricted to more recent years when there was less missing smoking status data.
All analyses were performed using Stata 13 (Stata Corp., College Station, TX). Statistical significance was defined as p <0.05.
RESULTS
Baseline characteristics
Across the three baseline calendar-time periods, HIV+ Veterans were mostly male (98%), almost half were black and over 50% in the later years were current smokers (Table 1). There was a downward trend in both alcohol and drug related diagnoses with time. Median age at HIV identification within the VA system increased from 44 to 50 years from the earliest to the most recent time period. The proportion of individuals with CD4+ ≥200 cells/μL and HIV viral load ≤500 copies/mL increased over time, while ART use at baseline was persistently low and declined from 29% in the earliest time period to 20% in the most recent period. Among those using ART at baseline, 49% had viral load ≤500. Overall, of HIV+ Veterans with CD4+ <200 cells/μL, 57% were prescribed PCP prophylaxis at baseline. The proportion who received influenza and pneumococcal vaccinations increased. The baseline prevalence of COPD and other chronic comorbidities generally increased over time; COPD prevalence rose from 3.7 to 6.0%. In contrast, the proportion of HIV+ Veterans with HCV co-infection declined from 37 to 24%.
Table 1.
Baseline characteristics and comorbidities by time period* (n = 41,993)
| Characteristic | 1996 – 2000 (n = 24,278) | 2001 – 2005 (n = 11,058) | 2006 – 2009 (n = 6,123) |
|---|---|---|---|
| Age (years), median (IQR) | 44 (39 – 50) | 48 (41 – 55) | 50 (42 – 57) |
| Male, % | 98 | 98 | 97 |
| Race/ethnicity, % | |||
| Black | 49 | 47 | 49 |
| White | 39 | 38 | 35 |
| Hispanic | 8 | 6 | 7 |
| Other | 4 | 9 | 9 |
| HIV-related variables | |||
| CD4+ cell count (cells/μL), median (IQR) | 280 (124 – 470) | 317 (124 – 524) | 325 (139 – 546) |
| CD4+ cell count <200 cells/μL, % | 37 | 35 | 32 |
| HIV viral load >500 copies/mL, % | 68 | 65 | 64 |
| ART use, % | 29 | 25 | 20 |
| Smoking status, % | |||
| Never smoker | 16 | 25 | 29 |
| Former smoker | 10 | 13 | 12 |
| Current smoker | 43 | 53 | 54 |
| Missing | 31 | 9 | 5 |
| Alcohol-related diagnoses, % | 17 | 15 | 14 |
| Drug use disorders, % | 20 | 16 | 16 |
| COPD, % | 3.7 | 5.0 | 6.0 |
| Comorbid conditions, % | |||
| CHF | 1.4 | 1.9 | 2.1 |
| CAD/MI | 13 | 19 | 23 |
| Renal insufficiency | 3.4 | 5.0 | 5.4 |
| ESLD | 1.5 | 1.5 | 1.6 |
| HCV infection | 37 | 32 | 24 |
| Any malignancy | 4.2 | 4.8 | 5.0 |
| Diabetes mellitus | 18 | 17 | 12 |
| Prior pneumonia, % | |||
| CAP | 6.4 | 6.0 | 6.0 |
| TB | 1.9 | 1.2 | 0.7 |
| PCP | 3.9 | 4.2 | 2.9 |
| Vaccinations, % | |||
| Influenza | 14 | 39 | 51 |
| Pneumococcal | 12 | 36 | 43 |
Chi-square tests or score tests for trends across time periods were statistically significant (p<0.05) for all variables except prior bacterial pneumonia and ESLD.
CAP = community-acquired pneumonia; TB = pulmonary tuberculosis; PCP = Pneumocystis jirovecii pneumonia; COPD = chronic obstructive pulmonary disease; CHF = congestive heart failure; CAD/MI = coronary artery disease/myocardial infarction; ESLD = end-stage liver disease
Incidence of CAP, TB and PCP requiring hospitalization over time by COPD status
Among individuals included in the earliest time period from 1996–2000, 4.1% developed CAP during the two-year follow-up period; this proportion decreased slightly to 3.1% in the most recent time period. A greater downward trend was observed for TB (0.7% to 0.3%) and PCP (2.1% to 0.8%). These declines in incidence were statistically significant for all three pulmonary infections (p ≤ 0.001). Overall, the median time from baseline to CAP, TB and PCP hospitalization among affected individuals was 519, 468 and 511 days, respectively.
COPD was associated with greater absolute rates for CAP, TB and PCP. Unadjusted incidence rates of CAP, TB and PCP requiring hospitalization were significantly higher among persons with COPD compared to rates in those without COPD (CAP: 53.9 vs. 19.4 per 1,000 person-years; TB: 8.7 vs. 2.8; PCP: 15.5 vs. 9.2; p ≤ 0.001 for all). Among individuals with pulmonary infections requiring hospitalization, CD4+ and viral load preceding hospitalization did not differ significantly by COPD. Median CD4+ cell count preceding hospitalization was 244 vs. 275 cells/μL prior to CAP, 241 vs. 272 cells/μL prior to TB and 170 vs. 116 cells/μL prior to PCP in those with and without COPD, respectively (p >0.05 for all). When stratified by time periods, the incidence of CAP and TB hospitalization remained significantly higher among those with COPD in each calendar-time period (Figure 1). The incidence of PCP was significantly higher in those with COPD in the first two time periods, but not in the most recent period when PCP was least common.
