Abstract
HIV-infection is an important risk factor for developing Kaposi sarcoma (KS), but it is unclear whether HIV-positive persons are also at increased risk of co-infection with human herpesvirus 8 (HHV-8), the infectious cause of KS. We systematically searched literature up to 12/2012 and included studies reporting HHV-8 seroprevalence for HIV-positive and HIV-negative persons. We used random-effects meta-analysis to combine odds ratios (ORs) of the association between HIV and HHV-8 seropositivity and conducted random-effects meta-regression to identify sources of heterogeneity. We included 93 studies with 58,357 participants from 32 countries in sub-Saharan Africa, North and South America, Europe, Asia, and Australia. Overall, HIV-positive persons were more likely to be HHV-8 seropositive than HIV-negative persons (OR 1.99, 95% confidence interval [CI] 1.70 - 2.34) with considerable heterogeneity among studies (I2 84%).The association was strongest in men who have sex with men (MSM, OR 3.95, 95% CI 2.92 - 5.35), patients with hemophilia (OR 3.11, 95%CI 1.19 - 8.11) and children (OR 2.45, 95% CI 1.58 - 3.81), but weaker in heterosexuals who engage in low-risk (OR 1.42, 95% CI 1.16 - 1.74) or high-risk sexual behavior (OR 1.66, 95% CI 1.27 - 2.17), persons who inject drugs (OR 1.66, 95% CI 1.28-2.14), and pregnant women (OR 1.68, 95% CI 1.15 - 2.47), p-value for interaction <0.001. In conclusion, HIV-infection was associated with an increased HHV-8 seroprevalence in all population groups examined. A better understanding of HHV-8 transmission in different age and behavioral groups is needed to develop strategies to prevent HHV-8 transmission.
Keywords: HIV, Human herpesvirus 8, co-infection, meta-analysis
Introduction
HIV-infection is an important risk factor for developing Kaposi sarcoma (KS), but to date, it is unclear whether HIV-positive persons are also at increased risk of co-infection with human herpesvirus 8 (HHV-8), the infectious cause of KS. Seroepidemiological studies have produced conflicting results concerning the HIV/HHV-8 association, possibly due to the wide range of populations with diverse transmission patterns studied.
HIV is transmitted through unprotected anal or vaginal intercourse in sexually active adults, and vertically from mother-to-child in children. HHV-8 transmission routes are less clear, and both sexual and horizontal non-sexual HHV-8 transmission routes have been reported.1 In sub-Saharan Africa, where HHV-8 prevalence reaches 50%,2-4 HHV-8 is primarily transmitted during childhood from mother to child and between siblings.5,6 In Europe and the United States the prevalence of HHV-8 is low in the general population (<4%)7,8 and high in men who have sex with men (MSM).9,10 In MSM HHV-8 seropositivity has been linked to sexual behavior, including the number of lifetime sexual partners, but the specific routes of transmission in these sexually active men are still unclear.10,11 In heterosexual adults in HHV-8 endemic and non-endemic regions HHV-8 might be transmitted through sexual contacts, but results from different studies have been inconsistent.7,12-14
We did a systematic review and meta-analysis to examine the association between HIV and HHV-8 seropositivity in different population groups and geographic regions.
Methods
Eligibility criteria
We included cross-sectional, cohort and case-control studies, as well as clinical trials that reported data on HHV-8 seropositivity on a total of at least 50 eligible HIV-positive and HIV-negative children or adults, published as full articles, short reports, letters or abstracts. We excluded items published before the first description of HHV-8 in 1994.15 HIV-positive and HIV-negative subjects had to be either recruited from the same source population, or matched for relevant confounding factors, and tested with the same HHV-8 assay. We excluded studies without HHV-8 positive participants in both the HIV-positive and HIV-negative groups.
Literature search
We searched MEDLINE and EMBASE up to December 21, 2012, without language restrictions. We combined keywords related to seropositivity and seroprevalence with keywords related to HHV-8 and Kaposi sarcoma associated herpes virus (see Appendix Box 1). We also screened reference lists of relevant papers and conference proceedings from the International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies (ICMAOI), the Conference on Retroviruses and Opportunistic Infections (CROI) and the annual meeting of the American Society of Clinical Oncology (ASCO). Where eligibility could not be determined from titles and abstracts, we retrieved full texts. Two out of three reviewers independently screened references for eligibility (ER, NW, ZH). When consensus could not be reached, a fourth reviewer (JB) was consulted.
Data extraction and quality assessment
We extracted study and participant characteristics, and assessed the study objective and primary outcome, the comparability of HIV-positive and HIV-negative persons, and the degree of adjustment for potential confounders. We extracted prevalence data, and unadjusted and confounder-adjusted odds ratios (ORs) for the association between HIV and HHV-8 seropositivity for each population group of interest: children, patients with hemophilia, pregnant women, persons who inject drugs (PWID), heterosexuals with low-risk or high-risk sexual behavior, and MSM. We extracted results as reported for different combined or single HHV-8 assays: latent or lytic antigens, enzyme immunoassay (EIA) or immunofluorescence assay (IFA). For study populations that were included in several publications, we chose the publication with the largest sample size. Data were extracted by one reviewer (ER, JB, NW, ZF, ZH) and checked for accuracy by a second reviewer (ER, JB, NW, ZF, ZH), using standardized, piloted data extraction forms. We then entered the data in duplicate into an electronic database created for this study.
