Abstract
Background
131I-metaiodobenzylguanidine (131I-MIBG) is a targeted radiopharmaceutical with significant activity in high-risk relapsed and chemotherapy-refractory neuroblastoma. Our primary aim was to determine if there are differences in response rates to 131I-MIBG between patients with relapsed and treatment-refractory neuroblastoma.
Methods
This was a retrospective cohort analysis of 218 patients with refractory or relapsed neuroblastoma treated with 131I-MIBG at UCSF between 1996 and 2014. Results were obtained by chart review and database abstraction. Baseline characteristics and response rates between relapsed patients and refractory patients were compared using Fisher exact and Wilcoxon rank sum tests, and differences in overall survival (OS) were compared using the log-rank test.
Results
The response rate (complete and partial response) to 131I-MIBG-based therapies for all patients was 27%. There was no difference in response rates between relapsed and refractory patients. However, after 131I-MIBG, 24% of relapsed patients had progressive disease compared to only 9% of refractory patients, and 39% of relapsed patients had stable disease compared to 59% of refractory patients (p = 0.02). Among all patients, the 24-month OS was 47.0% (95% CI 39.9%–53.9%). The 24-month OS for refractory patients was significantly higher at 65.3% (95% CI 51.8%–75.9%), compared to 38.7% (95% CI 30.4%–46.8%) for relapsed patients (p < 0.001).
Conclusions
Although there was no significant difference in overall response rates to 131I-MIBG between patients with relapsed vs. refractory neuroblastoma, patients with prior relapse had higher rates of progressive disease and had lower 2-year overall survival after 131I-MIBG compared to patients with refractory disease.
Keywords: neuroblastoma, 131I-MIBG, pediatric, radionuclide, relapse, refractory
1. Introduction
Neuroblastoma is the most common extra-cranial solid tumor in children. 1,2 At diagnosis, 50% of patients have high-risk disease, due to tumor MYCN amplification or metastatic disease in patients older than 18 months. 3 Approximately 20% of patients with high-risk neuroblastoma progress early or are refractory to standard induction therapy, and 50% of patients who achieve remission later relapse. 4,5 Five-year overall survival (OS) for patients with high-risk neuroblastoma, even when treated with myeloablative therapy, is only 40%. 4,6 Patients with relapsed and refractory neuroblastoma have even poorer outcomes, with a 5-year OS of less than 20%. 1,7
131I-metaiodobenzylguanidine (131I-MIBG), a norepinephrine analogue, is a promising therapy for patients with high-risk neuroblastoma. Neuroblastoma originates in neural crest cells of the peripheral nervous system, and 90% of neuroblastomas express human norepinephrine transporter (hNET). 8,9 When labeled with iodine-131, MIBG is a targeted radiopharmaceutical for high-risk neuroblastoma, with a response rate of 20–40% in early phase studies and a recent meta-analysis. 10–15 However, it is not well established if patients with relapsed disease respond differently to 131I-MIBG compared to patients with refractory disease.
Our primary aim was to investigate whether there are differences in overall response (OR) to 131I-MIBG alone or combined with other agents between relapsed and refractory neuroblastoma. Our secondary aims were to compare baseline clinical characteristics in these two cohorts as well as OS after therapy with 131I-MIBG.
2. Patients and Methods
2.1 Study Design
This was a retrospective cohort analysis of 218 patients with relapsed or refractory neuroblastoma treated with 131I-MIBG at UCSF Benioff Children’s Hospital on three local and six New Approaches to Neuroblastoma Therapy (NANT) clinical trials between August 30, 1996, and April 23, 2014 (Supplementary Table 1). Results were obtained by chart review and database abstraction.
131I-MIBG treatment protocols were approved by the UCSF institutional review board (IRB), and informed consent was obtained for all patients. The UCSF IRB approved this retrospective analysis.
2.2 Patient Eligibility and Treatment
Patients age >1 year with high-risk neuroblastoma treated on nine protocols (Supplementary Table 1) were eligible for this study. Of these patients, 154 have been included in primary trial publications 14–22, and 64 have not.
Patients were required to have MIBG-avid disease within 4–6 weeks before enrollment and to have failed to achieve a partial response (PR) to induction therapy, or have relapsed or progressive disease. Patients enrolled in NANT 1999-01, NANT 2001-02, NANT 2004-06, NANT 2007-03 and 131I-MIBG Vincristine/Irinotecan, were also eligible if they had PR but persistent active disease. Prior therapy must not have included 131I-MIBG but could include chemotherapy, surgical resection, radiation, and autologous stem cell transplant (ASCT) (except NANT 1999–01 and 2001–02, which excluded prior ASCT).
