Abstract
We have recently disclosed the discovery of the class of 1,2,4-oxadiazole antibiotics, which emerged from in silico docking and scoring efforts. This class of antibacterials exhibits Gram-positive activity, particularly against Staphylococcus aureus. We define the structure-activity relationship (SAR) of this class of antibiotics with the synthesis and evaluation of a series of 59 derivatives with variations in the C ring or C and D rings. A total of 17 compounds showed activity against S. aureus. Four derivatives were evaluated against a panel of 16 Gram-positive strains, inclusive of several methicillin-resistant S. aureus strains. These compounds are broadly active against Gram-positive bacteria.
Keywords: 1,2,4-oxadiazoles; structure-activity relationship; Antibiotics
Graphical Abstract
The Gram-positive bacterium Staphylococcus aureus is commensal to humans and exists on the skin and mucosa of 30% of the population. 1,2 It is a principal cause of hospital infections, the most frequent and serious of which are bacteremia and endocarditis in hospitalized patients.3–6 This organism has become resistant to many different classes of antibiotics.7,8 Of special concern are the strains designated as methicillin-resistant S. aureus (MRSA), which are broadly resistant to most β-lactam antibiotics, agents of historic choice for treatment of infections by S. aureus. There has been recent activity in discovery of novel antibiotics for treatment of S. aureus infections,9 but emergence of antibiotic-resistant variants is inevitable, necessitating search for novel classes of antibiotics effective against these organisms.
We recently reported the discovery of the 1,2,4-oxadiazole class of antibiotics, which emerged from in silico docking and scoring, followed by screening against the ESKAPE panel of bacteria.10,11 This class of antibiotics targets the cell wall for inhibition, it exhibits good in vitro and in vivo activity and it is orally bioavailable.10,11
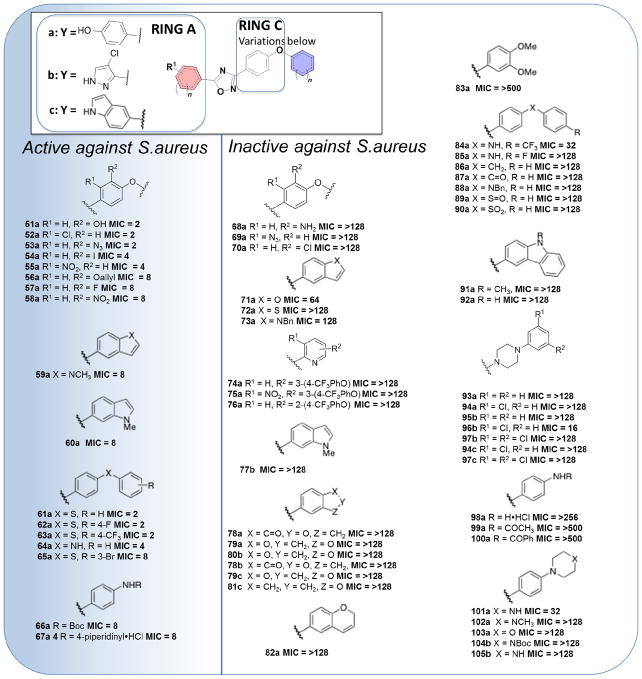
The 1,2,4-oxadiazoles are generally comprised of four rings, designated as A, B, C and D, as indicated by the representative compound 1a (Fig. 1). A hydrogen-bond donor in the A ring is necessary for antibacterial activity. The phenol, aniline and some heterocycles with hydrogen-bonding capability, such as pyrazoles, are tolerated. However, some substituents at this site such as sulfonamides, amides and carboxylic acids reduce the antibacterial activity or are inactive.11 Hydrogen-bond acceptors on the A ring are not favored. As indicated, pyrazoles with halogen substituents are all active, as is the indole at the A ring. Other variants with heteroaromatic systems such as pyridines, triazoles and pyroles generally lose activity, as do the ones with aliphatic heterocycles.11
Figure 1.
