Abstract
Objective
To assess the impact of illicit drug use on health-related quality of life (health utility) among opioid-dependent, HIV-infected patients.
Design
Secondary analyses of data from the Buprenorphine-HIV Evaluation and Support (BHIVES) cohort of HIV-infected patients with opioid dependence in 9 U.S. HIV clinics between 2004 and 2009. Health status (Short Form-12 (SF-12)), combination antiretroviral treatment (ART) status, CD4 cell count, HCV antibody status, current drug use, and demographics were assessed at an initial visit and quarterly follow-up visits for up to one year. Short Form-6D health utility scores were derived from the SF-12. Multivariate mixed effects regression models were used to assess the impact of illicit drug use on health utility controlling for demographic, clinical and social characteristics.
Results
Health utility was assessed among 307 participants, 67% male, with median age 46 at 1089 quarterly assessments. In multivariate analyses, illicit opioid use, non-opioid illicit drug use, not being on ART and being on ART with poor adherence were associated with lower health utility. The observed decrement in health utility associated with illicit opioid use was larger for those on ART with good adherence (beta = −0.067; p<0.01) or poor adherence (−0.049; p<0.01) than for those not on ART.
Conclusions
Illicit opioid and non-opioid drug use are negatively associated with health utility in patients with HIV, however the relative effect of illicit opioid use is smaller than that of not being on ART. Postponing ART until initiation of opioid substitution therapy or abstinence may have limited benefits from the perspective of maximizing health utility.
Keywords: Health Utility, HIV, Illicit drug use, Opioid, Opiate
Introduction
Drug use is associated with high levels of medical co-morbidities, including HIV infection and hepatitis C virus (HCV) infection. Approximately 8% of all annual incident cases and 16% of all prevalent cases of HIV/AIDS infections in the United States were acquired through injection drug use. [1, 2] Illicit drug use is frequently reported among persons in HIV treatment. [3, 4] Despite the substantial health benefits associated with initiation of combined antiretroviral therapy (ART), including improved health-related quality of life (HRQoL) for individual patients and benefits of reduced risk of HIV transmission to others [5, 6], some clinicians remain reluctant to initiate ART in active illicit drug users. [7–10] Clinicians cite real concerns about initiating ART including non-adherence, poor follow-up, and concern about poorer outcomes, though the evidence about outcomes in this population is conflicting. [11–18] Recent studies have examined integrated opiate substitution therapy (OST) in HIV treatment settings. One such study, the Buprenorphine-HIV Evaluation and Support (BHIVES) collaborative, found decreased illicit opioid use [19] and improvements in ART initiation and CD4 counts over time for patients receiving OST in integrated care settings. [20] BHIVES investigators reported an improvement in mental and physical HRQoL with buprenorphine treatment in this setting, but did not examine health utility. [21] Previous studies have shown that HIV/AIDS and opioid dependence are each associated with negative impacts on health utility. [22–25] We are only aware of one previous study in the US that examined the combined impact of treatment for HIV and opioid dependence on health utility, and this study was limited to HIV-infected women. [26] This study found that HIV disease had a greater negative impact on health utility than illicit drug use for women whose HIV disease was not well controlled. If confirmed in other populations, such findings have important implications for setting clinical priorities for treatment and for quantifying the value to patients of treatment programs targeting illicit substance-using HIV-infected individuals.
Health utilities are commonly used in the assessment of treatment of chronic diseases and are important for health economic evaluation and subsequent guidance for treatment and resource allocation decisions. [27] HRQoL assessments, such as the Short Form-12 (SF12) questionnaire [28–30], measure self-reported quality of life for various domains, such as physical function, pain and social function. HRQoL measures do not, however, assign value to the states described by these domains. In contrast, health utilities incorporate individuals’ preferences for particular states of health, meaning how they feel about living in such a health state as opposed to describing the experience of being in that state. Utilities, therefore, capture preferences for states of health, and can be used for comparisons across distinct health conditions. They are also used to calculate Quality Adjusted Life Years, the outcome measure used in cost-effectiveness analysis, and hence are necessary for economic evaluation. [31–33]
In this study we used data from the BHIVES collaborative, a prospective multisite demonstration project that evaluated the integration of buprenorphine treatment into HIV primary care sites to treat opioid-dependent HIV patients, to assess the impact of illicit drug use on health utility among opioid-dependent HIV patients.