Patients received 6.3–20.9 mCi/kg (233–773 MBq/kg) of 131I-MIBG, except for patients treated on NANT 2000–01, a double infusion protocol where patients received up to 50.1 mCi/kg (1854 MBq/kg) over two treatments at a two-week interval. 131I-MIBG intended dose levels were stratified into three categories for this analysis: a low dose of •12 mCi/kg (•444 MBq/kg), an intermediate dose of >12 to <18 (<666 MBq/kg), and a high dose of •18 mCi/kg. In the analysis, 13 mCi/kg (481 MBq/kg) was used as the lower threshold and 17 mCi/kg (629 MBq/kg) was used as the higher threshold to account for dosing variation.
2.3 Primary Predictor Variable
Patients were grouped by their response to prior therapy. Relapsed patients had disease recurrence or progression at any time prior to study enrollment. This included patients who achieved complete response (CR) or PR to prior induction therapy and then progressed, and patients who progressed without achieving CR or PR. Refractory patients included those who had not achieved at least a PR to induction therapy (minimum of four cycles), and had never progressed, and patients with persistent disease who had achieved PR to induction therapy but maintained MIBG- and biopsy-proven active disease. In this analysis, patients with refractory or persistent disease were combined into the “refractory” category. Patients with relapsed or progressive disease comprised the “relapsed” category. These designations do not reflect disease status at the start of 131I-MIBG, such that patients in the relapsed category were not necessarily actively progressing at time of therapy.
2.4 Primary Outcome Variable
Overall response to 131I-MIBG-based therapy was assessed according to NANT response criteria. These criteria modified the existing International Neuroblastoma Response Criteria (INRC) 23 to include Curie score for MIBG lesions 24 and the Response Evaluation Criteria in Solid Tumors (RECIST) 25 for soft tissue disease. Categories for bone marrow response included CR or progressive disease, defined as tumor seen in previously negative bone marrow or ≥25% bone marrow involvement that had at least doubled. 20 For patients on the UCSF institutional studies, response was assessed by review of CT scans, bone marrow biopsies, and MIBG scans before and approximately 8 weeks following 131I-MIBG therapy. Scans and reports were reviewed by UCSF radiologists and oncologists. For patients on NANT studies, response was graded by central review of MIBG scans, CT/MRI scans and bone marrow biopsy slides.20 For patients treated with multiple courses of 131I-MIBG therapy, only the response to the first treatment course was included in this analysis, with the exception of patients treated on the NANT 2000–01 double infusion protocol. 19
2.5 Statistical Methods
Fisher exact and Wilcoxon rank sum tests were used to compare the proportions of relapsed and refractory patients with key clinical and biological features in neuroblastoma. The Fisher exact test was also used to compare proportions of response to 131I-MIBG therapy between these cohorts in three analyses: 1) a primary analysis that included all eligible patients from all nine 131I-MIBG protocols; 2) a sub-analysis that included only patients treated on 131I-MIBG monotherapy protocols; and 3) a sub-analysis that included only patients treated on 131I-MIBG protocols that did not include myeloablative chemotherapy. Multivariate logistic regression was used to control for potential confounding by other variables that might impact response to 131I-MIBG therapy aside from our primary predictor variable of interest. OS for patients from date of 131I-MIBG administration was estimated using Kaplan-Meier methods and compared between the relapsed and refractory cohorts by the log-rank test.
3. Results
3.1 Patient Characteristics Differ by Response to Therapy Prior to 131I-MIBG Treatment
Characteristics of the 218 patients are outlined in Table 1. Of these, 82% had stage 4 disease at diagnosis and the rest had metastatic progression. All patients in the relapse category had received at least two prior regimens before 131I-MIBG therapy. Seventy-two patients comprised the refractory cohort (including 65 with refractory disease and 7 with persistent disease), and 146 patients comprised the relapsed cohort. Of patients with known MYCN gene copy number (n = 169), 25% had tumor MYCN amplification, with a greater proportion of relapsed patients having MYCN amplification (p = 0.06). Median age at diagnosis was significantly greater for refractory patients than relapsed patients (p = 0.003). The median time from diagnosis to 131I-MIBG study entry was shorter for refractory compared to relapsed patients (p<0.001). Other expected differences included a smaller proportion of patients with prior ASCT (p<0.001) and a larger proportion of patients who underwent combination therapy in the refractory cohort (p < 0.001). Similarly, a greater proportion of the relapsed cohort (56%) received a high (•18 mCi/kg) dose of 131I-MIBG compared to the refractory cohort (38%) because the tolerable dose of 131I-MIBG combined with myeloablative therapy was lower than in non-myeloablative protocols. The other significant difference between cohorts was in sites of disease at the start of 131I-MIBG therapy, with the refractory cohort having fewer patients with soft tissue involvement and more patients with bone or bone marrow involvement.