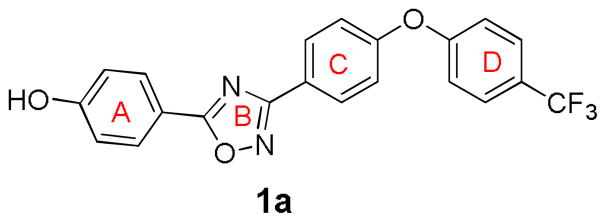
The structure of compound 1a
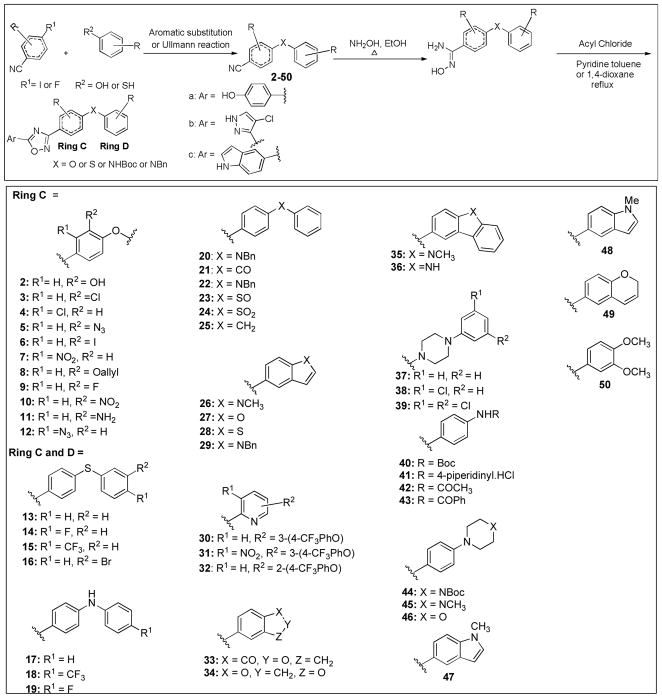
We outline here our preparation and evaluation of a series of 59 additional oxadiazole analogs. In general, the derivatives have attempted to explore the effect of structural diversity on the antibacterial activity on the right-hand side of the molecule in the perspective depicted in Fig. 1. The diverse analogs were selected for variation in rings C or in rings C and D, inclusive of fused-ring variants (Fig. 2). These studies further define the structure-activity relationship (SAR) for this class of antibacterials.
Figure 2.
Results of in vitro antibacterial activity against S. aureus ATCC29213 of the new 1,2,4-oxadiazole derivatives. The functional groups in ring C were changed to produce all the synthetic compounds in this series and Y was limited to the three indicated entities. MIC values were measured in μg/mL and active compounds have an MIC ≤ 8 μg/mL.
The general synthesis of this library followed the methodology reported earlier, as depicted in Scheme 1.5 Nitrile intermediates with the C and D rings fused were commercially available (examples 26 and 33). The nitrile intermediates 2–50 were key to the formation of the 1,2,4-oxadiazole derivatives. The biphenyl ether fragment can be formed through Ullmann reaction or aromatic substitution. The former takes place between aryl iodides and phenol in the presence of CuI, Cs2CO3 and N, N-dimethylglycine. HCl at 90 °C. Nucleophilic aromatic substitution between the aryl fluorides and phenol was accomplished using K2CO3 as base. With the nitriles 2–50 in hand, the amidoximes were easily generated from the reaction between the nitrile and hydroxylamine in ethanol. Under the standard conditions, the acyl chloride was allowed to react with amidoxime in the presence of pyridine under reflux to afford the key 1, 2, 4-oxadiazole intermediates. Removal of the protective groups furnished the final compounds. For example, the Boc group was removed by exposure to acid and deprotection of the benzyl was performed in the presence of BBr3.
Scheme 1.
General synthetic route to the 1,2,4-oxadiazoles, and the intermediates (2–50) used for variations within the C ring or the C and D rings fused. Aromatic substitution condition: K2CO3, DMF, 60–100 °C; Ullmann reaction condition: CuI, Cs2CO3, N,N-dimethylglycine. HCl, 1,4-dioxane 90 °C.
