Abstract
The formation of advanced glycation end-products (AGE) as a result of the action of reducing sugars on host macromolecules plays a role in increased morbidity of diabetic patients. There are currently no clinically available therapeutics for the prevention or eradication of AGEs. Following our previous identification of 2-aminoimidazole (2-AI) based AGE inhibitors and breakers, we now report the use of a rapid, scalable, two-step procedure to access a second generation of 2-AI based anti-AGE compounds from commercially available amino acids. Several second generation compounds exhibit increased AGE inhibition and breaking activty compared to the first generation compounds and to the known AGE inhibitor aminoguanidine.
Keywords: Advanced glycation end-product (AGE), 2-aminoimidazole, Akabori, Diabetes
Graphical Abstract

Advanced glycation end-products (AGE) are formed as a result of the random action of reducing sugars and reactive aldehydes upon host macromolecules, which can lead to protein misfolding.1,2 AGE formation and accumulation occurs naturally with aging but at an accelerated rate associated with uncontrolled hyperglycemia in people with diabetes. AGEs have been reported to play a role in a number of diabetes related complications including: retinopathy, cataract formation, atherosclerosis, neuropathy, nephropathy, diabetic embryopathy, and impaired wound healing.3 AGE formation contributes to the pathogenesis of these conditions in two major ways: 1) direct effects of covalent cross-linking and other chemical modifications of proteins including decreased proteolytic degradation, altered interactions with other molecules, and effects upon the biomechanical characteristics of tissues,4 and 2) receptor-mediated pathways, such as the binding of AGEs to the receptor for advanced glycation end-products (RAGE),5 which leads to the induction of proinflammatory and procoagulant responses. RAGE activation has been implicated in the pathogenesis of several diseases including diabetes, Alzheimer’s disease, and renal failure.6 The formation of AGEs in both aging and diabetes also leads to alterations in blood vasculature and contributes to the development of cardiovascular disease.7 The prevalence of diabetes, and the contribution of AGEs to the pathogenesis of diabetic complications mean that small molecules that inhibit or disrupt AGEs could have a broad-ranging therapeutic impact in the treatment of diabetes-related complications.
There are currently no drugs available that are effective at preventing AGE formation, or that target preformed AGEs clinically. Both aminoguanidine (AG) 18,9, and the thiazolium derived compound ALT-711 (algebrium) 210,11 showed potential as drugs that inhibit AGE formation, but in both cases further clinical development was discontinued due to safety and/or efficacy concerns.12 Our goal therefore was to develop small molecules with therapeutic potential to both inhibit AGE formation as well as break preformed AGEs. We previously reported the identification of a series of 2-aminoimidazoles (2-AIs) that possess the ability to both inhibit the formation of, and break preformed, AGEs.13 The lead compound identified in this study, the bis 2-AI 3 (Figure 1), possesses superior AGE inhibition and breaking activity compared to AG 1. Compound 3 inhibited AGE formation on bovine serum albumin by glycolaldehyde (GO) by 44.6% and 5.6% at 400 and 40 μM respectively, as measured by a standard fluorescence based assay. More significantly, when measured using a 2,4,6-trinitrobenzene sulfonic acid (TNBSA) based assay that quantifies total primary amines regardless of fluorescent properties, compound 3 protected a considerable proportion of reactive primary amines, with only a 3.6% reduction compared to a 18.3% and 19.4% reduction in AG-treated and untreated controls respectively.
Figure 1.
AGE inhibitors aminoguanidine 1, ALT-711 2, and bis-2-aminoimidazole 3.
Outside of its inhibitory activity, bis 2-AI 3 also demonstrated the ability to break preformed AGEs. The ability to reverse AGE formation is potentially more useful from a clinical standpoint as the ability to reverse mis-glycation may have the potential to delay or prevent disease progression. To this end, we established that compound 3 restored the primary amine content of glycated-BSA to 78.9% of the baseline value after 48 hours, exceeding the 71.9% restoration afforded by ALT-711 as quantified with the TNBSA assay, while AG 1 (as previously established) showed no significant breaking activity.
To further develop this new class of anti-AGE compounds, we set out to investigate the structure activity relationship of compound 3 in the context of its anti-glycating activity. We first explored the effect of the alkyl linker length between the two 2-AI heterocycles in compound 3. Compound 3, along with compound 4, which possesses an increased linker length of five methylene units as compared to four methylene units in compound 3, were readily synthesized from the corresponding di-acid chlorides via the Nierenstein reaction to the di-α-chloroketones 5 and 6, followed by cyclization with Boc-guanidine and subsequent Boc deprotection (Scheme 1). Compound 4 was then assayed for its ability to inhibit AGE formation on bovine serum albumin by glycolaldehyde (GO) using a standard fluorescence assay, and its activity compared to compound 3. Interestingly, we noted no significant decrease in activity between the two compounds (data not shown).