Table 1.
Characteristics of 218 patients treated with 131I-MIBG.
| Characteristic | All (n = 218) |
Relapsed (n = 146) |
Refractorya (n = 72) |
p-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 140 (64%) | 94 (64%) | 46 (64%) | 1.00 |
| Female | 78 (36%) | 52 (36%) | 26 (36%) | |
| Median age at diagnosis, years (range) | 4.8 (0.3–50.8) | 4.5 (0.3–39.4) | 5.6 (1.9–50.8) | 0.003 |
| <18 months | 13 (6%) | 13 (9%) | 0 | 0.01 |
| ≥18 months | 205 (94%) | 133 (91%) | 72 (100%) | |
| Median age at first 131I-MIBG, years (range) | 7.3 (1.9–51.2) | 7.8 (1.9–40.8) | 6.2 (2.4–51.2) | 0.14 |
| <12 years | 172 (79%) | 115 (79%) | 57 (79%) | 1.00 |
| ≥12 years | 46 (21%) | 31 (21%) | 15 (21%) | |
| Median time from diagnosis to 131I-MIBG treatment, years (range) | 1.8 (0.4–18.9) | 2.7 (0.4–18.9) | 0.6 (0.4–1.9) | < 0.001 |
| Stage at diagnosis b | ||||
| 1, 2, 3c | 38 (18%) | 38 (26%) | 0 | < 0.001 |
| 4 | 179 (82%) | 107 (74%) | 72 (100%) | |
| Amplified MYCN d | ||||
| Yes | 42 (25%) | 34 (30%) | 8 (15%) | 0.06 |
| No | 127 (75%) | 81 (70%) | 46 (85%) | |
| Prior ASCTe | 123 (56%) | 111 (76%) | 12 (17%) | < 0.001 |
| No prior ASCT | 95 (44%) | 35 (24%) | 60 (83%) | |
| Sites of disease involvement at first 131I-MIBG | ||||
| STf + Bg/BMh | 91 (42%) | 62 (43%) | 29 (40%) | 0.001 |
| ST only | 25 (11%) | 24 (16%) | 1 (2%) | |
| B/BM only | 102 (47%) | 60 (41%) | 42 (58%) | |
| Any ST sites | 116 (53%) | 86 (59%) | 30 (42%) | 0.02 |
| No ST sites | 102 (47%) | 60 (41%) | 42 (58%) | |
| Any B sites | 188 (86%) | 118 (81%) | 70 (97%) | 0.001 |
| No B sites | 30 (14%) | 28 (19%) | 2 (3%) | |
| Any BM sitesi | 113 (52%) | 67 (46%) | 46 (66%) | 0.01 |
| No BM sites | 103 (48%) | 79 (54%) | 24 (34%) | |
| Type of 131I-MIBG protocol | ||||
| Monotherapy | 133 (61%) | 106 (73%) | 27 (38%) | < 0.001 |
| Combination | 85 (39%) | 40 (27%) | 45 (62%) | |
| 131I-MIBG without ASCT | 184 (84%) | 138 (95%) | 46 (64%) | < 0.001 |
| 131I-MIBG with ASCT | 34 (16%) | 8 (5%) | 26 (36%) | |
| 131I-MIBG Intended Dose | ||||
| ≤12 mCi/kgj | 74 (34%) | 43 (30%) | 31 (43%) | 0.03 |
| >12 – <18 mCi/kg | 35 (16%) | 21 (14%) | 14 (19%) | |
| ≥18 mCi/kg | 109 (50%) | 82 (56%) | 27 (38%) | |
Includes refractory (n = 65) and persistent (n = 7) cases
Stage at diagnosis was not known for 1 patient (1 relapsed)
Stage 1: n = 6; Stage 2: n = 9; Stage 3: n = 23
MYCN status was not known for 48 patients (31 relapsed, 18 refractory)
ASCT = autologous stem cell transplant.
ST = soft tissue
B = bone
BM = bone marrow
Presence of disease in bone marrow was unknown for 2 patients (2 refractory)
12 mCi/kg = 444 MBq/kg; 18 mCi/kg = 666 MBq/kg
3.2 Pattern of Response to 131I-MIBG Differs by Response to Therapy Prior to 131I-MIBG Treatment
In the analysis of all 131I-MIBG protocols, overall response rate (CR, PR) across the relapsed and refractory cohorts was 27% (Table 2). The overall response rate for patients with prior relapse (29%) was not significantly different from that of refractory patients (23%; p = 0.25). In order to account for the potential impact of different 131I-MIBG protocols, we analyzed the response rates for the relapsed vs. refractory patients who received 131I-MIBG monotherapy and for those who received 131I-MIBG without myeloablative chemotherapy. We found no significant difference in response rates between the two cohorts in any of the analyses. A sensitivity analysis for response to 131I-MIBG excluding patients with persistent disease (n = 7) yielded similar results (Supplementary Table 2, p = 0.40).