Methods
Patient Population
We evaluated data collected by the BHIVES collaborative from 427 patients with opioid dependence who were recruited at 9 HIV treatment centers located in 9 cities (Baltimore, New York City, Chicago, Miami, New Haven, Providence, Portland, San Francisco, and Tucson) between 2004 and 2009 and assessed on a quarterly basis for up to one year. [34, 35] Patients were eligible if they were HIV infected, 18 years of age and met DSM-IV criteria for opioid dependence. [36] Participants with alcohol dependence or benzodiazepine dependence were excluded at enrollment, as these are contraindications for prescribing buprenorphine [37], along with participants with severe medical or psychiatric conditions. Patients’ demographic characteristics were collected at enrollment, including age, race/ethnicity, gender and marital status. Additional measures extracted from medical charts at enrollment and quarterly thereafter included HIV viral load, CD4 cell count, whether the patient had an incident opportunistic infection in the prior 3 months, ART status, buprenorphine treatment status, and HCV antibody status (at enrollment only). Self-reported measures obtained at enrollment and quarterly follow-up visits included substance use (opioids, alcohol, marijuana, cocaine, amphetamines, barbiturates, sedatives/hypnotics, and inhalants) in the prior 30 days, ART adherence, criminal justice involvement, employment status, housing status, and SF-12 health status questionnaire responses. [34, 35]
Utility Measure of Health-Related Quality of Life
We utilized the SF-6D, a generic community preference-weighted health utility measure, which can be derived from the SF-36 or SF-12 questionnaires. [28, 29] The SF-12 covers 8 health domains: 1) physical functioning; 2) role limitations due to physical health; 3) body pain; 4) social functioning; 5) general mental health; 6) role limitations due to emotional health; 7) vitality; and 8) general health perception. [38, 39] The SF-6D condenses these eight domains into 6 dimensions: physical functioning, role functioning, social functioning, pain, mental health and vitality. A sample of the 7500 possible health states describing combinations of levels for each of the 6 dimensions was directly assessed using a standard gamble health utility assessment methodology by a representative sample of the UK population, providing empirically-derived societal preference weights on a scale where 0=death and 1=perfect health. The standard gamble methodology directly assesses health preferences by providing an interviewee with a detailed description of a health state, in this case some combination of levels of the 6 health dimensions. The interviewee is asked to imagine being in the health state, and whether while in this health state he or she would take a pill that would result in either perfect health or death with a series of specified chances of each outcome. In effect the method elicits what risk of death the interviewee is willing to accept to avoid being in the health state, thereby assessing the value the interviewee places on that health state relative to perfect health. Health utility weights for the remaining states not directly assessed were derived using econometric methods. [28, 29]
Other Measures
Past 30-day use of a range of illicit substances was assessed [40], and patients reporting at least one day of use were classified as current drug users. Alcohol use was defined as a report of drinking to intoxication. Non-opioid illicit substances (cocaine, barbiturates, sedatives/hypnotics, amphetamines, hallucinogens, and inhalants) were examined individually and as a combined single variable (use of one or more of the non-opioid illicit substances), while marijuana, opioids and alcohol were considered separately. Marijuana was considered separately from other non-opioid illicit substances because it was more frequently used and its use may have been more socially acceptable to some participants.
Housing status was categorized as either stably housed (owned or renting) or unstably housed (homeless, living in a shelter, temporarily housed, or staying with friend/family). Patients were considered employed if they reported working either full-time or part-time in the prior 30 days. Patients were considered to have criminal justice involvement if they reported that they were facing criminal charges or were currently on parole.
ART status was extracted from the medical chart and was defined as being prescribed an ART regimen in the previous month. Patients were categorized as having good or poor adherence using the CASE adherence index, which measures adherence based on responses to three questions: 1) How often do you have difficulty taking your HIV medications?; 2) On average, how many days per week would you say that you missed at least one dose of your HIV medication?; and 3) When was the last time you missed at least one dose of HIV medications? [41] Each of the three questions has multiple possible responses (Q1–4 responses, Q2 and Q3–6 responses), with more points given to responses denoting fewer missed dosses. Patients reporting 0 missed doses receive a score of 16, while patients reporting frequent missed doses (Q1: all the time, Q2: daily, Q3: within the last week) receive the lowest score of 3. A score of ≥10 points (on a 16-point scale) was categorized as good adherence.