Scheme 1.
Synthetic route to 4 and 5 methylene linked bis-2-AIs. Reagents and conditions: i) CH2N2, Et2O, 0 °C; ii) HCl; iii) Boc-guanidine, NaI, DMF, rt; iv) TFA:DCM (1:2) then MeOH:HCl.
With no real activity differential between 3 and 4, we attempted to delineate the effect that shortening the tether had upon activity. To this end, we attempted to access compounds 7, 8, and 9 via Boc-guanidine cyclization of the corresponding bis-α-chloroketones 10, 11, and 12 (Scheme 1, n = 1, 2, and 3 respectively). Starting from the commercially available acid chlorides, we observed decomposition of compound 7 and 8 upon treatment with diazomethane, while we were able to access bis-α-chloroketone 12. Unfortunately, treatment of 12 with Boc-guanidine also led to decomposition.
With this failure, we re-designed our target molecules to bis-2-AIs 13 and 14 (Scheme 2). Our reasoning was that if proximity between the two 2-AI groups correlated with activity, then altering their bite angle through the Thorpe-Engold effect would deliver significantly more active compounds than 3, 4, or the activity that compounds 7-9 could have potentially possessed. However, similar to 10 and 11, treatment of acid chlorides 15 and 16 with diazomethane followed by an HCl quench in an effort to access bis- α-chloroketones 17 and 18 also led to decomposition.
Scheme 2.
Attempted synthesis of bis 2-AIs with shortened linkers. Reagents and Conditions: i) CH2N2, Et2O, 0 °C; ii) HCl; iii) TBDPSCl, imidazole, DCM, 0 °C; iv) (COCl)2, cat. DMF, DCM, 0 °C – rt; v) CH2N2, Et2O, 0 °C; vi) HBr; vii) Boc-guanidine, DMF, rt; viii)1M TBAF in THF, rt; ix) 5% Na/Hg, H2O, pH 1.5, 0 – 5 °C; x) NH2CN, H2O, pH 4.3, 95 °C; xi) Br2, MeSO4H then 2-aminoimidazole sulfate.
With repeated failures to access the shorter tethered 2-AIs through one-pot cyclizations, we next attempted to synthesize 13 and 14 through sequential installation of each 2-AI heterocycle. To this end (Scheme 2), we protected the hydroxyl group of 19 with TBDPS-Cl and converted the resulting carboxylic acid into the corresponding α-bromoketone 20 using a standard three-step sequence (oxalyl chloride, diazomethane, and then HBr quench). Cyclization with Boc-guanidine followed by TBAF mediated cleavage of the TBDPS silyl ether then delivered the corresponding Boc-protected 2-AI alcohol 21. Repetition of this reaction sequence to carboxylic acid 22 first generated the α-bromoketone 23 that, following cyclization and silyl ether cleavage, produced the corresponding Boc-protected 2-AI alcohol 24. With both compounds in hand, we simply needed to oxidize the primary alcohol to the corresponding carboxylic acid and then install the last 2-AI ring. Unfortunately, all attempts at oxidizing the primary alcohol of 20 or 22 to the carboxylic acid (directly via Jones or stepwise via Swern-Pinnick) also led to decomposition.
Finally, we tried to access compounds 13 and 14 through bromine-mediated oxidative heterodimerization.14 2-AIs 25 and 26 (Scheme 2) were accessed through standard Akabori reduction of the corresponding amino acid methyl esters followed by pH controlled cyanamide condensation. Treatment of each compound with 1 eq. of Br2 followed by attempted dimerization with 2-aminoimidazole under acidic conditions also failed to deliver compounds 13 and 14.
With repeated failures to access our targeted bis-2-AI derivatives, we elected to reconsider our AGE-inhibitor design. Although we are confident that synthetic procedures could be developed to access bis-2-AIs with compressed tether lengths (and these efforts are ongoing), our overarching goal is to develop potent anti-AGE compounds that can be evaluated for efficacy in vivo. In this regard, we considered the possibility that bis 2-AIs 3 and 4 could be simply viewed as a 2-AI group tethered to another reactive group (Figure 2) in which both entities were capable of sequestering reactive aldehyde species. If this were the case, then the reactive group could potentially be another heterocycle or even something as simple as an amine or guanidine.
Figure 2.