Table 2.
Response to 131I-MIBG by response to prior therapy before 131I-MIBG therapy.
| All 131I-MIBG Protocols, n = 214a | ||||
|---|---|---|---|---|
| All | Relapsed | Refractory | p-value | |
| Response | 57 (27%) | 41 (29%) | 16 (23%) | 0.25 |
| No response | 157 (73%) | 102 (71%) | 55 (77%) | |
| CR | 16 (8%) | 11 (8%) | 5 (7%) | 0.02 |
| PR | 41 (19%) | 30 (21%) | 11 (16%) | |
| SD | 99 (46%) | 56 (39%) | 42 (59%) | |
| MR | 17 (8%) | 11 (8%) | 7 (10%) | |
| PD | 41 (19%) | 35 (24%) | 6 (8%) | |
| 131I-MIBG Monotherapy Protocols, n = 131 | ||||
| All | Relapsed | Refractory | p-value | |
| Response | 40 (30%) | 32 (31 %) | 8 (30%) | 0.91 |
| No response | 91 (70%) | 72 (69%) | 19 (70%) | |
| CR | 7 (5%) | 6 (6%) | 1 (4%) | 0.03 |
| PR | 33 (25%) | 26 (25%) | 7 (26%) | |
| SD | 54 (42%) | 37 (35%) | 17 (63%) | |
| MR | 7 (5%) | 6 (6%) | 1 (4%) | |
| PD | 30 (23%) | 29 (28%) | 1 (4%) | |
| Non-ASCT Protocols (excludes NANT 99-01, NANT 01-02), n = 182 | ||||
| All | Relapsed | Refractory | p-value | |
| Response | 49 (27%) | 40 (29%) | 9 (20%) | 0.19 |
| No response | 133 (73%) | 96 (71%) | 37 (80%) | |
| CR | 12 (7%) | 11 (8%) | 1 (2%) | 0.11 |
| PR | 37 (20%) | 29 (21%) | 8 (18%) | |
| SD | 82 (45%) | 53 (39%) | 28 (61%) | |
| MR | 12 (7%) | 10 (8%) | 3 (6%) | |
| PD | 39 (21%) | 33 (24%) | 6 (13%) | |
Abbreviations: CR = complete response; PR = partial response; SD = stable disease; MR = mixed response; PD = progressive disease; ASCT = autologous stem cell transplant.
Response for 4 patients was not evaluable
When evaluating discrete categories of response, a significant difference was found in the pattern of non-response (Table 2). Specifically, 24% of patients in the relapse cohort had progressive disease at the time of response evaluation after 131I-MIBG compared to only 9% of refractory patients (p = 0.02). Only 39% of relapsed patients had stable disease following 131I-MIBG compared to 59% of refractory patients. A similar pattern was seen in the analyses of patients treated with 131I-MIBG monotherapy and non-myeloablative protocols.
Since patient and treatment characteristics differed between our cohorts, the lack of difference in overall response rate was potentially due to other confounding variables that influence response to 131I-MIBG. Univariate predictors of response to 131I-MIBG therapy (Supplementary Table 3) showed that the only significant predictor of response in this dataset was site of disease at the time of 131I-MIBG therapy. Patients with bone or bone marrow disease or any combination of disease sites had lower response rates compared to patients with isolated soft tissue disease. Other patient or treatment characteristics were not significant predictors of response in this study, including 131I-MIBG dose. We therefore performed multivariate logistic regression to control for differences in sites of disease involvement, but still confirmed that the relapsed or refractory category did not predict response to 131I-MIBG.