Statistical analyses
We pooled the unadjusted and, where available, adjusted ORs of the association between HIV and HHV-8 seropositivity using random-effects meta-analysis. We measured between-study heterogeneity with the I-squared statistic,16 and conducted stratified analyses and random-effects meta-regression to identify sources of heterogeneity and potential effect modifiers.17 The following variables were considered: population group (children, patients with hemophilia, pregnant women, PWID, heterosexuals with low-risk or high-risk sexual behavior, and MSM), sex (men/boys versus women/girls), HHV-8 tests used (EIA versus IFA), HHV-8 antigens used (latent versus lytic), source of study data (cross-sectional study versus cohort study versus clinical trial versus case-control study), combined antiretroviral therapy (cART) period (pre-cART versus cART period), geographic region (sub-Saharan Africa versus outside Africa), and country income level (low- versus middle- versus high-income). We used the World Bank classification of Gross National Income per Capita 2013, dividing countries into low-income (per capita income of US$ 1045 or less per year), middle income (US$ 1046 to 12,745 per year), or high income (US$ 12,746 or more per year).18 We defined pre-cART period as sample collection occurring before 2004 in low- and middle-income countries and before 1996 in high-income countries. The group of low-risk heterosexuals included patients from general hospitals, blood donors, members of the general population, and factory workers. The group of heterosexuals with high-risk sexual behavior included patients attending sexually transmitted infections clinics, female sex workers, male clients of sex workers, and wives of HIV-positive men. For publications that reported results for several distinct population groups, we considered each population as a separate study. We selected the results from lytic over latent antigen tests, EIA over IFA, and from combined tests over single test if studies reported results for several HHV-8 testing strategies. We used meta-regression to examine differences in the strength of the association between HIV and HHV-8 seropositivity and funnel plots and a regression test for funnel plot asymmetry to assess potential publication and small study bias.19 Results of the meta-analysis are presented in forest plots. All data analyses were done in Stata version 12.1 (Stata Corporation, College Station, Texas, USA).
To assess the impact of confounder-adjustment on the effect estimate, we restricted the analyses to studies that reported confounder-adjusted estimates in a sensitivity analysis. In further sensitivity analyses we excluded studies where HIV-positive and HIV-negative persons had not been recruited from the same source population; we included studies that did not report results for a distinct population group; we selected results from latent over lytic antigen tests and IFA over EIA; and used a fixed-effects model to combine results.
Changes and amendments to the published protocol of this systematic review20 are as follows: we excluded studies with less than 10 HIV-positive or HIV-negative persons, studies with no HHV-8 positive persons in both groups, and studies which did not present results for a distinct population group. We also included studies that recruited study participants from different sources and matched these participants for relevant confounding factors.
Results
Number of eligible, included and excluded studies
The literature searches identified 1,957 references in MEDLINE and 1,820 in EMBASE. After removing duplicates, we screened 2,760 references for eligibility. We excluded 1,572 irrelevant records based on titles and abstracts, and assessed full text papers for the remaining 1,188 references. Of these, 1,124 study reports were excluded for the reasons described in Figure 1, including: no comparison group (334 references); review articles, book chapters or editorials (250 references); studies where HHV-8 antibodies were measured in tissue/body fluids other than blood (167 references). By searching for related publications of eligible studies and screening reference lists we identified two additional eligible publications.21,22 The 66 publications eligible for inclusion contained a total of 160 studies in different population groups, of which we excluded 67 studies mainly because they had no comparison group (40 studies), were restricted to patients with KS (10 studies), or had a sample size below 10 in the HIV-positive or HIV-negative group (5 studies). We therefore included 66 publications that described 93 relevant studies in our systematic review. These 93 studies represent the denominator of our meta-analysis.
Figure 1. Inclusion of studies (flow diagram).
The flow diagram shows the process of selecting eligible studies. HHV-8, human herpesvirus 8; KS, Kaposi sarcoma; PCR, polymerase chain reaction.
Characteristics of included studies
The 93 studies included 58,357 participants; the median number of participants per study was 287 (interquartile range [IQR] 120-556); for details see Table 1 and Appendix Table 1. Thirty-eight studies (41%) were conducted in sub-Saharan Africa and 55 studies (59%) in Europe, North and South America, Asia, and Australia. Study subjects were drawn from 32 different countries (13 sub-Saharan African and 19 non-sub-Saharan African countries). Five countries contributed about half of the studies: the USA (16 studies), Italy (10 studies), South Africa (8 studies), Uganda (7 studies), and Brazil (5 studies). Studies in sub-Saharan Africa mainly examined heterosexuals with low-risk sexual behavior (19 studies), pregnant women (9 studies), and children (6 studies). Outside Africa, the most commonly studied population groups were MSM (16 studies), heterosexuals with high-risk sexual behavior (15 studies), and PWID (10 studies). Two of the 10 PWID studies included up to 20% of persons who did not inject drugs.22,23 Half of the studies (47 studies) used only one HHV-8 assay; the other half combined different HHV-8 assays. Sera were specifically collected for HHV-8 analyses in 24 studies; 63 studies used stored blood samples that had been collected for other purposes. In six studies it was not clear if blood samples were specifically collected for HHV-8 analyses or not. All HHV-8 seroprevalence data included in our meta-analysis were cross-sectional, however, in several instances the data were taken from other study design types, for example, cohort studies (28 studies), clinical trials (3 studies), or case-control studies (3 studies). The association between HIV and HHV-8 seropositivity was the primary outcome in one study.24 In the other studies, HHV-8 seroprevalence (68 studies), risk factors for HHV-8 infection in general (40 studies), and HHV-8 transmission modalities (11 studies) were primary outcomes. Confounder-adjusted ORs were available for 24 studies. Sociodemographic, behavioral, or medical characteristics were reported separately for HIV-positive and HIV-negative persons in 27 studies. Sixty-six studies did not compare HIV-positive and HIV-negative groups.
Table 1.
