Abstract
The T-cell/transmembrane, mucin and immunoglobulin domain protein 1 (TIM-1) is a phosphatidlyserine (PtdSer) receptor and a T cell costimulatory molecule linked to the development of atopic diseases. TIM-1 locates preferentially in intracellular compartments. Here we show that in human and mouse lymphoid cells, TIM-1 localizes in different types of endosomes and that its domain structure is important for protein sorting to intracellular vesicles. The BALB/c mouse TIM-1 protein, which has a longer mucin domain, is sorted more efficiently to endosomes than the shorter C57BL/6 variant. High affinity ligands such as PtdSer increase the amount of cell surface TIM-1; the protein also polarizes toward cell contacts with apoptotic cells. The large pool of intracellular TIM-1 translocates to the immune synapse (IS) with the CD3-TCR (T cell receptor) complex and colocalizes to the central supramolecular activation cluster (cSMAC). In contrast, cell surface TIM-1 does not traffic to the IS, but is located away from it. The bipolar TIM-1 sorting observed during IS formation is determined by differences in its subcellular location, and might modulate antigen-driven immune responses.
Keywords: TIM family, T-cell, immune synapse, protein sorting, structural immunology, microscopy
Graphical abstract

Introduction
The T cell/transmembrane, immunoglobulin (Ig) and mucin domain (TIM) proteins are type I membrane proteins with an N-terminal Ig variable (IgV) domain, followed by a glycosylated mucin domain, a single transmembrane region, and a cytoplasmic domain with tyrosine phosphorylation motifs (1). Numerous studies link the TIM family with immune tolerance, autoimmunity, atopic diseases, cell death, tumor growth and control of viral infections (2–7), indicating that these proteins regulate innate and adaptive immunity. Genetic studies show that certain alleles, HAVCR1 (hepatitis A virus cellular receptor 1, for TIM-1) and HAVCR2 (TIM-3), are associated with high asthma susceptibility in humans and mouse (8, 9). The HAVCR1 alleles differ in single residue polymorphisms in the signal peptide, IgV and mucin domains, as well as insertion/deletions in the mucin domain; polymorphisms in murine HAVCR2 are in the IgV domain.
Three TIM proteins are described in man (hTIM-1, hTIM-3, hTIM-4) and four in mice (mTIM-1 to mTIM-4). There is considerable sequence identity (~50%) among TIM IgV domains, but substantial diversity in the mucin domains. TIM IgV domains have a unique pocket with a conserved metal-ion coordination site termed the metal ion-dependent ligand binding site (MILIBS), absent only in TIM-2 (10). The MILIBS pocket accommodates the hydrophilic head of phosphatidylserine (PtdSer), whereas the hydrophobic or polar walls of the pocket probably penetrate the lipid bilayer (10, 11). TIM proteins are receptors of PtdSer (1), a lipid that signals cell death and is exposed on the outer leaflet of the apoptotic cell membrane (12). Cells that express TIM-1, TIM-3 and TIM-4 proteins can engulf and eliminate apoptotic cells (11, 13–15), a process essential for tissue homeostasis and prevention of autoimmunity (16, 17). mTIM-3 variants bind PtdSer with distinct affinities (11).
TIM-1 is expressed in several B- and T-cell subsets and is a marker of kidney injury and renal carcinoma (1, 4, 7, 18). TIM-1 is an entry receptor for the hepatitis A virus (HAV) (19), and can mediate T cell trafficking and function as a costimulatory molecule (2, 20). Ligand binding to TIM-1 can trigger T cell activation, mediating their proliferation and cytokine production (21–23). These functions are linked to signaling events by engaging several protein kinases; they are triggered by Tyr phosphorylation in the TIM-1 cytoplasmic domain (3). TIM-1 associates with the TCR complex components ZAP-70 and CD3 (3, 7, 24); some reports indicate that TIM-1 acts as a costimulatory molecule during antigen (Ag) presentation and that it can amplify TCR signaling.
In mouse T cells, mTIM-1 monoclonal antibodies (mAb) can trigger different types of Ag-dependent costimulatory signals and control the type of cytokines released. TIM-1 engagement with RMT1-10 and 1H8.2 mAb on T cells preferentially induces production of Th2 cytokines (IL4, IL5, IL10 and IL13) (25, 26), whereas high affinity mTIM-1 mAb such as 3B3 induce secretion of Th1/Th17 cytokines (IFN-γ and IL17) (26); other mAb (HA2.2 and 3A2.5) decrease Th2 cytokine production and lung inflammation in mouse models of asthma (25). HAVCR1 BALB/c and C57BL/6 alleles in congenic HBA mice are also linked to Th2- and Th1-biased immune responses, respectively (8). The basis for this divergence in TIM-1-mediated T cell costimulation is currently unclear.