3.3 OS Differs by Response to Therapy Prior to 131I-MIBG Treatment
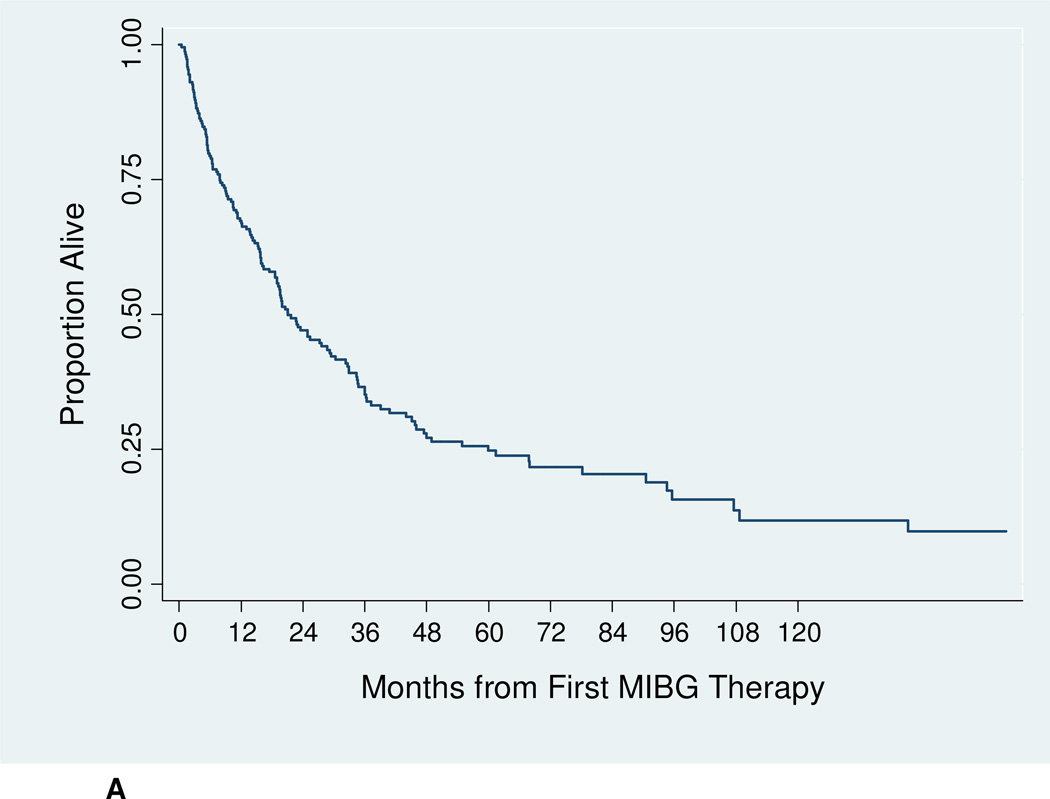
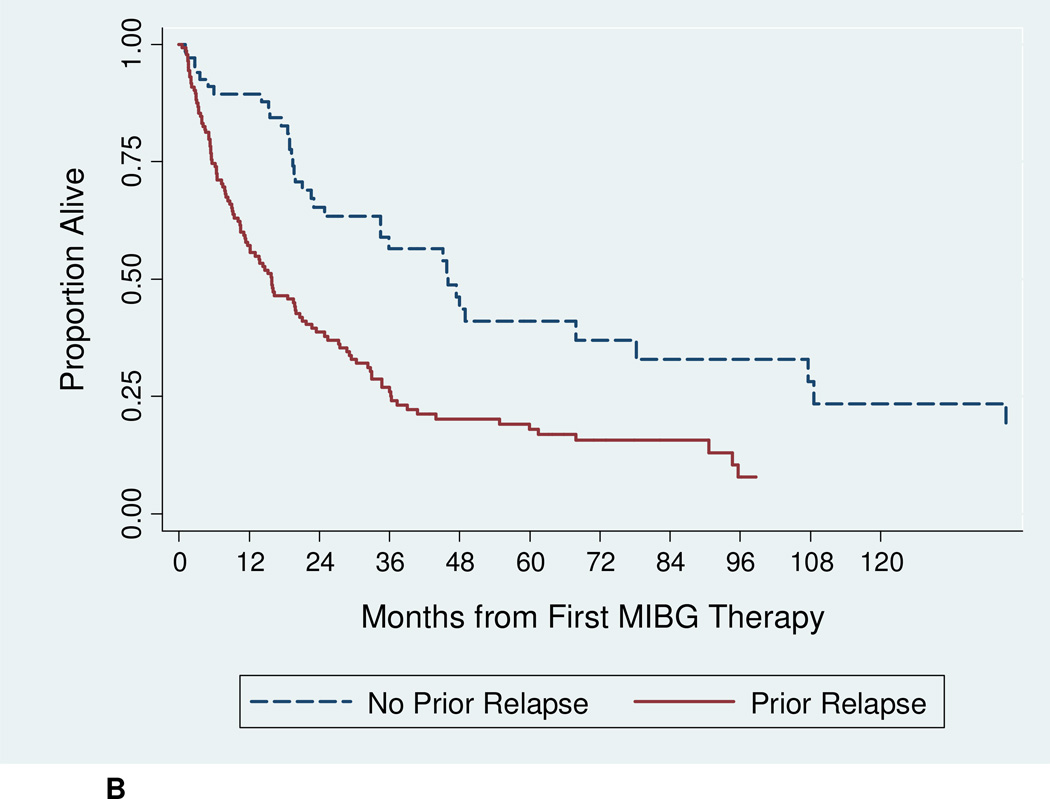
The median follow-up for surviving patients was 61.4 months. Among all patients, median OS was 21 months (Figure 1A). The 12-month OS was 67.3% (95% CI 60.4%–73.3%), and the 24-month OS was 47.0% (95% CI 39.9%–53.9%). The 24-month OS for refractory patients was significantly higher at 65.3% (95% CI 51.8%–75.9%), compared to 38.7% (95% CI 30.4%–46.8%) for relapsed patients (p < 0.001) (Figure 1B). An estimated 41% of refractory patients were alive 5 years after first 131I-MIBG therapy.