Figure 1.
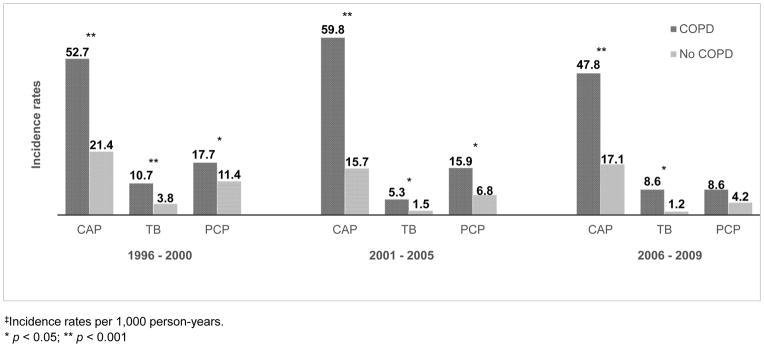
Incidence rates‡ of CAP, TB and PCP requiring hospitalization by COPD status and calendar-time
‡Incidence rates per 1,000 person-years.
* p < 0.05; ** p < 0.001
NOTE: There was a significant decline in incidence of each type of pulmonary infection over time (p ≤ 0.001). CAP = community-acquired pneumonia; TB = pulmonary tuberculosis; PCP = Pneumocystis jirovecii pneumonia
Unadjusted risk of COPD for CAP, TB and PCP requiring hospitalization
Using unadjusted Poisson regression models, COPD was associated with a greater risk of hospitalization for CAP (2.79, 95% CI 2.37–3.27), TB (3.09, 95% CI 2.07–4.61) and PCP (1.68, 95% CI 1.25–2.25, Table 2). Smoking and other comorbidities, such as CHF, renal insufficiency, ESLD and malignancy were also associated with increased risk for pulmonary infections requiring hospitalization. Missing smoking status was most strongly associated with risk of each event (p <0.001). Statistically, the unadjusted risk of COPD for each of these outcomes was relatively similar over time (Table 3).
Table 2.
Unadjusted incidence rate ratios for baseline COPD, other comorbidities and risk factors associated with CAP, TB and PCP requiring hospitalization in Poisson regression models
| Incidence rate ratios (95% confidence intervals)
|
|||
|---|---|---|---|
| CAP | TB | PCP | |
| COPD | 2.79 (2.37 – 3.27)** | 3.09 (2.07 – 4.61)** | 1.68 (1.25 – 2.25)* |
| Age | 1.01 (1.01 – 1.02)** | 1.00 (0.99 – 1.02) | 0.99 (0.98 – 1.00)* |
| Male | 1.17 (0.83 – 1.65) | 0.95 (0.42 – 2.13) | 2.56 (1.22 – 5.40)* |
| Race/ethnicity | |||
| White | Referent | Referent | Referent |
| Black | 1.39 (1.25 – 1.55)** | 2.86 (2.07 – 3.95)** | 1.57 (1.33 – 1.85)** |
| Hispanic | 1.29 (1.06 – 1.56)* | 2.06 (1.20 – 3.55)* | 1.82 (1.39 – 2.37)** |
| Other | 0.43 (0.30 – 0.62)** | -- | 0.52 (0.31 – 0.88)* |
| HIV-related variables | |||
| CD4+ cell count, cells//μL | |||
| <50 | 3.59 (2.92 – 4.40)** | 2.90 (1.77 – 4.74)** | 13.2 (8.69 – 20.1)** |
| 50–99 | 2.76 (2.16 – 3.52)** | 2.44 (1.36 – 4.38)* | 10.8 (6.88 – 16.8)** |
| 100–199 | 2.25 (1.81 – 2.80)** | 2.37 (1.44 – 3.91)* | 6.13 (3.94 – 9.52)** |
| HIV viral load >500 copies/mL | 1.51 (1.31 – 1.75)** | 1.41 (0.98 – 2.03) | 1.82 (1.45 – 2.29)** |
| ART use | 0.90 (0.79 – 1.03) | 0.56 (0.37 – 0.85)* | 1.00 (0.83 – 1.22) |
| Smoking status | |||
| Never smoked | Referent | Referent | Referent |
| Former smoker | 1.37 (1.08 – 1.74)** | 1.28 (0.57 – 2.87) | 0.88 (0.60 – 1.29) |
| Current smoker | 1.80 (1.51 – 2.14)* | 3.55 (2.04 – 6.18)** | 1.38 (1.07 – 1.77)* |
| Missing status | 5.40 (4.53 – 6.43)** | 8.39 (4.79 – 14.7)** | 5.25 (4.12 – 6.69)** |
| Alcohol-related diagnoses | 1.50 (1.33 – 1.69)** | 1.66 (1.23 – 2.25)* | 1.16 (0.96 – 1.40) |