Characteristics of included studies.
| Sub-Saharan Africa | Europe | North America | South America | Asia | Australia | All regions | |
|---|---|---|---|---|---|---|---|
| All comparisons | 38 (100%) | 22 (100%) | 20 (100%) | 7 (100%) | 5 (100%) | 1 (100%) | 93 (100%) |
| Population group | |||||||
| Children | 6 (16%) | 2 (9%) | 0 | 3 (43%) | 0 | 0 | 11 (12%) |
| Patients with hemophilia | 0 | 3 (14%) | 0 | 0 | 0 | 0 | 3 (3%) |
| Pregnant women | 9 (24%) | 0 | 1 (5%) | 0 | 0 | 0 | 10 (11%) |
| Low-risk heterosexuals | 19 (50%) | 0 | 2 (10%) | 1 (14%) | 2 (40%) | 0 | 24 (26%) |
| High-risk heterosexuals | 4 (11%) | 7 (32%) | 5 (25%) | 1 (14%) | 2 (40%) | 0 | 19 (20%) |
| Persons who inject drugs | 0 | 6 (27%) | 3 (15%) | 0 | 1 (20%) | 0 | 10 (11%) |
| Men who have sex with men | 0 | 4 (18%) | 9 (45%) | 2 (29%) | 0 | 1 (100%) | 16 (17%) |
| Country income level* | |||||||
| Low | 20 (53%) | 0 | 0 | 0 | 1 (20%) | 0 | 21 (23%) |
| Middle | 18 (47%) | 0 | 3 (15%) | 7 (100%) | 4 (80%) | 0 | 32 (34%) |
| High | 0 | 22 (100%) | 17 (85%) | 0 | 0 | 1 (100%) | 40 (43%) |
| Sex | |||||||
| Men/boys | 3 (8%) | 8 (36%) | 11 (55%) | 3 (43%) | 0 | 1 (100%) | 26 (28%) |
| Women/girls | 18 (47%) | 4 (18%) | 6 (30%) | 0 | 1 (20%) | 0 | 29 (31%) |
| Both sexes | 14 (37%) | 6 (27%) | 2 (10%) | 2 (29%) | 4 (80%) | 0 | 28 (30%) |
| Not reported | 3 (8%) | 4 (18%) | 1 (5%) | 2 (29%) | 0 | 0 | 10 (11%) |
| HHV-8 tests used | |||||||
| EIA | 16 (42%) | 3 (14%) | 8 (40%) | 1 (14%) | 3 (60%) | 0 | 31 (33%) |
| IFA | 14 (37%) | 15 (68%) | 9 (45%) | 3 (43%) | 1 (20%) | 0 | 42 (45%) |
| EIA and IFA | 8 (21%) | 4(18%) | 3 (15%) | 3 (43%) | 1 (20%) | 1 (100%) | 20 (22%) |
| HHV-8 antigens used for testing | |||||||
| Latent | 5 (13%) | 4 (18%) | 4 (20%) | 1 (14%) | 0 | 0 | 14 (15%) |
| Lytic | 21 (55%) | 6 (27%) | 9 (45%) | 1 (14%) | 1 (20%) | 0 | 38 (41%) |
| Latent and lytic | 12 (32%) | 12 (55%) | 7 (35%) | 5 (71%) | 4 (80%) | 1 (100%) | 41 (44%) |
| Source of study data | |||||||
| Cross-sectional study | 23 (61%) | 18 (82%) | 2 (10%) | 5 (71%) | 4 (80%) | 0 | 52 (56%) |
| Cohort study | 6 (16%) | 4 (18%) | 15 (75%) | 1 (14%) | 1 (20%) | 1 (100%) | 28 (30%) |
| Clinical trial | 3 (8%) | 0 | 0 | 0 | 0 | 0 | 3 (3%) |
| Case -control study | 3 (8%) | 0 | 0 | 0 | 0 | 0 | 3 (3%) |
| Other/unclear | 3 (8%) | 0 | 3 (15%) | 1 (14%) | 0 | 0 | 7 (8%) |
| cART period** | |||||||
| Pre-cART period | 25 (66%) | 1 (5%) | 6 (30%) | 5 (71%) | 1 (20%) | 0 | 38 (41%) |
| cART period | 6 (16%) | 3 (14%) | 6 (30%) | 0 | 2 (40%) | 1 (100%) | 18 (19%) |
| Pre-cART and cART period | 4 (11%) | 12 (55%) | 3 (15%) | 0 | 0 | 0 | 19 (20%) |
| Unclear/unknown | 3 (8%) | 6 (27%) | 5 (25%) | 2 (29%) | 2 (40%) | 0 | 18 (19%) |
as defined by the World Bank classification
Study samples collected in pre-cART period defined as before 1996 for high-income countries and before 2004 for low- and middle-income countries; cART period starts thereafter.
cART, combined antiretroviral therapy; EIA, enzyme immunoassay; HHV-8, human herpesvirus 8; IFA, immunofluorescence assay.
Main findings
HIV-positive persons were more likely to be HHV-8 seropositive than HIV-negative persons (OR 1.99, 95% confidence interval [CI] 1.70-2.34). Studies were highly heterogeneous (I2 84%, p<0.001), but there was no evidence for funnel plot asymmetry (Egger's test p=0.14), see Appendix Figure 1. The weighted median HHV-8 seroprevalence was 47% (IQR 26-68%) in HIV-positive populations and 24% (IQR 11-37%) in HIV-negative populations.