Figure 1.
A: Overall survival for all study patients treated with 131I-MIBG (n = 218).
B: Overall survival according to disease status at time of 131I-MIBG therapy [relapsed (n = 146) vs. refractory (n = 72); p < 0.001 by log-rank test].
4. Discussion
This study provides important new data on response rates and survival after 131I-MIBG therapy between patients with relapsed and refractory neuroblastoma. We found that there was not a significant difference in response rates between the two cohorts. This result is similar to a recent meta-analysis, which included some overlapping data from our patient population, and showed a response rate of 37% vs. 38% for refractory vs. relapsed disease, respectively. 15 However, in our study, we noted a significant difference in the pattern of non-response between the cohorts. Specifically, a significantly larger proportion of relapsed patients had a response of progressive disease after 131I-MIBG compared to patients with refractory disease, who were more likely to have stable disease after 131I-MIBG. These results suggest that patients who experienced recurrent or progressive disease prior to 131I-MIBG were at increased risk to continue to undergo disease progression, whereas patients who were refractory to initial therapy continued to be refractory after 131I-MIBG. This study also investigated characteristics that distinguish relapsed from refractory neuroblastoma. We found that a significantly greater proportion of relapsed patients had isolated soft tissue disease compared to refractory patients, suggesting that site of disease may play a role in disease responsiveness, or be a surrogate for unfavorable biology.
In this study, only sites of disease at time of 131I-MIBG therapy were shown to be a significant predictor of response rate. Specifically, having only soft tissue disease was shown to be significantly favorable for response to 131I-MIBG on univariate analysis compared to patients with a combination of soft tissue and bone/bone marrow disease, or only bone/bone marrow disease. This result supports previous observations that isolated soft tissue disease is a favorable prognostic factor for response to 131I-MIBG, but contrasts with previous reports that disease limited to only bone/bone marrow is a favorable factor for response. 14 Combined with the decreased rate of response among patients who had any bone or bone marrow disease, these results suggest that 131I-MIBG may be more effective in reducing soft tissue disease than other sites of disease. Previous studies have also identified older age as a favorable factor for response; 14,27 however in this study, age at 131I-MIBG treatment was not identified as a significant predictor of response. Although prior studies have suggested a correlation between response and 131I-MIBG dose 13,16,27–29, the lack of correlation in our study may reflect the fact that this study included patients treated on various protocols with a range of responses to prior therapies.
Refractory patients had significantly superior 2-year OS after 131I-MIBG compared to relapsed patients, despite the slightly higher rate of response to 131I-MIBG demonstrated in the relapsed cohort. This is likely due to the increased proportion of progressive disease after 131I-MIBG among relapsed patients, as well as the tendency of refractory disease to remain stable. The lack of data on therapies administered after 131I-MIBG may confound this analysis. In addition, for patients who received multiple courses of 131I-MIBG (n = 44), the dose, disease status, and response to only the first 131I-MIBG treatment are reported here. The course of disease in relapsed patients may be less favorable than that of refractory patients due to factors such as increased tumor MYCN amplification and older age at 131I-MIBG treatment. A study investigating prognostic factors among patients post-relapse found that time from diagnosis to first relapse has a significant effect on OS, with patients who relapsed 6 to 18 months after diagnosis having the lowest survival rates of this cohort. 30 Since time from diagnosis to relapse was not accounted for in this study, the lower OS among the relapsed cohort may be skewed by a subset of relapsed patients who had decreased time from diagnosis to relapse.
To assess the robustness of our primary analysis, we completed two additional analyses. First, we compared response rates between relapsed and refractory patients focusing on either 131I-MIBG monotherapy protocols or on 131I-MIBG protocols without myeloablative therapy. These sensitivity analyses yielded results that were similar to our primary analysis. Second, we performed multivariate logistic regression to control for differences in sites of disease between relapsed and refractory patients, which also yielded a similar conclusion to our primary analysis. Nevertheless, any differences in response to 131I-MIBG may have been confounded by variables that were not assessed in this study, such as differences between relapsed compared to progressive disease, or details of prior therapy regimens (significant in other studies 14 and not available for analysis in this study).