| Drug use disorders | 1.49 (1.33 – 1.67)** | 1.67 (1.26 – 2.23)** | 1.20 (1.00 – 1.43) |
| Comorbid conditions | |||
| CHF | 2.83 (2.18 – 3.67)** | 2.57 (1.27 – 5.20)* | 2.39 (1.58 – 3.62)** |
| CAD/MI | 1.18 (1.04 – 1.34)* | 0.75 (0.51 – 1.11) | 0.89 (0.72 – 1.09) |
| Renal insufficiency | 2.97 (2.50 – 3.53)** | 2.07 (1.23 – 3.50)* | 1.87 (1.37 – 2.55)** |
| ESLD | 2.72 (1.99 – 3.71)** | 2.20 (0.91 – 5.34) | 1.70 (0.96 – 3.01) |
| HCV infection | 1.28 (1.15 – 1.41)** | 1.33 (1.02 – 1.73)* | 1.01 (0.87 – 1.18) |
| Any malignancy | 2.16 (1.80 – 2.58)** | 1.54 (0.90 – 2.64) | 1.64 (1.22 – 2.21)* |
| Diabetes mellitus | 0.92 (0.81 – 1.05) | 1.08 (0.78 – 1.50) | 0.69 (0.55 – 0.85)* |
| Vaccinations | |||
| Influenza | 0.82 (0.73 – 0.93)* | 0.57 (0.41 – 0.81)* | 0.50 (0.41 – 0.62)** |
| Pneumococcal | 0.97 (0.86 – 1.09) | 0.80 (0.58 – 1.11) | 0.71 (0.59 – 0.86)** |
p < 0.05;
p < 0.001
CAP = community-acquired pneumonia; TB = pulmonary tuberculosis; PCP = Pneumocystis jirovecii pneumonia; COPD = chronic obstructive pulmonary disease; CHF = congestive heart failure; CAD/MI = coronary artery disease/myocardial infarction; ESLD = end-stage liver disease
Table 3.
Unadjusted incidence rate ratios for the association of baseline COPD with CAP, TB and PCP requiring hospitalization by time period in Poisson regression models
| Incident rate ratios (95% confidence intervals)
|
|||
|---|---|---|---|
| CAP | TB | PCP | |
| 1996 – 2000 | 2.46 (1.94 – 3.08)** | 2.86 (1.63 – 4.72)** | 1.56 (1.02 – 2.28)* |
| 2001 – 2005 | 3.80 (2.80 – 5.08)** | 3.57 (1.08 – 9.34)* | 2.36 (1.28 – 4.03)* |
| 2006 – 2009 | 2.79 (1.78 – 4.21)** | 6.99 (1.93 – 21.3)* | 2.04 (0.63 – 5.16) |
p < 0.05;
p < 0.001
Adjusted risk of COPD for CAP, TB and PCP requiring hospitalization
In final multivariable Poisson regression models, COPD was independently associated with an increased risk of CAP, TB, and PCP hospitalization (IRR 1.94, 95% CI 1.64–2.30; IRR 2.60, 95% CI 1.70–3.97, and IRR 1.48, 95% CI 1.10–2.01, respectively), adjusting for demographics, time periods, smoking status, alcohol and drug related diagnoses, other comorbidities, CD4+ cell count, HIV viral load, and vaccination status (Table 4). When evaluating the effects of different risk factors entered en bloc into the models (see eTable 1, Supplemental Digital Content), the addition of demographics, risk behaviors and comorbid conditions resulted in the greatest attenuation of the association of COPD with CAP. In contrast, adjusting for HIV-related variables resulted in greater attenuation for the association of COPD with TB and PCP. The IRR for CAP requiring hospitalization was increased over time, and though not statistically significant, the IRRs for TB and PCP were decreased in the most recent era of 2006–2009 compared to the referent time period of 1996–2000 (Table 4).
Table 4.