Differences in strength of association
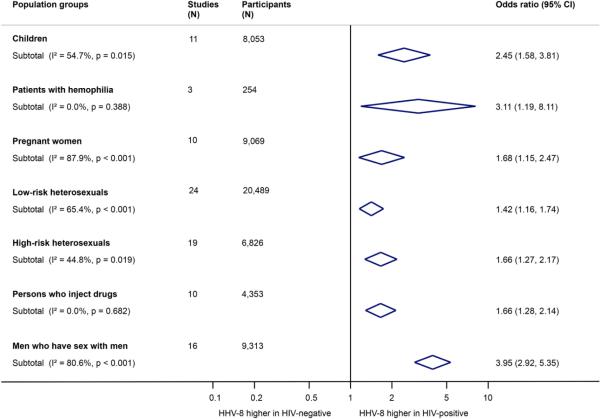
Consistent with the overall pattern, HIV-infection was associated with an increased risk of HHV-8 seropositivity in all examined population groups (children, patients with hemophilia, pregnant women, heterosexuals with low-risk or high-risk sexual behavior, PWID, and MSM), see Figure 2 and Appendix Figure 2. However, the strength of the association varied between different population groups (p-value for interaction <0.001), see Table 2. The strongest association was found in MSM (OR 3.95, 95%CI 2.92-5.35), patients with hemophilia (OR 3.11, 95%CI 1.19-8.11), and children (OR 2.45, 95%CI 1.58-3.81). The association was weaker in heterosexuals with low-risk (OR 1.42, 95%CI 1.16-1.74) or high-risk sexual behavior (OR 1.66, 95% CI 1.27-2.17), PWID (OR 1.66, 95% CI 1.28-2.14), and pregnant women (OR 1.68, 95% CI 1.15-2.47). The association between HIV and HHV-8 seropositivity was similar in pregnant women (OR 1.68, 95% CI 1.15-2.47) and other women from low-risk groups (OR 1.55, 95% CI 1.10-2.18). There was some evidence for interaction with sex (p-value for interaction=0.001) but this disappeared when we excluded MSM (p-value for interaction=0.57). The association between HIV and HHV-8 seropositivity was less pronounced in studies from sub-Saharan Africa (OR 1.59, 95% CI 1.34-1.90) than in studies from outside Africa (OR 2.50, 95% CI 1.99-3.14; p-value for interaction=0.001). Similarly, the association was also weaker in low-income countries than in middle- and high-income countries (p-value for interaction=0.007). Heterogeneity between studies was not explained by the type of HHV-8 test(s) used (EIA or IFA; p-value for interaction=0.79), the HHV-8 antigens used for testing (latent or lytic; p-value for interaction=0.99), or source of study data (cross-sectional study, cohort study, clinical trial, or case-control study; p-value for interaction=0.36). The strength of the HIV/HHV-8 association was also not modified by the sample collection period (pre-cART or cART period; p-value for interaction=0.89).
Figure 2. Association between HIV and HHV-8 seropositivity in different population groups.
The center of the diamonds is the pooled point estimate of the studies included in each population group, and the width of the diamonds represents the 95% confidence interval (CI) of the pooled odds ratio.
Table 2.
Association between HIV and HHV-8 seropositivity, stratified by different explanatory variables.
| Studies (N) | Odds ratio (95% CI) | p-value for interaction | ||
|---|---|---|---|---|
| Overall | 93 | 1.99 (1.70-2.34) | ||
| Population group | Children | 11 | 2.45 (1.58-3.81) | <0.001 |
| Patients with hemophilia | 3 | 3.11 (1.19-8.11) | ||
| Pregnant women | 10 | 1.68 (1.15-2.47) | ||
| Low-risk heterosexual | 24 | 1.42 (1.16-1.74) | ||
| High-risk heterosexuals | 19 | 1.66 (1.27-2.17) | ||
| Persons who inject drugs | 10 | 1.66 (1.28-2.14) | ||
| Men who have sex with men | 16 | 3.95 (2.92-5.35) | ||
| Region | Sub-Saharan Africa | 38 | 1.59 (1.34-1.90) | <0.001 |
| Europe | 22 | 2.06 (1.60-2.66) | ||
| North America | 20 | 2.70 (1.91-3.83) | ||
| South America | 7 | 4.33 (3.13-5.99) | ||
| Asia | 5 | 1.26 (0.75-2.14) | ||
| Australia | 1 | 8.60 (3.55-20.86) | ||
| Country income level* | Low | 21 | 1.43 (1.12-1.82) | 0.007 |
| Middle | 32 | 1.98 (1.59-2.46) | ||
| High | 40 | 2.47 (1.90-3.21) | ||
| Sex | Men/boys | 26 | 2.93 (2.12-4.06) | <0.001 |
| Women/girls | 29 | 1.61 (1.32-1.96) | ||
| Both sexes† | 28 | 1.54 (1.20-1.98) | ||
| Not reported† | 10 | 3.62 (2.78-4.73) | ||
| HHV-8 tests used | EIA | 31 | 2.09 (1.62-2.71) | 0.79 |
| IFA | 42 | 2.03 (1.65-2.50) | ||
| EIA and IFA† | 20 | 1.69 (1.16-2.47) | ||
| HHV-8 antigens used for testing | Latent | 14 | 1.78 (1.21-2.64) | 0.99 |
| Lytic | 38 | 1.78 (1.49-2.13) | ||
| Latent and lytic† | 41 | 2.32 (1.80-2.98) | ||
| Source of study data | Cross-sectional study | 52 | 1.93 (1.59-2.33) | 0.36 |
| Cohort study | 28 | 2.33 (1.72-3.17) | ||
| Clinical trial | 3 | 1.35 (0.77-2.38) | ||
| Case-control study | 3 | 1.94 (0.93-4.05) | ||
| Other/unclear† | 7 | 1.45 (0.68-3.10) | ||
| cART period** | Pre-cART period | 38 | 1.87 (1.55-2.24) | 0.89 |
| cART period | 18 | 1.81 (1.29-2.53) | ||
| Pre-cART and cART period† | 19 | 2.33 (1.51-3.60) | ||
| Unclear/unknown† | 18 | 2.46 (1.64-3.69) | ||
as defined by the World Bank classification
Study samples collected in pre-cART period defined as before 1996 for high-income countries and before 2004 for low- and middle-income countries; cART period starts thereafter.
excluded from test for interaction
cART, combined antiretroviral therapy; CI, confidence interval; EIA, enzyme immunoassay; HHV-8, human herpesvirus 8; IFA, immunofluorescence assay.