In conclusion, this study demonstrated no significant difference in overall response rates to 131I-MIBG between patients with relapsed and refractory neuroblastoma, although the pattern of nonresponse differed between these two cohorts. Additionally, OS among the refractory cohort was superior to that of the relapsed cohort. Our results can be used to guide stratification on future 131I-MIBG clinical trials.
Supplementary Material
Highlights.
We found no difference in response rate to 131I-MIBG between relapsed vs. refractory neuroblastoma patients.
Relapsed patients were more likely to develop progressive disease after 131I-MIBG.
Refractory patients were more likely to have stable disease after 131I-MIBG.
2-year OS was greater for refractory patients than for relapsed patients.
These differences must be taken into account in evaluating new therapies.
Acknowledgements
Alex Lemonade Stand Foundation Infrastructure grant, Dougherty Foundation, UCSF School of Medicine Resource Allocation Program for Trainees (RAPtr) Dean’s Summer Fellowship, PO1 CA81403, Mildred V. Strouss Chair, Conner Fund, and Frank A. Campini Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mueller SMMK. Neuroblastoma: Biology and staging. Curr Oncol Rep. 2009;11(6):431–438. doi: 10.1007/s11912-009-0059-6. [DOI] [PubMed] [Google Scholar]
- 2.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics. CA Cancer J Clin. 2014 Mar-Apr;64(2):83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 3.DuBois SG, Matthay KK. 131I-metaiodobenzylguanidine therapy in children with advanced neuroblastoma. Q J Nucl Med Mol Imaging. 2013 Mar;57(1):53–65. [PubMed] [Google Scholar]
- 4.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. children's cancer group. N Engl J Med. 1999;341(16):1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 5.Matthay KK, Atkinson JB, Stram DO, Selch M, Reynolds CP, Seeger RC. Patterns of relapse after autologous purged bone marrow transplantation for neuroblastoma: A childrens cancer group pilot study. J Clin Oncol. 1993;11(11):2226–2233. doi: 10.1200/JCO.1993.11.11.2226. [DOI] [PubMed] [Google Scholar]
- 6.Berthold F, Boos J, Burdach S, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: A randomised controlled trial. Lancet Oncol. 2005;6(9):649–658. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 7.London WB, Castel V, Monclair T, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: A report from the international neuroblastoma risk group project. J Clin Oncol. 2011;29(24):3286–3292. doi: 10.1200/JCO.2010.34.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treuner J, Feine U, Niethammer D, et al. Scintigraphic imaging of neuroblastoma with [131-I]iodobenzylguanidine. Lancet. 1984;1(8372):333–334. doi: 10.1016/s0140-6736(84)90375-1. [DOI] [PubMed] [Google Scholar]
- 9.Carlin S, Mairs RJ, McCluskey AG, et al. Development of a real-time polymerase chain reaction assay for prediction of the uptake of meta-[(131)I]iodobenzylguanidine by neuroblastoma tumors. Clin Cancer Res. 2003 Aug 15;9(9):3338–3344. [PubMed] [Google Scholar]
- 10.Wieland DM, Wu J, Brown LE, Mangner TJ, Swanson DP, Beierwaltes WH. Radiolabeled adrenergi neuron-blocking agents: Adrenomedullary imaging with [131I]iodobenzylguanidine. J Nucl Med. 1980;21(4):349–353. [PubMed] [Google Scholar]
- 11.Hutchinson RJ, Sisson JC, Miser JS, et al. Long-term results of [131I]metaiodobenzylguanidine treatment of refractory advanced neuroblastoma. J Nucl Biol Med. 1991;35(4):237–240. [PubMed] [Google Scholar]
- 12.Klingebiel T, Berthold F, Treuner J, et al. Metaiodobenzylguanidine (mIBG) in treatment of 47 patients with neuroblastoma: Results of the german neuroblastoma trial. Med Pediatr Oncol. 1991;19(2):84–88. doi: 10.1002/mpo.2950190203. [DOI] [PubMed] [Google Scholar]
- 13.Matthay KK, DeSantes K, Hasegawa B, et al. Phase I dose escalation of 131-Imetaiodobenzylguanidine with autologous bone marrow support in refractory neuroblastoma. J Clin Oncol. 1998;16(1):229–236. doi: 10.1200/JCO.1998.16.1.229. [DOI] [PubMed] [Google Scholar]
- 14.Matthay KK, Yanik G, Messina J, et al. Phase II study on the effect of disease sites age, prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol. 2007;25(9):1054–1060. doi: 10.1200/JCO.2006.09.3484. [DOI] [PubMed] [Google Scholar]