Analysis
We compared mean health utilities by age, race/ethnicity, gender, and marital status at baseline visit using t-test and ANOVA testing. We also compared mean health utilities by current HIV viral load, CD4 cell count, ART status, opportunistic infection, housing status, employment status, and criminal justice involvement across all visits. We also evaluated health utilities for current users versus non-users of each substance and for users versus non-users of any non-opioid substance other than alcohol and marijuana. Differences in utility for these comparisons were done using mixed effect regression to control for intra-individual correlation for repeated measures.
We then conducted multivariate analyses we also used mixed effects regression methods to control for intra-individual correlation. We conducted backward selection model building and retained variables with p-values <0.1, except CD4 cell count, which was included in the model a priori as an indicator of eligibility for ART. We tested for interactions between: (a) illicit opioid use and CD4 cell count, (b) illicit opioid use and ART treatment status, (c) illicit opioid use and buprenorphine treatment, (d) non-opioid drug use and CD4 cell count, and (e) non-opioid drug use and ART status. All analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC).
Results
Patient Characteristics
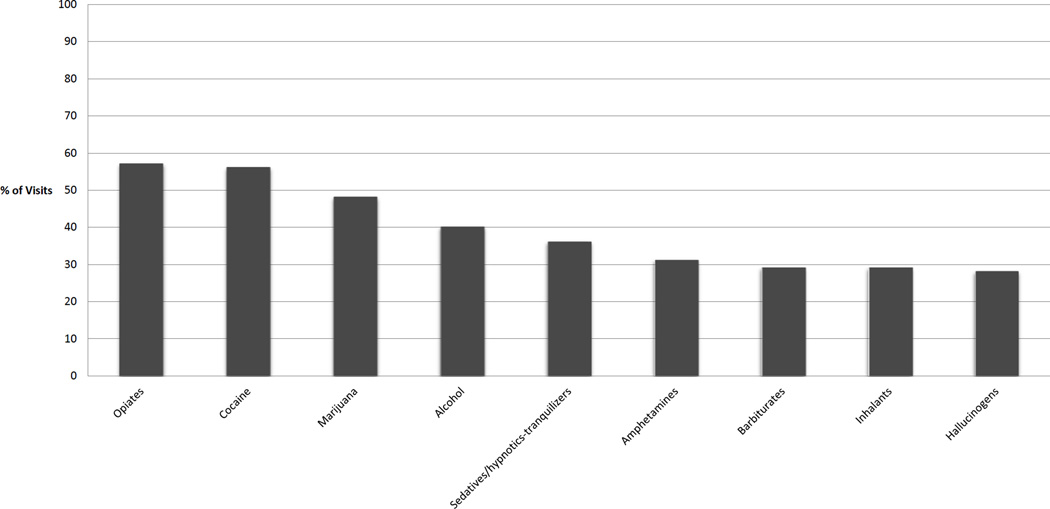
We analyzed data from 307 baseline visits and data from 782 (Median=2, range 1–4) follow up visits after excluding 120 patients who were missing variables of interest. At the baseline visit, approximately 80% of the patients were over 40 years old, two-thirds were male, and approximately half were African American. (Table 1) Approximately 60% of the patients were HCV antibody positive. Across all visits, most were unemployed (76%) and unstably housed (55%). Criminal justice involvement (26%), not being on ART (33%), and being on ART with poor adherence (31%) were also frequently observed. At approximately 40% of visits patients had CD4 cell count ≤350 cells/ul. (Table 2) Patients frequently reported substance use in the past 30 days, including reporting illicit opioid use at 57% of visits, marijuana at 48% of visits and alcohol use at 40% of visits (Figure 1). Patients reported use of at least one non-opioid drug in the past 30 days at 61% of visits. Baseline characteristics were comparable between the 120 excluded and the 307 included patients in our analyses (age, race/ethnicity, marital status, housing status, baseline drug use, baseline SF-6D scores), except gender (excluded more likely to be male, 77% vs 67%, P<0.01).
Table 1.