The interaction between population group and the HIV/HHV-8 association remained statistically significant (p-value for interaction <0.001) when we included geographic region (sub-Saharan Africa versus outside sub-Saharan Africa), or country income level (high versus middle versus low) in the regression model. In contrast, after adjustment for population group there was no longer evidence for a statistically significant interaction of geographic location or country income level with the HIV/HHV-8 association (p-values for interaction=0.44 and 0.52, respectively). The meta-regression coefficients and the modeled effect estimates of these analyses are shown in the Appendix Tables 2+3.
Sensitivity analyses
The overall association between HIV and HHV-8 seropositivity and the interaction with population group remained similar when we selected results from IFA over EIA, latent antigens over lytic antigens, or used fixed-effects instead of random-effects models for analyses (Appendix Table 4). Exclusion of four studies25,26 that recruited HIV-positive and HIV-negative participants from different sources (but matched them for confounders), and inclusion of small and mixed study populations did also not affect the overall result or the interaction between population group and the HIV/HHV-8 association (Appendix Table 4). The pooled estimate in the 24 studies that reported confounder-adjusted ORs (OR 1.80, 95% CI 1.42-2.28) was slightly weaker than the pooled estimate in the same set of studies when we used unadjusted ORs (OR 1.88, 95% CI 1.48-2.39). Both pooled estimates were similar to the result from the main meta-analysis for which we pooled both confounder-adjusted and unadjusted ORs (OR 1.99, 95% CI 1.70-2.34). The number of studies with adjusted ORs per population group was too small to allow meaningful comparisons (Appendix Table 4). In the 24 studies that reported both confounder-adjusted and unadjusted odds ratios, the two estimates were mostly similar (Appendix Table 5). The variables used for adjustment in the individual studies are listed in the Appendix Table 1.
Discussion
Our meta-analysis shows that HIV-infection is associated with an increased HHV-8 seroprevalence. The association was strongest in MSM, patients with hemophilia, and children, but weaker in heterosexual adults with low-risk or high-risk sexual behavior, in pregnant women, and in PWID. There was some evidence for a geographic variation of the HIV/HHV-8 association in univariable analysis; however, this was mainly explained by the different population groups studied in these regions. When we adjusted for population group the effect of geographic region disappeared.
Our findings extend those of a systematic review of the HIV/HHV-8 association done for China,27 which reported a pooled OR of 2.97 (95% CI 2.22-3.97). Our study was based on an extensive literature search that covered all regions and countries. We developed a study protocol20 with rigorous inclusion criteria and assessed the quality of studies carefully. To reduce potential confounding and selection biases, we only included studies that recruited HIV-positive and HIV-negative participants from the same population and studies that recruited participants from different sources, but matched them for relevant confounding factors. Because we identified a large number of eligible studies, we could assess the association between HIV and HHV-8 seropositivity within different age and behavioral subgroups.
Our results are based on cross-sectional data, so we cannot determine the temporal sequence of infection with the two viruses. Also, most of the studies we included were not designed to assess the association between HIV and HHV-8 seropositivity. Their sampling methods and the comparability of HIV-positive and HIV-negative participants with respect to relevant confounders were often reported incompletely. This limited our ability to assess potential confounding and selection bias. However, in the studies that reported both adjusted and unadjusted effect estimates, adjustment for confounders had little effect on the association between HIV and HHV-8 seropositivity. We observed substantial heterogeneity between studies and explored sources of heterogeneity using meta-regression analysis. Findings from meta-regressions of aggregate data, however, have to be interpreted cautiously. For example, grouping studies into low-risk or high-risk sexual behavior categories will not be a perfect reflection of the risk status of the individual persons studied. High-risk behaviors may overlap across groups; for example injection drug use may be common among commercial sex workers. More detailed data on risk factors, such as number of sexual partners or histories of sexually transmitted infections stratified by HIV and HHV-8 infection status were available only for few studies. Ideally, analyses of the HIV/HHV-8 association should therefore be based on individual participant data, but such data were not available for the current meta-analysis. Only three studies in patients with hemophilia and no studies in MSM or PWID from sub-Saharan Africa were included in the analysis, limiting the precision of estimates for these groups. Because only one of the included studies reported whether HIV-positive participants received cART or not,26 we could not assess whether cART modified the HIV/HHV-8 association. Finally, we will have missed studies that measured HHV-8 seropositivity in HIV-positive and HIV-negative persons but failed to report the results.
Because there is no gold standard for HHV-8 serological testing, we were confronted with different tests of varying sensitivity and specificity.28,29 However, within each study the same HHV-8 test was used in HIV-positive and HIV-negative groups and the odds ratios will therefore be more comparable across studies than absolute measures, such as seroprevalence, would have been. Indeed, we found no statistical evidence for differences in odds ratios by type of HHV-8 test (IFA versus EIA) or antigens (latent versus lytic) used. Only two of the included studies reported data on HHV-8 DNA in peripheral blood mononuclear cells30 or saliva31 for HIV-positive and HIV-negative persons. In both studies HHV-8 DNA was detected slightly more often in the HIV-positive group compared to the HIV-negative group, but these findings were not statistically significant.