Adjusted incidence rate ratios for the association of baseline COPD, other comorbidities and risk factors with CAP, TB and PCP requiring hospitalization in final multivariable Poisson regression models (n = 41,993)
| Incident rate ratios (95% confidence intervals)
|
|||
|---|---|---|---|
| CAP | TB | PCP | |
| COPD | 1.94 (1.64 – 2.30)** | 2.60 (1.70 – 3.97)** | 1.48 (1.10 – 2.01)* |
| Age | 1.01 (1.00 – 1.01)* | 1.01 (0.99 – 1.02) | 0.99 (0.98 – 0.99)** |
| Race/ethnicity | |||
| White | Referent | Referent | Referent |
| Black | 1.24 (1.11 – 1.38)** | 2.57 (1.84 – 3.58)** | 1.44 (1.21 – 1.70)** |
| Hispanic | 1.21 (1.00 – 1.48) | 1.98 (1.15 – 3.43)* | 1.69 (1.29 – 2.21)** |
| Other | 0.41 (0.28 – 0.59)** | -- | 0.49 (0.29 – 0.82)* |
| HIV-related variables | |||
| CD4+ <200 cells/μL | 1.76 (1.55 – 2.00)** | 2.30 (1.66 – 3.19)** | 4.11 (3.33 – 5.08)** |
| HIV viral load >500 copies/mL | 1.21 (1.04 – 1.40)* | 1.01 (0.70 – 1.47) | 1.23 (0.98 – 1.55) |
| Smoking status | |||
| Never smoker | Referent | Referent | Referent |
| Former smoker | 1.25 (0.98 – 1.60) | 1.14 (0.50 – 2.57) | 0.90 (0.61 – 1.31) |
| Current smoker | 1.58 (1.32 – 1.89)** | 2.98 (1.70 – 5.23)** | 1.32 (1.02 – 1.70)* |
| Missing | 4.96 (4.12 – 5.97)** | 5.69 (3.19 – 10.1)** | 4.39 (3.39 – 5.66)** |
| Alcohol-related diagnoses | 1.13 (0.97 – 1.31) | 1.18 (0.81 – 1.72) | 1.04 (0.82 – 1.32) |
| Drug use disorders | 1.16 (1.00 – 1.34)* | 1.10 (0.76 – 1.59) | 1.00 (0.80 – 1.25) |
| Comorbid conditions | |||
| CHF | 1.35 (1.02 – 1.78)* | 1.55 (0.73 – 3.27) | 1.76 (1.14 – 2.73)* |
| CAD/MI | 1.02 (0.89 – 1.17) | 0.71 (0.47 – 1.06) | 0.98 (0.79 – 1.22) |
| Renal insufficiency | 1.83 (1.52 – 2.19)** | 1.23 (0.71 – 2.13) | 1.17 (0.85 – 1.62) |
| ESLD | 1.29 (0.94 – 1.79) | 1.22 (0.49 – 3.07) | 1.01 (0.56 – 1.81) |
| HCV infection | 1.21 (1.09 – 1.35)** | 1.02 (0.77 – 1.34) | 1.01 (0.86 – 1.19) |
| Any malignancy | 1.61 (1.34 – 1.93)** | 1.27 (0.73 – 2.21) | 1.30 (0.96 – 1.76) |
| Vaccinations | |||
| Influenza | 0.86 (0.75 – 0.98)* | 0.75 (0.51 – 1.09) | 0.66 (0.53 – 0.82)** |
| Pneumococcal | 1.09 (0.96 – 1.24) | 1.14 (0.80 – 1.62) | 1.06 (0.86 – 1.31) |
| Time periods | |||
| 1996 – 2000 | Referent | Referent | Referent |
| 2001 – 2005 | 1.17 (1.02 – 1.34)* | 0.57 (0.38 – 0.85)* | 1.11 (0.90 – 1.36) |
| 2006 – 2009 | 1.33 (1.12 – 1.59)** | 0.60 (0.35 – 1.03) | 0.82 (0.59 – 1.14) |
p < 0.05;
p < 0.001
Individually, comorbidities other than COPD were also significantly associated with CAP requiring hospitalization, but with the exception of CHF, were not significantly associated with TB or PCP (Table 4). After COPD, malignancy and renal insufficiency were most strongly associated with increased risk for CAP among comorbidities. Current smoking was also significantly associated with CAP, TB, and PCP, although missing smoking status had the highest IRR for these events. In multivariable models restricted to recent years without substantial missing smoking status, associations of COPD with greater CAP, TB and PCP risk were similar (data not otherwise shown).
DISCUSSION
In a longitudinal, national cohort of more than 40,000 HIV+ Veterans, we found that COPD was an independent risk factor for CAP, TB and PCP requiring hospitalization after adjusting for CD4+ cell count, HIV viral load, cigarette smoking, substance abuse, other comorbidities, vaccinations and time periods. In the current ART era, COPD and other chronic comorbidities contribute substantially to the burden of morbidity and mortality amongst HIV+ populations.3–6,34 In fact, we found that the magnitude of risk associated with COPD for CAP and TB was similar to the magnitude of risk associated with baseline CD4+ <200 cells/μL. Our findings suggest that one of the ways COPD contributes to morbidity in HIV is by increasing risk for different types of pulmonary infections.
To our knowledge, our study is the first to document an association between COPD and risk of CAP, TB and PCP in HIV+ patients. Our primary exposure, COPD, is a risk factor for CAP in the general population,19–23 with a reported incidence of 22 events per 1,000 person-years among those with COPD.22 Another study found that individuals with COPD have a threefold increased risk of tuberculosis, primarily attributed to pulmonary involvement.35 The substantial burden of pneumonia among individuals with COPD is associated with higher all-cause hospitalizations and markedly increased healthcare costs.36 In our cohort, HIV+ individuals with COPD experienced >50 CAP events requiring hospitalization per 1,000 person-years, more than double the rate among those without COPD, underscoring the necessity of understanding the excess risk of pulmonary infection in this high-risk population.