Baseline Characteristics of BHIVES Cohort
| Number of Participants N (%) |
SF-6D Score Mean (SD) |
P- Value |
|
|---|---|---|---|
| Overall | 307 | 0.62 (0.13) | |
| Age | |||
| <30 | 10 (3) | 0.58 (0.13) | 0.51 |
| 30–39 | 49 (16) | 0.63 (0.13) | |
| 40–49 | 144 (47) | 0.62 (0.13) | |
| 50+ | 104 (34) | 0.63 (0.13) | |
| Gender | |||
| Male | 206 (67) | 0.63 (0.14) | 0.16 |
| Female | 101 (33) | 0.61 (0.12) | |
| Race/Ethnicity | |||
| Caucasian | 64 (21) | 0.60 (0.12) | <0.01 |
| African American | 163 (54) | 0.65 (0.13) | |
| Hispanic | 56 (19) | 0.57 (0.11) | |
| Asian/Pacific Islander/Native American | 17 (6) | 0.65 (0.14) | |
| Marital Status | |||
| Married | 45 (15) | 0.66 (0.17) | 0.1 |
| Single/Divorced/Widowed | 262 (85) | 0.62 (0.12 |
Table 2.
Bivariate Analyses of SF6D Scores Across All Visits
| Number Of Visits N (%) |
SF-6D Score Mean (SE) |
P- Value |
|
|---|---|---|---|
| Overall | 1089 | 0.65 | |
| Employment | |||
| Working part-time or full-time | 255 (24) | 0.70 (0.005) | <0.01 |
| Not working | 830 (76) | 0.63 (0.009) | |
| Housing | |||
| Stable Housing | 489 (45) | 0.66 (0.007) | < 0.01 |
| Unstable Housing/Homeless | 600 (55) | 0.64 (0.006) | |
| Involvement with Criminal Justice | |||
| Face Charges or on Parole | 278 (26) | 0.61 (0.009) | |
| No Pending charges/parole | 811 (74) | 0.66 (0.005) | <0.01 |
| CD4 Count | |||
| ≤350 cells/µL | 425 (39) | 0.66 (0.007) | 0.38 |
| >350 cells/µL | 395 (36) | 0.65 (0.007) | |
| Missing | 273 (25) | ||
| ART Therapy | |||
| Not on Treatment | 357 (33) | 0.63 (0.008) | <0.01 |
| On Treatment with Poor Adherence | 338 (31) | 0.63 (0.008) | |
| On Treatment with Good Adherence | 386 (36) | 0.69 (0.007) | |
| History of Opportunistic Infection in last 3 months | |||
| Yes | 25 (2) | 0.63 (0.030) | 0.30 |
| No | 753 (69) | 0.66 (0.005) | |
| Missing | 311 | ||
| HCV Status | |||
| Antibody Positive | 655 (60) | 0.65 (0.006) | 0.73 |
| Negative | 434 (40) | 0.65 (0.007) | |
| Alcohol Use (Drinking to Intoxication) | |||
| Yes | 435 (40) | 0.63 (0.007) | <0.01 |
| No | 651 (60) | 0.66 (0.006) | |
| Marijuana Use | |||
| Yes | 517 (48) | 0.64 (0.006) | <0.01 |
| No | 570 (52) | 0.66 (0.006) | |
| Other Non-Opioid Drug use‡ | |||
| Yes | 664 (61) | 0.63 (0.006) | <0.01 |
| No | 425 (39) | 0.69 (0.007) | |
| Prescription Opioid or Heroin Use | |||
| Yes | 624 (57) | 0.62 (0.006) | <0.01 |
| No | 465 (43) | 0.69 (0.007) | |
| Opioid Dependence Treatment | |||
| Not on Buprenorphine* | 783 (72) | 0.64 (0.005) | 0.04 |
| Buprenorphine | 306 (28) | 0.67 (0.008) |
634 visits with patients not on opioid substitution treatment, 149 visits with patients on methadone
Includes cocaine (includes crack cocaine), barbiturates, sedative/hypnotics, amphetamines (includes methamphetamines), hallucinogens, and inhalants
Figure 1.