- 15.Wilson JS, Gains JE, Moroz V, Wheatley K, Gaze MN. A systematic review of 131I-meta iodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur J Cancer. 2014 Mar;50(4):801–815. doi: 10.1016/j.ejca.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 16.DuBois SG, Groshen S, Park JR, Haas-Kogan DA, Yang X, Geier E, Chen E, Giacomini Weiss B, Cohn SL, Granger MM, Yanik GA, Hawkins R, Courtier J, Jackson H, Goodarzian F, Shimada H, Czarnecki S, Tsao-Wei D, Villablanca JG, Marachelian A, Matthay KK. Phase 1 study of vorinostat as a radiation sensitizer with 131-I-MIBG for patients with relapsed or refractory neuroblastoma. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-3240. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DuBois SG, Allen S, Bent M, Hilton JF, Hollinger F, Hawkins R, Courtier J, Mosse YP, Matthay KK. Phase I/II study of 131-I-MIBG with vincristine and five days of irinotecan for advanced neuroblastoma. British Journal of Cancer, in press. 2015 doi: 10.1038/bjc.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthay KK, Tan JC, Villablanca JG, et al. Phase I dose escalation of iodine-131-metaiodobenzylguanidine with myeloablative chemotherapy and autologous stem-cell transplantation in refractory neuroblastoma: A new approaches to neuroblastoma therapy consortium study. J Clin Oncol. 2006;24(3):500–506. doi: 10.1200/JCO.2005.03.6400. [DOI] [PubMed] [Google Scholar]
- 19.Matthay KK, Quach A, Huberty J, et al. Iodine-131--metaiodobenzylguanidine double infusion with autologous stem-cell rescue for neuroblastoma: A new approaches to neuroblastoma therapy phase I study. J Clin Oncol. 2009;27(7):1020–1025. doi: 10.1200/JCO.2007.15.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DuBois SG, Chesler L, Groshen S, et al. Phase I study of vincristine, irinotecan, and (1)(3)(1)I-metaiodobenzylguanidine for patients with relapsed or refractory neuroblastoma: A new approaches to neuroblastoma therapy trial. Clin Cancer Res. 2012;18(9):2679–2686. doi: 10.1158/1078-0432.CCR-11-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthay KK, Weiss B, Villablanca JG, et al. Dose escalation study of no-carrier-added 131I-metaiodobenzylguanidine for relapsed or refractory neuroblastoma: New approaches to neuroblastoma therapy consortium trial. J Nucl Med. 2012;53(7):1155–1163. doi: 10.2967/jnumed.111.098624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanik GA, Villablanca JG, Maris JM, Weiss B, Groshen S, Marachelian A, Park JR, Hawkins R, Shulkin BL, Jackson H, Goodarzian F, Shimada H, Courtier J, Hutchinson R, Haas-Kogan D, Hasenauer CB, Czarnecki S, Tsao-Wei D, Katzenstein HM, Matthay KK. 131I-metaiodobenzylguanidine with intensive chemotherapy and autologous stem cell transplant for high risk neuroblastoma. A new approaches to neuroblastoma therapy (NANT) phase II study. Biology of Blood and Bone Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2014.12.008. in press. [DOI] [PubMed] [Google Scholar]
- 23.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11(8):1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 24.Matthay KK, Shulkin B, Ladenstein R, et al. Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: A report for the international neuroblastoma risk group (INRG) task force. Br J Cancer. 2010;102(9):1319–1326. doi: 10.1038/sj.bjc.6605621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. european organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 26.Kushner BH, Modak S, Kramer K, et al. Striking dichotomy in outcome of MYCN-amplified neuroblastoma in the contemporary era. Cancer. 2014;120(13):2050–2059. doi: 10.1002/cncr.28687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polishchuk AL, Dubois SG, Haas-Kogan D, Hawkins R, Matthay KK. Response, survival, and toxicity after iodine-131-metaiodobenzylguanidine therapy for neuroblastoma in preadolescents, adolescents, and adults. Cancer. 2011;117(18):4286–4293. doi: 10.1002/cncr.25987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson K, McGlynn B, Saggio J, et al. Safety and efficacy of tandem 131I-metaiodobenzylguanidine infusions in relapsed/refractory neuroblastoma. Pediatr Blood Cancer. 2011;57(7):1124–1129. doi: 10.1002/pbc.23062. [DOI] [PubMed] [Google Scholar]
- 29.Howard JP, Maris JM, Kersun LS, et al. Tumor response and toxicity with multiple infusions of high dose 131I-MIBG for refractory neuroblastoma. Pediatr Blood Cancer. 2005;44(3):232–239. doi: 10.1002/pbc.20240. [DOI] [PubMed] [Google Scholar]
- 30.London WB, Castel V, Monclair T, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: A report from the international neuroblastoma risk group project. J Clin Oncol. 2011;29(24):3286–3292. doi: 10.1200/JCO.2010.34.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.