Although the absolute incidence of CAP, TB and PCP requiring hospitalization decreased over time from 1996 to 2009, the adjusted risk for CAP generally increased over time when examining trends. The decreased incidence of pulmonary infections parallels the increasing proportion of individuals over time who had less advanced HIV based on higher CD4+ cell counts and lower HIV viral load at entry into the VACS-VC, likely related to increased testing and earlier detection of HIV within the VA, as well as improving care for HIV.37 The VACS-VC identifies all individuals at the time of the first ICD-9 diagnosis of HIV within the VA; once engaged in care, published data from 2008 support that 80% of HIV-infected Veterans are prescribed ART, only 14% have CD4+ <200 cells/μL, and 86% of eligible patients receive PCP prophylaxis.38 Additionally, influenza and pneumococcal vaccination rates increase, likely also reflecting better engagement in and greater utilization of healthcare.38 In our analyses, baseline CD4+ cell count remained significantly associated with CAP, TB and PCP risk. We also compared CD4+ prior to hospitalization and found no significant difference between those with and without COPD, suggesting that the association of COPD with risk for these pulmonary infections is less likely to be explained by differences in CD4+ cell count.
In addition to greater prevalence of COPD and smoking in the most recent time period, both of which were significantly associated with all three pulmonary infections in adjusted analyses, Veterans in the most recent era were also older and had more chronic, non-infectious comorbidities. When the association of COPD with pulmonary infections requiring hospitalization was incrementally adjusted, addition of demographic and behavioral risk factors as well as chronic comorbidities en bloc resulted in more marked attenuation of CAP risk, with lesser attenuation of TB and PCP risk. These data suggest that if smoking rates do not decrease substantially amongst HIV+ populations, COPD and other comorbidities will be increasingly important drivers of CAP risk. Thus, while CAP has decreased in absolute incidence in the most recent era, the incidence may again increase due to advancing age, continued smoking, and increasing prevalence of comorbidities such as COPD.
Several potential mechanisms may explain the increased risk of pulmonary infections associated with COPD in HIV. Phagocytosis of pathogens by alveolar macrophages, a primary reservoir of HIV in the lung, may be defective in the lungs of patients with COPD.39,40 This macrophage defect may be an important factor in microbial colonization of the lungs, potentially acting as a chronic antigenic driver of pulmonary inflammation with increased lymphocytes in the lower airways.40,41 Mucociliary clearance and related mechanical lung defenses are also impaired in patients with COPD.42 Treatment of COPD with inhaled corticosteroids (ICS) has been linked with increased risk of both CAP and TB among uninfected individuals with COPD,43–45 and may be related to local suppressive effects on the innate immune system.44 In our cohort, relatively few patients were prescribed ICS at baseline, so we could not examine the risk of pulmonary infections associated with baseline ICS use.
In turn, although COPD has traditionally been considered a chronic, non-infectious disease, the “vicious circle hypothesis” suggests that acute pulmonary infections and chronic colonization perpetuate inflammation and airway epithelial injury, resulting in progression of COPD.46 Data suggest an association of TB with chronic airflow obstruction, but whether this reflects the same pathophysiology as COPD from causes, such as smoking, is unknown.47 Bacterial pneumonia and PCP are also associated with permanent declines in lung function among HIV+ individuals, supporting a potential role in fueling COPD pathogenesis.48 The greater risk of pulmonary infections in those with COPD may thus contribute to the greater risk of COPD and accelerated decline in lung function observed in individuals with poorly controlled HIV.49
Our study has a number of strengths. The cohort is large, allowing us to impose strict criteria to define COPD at baseline as our primary exposure despite its relatively low prevalence, ensuring that identification of COPD precedes our outcome of incident pulmonary infection during the two-year follow-up period. As previously demonstrated in the VACS-VC, 16% of HIV+ patients had a baseline or incident diagnosis of COPD,4 suggesting that our estimates of the burden of COPD and pulmonary infections are conservative, as more patients are newly diagnosed with COPD during follow-up within the VA. Additionally, we examined data spanning a long time interval, from the earliest era shortly after the initiation of combination ART and including contemporary management. Also, the VACS-VC comprises a nationally representative sample of HIV+ Veterans in care at multiple institutions and includes a substantial proportion of minority patients.