Percent of patient visits (N=1089) with report of drug use in prior 30 days
Health Utility
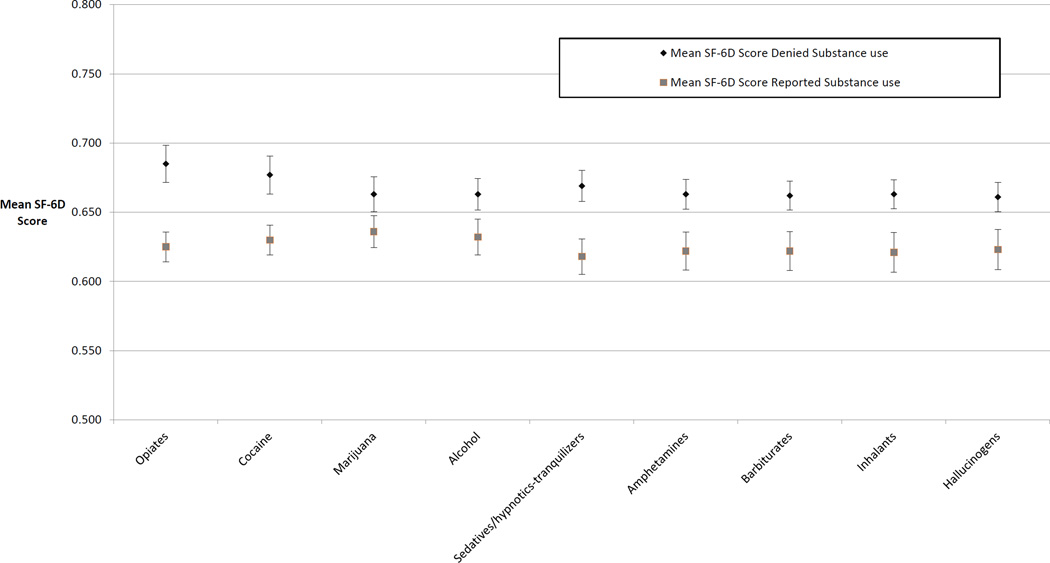
The unadjusted mean utility score across all visits was 0.65. Lower (worse) utility scores were associated with self-reported use of any substance (Figure 2). In unadjusted analyses, a higher (better) utility score was associated with being on ART with good adherence compared to not being on ART or being on ART with poor adherence (0.69 vs 0.63; p<0.01). Being on treatment with buprenorphine was associated with higher utility (0.67 vs 0.64; p=0.04) whereas HCV status, CD4 cell count and opportunistic infection in the past 3 months were not associated with significant differences in utility scores. Non-health factors associated with a lower utility score included being unemployed (0.63 vs 0.70; p<0.01), being unstably housed (0.64 vs 0.66; p<0.01), and having criminal justice involvement (0.61 vs 0.66; P<0.01). (Table 2)
Figure 2.
Mean SF-6D score by drug use in prior 30 days†
The adjusted association of marijuana use, alcohol use, and HCV status with health utility did not reach statistical significance at p-value<0.1 and were not included in the multivariate model. The current CD4 cell count (≤350 cells/ul versus >350 cells/ul) variable had a p-value>0.1, but was retained in the model because this threshold indicates patients who were or were not eligible for ART therapy according to national guidelines at time of the study. All other associations observed in the bivariate analyses were significant and were retained in our final multivariate model (Appendix).
There was a significant interaction between illicit opioid use and ART treatment status such that the negative association between illicit opioid use and health utility was only seen among those on ART (Table 3). Moreover, this negative association, was greatest among those with good ART adherence (Table 3). Specifically, opioid use was not associated with any change in health utility among patients not on ART and not using illicit opioids (ref) compared to those not on ART and using illicit opioids (−0.000). However, those on ART with poor adherence and using illicit opioids [0.022+(−0.000)+(−0.049)=−0.027] have significantly lower health utility compared to those on ART with poor adherence not using opioids (0.022). Additionally, those on ART with good adherence and using illicit opioids [+0.082+(−0.000)+(−0.067)=0.015] have significantly lower health utility compared to those on ART with good adherence not using opioids (0.082). The associations of all other interactions were not statistically significant.
Table 3.
Multivariate Linear Model of SF6D Scores for BHIVES Cohort
| Model variable | Estimate | p-value |
|---|---|---|
| Intercept | 0.738 | |
| Unstably housed | ref | |
| Stably housed | 0.023 | 0.02 |
| Not working | ref | |
| Working | 0.067 | <0.01 |
| No criminal charges and not on parole | ref | |
| Facing criminal charges or on parole | −0.028 | 0.01 |
| CD4 Count>350 cells/µL | ref | |
| CD4 Count ≤350 cells/µL | −0.011 | 0.23 |
| Not on ART | ref | |
| On ART with poor adherence | 0.022 | 0.26 |
| On ART with good adherence | 0.082 | <0.01 |
| Not using other drugs | ref | |
| Using other drugs‡ | −0.028 | 0.02 |
| Not using illicit opioids | Ref | |
| Using illicit opioids | 0.000 | 0.98 |
| Not in Buprenorphine treatment | ref | |
| Buprenorphine treatment | 0.026 | 0.03 |
| On ART with poor adherence *Using illicit opioids | −0.049 | 0.04 |
| On ART with good adherence*Using illicit opioids | −0.067 | <0.01 |
Includes cocaine (includes crack cocaine), barbiturates, sedative/hypnotics, amphetamines (includes methamphetamines), hallucinogens, and inhalants
Model Controlling for age, ethnicity, gender, marital status, site
Discussion
In this cohort of HIV-infected opioid dependent individuals, not being on ART, being poorly adherent to ART, current illicit opioid and non-opioid drug use were all independently associated with lower health utility. Conversely, current buprenorphine treatment was independently associated with higher health utility. These differences were all significant when compared to reported estimated mean minimally important differences (MID) in SF-6D scores of 0.03–0.04. [42, 43] However, the negative association of illicit opioid with health utility was not significant for patients not on ART, whereas it was significant for patients on ART with poor or good adherence.