TIM-1 resides mainly inside transfected cells and polarizes to intercellular junctions in TIM-1-expressing cells (10, 27); it is internalized by clathrin-mediated endocytosis (28). Here we show that endogenous TIM-1 protein is located preferentially in intracellular compartments in human and in mouse primary lymphoid cells. TIM-1 domains and high affinity ligands modulate the proportion of cell surface versus intracellular protein. The protein pool that accumulates in endosomes migrates to cell contact sites with apoptotic cells and toward the immune synapse (IS), where it accumulates at the central supramolecular activation cluster (cSMAC) together with CD3. In contrast, protein at the plasma membrane does not migrate to the IS; stimuli that increase the amount of cell surface protein prevent TIM-1 trafficking to the IS. These results indicate that TIM-1 translocation to the IS relies on the cell compartment in which the protein locates.
Results
Influence of protein domains on mTIM-1 subcellular distribution
We previously observed that mTIM-1 is mainly inside transfected cells (27), whereas MILIBS mutants that do not bind PtdSer are on the cell surface (10). These differences in cell trafficking might be linked to two distinct TIM-1 conformations on membrane surfaces (Supplementary Fig. 1A). The PtdSer-bound “bent” TIM-1 is thought to be mainly intracellular, whereas an extended form of the protein resides at the cell surface (Supplementary Fig. 1A). Polymorphisms in mTIM-1 mucin and signal peptide are supposed to affect protein conformation and cell trafficking (10).
Here we used flow cytometry to analyze the contribution of the distinct protein domains to TIM-1 subcellular distribution in the 300.19 preB cell line. In 300.19 cells transfected with mTIM-1-YFP (yellow fluorescent protein fused to the mTIM-1 C terminus)(Fig. 1A), mTIM-1 3B3 mAb analysis showed ~20% of the mTIM-1-YFP on the surface of 30% of cells (Fig. 1B). In contrast, 100% of the MILIBS mutant (ND/AA) was found on the cell surface. We prepared TIM-1-YFP fusion proteins with substitutions or deletions in the signal peptide, mucin, or cytoplasmic regions (Supplementary Fig. 1B), and analyzed its subcellular distribution in transfected 300.19 cells (Fig. 1A, 1B). A protein mutant (mT1(IgV)-IC1) with the mTIM-1 IgV domain at the N terminus of intercellular adhesion molecule-1 (ICAM-1) was located mainly on the cell surface, indicating that the IgV domain is insufficient to maintain the protein within intracellular compartments. Replacement of the endogenous signal peptide and inclusion of an HA peptide at the TIM-1 N terminus (SpHA mutant) doubled the amount of cell surface protein (Fig. 1B). A similar phenotype was observed after deletion of the TIM-1 cytoplasmic tail (Fig. 1A, 1B), which could indicate defective mTIM-1 endocytosis (28). These data show that the TIM-1 MILIBS and mucin domain critically influence its subcellular distribution, whereas extension of the N-terminal region and deletion of the cytoplasmic tail partially reduced protein sorting to intracellular compartments.
Figure 1. Influence of mTIM-1 domains on its subcellular distribution.
A. Fluorescence of 300.19 preB cells transfected with indicated YFP-tagged mTIM-1 (see Supplementary Fig. 1B); wild type protein (mTIM-1), mTIM-1 with an exogenous signal peptide (SpHAmut), a MILIBS mutant (ND/AA) of mTIM-1 (10), a fusion with the mTIM- 1 IgV domain at the ICAM-1 N terminus (mT1(IgV)-IC1), and mTIM-1 lacking the cytoplasmic domain (ΔCyt). Confocal images are shown of representative cells. Bar = (5 μm). B. Quantification of mTIM-1 protein localization in transfected 300.19 cells using flow cytometry. Mean fluorescent intensity (MFI, top) or percentage of stained cells (bottom) with mTIM-1 3B3 mAb in permeabilized (total cell protein) and non-permeabilized (cell surface protein) conditions. Cell surface:cell ratios are shown above each sample. Student’s t-test; *P <0.05, **P <0.01. C. Cell surface distribution of mTIM-1 C57BL/6 and BALB/c variants. Ratios were determined as in B using the percentage of 3B3-positive cells expressing increasing amounts of mTIM-1-YFP protein. B, C. Mean + SD (n = 5).
We compared the distribution of two mTIM-1 variants (C57BL/6 and BALB/c) that differ in single residues at the signal peptide and in mucin, as well as in a 23-residue insertion/deletion in the mucin domain (8). The longer BALB/c variant was sorted more efficiently inside cells (Fig. 1C), which suggest that mucin length modulates protein distribution in the cell.