AGE inhibitor redesign
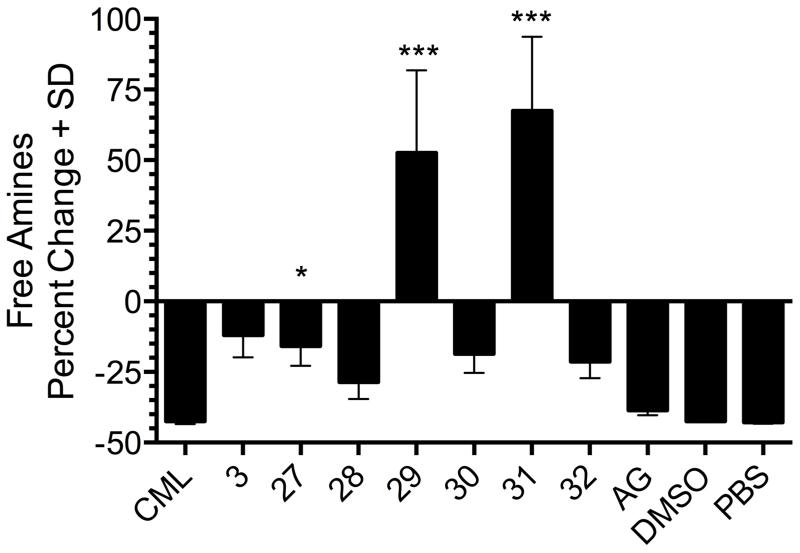
To test this hypothesis, we again employed the Akabori reduction/cyanamide condensation to rapidly access potential new 2-AI AGE inhibitors derived from a series of six amino acid methyl esters (Scheme 3). The ability of these amino acid-derived 2-AI compounds 27-32 to inhibit AGE formation was first assessed by reacting BSA with glycolaldehyde in the absence or presence of potential inhibitors for seven days followed by dialysis purification of the AGE-BSA and quantification of the remaining free amines by reaction with TNBSA, as previously reported.13 All compounds were biologically active as evidenced by a significant increase in the number of primary amines as compared to the unmodified control BSA and glycolaldehyde BSA (Figure 3). The lysine derived 2-AI 29 exhibited the most potent activity, increasing the number of primary amines by 44.6% relative to control BSA. Similar to 29, compounds 28, 30, 31, and 32 all increased the number of free amines above that of BSA alone, suggesting that these compounds not only inhibit AGE formation in the presence of glycolaldehyde, but also possess the ability to break preformed AGEs. As a product of natural aging, the presence of AGEs in purified BSA has been demonstrated previously, and restoration of free amines at these sites may lead to a proportion in excess of basal levels.15-17 Furthermore, it is possible that in the presence of 2-AI compounds, chemical modifications are created on BSA that lead to increased reactivity in the TNBSA assay. The chemical products characteristic of 2-AI treated BSA with increased amine content are currently under further investigation. Furthermore, the previously reported result was reproduced with compound 3, although to a lesser extent than was achieved with these 2nd generation compounds.13
Scheme 3.
Akabori reduction and cyanamide cyclization to produce amino acid derived 2-AIs. Reagents and conditions: i) Na/Hg, H2O, pH 1.5, 0-5 °C; ii) NH2CN; ii) NH2CN, H2O, pH 4.3, 95 °C.
Figure 3.
2nd generation bis 2-AI compounds effectively inhibit the formation of AGE-BSA. The line represents basal frequency of free amines in BSA prior to GO and 2-AI treatment. Inhibition of AGE formation is reflected as a preservation of free amine groups compared to GO-modified BSA. DMSO and PBS are vehicle controls. AG aminoguanidine. *P<0.05, ***P<0.001.
To further assess the breaking ability of this series of 2-AIs, we first generated preformed carboxymethyl lysine (CML) - enriched AGEs made by incubation of 50 mg/mL BSA with 25 mM glyoxylic acid in the presence of sodium cyanoborohydride for 48 hours. Dialysis-purified glycated proteins were then treated with 2-AIs for 24 hours and the change in the number of primary amines was quantified with TNBSA (Figure 4). As was the case for the inhibition assay, every member of the series, as well as 1st generation compound 3, caused an increase in the number of primary amines, some in excess of basal levels, suggesting AGE displacement or breaking. By this metric, ornithine derivative 31 was the most potent AGE-breaker, producing a 67.5% increase in primary amines relative to BSA alone, a 109.8% increase relative to the preformed CML-enriched AGE reference, and a 79.4% improvement over compound 3. Lysine derivative 29 also exhibited potent activity, with a 52.5% increase in the number of primary amines relative to BSA, a 97.9% increase relative to the preformed CML-enriched AGE reference, and a 64.5% improvement over compound 3. In the same assay, AG was again devoid of breaking activity, causing no increase in the number of primary amines.13
Figure 4.