Our previous research examined the combined effect of these co-occurring chronic diseases on health utilities in women with HIV and also found that drug use, specifically heroin and cocaine use, had a differential impact on health utility. In that study we found that drug use had less impact on health utility in patients who had AIDS compared to those with well controlled HIV infection. [26] In the current study, which included both genders but was limited to patients in HIV care, illicit opioid use also had less impact on patients who were not on ART, many of whom had low CD4 counts. Our previous study taken together with these findings have important clinical and policy implications for treating this population. HIV-infected patients with illicit substance use are often marginalized and have limited access to care. [7–10] These findings suggest that treating HIV, even in the context of continued illicit substance use, may improve the preference based outcome of health utility. Other factors may play a role in the decision to start ART, such as a patient’s willingness to start medication, ability to obtain medication (e.g. insurance coverage), and motivation to take the medication, but treatment delay awaiting OST or stopping drug use may also adversely affect health utility. Patients not on ART in this study, but meeting clinical criteria to start treatment, reported high levels of use of illicit opioids and non-opioid substance use. If treatment of these patients was being delayed solely because of their substance use, as suggested by other studies of HIV providers’ willingness to prescribe ART to patient with illicit drug use [10], delaying ART may represent a missed opportunity to improve their health utility.
Social factors that are commonly associated with substance use also had a significant and independent negative effect on health utility, including unstable housing, unemployment, and criminal justice involvement. The mean health utility for all respondents in our sample was lower than that of a representative sample of the US population of similar age and gender (which range from 0.76–0.80). [44] The mean utility was also lower than utilities previously reported for patients with HIV in the US (0.72–0.87). [22, 23, 45] The mean utility is comparable, however, to previously reported utilities for patients with chronic heroin use (0.67) or HIV and heroin/cocaine use (0.68). [26, 46]
Our study has both sample and data limitations. The sample population was limited to those already in HIV care and was predominately African American and male, which limits its generalizability. Those excluded from our analysis do to missing variable were more likely to be male than those included, though the populations did not differ with regard to any of the other demographics or health states. Data limitations include that patients reported use of multiple substances on any single visit, which limited our ability to assess the impact of each drug individually. Polysubstance use, however, reflects the reality of clinical practice in the U.S. We included other drug use as a combined variable in our model to account for the potential impact of these other illicit substances separately from the impact of illicit opioid use. Substance use was self-reported and while it is a commonly-used measure in other studies and generally found to have high reliability and validity, it may have been underreported in our study. [47–49] However, if underreported, it would likely have resulted in an underestimation of associated decrease in health utility. We were also not able to fully assess the impact of HCV co-infection because we did not have HCV viral load data available to identify which patients had chronic HCV disease.
In conclusion, starting ART, even in the context of continued drug use, may have significant benefits from the perspective of maximizing health utility. Ultimately, it is important to address both comorbid conditions simultaneously as both ART and OST are associated with improved health utilities.
Supplementary Material
AKNOWLEDGEMENTS
The authors thank Linda Weiss, PhD and Tongtan Chantarat, MPH at the New York Academy of Medicine for their assistance with the BHIVES data. We would also like to thank P. Todd Korthuis, MD, MPH for his comments on a previous version of the manuscript. Financial support for this study was provided in part by a grant from the National Institute on Drug Abuse R01 DA033424. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Footnotes
Portions of this manuscript were previously presented at the Society for Medical Decision Making Annual Meeting, October 19–23, 2013, Baltimore, MD.