Subcellular distribution of endogenous TIM-1 in lymphocytes
We examined mouse and human TIM-1 location in naïve and activated T and B cells. Mouse naïve spleen cells expressed small amounts of TIM-1 at the surface (Fig. 2A), and the protein localized mainly inside cells. mTIM-1 expression increased after cell stimulation (Fig. 2A). Approximately 50% of T cells treated with phorbol myristate acetate (PMA) showed intracellular mTIM-1 expression. B cells exposed overnight to lipopolysaccharide (LPS) gave equivalent results (Fig. 2A).
Figure 2. Subcellular TIM-1 protein distribution in lymphocytes.
A. Intracellular mTIM-1 expression in mouse splenocytes. Flow cytometry of naïve or activated mouse splenocytes, stimulated overnight with PMA (50 ng/ml) or LPS (1 μg/ml) (see Materials and Methods). Representative histograms are shown; n = 8 (for T cells) or n = 3 (B cells). Shaded profiles represent isotype controls. B. Activated human T cells show intracellular hTIM-1 expression. Flow cytometry of naïve CD4+ T cells purified from PBMC (left, donor A) and cells from three different donors stimulated with CD3/CD28 mAb (1 μg/ml). Cells were stained for cell surface and intracellular TIM-1 expression at 5 and 7 days post-stimulation. C. TIM-1 ligands mediate increase in cell surface protein. Percentage of mTIM-1-YFP-transfected 300.19 cells stained with mTIM-1 3B3 mAb after overnight incubation with the indicated molecule; no treatment (−), isotype control (Ab), mTIM-1 RMT1-10 and 3B3 mAb, liposomes of phosphatidylcholine (PC) or a 1:1 mixture of PC and phosphatidylserine (PS). Antibody (μg/ml) and lipid (μM) concentrations in culture media are shown. Mean + SD (n ≥3).
Findings were similar for hTIM-1 in human CD4+ T cells (Fig. 2B). Naïve human CD4+ T cells express a small amount of intracellular TIM-1, which increases after CD3/CD28 antibody stimulation. Whereas we detected increased intracellular TIM-1 by day 5 post-stimulation, the protein was not detected on the plasma membrane until day 7 and was T cell-donor-dependent (Fig. 2B). Cell surface TIM-1 expression was preceded by an increase in mRNA expression (Supplementary Fig. 2). Endogenous human and mouse TIM-1 is thus located preferentially inside lymphoid cells and can access the plasma membrane after sustained cell activation.
High affinity ligands increase cell surface TIM-1 levels
TIM-1 ligands such as high affinity mAb can trigger intracellular signals that lead to T cell activation (21, 23). To analyze the effect of its ligands on intracellular or cell surface sorting of mTIM-1, we determined the amount of cell surface TIM-1-YFP after overnight treatment of transfected 300.19 cells with several ligands (Fig. 2C). Whereas only 30% of untreated cells expressed surface TIM-1, the protein was on the surface of most cells treated with the high affinity 3B3 mAb (80%) or PtdSer-bearing liposomes (90%); the percentage of cells showing surface TIM-1 was ligand concentration-dependent. At 5 μg/ml, the low affinity RMT1-10 mAb triggered some increase in surface mTIM-1, similar to PtdChol-liposomes at high concentration (Fig. 2C). Thus, only high affinity ligands notably increased (>2x) cell surface TIM-1 levels.
Characterization of the intracellular compartments to which TIM-1 locates
To test for colocalization of intracellular compartment markers with mouse and human TIM-1 in lymphoid cells, we used specific anti-marker antibodies in confocal and electron microscopy studies (Fig. 3–5). In light microscopy, a cell surface MILIBS mutant was used as a distinguishing negative control, whereas TIM-1 antibodies that bind the IgV domain were used as a positive control in colocalization experiments with TIM-1-YFP (Fig. 3). Colocalization was quantified by determining Mander’s overlap coefficient in confocal images (Fig. 4), which indicated TIM-1 accumulation in recycling, early and late endosomes. The coefficients were significantly higher for endosome markers than for markers of other subcellular compartments (P <0.001 in Student’s t-test).
Figure 3. Identification of intracellular compartments with TIM-1.
Colocalization (yellow) of human and mouse TIM-1-YFP (green) in human Jurkat and murine 300.19 preB cells with markers of intracellular compartments (red). Confocal images show cells transfected with human or mouse TIM-1-YFP, or with a hTIM-1 MILIBS mutant. Representative images from at least 3 experiments are shown. Cells were stained with hTIM-1 (1D12), mTIM-1 (3B3) or antibodies to protein markers of recycling endosomes (RE), early endosomes (EE), late endosomes (LE), lysosomes (LYS), Golgi, or endoplasmic reticulum (ER). Bar = (5 μm).
Figure 5. Electron microscopy of intracellular TIM-1.