These compounds were screened for antibacterial activity by determination of minimal-inhibitory concentrations (MICs) against the ESKAPE panel of bacteria, including S. aureus ATCC29213. Active compounds were designated as those with MIC values of ≤8 μg/mL, which encompassed 17 of the synthetic compounds. The MIC data and the corresponding structures are listed in Fig. 2. All the active derivatives (51a–67a) have the phenol moiety as the A ring. Compounds 51a and 52a displayed identical antibacterial potency with an MIC value of 2 μg/mL, which indicated both the electron-withdrawing group chlorine or the electron-donating hydroxyl were well tolerated. Other substituents such as iodine, fluorine and the nitro group at the R1 and R2 positions showed the same trend. Interestingly, compound 68a with the NH2 group at R2 did not have any antibacterial activity. A small difference in antibacterial activity was observed between 55a and 58a with the switching of the NO2 group between the R1 and R2 positions (MIC = 4 μg/mL versus MIC = 8 μg/mL). On the contrary, when the position of the azide was changed between R1 and R2, one compound (53a) demonstrated activity and the other (69a) was inactive. Intriguingly, chlorine as a substituent exhibited the opposite trend between the R1 and R2 positions (52a and 70a). Replacement of the bridging oxygen with sulfur, compounds 61a, 62a, 63a and 65a, did not alter the activity. Also, substitution of oxygen by the NH group (64a) retained activity, although there was a two-fold effect on the MIC value. It is of note that, compound 86a with the oxygen substituted by CH2 resulted in inactivity, so did replacement of oxygen with NH (85a). The CO, SO and SO2 groups at the same location behaved the same way, resulting in the loss of activity (87a, 89a and 90a). The more polar piperidine derivative 67a was active (MIC = 8 μg/mL).
Other attempts to introduce piperidine (93a) or piperazine (102a) failed to produce active compounds. Compounds 74a–76a with pyridine rings were devoid of activity. Most of the compounds with the C and D rings fused were inactive, except 59a and 60a, which exhibited activity with an MIC of 8 μg/mL. Isomeric compounds 59a and 60a showed the same activity, indicating that the different positions for the indole nitrogen did not affect the antibacterial activity. The substitution of the indole nitrogen by O, S or NBn resulted in inactive compounds (71a, 72a and 73a). Our previous work indicated that several derivatives with 4-chloro pyrazole or indole as the A ring and the biphenyl ether for the C and D rings have potent antibacterial activity5 However, the variants prepared in the present study in which the C and D rings were fused did not result in active compounds.
This SAR effort based on a library of 59 compounds established a number of important observations on the oxadiazole antibiotics. These are: (i) structural variations on the C ring can support antibacterial activity; (ii) substitutions of oxygen for sulfur at the bridging moiety between rings C and D can generally be tolerated, but other moieties at the same site are detrimental; (iii) fusion of rings C and D with the phenol as the A ring retains activity; (iv) variations on the C ring abolish activity, if the A ring is either pyrazole or indole.
Antibiotics 51a–53a and 63a were evaluated with a larger panel of 16 Gram-positive bacteria, including antibiotic-resistant strains (Table 1). The properties of these strains are given in the footnotes to the table. These compounds in general exhibit broad activity against many of these leading Gram-positive bacterial pathogens.
Table 1.
Minimal-Inhibitory Concentrations (MICs) of Oxadiazolesa
| microorganism | MIC (μg/mL)
|
||||
|---|---|---|---|---|---|
| 51a | 52a | 53a | 63a | vancomycinj | |
| S.aureus ATCC 29213b | 2 | 2 | 2 | 2 | 1 |
| S.aureus ATCC 27660c | 16 | 8 | 4 | 2 | 1 |
| S.aureus NRS100 (COL)c | 16 | 8 | 4 | 2 | 2 |
| S.aureus NRS119d | 8 | 4 | 4 | 2 | 2 |
| S.aureus NRS120d | 8 | 4 | 4 | 2 | 2 |
| S.aureus VRS1e | 8 | 2 | 2 | 2 | 512 |
| S.aureus VRS2f | 8 | 2 | 2 | 2 | 64 |
| S.epidermis ATCC 35547 | 16 | 4 | 2 | 2 | 16 |
| S.hemolyticus ATCC 29970 | 8 | 4 | 2 | 2 | 2 |
| B. cereus ATCC 13061 | 8 | 4 | 4 | 8 | 1 |
| B. licheniformis ATCC 12759 | 16 | 2 | 4 | 1 | 0.5 |
| E.faecalis ATCC 29212b | 8 | 4 | 2 | 2 | 2 |
| E.faecalis 201(Van S)g | 8 | 4 | 4 | 2 | 1 |
| E.faecalis 99(Van R)h | 16 | 4 | 4 | 2 | 128 |
| E.faecium 119-39A (Van S)g | 8 | 4 | 4 | 2 | 0.5 |
| E.faecium 106 (Van R)h | 8 | 4 | 4 | 2 | 256 |
| E.faecium C68i | 8 | 4 | 4 | 2 | 64k |
Whereas the compounds were screened against the ESKAPE panel of bacteria, they exhibited antibacterial activity only against Gram-positive bacteria.