Conflicts of Interest: None to declare
References
- 1.CDC, editor. CDC. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas—2011. HIV Surveillance Supplemental Report. 2013 [Google Scholar]
- 2.CDC. Estimated HIV incidence in the United States, 2007–2010. 2013 [Google Scholar]
- 3.Mimiaga MJ, et al. Substance use among HIV-infected patients engaged in primary care in the United States: findings from the Centers for AIDS Research Network of Integrated Clinical Systems cohort. Am J Public Health. 2013;103(8):1457–1467. doi: 10.2105/AJPH.2012.301162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohler NL, et al. Type and pattern of illicit drug use and access to health care services for HIV-infected people. AIDS Patient Care STDS. 2007;21(Suppl 1):S68–S76. doi: 10.1089/apc.2007.9985. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MS, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathers BM, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2010;375(9719):1014–1028. doi: 10.1016/S0140-6736(10)60232-2. [DOI] [PubMed] [Google Scholar]
- 7.Hanna DB, et al. Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001–2009. Clin Infect Dis. 2013;56(8):1174–1182. doi: 10.1093/cid/cit003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milloy MJ, Montaner J, Wood E. Barriers to HIV treatment among people who use injection drugs: implications for 'treatment as prevention'. Curr Opin HIV AIDS. 2012;7(4):332–338. doi: 10.1097/COH.0b013e328354bcc8. [DOI] [PubMed] [Google Scholar]
- 9.Volkow ND, Montaner J. The urgency of providing comprehensive and integrated treatment for substance abusers with HIV. Health Aff (Millwood) 2011;30(8):1411–1419. doi: 10.1377/hlthaff.2011.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westergaard RP, et al. Provider and clinic-level correlates of deferring antiretroviral therapy for people who inject drugs: a survey of North American HIV providers. J Int AIDS Soc. 2012;15(1):10. doi: 10.1186/1758-2652-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruce RD, Altice FL. Clinical care of the HIV-infected drug user. Infect Dis Clin North Am. 2007;21(1):149–179. doi: 10.1016/j.idc.2007.03.009. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients: epidemiology and impact on antiretroviral therapy. Drugs. 2006;66(6):769–789. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- 13.Krusi A, et al. Ongoing drug use and outcomes from highly active antiretroviral therapy among injection drug users in a Canadian setting. Antivir Ther. 2010;15(5):789–796. doi: 10.3851/IMP1614. [DOI] [PubMed] [Google Scholar]
- 14.Lert F, Kazatchkine MD. Antiretroviral HIV treatment and care for injecting drug users: an evidence-based overview. Int J Drug Policy. 2007;18(4):255–261. doi: 10.1016/j.drugpo.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malta M, et al. Adherence to antiretroviral therapy among HIV-infected drug users: a meta-analysis. AIDS Behav. 2010;14(4):731–747. doi: 10.1007/s10461-008-9489-7. [DOI] [PubMed] [Google Scholar]
- 16.Montaner JS, et al. Expanded highly active antiretroviral therapy coverage among HIV-positive drug users to improve individual and public health outcomes. J Acquir Immune Defic Syndr. 2010;55(Suppl 1):S5–S9. doi: 10.1097/QAI.0b013e3181f9c1f0. [DOI] [PubMed] [Google Scholar]
- 17.Wood E, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300(5):550–554. doi: 10.1001/jama.300.5.550. [DOI] [PubMed] [Google Scholar]
- 18.Wood E, Kerr T, Montaner JS. HIV treatment, injection drug use, illicit drug policies. Lancet. 2007;370(9581):8–10. doi: 10.1016/S0140-6736(07)61025-3. [DOI] [PubMed] [Google Scholar]
- 19.Fiellin DA, et al. Drug treatment outcomes among HIV-infected opioid-dependent patients receiving buprenorphine/naloxone. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S33–S38. doi: 10.1097/QAI.0b013e3182097537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altice FL, et al. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S22–S32. doi: 10.1097/QAI.0b013e318209751e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korthuis PT, et al. Improved quality of life for opioid-dependent patients receiving buprenorphine treatment in HIV clinics. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S39–S45. doi: 10.1097/QAI.0b013e318209754c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schackman BR, et al. Comparison of health state utilities using community and patient preference weights derived from a survey of patients with HIV/AIDS. Med Decis Making. 2002;22(1):27–38. doi: 10.1177/0272989X0202200103. [DOI] [PubMed] [Google Scholar]
- 23.Tengs TO, Lin TH. A meta-analysis of utility estimates for HIV/AIDS. Med Decis Making. 2002;22(6):475–481. doi: 10.1177/0272989X02238300. [DOI] [PubMed] [Google Scholar]