2nd generation bis 2-AI compounds are effective at breaking preformed CML-BSA. The line represents basal frequency of free amines in BSA prior to formation of CML-AGEs. Breaking of preformed CML by bis 2-AI is reflected as an increase in free amine groups compared to CML-BSA in the absence of 2-AI compounds (CML). DMSO and PBS are vehicle controls. AG, aminoguanidine. *P<0.05, ***P<0.001.
In conclusion, we have demonstrated the versatility of the 2-AI scaffold for the generation of novel anti-AGE compounds. By simplifying the design of our lead compound 3, we were able to demonstrate that 2-AIs derived from commercially available α-amino acids that contain a side chain nucleophile surpass the activity of compound 3, which itself is significantly more active than the gold standard in the field aminoguanidine. The additional advantages of these next generation anti-AGE compounds are that they are rapidly generated in a two-step process from α-amino acids and the synthetic approach is scalable such that 50-100 g quantities of material are readily available in a single run under a standard academic laboratory setting. Efforts to further augment activity with non-natural α-amino acid precursors are underway as well as evaluation of lead anti-AGE compounds in various animal models.
Supplementary Material
Acknowledgments
These studies were supported by funding from NIH grants 1R01AI106733-01 (RJB, CM), R21AI107254-02 (RJB) and 1K01OD016997 (BKP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Synthetic procedures, biological assay conditions and compound characterization can be found in the supplementary material.
References and notes
- (1).Hellwig M, Henle T. Angew. Chem. Int. Ed. Engl. 2014;53:10316. doi: 10.1002/anie.201308808. [DOI] [PubMed] [Google Scholar]
- (2).Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Glycobiology. 2005;15:16R. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- (3).Ahmed N. Diabetes Res. Clin. Pract. 2005;67:3. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- (4).Aronson D. J. hypertens. 2003;21:3. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- (5).Schmidt AM, Yan SD, Yan SF, Stern DM. J. Clin. Invest. 2001;108:949. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, Thiyagarajan M, Zarcone T, Fritz G, Friedman AE, Miller BL, Zlokovic BV. J. Clin. Invest. 2012;122:1377. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Goldin A, Beckman JA, Schmidt AM, Creager MA. Circulation. 2006;114:597. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- (8).Freedman BI, Wuerth JP, Cartwright K, Bain RP, Dippe S, Hershon K, Mooradian AD, Spinowitz BS. Control Clin. Trials. 1999;20:493. doi: 10.1016/s0197-2456(99)00024-0. [DOI] [PubMed] [Google Scholar]
- (9).Tilton RG, Chang K, Hasan KS, Smith SR, Petrash JM, Misko TP, Moore WM, Currie MG, Corbett JA, McDaniel ML, et al. Diabetes. 1993;42:221. doi: 10.2337/diab.42.2.221. [DOI] [PubMed] [Google Scholar]
- (10).Hartog JW, Willemsen S, van Veldhuisen DJ, Posma JL, van Wijk LM, Hummel YM, Hillege HL, Voors AA. Eur. J. Heart Fail. 2011;13:899. doi: 10.1093/eurjhf/hfr067. [DOI] [PubMed] [Google Scholar]
- (11).Willemsen S, Hartog JW, Hummel YM, Posma JL, van Wijk LM, van Veldhuisen DJ, Voors AA. Eur. J. Heart Fail. 2010;12:294. doi: 10.1093/eurjhf/hfp207. [DOI] [PubMed] [Google Scholar]
- (12).Engelen L, Stehouwer CD, Schalkwijk CG. Diabetes Obes. Metab. 2013;15:677. doi: 10.1111/dom.12058. [DOI] [PubMed] [Google Scholar]
- (13).Richardson MA, Furlani RE, Podell BK, Ackart DF, Haugen JD, Melander RJ, Melander C, Basaraba RJ. Tetrahedron Lett. 2015;56:3406. doi: 10.1016/j.tetlet.2015.01.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Xu YZ, Yakushijin K, Horne DA. J. Org. Chem. 1996;61:9569. [Google Scholar]
- (15).Xue J, Rai V, Singer D, Chabierski S, Xie J, Reverdatto S, Burz DS, Schmidt AM, Hoffmann R, Shekhtman A. Structure. 2011;19:722. doi: 10.1016/j.str.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Coughlan MT, Yap FY, Tong DC, Andrikopoulos S, Gasser A, Thallas-Bonke V, Webster DE, Miyazaki J, Kay TW, Slattery RM, Kaye DM, Drew BG, Kingwell BA, Fourlanos S, Groop PH, Harrison LC, Knip M, Forbes JM. Diabetes. 2011;60:2523. doi: 10.2337/db10-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Waanders F, van den Berg E, Schalkwijk C, van Goor H, Navis G. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2007;22:3093. doi: 10.1093/ndt/gfm398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.