Biological mechanisms may account for the positive association between HIV and HHV-8 seropositivity. These mechanisms include reactivation of pre-existing HHV-8 infections in HIV-positive persons,32 increased susceptibility for HHV-8 infection in the presence of HIV co-infection9,33,34 or shared routes of transmission for HIV and HHV-8. Combined ART might influence the HIV/HHV-8 association by stimulating the immune response against HHV-8 and reducing HHV-8 viremia.35,36 However, we found no evidence that the strength of the HIV/HHV-8 association varied between the pre-cART and the cART period. Reactivation and increased susceptibility are expected to lead to similar magnitudes of the association between HIV and HHV-8 seropositivity in different population groups. Conversely, shared transmission routes, for example through anal intercourse, would lead to stronger associations in MSM than in heterosexual populations, as observed in our study. Interestingly, associations were similar in high-risk and low-risk heterosexual populations, indicating that vaginal intercourse may be less important in this context. The finding of a strong association between HIV and HHV-8 in children could be explained by transmission through saliva: HHV-8 DNA is detected more frequently and at a higher viral load in saliva than in semen, or vaginal and prostatic secretions.37,38 Of note, HIV- infection may increase HHV-8 shedding in different body fluids, including saliva38-40 and semen.41 Further studies are needed to clarify what exposures may explain the strong association between HIV and HHV-8 seropositivity in MSM and children.42,43
In conclusion, this large-scale systematic review and meta-analysis found that HIV-infection was associated with an increased HHV-8 seroprevalence in all population groups examined. The association was strongest in MSM and children. Shared routes of sexual transmission of HIV and HHV-8 in MSM, and shedding of HHV-8 in HIV-positive mothers could explain these associations. Interventions that prevent the spread of HHV-8 are likely to reduce the burden of KS, however, a better understanding of HHV-8 transmission in different age and behavioral groups will be required before such preventive strategies can be developed and evaluated.
Supplementary Material
What's new?
Human herpesvirus 8 (HHV-8) is the infectious cause of Kaposi sarcoma (KS) and HIV-infection is an important risk factor for developing KS. To date, it is unclear whether HIV-positive persons are at increased risk of co-infection with HHV-8. This large-scale systematic review and meta-analysis found that HIV-infection was associated with an increased HHV-8 seroprevalence in all population groups examined. The association was strongest in men who have sex with men and children.
Acknowledgements
We thank Sven Trelle for statistical support, and Jingying Wang for her help with studies published in Chinese. We also thank Kali Tal for her editorial suggestions. This study was done on behalf of The International epidemiologic Database to Evaluate AIDS (IeDEA). Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health [award number U01AI069924 to M.E.] and also supported by the National Cancer Institute [grant number 5U01A1069924-05 to M.E.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional funding was received from the Swiss Bridge Foundation, the Swiss Cancer League [Robert Wenner Award to J.B.], and the Swiss National Science Foundation [Ambizione-PROSPER PZ00P3_136620_3 to J.B.; Marie Heim-Vögtlin grant PMCDP3_145489 to N.W.].
Abbreviations used
- CI
confidence interval
- EIA
enzyme immunoassay
- HHV-8
human herpesvirus 8
- HIV
human immunodeficiency virus
- IFA
immunofluorescence assay
- IQR
interquartile range
- KS
Kaposi sarcoma
- MSM
men who have sex with men
- OR
odds ratio
- PWID
persons who inject drugs
Footnotes
- 14th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies (ICMAOI); November 12-13, 2013; Bethesda, Maryland, USA.
- 18th International Workshop on HIV Observational Databases (IWHOD); March 27-29, 2014; Sitges, Spain.
- 18th International Workshop on Kaposi's Sarcoma Herpesvirus and Related Agents; June 30 - July 3, 2015; Miami, Florida, USA.
The authors have no conflicts of interest to declare.
References
- 1.Bhutani M, Polizzotto MN, Uldrick TS, Yarchoan R. Kaposi sarcoma-associated herpesvirus-associated malignancies: epidemiology, pathogenesis, and advances in treatment. Semin.Oncol. 2015;42:223–246. doi: 10.1053/j.seminoncol.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler LM, Were WA, Balinandi S, Downing R, Dollard S, Neilands TB, Gupta S, Rutherford GW, Mermin J. Human herpesvirus 8 infection in children and adults in a population-based study in rural Uganda. J Infect Dis. 2011 Mar 1;203(5):625–34. doi: 10.1093/infdis/jiq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dollard SC, Butler LM, Jones AM, Mermin JH, Chidzonga M, Chipato, Shiboski CH, Brander C, Mosam A, Kiepiela P, Hladik W, Martin JN. Substantial regional differences in human herpesvirus 8 seroprevalence in sub-Saharan Africa: insights on the origin of the “Kaposi's sarcoma belt”. Int J Cancer. 2010 Nov 15;127(10):2395–401. doi: 10.1002/ijc.25235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsen SJ, Chang Y, Moore PS, Biggar RJ, Melbye M. Increasing Kaposi's sarcoma-associated herpesvirus seroprevalence with age in a highly Kaposi's sarcoma endemic region, Zambia in 1985. AIDS. 1998 Oct 1;12(14):1921–5. doi: 10.1097/00002030-199814000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Mbulaiteye SM, Pfeiffer RM, Whitby D, Brubaker GR, Shao J, Biggar RJ. Human herpesvirus 8 infection within families in rural Tanzania. J Infect Dis. 2003 Jun 1;187(11):1780–5. doi: 10.1086/374973. [DOI] [PubMed] [Google Scholar]