- 24.Tran B, et al. Longitudinal and cross sectional assessments of health utility in adults with HIV/AIDS: a systematic review and meta-analysis. BMC Health Serv Res. 2015;15(1):7. doi: 10.1186/s12913-014-0640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pyne JM, et al. Longitudinal association of preference-weighted health-related quality of life measures and substance use disorder outcomes. Addiction. 2011;106(3):507–515. doi: 10.1111/j.1360-0443.2010.03299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aden B, et al. Health-Related Quality of Life in HIV-Infected and At-Risk Women: The Impact of Illicit Drug Use and Hepatitis C on a Community Preference Weighted Measure. Med Decis Making. 2013 doi: 10.1177/0272989X13507340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husereau D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)--explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–292. doi: 10.1016/s0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 29.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42(9):851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 30.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Torrance GW. Measurement of health state utilities for economic appraisal. J Health Econ. 1986;5(1):1–30. doi: 10.1016/0167-6296(86)90020-2. [DOI] [PubMed] [Google Scholar]
- 32.Torrance GW. Utility approach to measuring health-related quality of life. J Chronic Dis. 1987;40(6):593–603. doi: 10.1016/0021-9681(87)90019-1. [DOI] [PubMed] [Google Scholar]
- 33.Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96:5–21. doi: 10.1093/bmb/ldq033. [DOI] [PubMed] [Google Scholar]
- 34.Cheever LW, et al. A model federal collaborative to increase patient access to buprenorphine treatment in HIV primary care. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S3–S6. doi: 10.1097/QAI.0b013e318209740f. [DOI] [PubMed] [Google Scholar]
- 35.Weiss L, et al. The BHIVES collaborative: organization and evaluation of a multisite demonstration of integrated buprenorphine/naloxone and HIV treatment. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S7–S13. doi: 10.1097/QAI.0b013e3182097426. [DOI] [PubMed] [Google Scholar]
- 36.American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. p. 370. xii. [Google Scholar]
- 37.Korthuis PT, et al. Improving adherence to HIV quality of care indicators in persons with opioid dependence: the role of buprenorphine. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S83–S90. doi: 10.1097/QAI.0b013e31820bc9a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 40.Cacciola JS, et al. Initial evidence for the reliability and validity of a "Lite" version of the Addiction Severity Index. Drug Alcohol Depend. 2007;87(2–3):297–302. doi: 10.1016/j.drugalcdep.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Mannheimer SB, et al. The CASE adherence index: A novel method for measuring adherence to antiretroviral therapy. AIDS Care. 2006;18(7):853–861. doi: 10.1080/09540120500465160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo N, Johnson J, Coons SJ. Using instrument-defined health state transitions to estimate minimally important differences for four preference-based health-related quality of life instruments. Med Care. 2010;48(4):365–371. doi: 10.1097/mlr.0b013e3181c162a2. [DOI] [PubMed] [Google Scholar]
- 43.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14(6):1523–1532. doi: 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- 44.Fryback DG, et al. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med Care. 2007;45(12):1162–1170. doi: 10.1097/MLR.0b013e31814848f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isogai PK, et al. Prediction of health preference values from CD4 counts in individuals with HIV. Med Decis Making. 2013;33(4):558–566. doi: 10.1177/0272989X12453499. [DOI] [PubMed] [Google Scholar]
- 46.Nosyk B, et al. The quality of eight health status measures were compared for chronic opioid dependence. J Clin Epidemiol. 2010;63(10):1132–1144. doi: 10.1016/j.jclinepi.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Amato L, et al. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: Available evidence to inform clinical practice and research. Journal of Substance Abuse Treatment. 2005;28(4):321–329. doi: 10.1016/j.jsat.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Napper LE, et al. The reliability and validity of drug users' self reports of amphetamine use among primarily heroin and cocaine users. Addictive Behaviors. 2010;35(4):350–354. doi: 10.1016/j.addbeh.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Farrell TJ, Fals-Stewart W, Murphy M. Concurrent validity of a brief self-report Drug Use Frequency measure. Addictive Behaviors. 2003;28(2):327–337. doi: 10.1016/s0306-4603(01)00226-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.