This study also has several limitations. First, VACS-VC does not contain spirometry data to allow physiologic assessment of COPD severity; however, we used a previously validated approach for identifying clinical diagnoses of COPD.27 Other large-scale epidemiologic studies have similarly used administrative data for the diagnosis of COPD in the absence of spirometry with fair accuracy.50–52 Similarly, we used ICD-9 codes to identify CAP, TB and PCP; episodes of COPD exacerbation could have been misclassified as CAP, though this seems less likely for TB or PCP. Second, smoking status was missing in a substantial proportion of patients in the earliest time period. However, in sensitivity analyses including only individuals with non-missing smoking status, associations were essentially unchanged. We suspect that individuals with missing smoking status represented a group of Veterans with overall greater severity of illness as this group had higher rates of death and fewer VA visits, resulting in fewer opportunities for documentation of smoking status. Nonetheless, although we adjusted for smoking status at baseline, there may still be residual confounding of the association between COPD and pulmonary infections by cigarette smoking. Third, we may not have ascertained all hospitalizations for our three pulmonary infections of interest because Veterans might have been admitted to non-VA facilities, particularly in cases of greater acuity or in areas where VA hospitals are difficult to access. This underestimation of outcome events would likely bias our results to the null, suggesting that detected associations represent conservative estimates of pneumonia hospitalization risk. Finally, we only report CD4+ cell counts and HIV viral load prior to pulmonary infection events requiring hospitalization as there is no equivalent time period for comparison of CD4+ cell count and viral load among those who were not hospitalized; however, we restricted analyses to two years of follow-up.
In conclusion, we found that COPD is an independent risk factor for CAP, TB, and PCP requiring hospitalization among HIV+ individuals, adjusting for CD4+ cell count, HIV viral load, cigarette smoking, substance abuse, other comorbidities, vaccinations and time periods. In an era of increasing comorbidities amongst HIV+ populations, our analysis highlights the importance of considering the impact of these chronic conditions on risk for HIV-related infections. Despite a decline in the absolute incidence of CAP, TB and PCP, we found a greater IRR for CAP requiring hospitalization in more recent time periods. Taken together, these data suggest that COPD is an important contributor to the risk for CAP, TB and PCP requiring hospitalization in HIV+ cohorts in the current ART era. Increasingly prevalent chronic comorbidities such as COPD will likely have significant impacts on health outcomes of aging HIV+ populations. Longitudinal investigations are underway and are expected to contribute to understanding of associations of HIV with chronic lung disease development.53 Additional studies are needed to assess whether the risk associated with COPD for pulmonary infection is mediated by the severity of underlying lung disease or the use of COPD therapies such as ICS, and to determine whether COPD affects severity and outcomes of pulmonary infections amongst HIV+ individuals.
Supplementary Material
Acknowledgments
source of funding: This work was supported by the National Institutes of Health (R01 HL090342, F32 HL125031-01, K23 HL111116, K24 HL24087713 and U24-AA020794).
We acknowledge the Veterans who participate in the VACS-VC and the coordinators and data managers who make the study possible.
Footnotes
Conflicts of interest No conflicts of interest were declared by any of the authors.
Prior abstract presentation: Parts of the data were presented at the American Thoracic Society International Conference on May 20, 2013; Philadelphia, Pennsylvania.
References
- 1.Grubb JR, Moorman AC, Baker RK, et al. for the HOPS investigators. The changing spectrum of pulmonary disease in patients with HIV infection on antiretroviral therapy. AIDS. 2006;20:1095–1107. doi: 10.1097/01.aids.0000226949.64600.f9. [DOI] [PubMed] [Google Scholar]
- 2.Segal LN, Methé BA, Nolan A, et al. HIV-1 and bacterial pneumonia in the era of antiretroviral therapy. Proc Am Thorac Soc. 2011;8:282–287. doi: 10.1513/pats.201006-044WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akgün KM, Pisani M, Crothers K. the changing epidemiology of HIV-infected patients in the intensive care unit. J Intensive Care Med. 2011;26:151–164. doi: 10.1177/0885066610387996. [DOI] [PubMed] [Google Scholar]
- 4.Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183:388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith CJ, Ryom L, Weber R, et al. for the D:A:D Study Group. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384:241–248. doi: 10.1016/S0140-6736(14)60604-8. [DOI] [PubMed] [Google Scholar]