- 6.Plancoulaine S, Abel L, Tregouet D, Duprez R, van Beveren M, Tortevoye P, Froment A, Gessain A. Respective roles of serological status and blood specific antihuman herpesvirus 8 antibody levels in human herpesvirus 8 intrafamilial transmission in a highly endemic area. Cancer Res. 2004 Dec 1;64(23):8782–7. doi: 10.1158/0008-5472.CAN-04-2000. [DOI] [PubMed] [Google Scholar]
- 7.Engels EA, Atkinson JO, Graubard BI, McQuillan GM, Gamache C, Mbisa G, Cohn S, Whitby D, Goedert JJ. Risk factors for human herpesvirus 8 infection among adults in the United States and evidence for sexual transmission. J Infect Dis. 2007 Jul 15;196(2):199–207. doi: 10.1086/518791. [DOI] [PubMed] [Google Scholar]
- 8.Mesri EA, Cesarman E, Boshoff C. Kaposi's sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010 Oct;10(10):707–19. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melbye M, Cook PM, Hjalgrim H, Begtrup K, Simpson GR, Biggar RJ, Ebbesen P, Schulz TFl. Risk factors for Kaposi's-sarcoma-associated herpesvirus (KSHV/HHV-8) seropositivity in a cohort of homosexual men, 1981-1996. Int J Cancer. 1998 Aug 12;77(4):543–8. doi: 10.1002/(sici)1097-0215(19980812)77:4<543::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Martin JN, Ganem DE, Osmond DH, Page-Shafer KA, Macrae D, Kedes DH. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med. 1998 Apr 2;338(14):948–54. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 11.Dukers NH, Renwick N, Prins M, Geskus RB, Schulz TF, Weverling GJ, Coutinho RA, Goudsmit J. Risk factors for human herpesvirus 8 seropositivity and seroconversion in a cohort of homosexual men. Am J Epidemiol. 2000 Feb 1;151(3):213–24. doi: 10.1093/oxfordjournals.aje.a010195. [DOI] [PubMed] [Google Scholar]
- 12.Eltom MA, Mbulaiteye SM, Dada AJ, Whitby D, Biggar RJ. Transmission of human herpesvirus 8 by sexual activity among adults in Lagos, Nigeria. AIDS. 2002 Dec 6;16(18):2473–8. doi: 10.1097/00002030-200212060-00014. [DOI] [PubMed] [Google Scholar]
- 13.Shebl FM, Dollard SC, Pfeiffer RM, Biryahwaho B, Amin MM, Munuo SS, Hladik W, Parsons R, Graubard BI, Mbulaiteye SM. Human herpesvirus 8 seropositivity among sexually active adults in Uganda. PLoS One. 2011;6(6):e21286. doi: 10.1371/journal.pone.0021286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Sanjose S, Mbisa G, Perez-Alvarez S, Benavente Y, Sukvirach S, Hieu NT, Shin HR, Anh PT, Thomas J, Lazcano E, Matos E, Herrero R, et al. Geographic variation in the prevalence of Kaposi sarcoma-associated herpesvirus and risk factors for transmission. J Infect Dis. 2009 May 15;199(10):1449–56. doi: 10.1086/598523. [DOI] [PubMed] [Google Scholar]
- 15.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994 Dec 16;266(5192):1865–9. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002 Jun 15;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17.Sterne JA, Juni P, Schulz KF, Altman DG, Bartlett C, Egger M. Statistical methods for assessing the influence of study characteristics on treatment effects in 'meta-epidemiological' research. Stat Med. 2002 Jun 15;21(11):1513–24. doi: 10.1002/sim.1184. [DOI] [PubMed] [Google Scholar]
- 18.The World Bank Country and Lending Groups [cited 2014 Nov 3] http://data.worldbank.org/about/country-and-lending-groups.
- 19.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997 Sep 13;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohner E, Wyss N, Trelle S, Mbulaiteye SM, Egger M, Novak U, Zwahlen M, Bohlius J. HHV-8 seroprevalence: a global view. Syst Rev. 2014;3:11. doi: 10.1186/2046-4053-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakeham K, Webb EL, Sebina I, Nalwoga A, Muhangi L, Miley W, Johnston WT, Ndibazza J, Whitby D, Newton R, Elliott AM. Risk factors for seropositivity to Kaposi sarcoma-associated herpesvirus among children in Uganda. J Acquir Immune Defic Syndr. 2013 Jun 1;63(2):228–33. doi: 10.1097/QAI.0b013e31828a7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang PR, Tan XH, Guo SX, Yang L, Zeng Z, Fu B, Wang L, Chen B. Research of Kaposi's sarcoma-associated herpesvirus in drug users in one city of Xinjiang. Modern Preventive Medicine. 2010;37(1):107–9. [Google Scholar]
- 23.Renwick N, Dukers NH, Weverling GJ, Sheldon JA, Schulz TF, Prins M, Coutinho RA, Goudsmit J. Risk factors for human herpesvirus 8 infection in a cohort of drug users in the Netherlands, 1985-1996. J Infect Dis. 2002 Jun 15;185(12):1808–12. doi: 10.1086/340817. [DOI] [PubMed] [Google Scholar]
- 24.He J, Bhat G, Kankasa C, Chintu C, Mitchell C, Duan W, Wood C. Seroprevalence of human herpesvirus 8 among Zambian women of childbearing age without Kaposi's sarcoma (KS) and mother-child pairs with KS. J Infect Dis. 1998 Dec;178(6):1787–90. doi: 10.1086/314512. [DOI] [PubMed] [Google Scholar]
- 25.Cannon MJ, Dollard SC, Smith DK, Klein RS, Schuman P, Rich JD, Vlahov D, Pellett PE. Blood-borne and sexual transmission of human herpesvirus 8 in women with or at risk for human immunodeficiency virus infection. N Engl J Med. 2001 Mar 1;344(9):637–43. doi: 10.1056/NEJM200103013440904. [DOI] [PubMed] [Google Scholar]