A quality–control strain susceptible to methicillin to monitor accuracy of MIC testing.
mecA positive, resistant to methicillin, oxacillin, and tetracycline; susceptible to vancomycin and linezolid.
mecA positive, resistant to ciprofloxacin, gentamicin, oxacillin, penicillin, and linezolid.
Vancomycin-resistant MRSA (vanA) clinical isolate from Michigan.
Vancomycin-resistant MRSA (vanA) clinical isolate from Pennsylvania.
Vancomycin-susceptible clinical isolate.
Vancomycin-resistant clinical isolate.
Clinical strain isolated in Cleveland hospitals; most prevalent vancomycin-resistant E.faecium strain from Cleveland hospitals.
Data from reference 10; reproduced here for the sake of comparison.
Data from reference 12; reproduced here for the sake of comparison.
We have described in this report the design, synthesis and the antibacterial activity against Gram-positive bacteria of a series of 1,2,4-oxadiazole analogs with modifications on the C ring or on the fused C and D rings. This study defines the structural properties of this novel class of antibacterials, which emerged from an in silico search and screening.
Supplementary Material
Acknowledgments
This research was financially supported by grant AI090818 from the National Institutes of Health (to M.C. and S.M.).
Footnotes
Supplementary data Supplementary data (synthetic information and the NMR spectra of representative compounds) associated with this article can be found, in the online version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, McDougal LK, Chaitram J, Jensen B, Fridkin SK, Killgore G, Tenover FC. J Infect Dis. 2006;193:172. doi: 10.1086/499632. [DOI] [PubMed] [Google Scholar]
- 2.Ryu S, Song PI, Seo CH, Cheong H, Park Y. Int J Mol Sci. 2014;15:8753. doi: 10.3390/ijms15058753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watkins RR, David MZ, Salata RA. J Med Microbiol. 2012;61:1179. doi: 10.1099/jmm.0.043513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García-Álvarez L, Holden MTG, Lindsay H, Webb CR, Brown DFJ, Curran MD, Walpole E, Brooks K, Pickard DJ, Teale C, Parkhill J, Bentley SD, Edwards GF, Girvan EK, Kearns AM, Pichon B, Hill RLR, Larsen AR, Skov RL, Peacock SJ, Maskell DJ, Holmes MA. Lancet Infect Dis. 2011;11:595. doi: 10.1016/S1473-3099(11)70126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ippolito G, Leone S, Lauria FN, Nicastri E, Wenzel RP. Int J Infect Dis. 2010;14S4:S7. doi: 10.1016/j.ijid.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Gould IM, Reilly J, Bunyan D, Walker A. Clin Microbiol Infect. 2010;16:1721. doi: 10.1111/j.1469-0691.2010.03365.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhanel GG, DeCorby M, Adam H, Mulvey MR, McCracken M, Lagace-Wiens P, Nichol KA, Wierzbowski A, Baudry PJ, Tailor F, Karlowsky JA, Walkty A, Schweizer F, Johnson J, Hoban DJ Canadian Antimicrobial Resistance Alliance. Antimicrob Agents Chemother. 2010;54:4684. doi: 10.1128/AAC.00469-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Department of Health and Human Services Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. 2013 http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf.
- 9.Tran MCN, Naumovski S, Goldstein EJC. Am J Clin Dermatol. 2015 doi: 10.1007/S40257-015-0125-9. [DOI] [PubMed] [Google Scholar]
- 10.O’Daniel PI, Peng Z, Pi H, Testero SA, Ding D, Spink E, Leemans E, Boudreau MA, Yamaguchi T, Schroeder VA, Wolter WR, Llarrull LI, Song W, Lastochkin E, Kumarasiri M, Antunes NT, Espahbodi M, Lichtenwalter K, Suckow MA, Vakulenko S, Mobashery S, Chang M. J Am Chem Soc. 2014;136:3664. doi: 10.1021/ja500053x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spink E, Ding D, Peng Z, Boudreau MA, Leemans E, Lastochkin E, Song W, Lichtenwalter K, O’Daniel PI, Testero SA, Pi H, Schroeder VA, Wolter WR, Antunes NT, Suckow MA, Vakulenko S, Chang M, Mobashery S. J Med Chem. 2015;58:1380. doi: 10.1021/jm501661f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Lee M, Peng Z, Blazquez B, Lastochkin E, Kumarasiri M, Bouley R, Chang M, Mobashery S. J Med Chem. 2015 doi: 10.1021/jm501831g. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.