- 6.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet F, Chêne G, Thiébaut R, et al. for the Groupe d’Epidémiologie Clinique du SIDA en Aquitaine (GECSA) Trends and determinants of severe morbidity in HIV-infected patients: the ANRS CO3 Aquitaine Cohort, 2000–2004. HIV Med. 2007;8:547–554. doi: 10.1111/j.1468-1293.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 8.Berry SA, Fleishman JA, Moore RD, et al. for the HIV Research Network. Trends in reasons for hospitalization in a multisite United States cohort of persons living with HIV, 2001–2008. J Acquir Immune Defic Syndr. 2012;59:368–375. doi: 10.1097/QAI.0b013e318246b862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirschtick RE, Glassroth J, Jordan MC, et al. for the Pulmonary Complications of HIV Infection Study Group. Bacterial pneumonia in persons infected with the human immunodeficiency virus. N Engl J Med. 1995;333:845–851. doi: 10.1056/NEJM199509283331305. [DOI] [PubMed] [Google Scholar]
- 10.Gordin FM, Roediger MP, Girard P-M, et al. Pneumonia in HIV-infected persons: increased risk with cigarette smoking and treatment interruption. Am J Respir Crit Care Med. 2008;178:630–636. doi: 10.1164/rccm.200804-617OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saindou M, Chidiac C, Miailhes P, et al. Pneumococcal pneumonia in HIV-infected patients by antiretroviral therapy periods. HIV Med. 2008;9:203–207. doi: 10.1111/j.1468-1293.2008.00546.x. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Barradas MC, Goulet J, Brown S, et al. Impact of pneumococcal vaccination on the incidence of pneumonia by HIV infection status among patients enrolled in the Veterans Aging Cohort 5-Site Study. Clin Infect Dis. 2008;46:1093–1100. doi: 10.1086/529201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mocroft A, Reiss P, Kirk O, et al. Is it safe to discontinue primary Pneumocystis jiroveci pneumonia prophylaxis in patients with virologically suppressed HIV infection and a CD4 cell count <200 cells/microL? Clin Infect Dis. 2010;51:611–619. doi: 10.1086/655761. [DOI] [PubMed] [Google Scholar]
- 14.Huang L, Cattamanchi A, Davis JL, et al. HIV-associated Pneumocystis pneumonia. Proc Am Thorac Soc. 2011;8:294–300. doi: 10.1513/pats.201009-062WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kendall CE, Wong J, Taljaard M, et al. A cross-sectional, population-based study measuring comorbidity among people living with HIV in Ontario. BMC Public Health. 2014;14:161. doi: 10.1186/1471-2458-14-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gingo MR, Balasubramani GK, Rice TB, et al. Pulmonary symptoms and diagnoses are associated with HIV in the MACS and WIHS cohorts. BMC Pulm Med. 2014;14:75. doi: 10.1186/1471-2466-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drummond MB, Kirk GD, Astemborski J, et al. Prevalence and risk factors for unrecognized obstructive lung disease among urban drug users. Int J Chron Obstruct Pulmon Dis. 2011;6:89–95. doi: 10.2147/COPD.S15968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrache I, Diab K, Knox KS, et al. HIV associated pulmonary emphysema: a review of the literature and inquiry into its mechanism. Thorax. 2008;63:463–469. doi: 10.1136/thx.2007.079111. [DOI] [PubMed] [Google Scholar]
- 19.Almirall J, Bolibar I, Balanzó X, et al. Risk factors for community-acquired pneumonia in adults: a population-based case-control study. Eur Respir J. 1999;13:349–355. doi: 10.1183/09031936.99.13234999. [DOI] [PubMed] [Google Scholar]
- 20.Gómez-Junyent J, Garcia-Vidal C, Viasus D, et al. Clinical features, etiology and outcomes of community-acquired pneumonia in patients with chronic obstructive pulmonary disease. PLoS One. 2014;9:e105854. doi: 10.1371/journal.pone.0105854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liapikou A, Polverino E, Ewig S, et al. Severity and outcomes of hospitalised community- acquired pneumonia in COPD patients. Eur Respir J. 2012;39:855–861. doi: 10.1183/09031936.00067111. [DOI] [PubMed] [Google Scholar]
- 22.Müllerova H, Chigbo C, Hagan GW, et al. The natural history of community-acquired pneumonia in COPD patients: A population database analysis. Respir Med. 2012;106:1124–1133. doi: 10.1016/j.rmed.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Almirall J, Bolibar I, Serra-Prat M, et al. New evidence of risk factors for community-acquired pneumonia: a population-based study. Eur Respir J. 2008;31:1274–1284. doi: 10.1183/09031936.00095807. [DOI] [PubMed] [Google Scholar]
- 24.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44:S25–S30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 25.McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13:1233–1239. doi: 10.1093/ntr/ntr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraemer KL, McGinnis KA, Skanderson M, et al. Alcohol problems and health care services use in human immunodeficiency virus (HIV)-infected and HIV-uninfected veterans. Med Care. 2006;44:S44–S51. doi: 10.1097/01.mlr.0000223703.91275.78. [DOI] [PubMed] [Google Scholar]