- 26.Casper C, Meier AS, Wald A, Morrow RA, Corey L, Moscicki AB. Human herpesvirus 8 infection among adolescents in the REACH cohort. Arch Pediatr Adolesc Med. 2006 Sep;160(9):937–42. doi: 10.1001/archpedi.160.9.937. [DOI] [PubMed] [Google Scholar]
- 27.Zhang T, Shao X, Chen Y, Zhang T, Minhas V, Wood C, He N. Human herpesvirus 8 seroprevalence, China. Emerg Infect Dis. 2012 Jan;18(1):150–2. doi: 10.3201/eid1801.102070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engels EA, Whitby D, Goebel PB, Stossel A, Waters D, Pintus A, Contu L, Biggar RJ, Goedert JJ. Identifying human herpesvirus 8 infection: performance characteristics of serologic assays. J Acquir Immune Defic Syndr. 2000 Apr 1;23(4):346–54. doi: 10.1097/00126334-200004010-00011. [DOI] [PubMed] [Google Scholar]
- 29.Rabkin CS, Schulz TF, Whitby D, Lennette ET, Magpantay LI, Chatlynne L, Biggar RJ. Interassay correlation of human herpesvirus 8 serologic tests. HHV-8 Interlaboratory Collaborative Group. J Infect Dis. 1998 Aug;178(2):304–9. doi: 10.1086/515649. [DOI] [PubMed] [Google Scholar]
- 30.Ariyoshi K, Schim van der Loeff M, Cook P, Whitby D, Corrah T, Jaffar S, Cham F, Sabally S, O'Donovan D, Weiss RA, Schulz TF, Whittle H. Kaposi's sarcoma in the Gambia, West Africa is less frequent in human immunodeficiency virus type 2 than in human immunodeficiency virus type 1 infection despite a high prevalence of human herpesvirus 8. J Hum.Virol. 1998;1:193–199. [PubMed] [Google Scholar]
- 31.Dedicoat M, Newton R, Alkharsah KR, Sheldon J, Szabados I, Ndlovu B, Page T, Casabonne D, Gilks CF, Cassol SA, Whitby D, Schulz TF. Mother-to-child transmission of human herpesvirus-8 in South Africa. J Infect Dis. 2004;190:1068–1075. doi: 10.1086/423326. [DOI] [PubMed] [Google Scholar]
- 32.Mercader M, Taddeo B, Panella JR, Chandran B, Nickoloff BJ, Foreman KE. Induction of HHV-8 lytic cycle replication by inflammatory cytokines produced by HIV-1-infected T cells. Am J Pathol. 2000 Jun;156(6):1961–71. doi: 10.1016/S0002-9440(10)65069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aoki Y, Tosato G. HIV-1 Tat enhances Kaposi sarcoma-associated herpesvirus (KSHV) infectivity. Blood. 2004 Aug 1;104(3):810–4. doi: 10.1182/blood-2003-07-2533. [DOI] [PubMed] [Google Scholar]
- 34.Caselli E, Galvan M, Santoni F, Rotola A, Caruso A, Cassai E, Luca DD. Human herpesvirus-8 (Kaposi's sarcoma-associated virus) ORF50 increases in vitro cell susceptibility to human immunodeficiency virus type 1 infection. J Gen Virol. 2003 May;84(Pt 5):1123–31. doi: 10.1099/vir.0.18799-0. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan SG, Hirsch HH, Franceschi S, Steffen I, Amari EB, Mueller NJ, Magkouras I, Biggar RJ, Rickenbach M, Clifford GM. Swiss HIV Cohort Study. Kaposi sarcoma herpes virus antibody response and viremia following highly active antiretroviral therapy in the Swiss HIV Cohort study. AIDS. 2010;24:2245–2252. doi: 10.1097/QAD.0b013e32833b7830. [DOI] [PubMed] [Google Scholar]
- 36.Bourboulia D, Aldam D, Lagos D, Allen E, Williams I, Cornforth D, Copas A, Boshoff C. Short- and long-term effects of highly active antiretroviral therapy on Kaposi sarcoma-associated herpesvirus immune responses and viraemia. AIDS. 2004;18:485–493. doi: 10.1097/00002030-200402200-00015. [DOI] [PubMed] [Google Scholar]
- 37.Pauk J, Huang ML, Brodie SJ, Wald A, Koelle DM, Schacker T, Celum C, Selke S, Corey L. Mucosal shedding of human herpesvirus 8 in men. N Engl J Med. 2000 Nov 9;343(19):1369–77. doi: 10.1056/NEJM200011093431904. [DOI] [PubMed] [Google Scholar]
- 38.Taylor MM, Chohan B, Lavreys L, Hassan W, Huang ML, Corey L, Ashley Morrow R, Richardson BA, Mandaliya K, Ndinya-Achola J, Bwayo J, Kreiss J. Shedding of human herpesvirus 8 in oral and genital secretions from HIV-1-seropositive and - seronegative Kenyan women. J Infect Dis. 2004 Aug 1;190(3):484–8. doi: 10.1086/421466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Franca TR, de Araujo RA, Ribeiro CM, Leao JC. Salivary shedding of HHV-8 in people infected or not by human immunodeficiency virus 1. J Oral Pathol Med. 2011 Jan;40(1):97–102. doi: 10.1111/j.1600-0714.2010.00959.x. [DOI] [PubMed] [Google Scholar]
- 40.Miller CS, Berger JR, Mootoor Y, Avdiushko SA, Zhu H, Kryscio RJ. High prevalence of multiple human herpesviruses in saliva from human immunodeficiency virus-infected persons in the era of highly active antiretroviral therapy. J Clin Microbiol. 2006 Jul;44(7):2409–15. doi: 10.1128/JCM.00256-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howard MR, Whitby D, Bahadur G, Suggett F, Boshoff C, Tenant-Flowers M, Schulz TF, Krik S, Matthews S, Weller IV, Tedder RS, Weiss RA. Detection of human herpesvirus 8 DNA in semen from HIV-infected individuals but not healthy semen donors. AIDS. 1997 Feb;11(2):F15–F19. doi: 10.1097/00002030-199702000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Crabtree KL, Wojcicki JM, Minhas V, Smith DR, Kankasa C, Mitchell CD, Wood C. Risk factors for early childhood infection of human herpesvirus-8 in Zambian children: the role of early childhood feeding practices. Cancer Epidemiol Biomarkers Prev. 2014 Feb;23(2):300–8. doi: 10.1158/1055-9965.EPI-13-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butler LM, Osmond DH, Jones AG, Martin JN. Use of saliva as a lubricant in anal sexual practices among homosexual men. J Acquir Immune Defic Syndr. 2009 Feb 1;50(2):162–7. doi: 10.1097/QAI.0b013e31819388a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.