- 27.McGinnis KA, Fine MJ, Sharma RK, et al. Understanding racial disparities in HIV using data from the veterans aging cohort 3-site study and VA administrative data. Am J Public Health. 2003;93:1728–1733. doi: 10.2105/ajph.93.10.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mor A, Thomsen RW, Ulrichsen SP, et al. Chronic heart failure and risk of hospitalization with pneumonia: a population-based study. Eur J Intern Med. 2013;24:349–353. doi: 10.1016/j.ejim.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Sibila O, Mortensen EM, Anzueto A, et al. Prior cardiovascular disease increases long-term mortality in COPD patients with pneumonia. Eur Respir J. 2014;43:36–42. doi: 10.1183/09031936.00117312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinogradova Y, Hippisley-Cox J, Coupland C. Identification of new risk factors for pneumonia: population-based case-control study. Br J Gen Pract. 2009;59:e329–e338. doi: 10.3399/bjgp09X472629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kornum JB, Thomsen RW, Riis A, et al. Diabetes, Glycemic Control, and Risk of Hospitalization With Pneumonia. Diabetes Care. 2008;31:1541–1545. doi: 10.2337/dc08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cilloniz C, Torres A, Polverino E, et al. Community-acquired lung respiratory infections in HIV-infected patients: microbial aetiology and outcome. Eur Respir J. 2014;43:1698–1708. doi: 10.1183/09031936.00155813. [DOI] [PubMed] [Google Scholar]
- 34.Palella FJ, Jr, Baker RK, Moorman AC, et al. for the HIV Outpatient Study Investigators. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 35.VA Policies and Reports on HIV/AIDS. U.S. Department of Veterans Affairs; Oct 27, 2014. Available at: http://www.hiv.va.gov/provider/policy/index.asp. [Google Scholar]
- 36.The State of Care for Veterans with HIV/AIDS. U.S. Department of Veterans Affairs; Dec, 2009. Available at: http://www.hiv.va.gov/pdf/state-of-care.pdf. [Google Scholar]
- 37.Inghammar M, Ekbom A, Engström G, et al. COPD and the risk of tuberculosis—a population-based cohort study. PLoS One. 2010;5:e10138. doi: 10.1371/journal.pone.0010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin J, Li Y, Tian H, et al. Costs and health care resource utilization among chronic obstructive pulmonary disease patients with newly acquired pneumonia. Clinicoecon Outcomes Res. 2014;6:349–356. doi: 10.2147/CEOR.S65824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beck JM. Abnormalities in host defense associated with HIV infection. Clin Chest Med. 2013;34:143–153. doi: 10.1016/j.ccm.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor AE, Finney-Hayward TK, Quint JK, et al. Defective macrophage phagocytosis of bacteria in COPD. Eur Respir J. 2010;35:1039–1047. doi: 10.1183/09031936.00036709. [DOI] [PubMed] [Google Scholar]
- 41.Sethi S, Maloney J, Grove L, et al. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:991–998. doi: 10.1164/rccm.200509-1525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sethi S. Infection as a comorbidity of COPD. Eur Respir J. 2010;35:1209–1215. doi: 10.1183/09031936.00081409. [DOI] [PubMed] [Google Scholar]
- 43.Kim JH, Park JS, Kim KH, et al. Inhaled corticosteroid is associated with an increased risk of TB in patients with COPD. Chest. 2013;143:1018–1024. doi: 10.1378/chest.12-1225. [DOI] [PubMed] [Google Scholar]
- 44.Finney L, Berry M, Singanayagam A, et al. Inhaled corticosteroids and pneumonia in chronic obstructive pulmonary disease. Lancet Respir Med. 2014;2:919–932. doi: 10.1016/S2213-2600(14)70169-9. [DOI] [PubMed] [Google Scholar]
- 45.Ernst P, Gonzalez AV, Brassard P, et al. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med. 2007;176:162–166. doi: 10.1164/rccm.200611-1630OC. [DOI] [PubMed] [Google Scholar]
- 46.Sethi S. Bacterial infection and the pathogenesis of COPD. Chest May. 2000;117(5 Suppl 1):286S–291S. doi: 10.1378/chest.117.5_suppl_1.286s. [DOI] [PubMed] [Google Scholar]
- 47.Allwood BW, Myer L, Bateman ED. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration. 2013;86:76–85. doi: 10.1159/000350917. [DOI] [PubMed] [Google Scholar]
- 48.Morris AM, Huang L, Bacchetti P, et al. for the Pulmonary Complications of HIV Infection Study Group. Permanent declines in pulmonary function following pneumonia in human immunodeficiency virus-infected persons. Am J Respir Crit Care Med. 2000;162:612–616. doi: 10.1164/ajrccm.162.2.9912058. [DOI] [PubMed] [Google Scholar]
- 49.Drummond MB, Merlo CA, Astemborski J, et al. The effect of HIV on longitudinal lung function decline amond IDUs: a prospective cohort. AIDS. 2013;27:1303–1311. doi: 10.1097/QAD.0b013e32835e395d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooke CR, Joo MJ, Anderson SM, et al. The validity of using ICD-9 codes and pharmacy records to identify patients with chronic obstructive pulmonary disease. BMC Health Serv Res. 2011;11:37. doi: 10.1186/1472-6963-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gershon AS, Wang C, Guan J, et al. Identifying individuals with physcian diagnosed COPD in health administrative databases. COPD. 2009;6:388–394. doi: 10.1080/15412550903140865. [DOI] [PubMed] [Google Scholar]
- 52.Lacasse Y, Daigle JM, Martin S, et al. Validity of chronic obstructive pulmonary disease diagnoses in a large administrative database. Can Respir J. 2012;19:e5–e9. doi: 10.1155/2012/260374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kunisaki KM, Niewoehner DE, Collins G, et al. for the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) START Study Group. Pulmonary function in an international sample of HIV-positive, treatment-naïve adults with CD4 counts >500 cells/μL: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med. 2015;16(Suppl S1):119–128. doi: 10.1111/hiv.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