Colocalization by immuno-EM of mTIM-1-YFP in murine 300.19 preB cells with markers of intracellular compartments (see Figure 3). Two mTIM-1- and Lamp1-positive vesicles with distinct morphology are shown at the bottom; on the right, LE with intraluminal vesicles, characteristic of multivesicular bodies (MVB). Double immunogold labeling of mTIM-1 with the RMT1-4 mAb (5 nm gold) and antibodies to protein markers of the indicated endolysosomal vesicles (10–12 nm gold). Bar = (50 nm). Representative images are shown; n = 14 (TfR), n = 17 (EEA1) or n=21 (Lamp1)
Figure 4. Colocalization of TIM-1 proteins with marker of intracellular compartments.
Confocal images (n ≥10) shown in Figure 3 were quantified by determining Mander’s overlap coefficients. Application of Costes methods gave P values ~100% except for GM130 (75%) colocalization. Wild type TIM-1 Student’s t-test; *** P <0.001.
In the 300.19 cells, mTIM-1 colocalized preferentially with transferrin receptor (TfR), a marker of recycling endosomes, and with EEA1, an early endosomal vesicle marker (Fig. 3, 4). In Jurkat T cells, hTIM-1 also colocalized with EEA1 and TfR markers; a mAb showed clear colocalization with human CD63 (Fig. 3,4), which accumulates in late endosomes and lysosomes (29). There was nonetheless no appreciable colocalization with LysoTracker Red, a marker of lysosomes and other acidic vesicles (30), or with markers of Golgi and endoplasmic reticulum. Taken together, these results indicated that in these cells, TIM-1 primarily localized to early and late endosomal compartments, and was absent from lysosomes. The MILIBS mutants were found on the cell surface and showed no preferential colocalization with any intracellular compartment marker (Fig. 3, 4).
We used immunoelectron microscopy to further study mTIM-1 location in endosomes of transfected 300.19 cells (Fig. 5). The RMT1-4 mAb showed mTIM-1 in endosomal compartments identified by specific markers. The Lamp1-positive had two distinct morphologies (Fig. 5, bottom). In their luminal side they contained either multilamellar structures commonly seen in late endosomes (LE), or numerous vesicles characteristic of LE known as multivesicular bodies (MVB) (29). TIM-1 and Lamp1 were preferentially in the luminal side of the endosomes, as reported for CD63 in LE/MVB (29). This likely indicated that the proteins became intraluminal by inward-budding of the limiting endosomal membrane (31). TIM-1 was thus located in endosomes by light (B and T cells) and by electron microscopy (B cells).
PtdSer-mediated TIM-1 traffic
TIM-1 is a PtdSer receptor (1), and TIM-1-expressing cells recognize apoptotic cells that expose PtdSer on the outer membrane leaflet (13). We used confocal microscopy to monitor TIM-1-YFP traffic in transfected 300.19 pre-B cells following interaction with apoptotic cells (Fig. 6). After interaction with apoptotic cells, however, more than 50% of the protein accumulated on the cell surface at the cell-cell contact sites (graph in Fig. 6). This result agreed with the increase in surface TIM-1 in PtdSer-treated 300.19 cells (Fig. 2C). A MILIBS mutant that does not bind PtdSer was observed on the cell surface, but did not translocate to the point of contact with apoptotic cells (Fig. 6). Some apoptotic cells not in contact with transfected 300.19 cells showed YFP-labeled vesicles (Fig. 6, center), possibly released by TIM-1-YFP-expressing cells. We identified TIM-1 in purified extracellular vesicles and exosomes in Western blot (Supplementary Fig. 3).
Figure 6. TIM-1 trafficking following recognition of apoptotic cells.
Confocal images of 300.19 preB cells transfected with mTIM-1-YFP or a MILIBS mutant (both in green) before (left) or after addition of 7AAD-labelled apoptotic 300.19 cells (red; center and right). Conjugates formed 4 h after addition of apoptotic cells are shown. The plot shows the percentage of TIM-1 protein that accumulated at the contacts of transfected and apoptotic cells. Bar = (5 μm). Student’s t-test; *** P <0.001. Representative images from 3 experiments are shown.
TIM-1 translocation to the immune synapse
hTIM-1 colocalization with TfR and CD63, markers of vesicles that translocate to the IS (32, 33), led us to analyze whether TIM-1 also polarized to the IS when expressed in T cells, for which we used Jurkat T cells transiently transfected with hTIM-1-YFP and Raji B cells loaded with Staphylococcal enterotoxin E (SEE) superantigen. Time-lapse confocal images recorded during Jurkat-Raji cell conjugate formation showed that hTIM-1-containing vesicles polarized to the cell-cell contact area in ~70% of conjugates (Fig. 7A, top; Video 1), whereas they moved randomly toward the cell surface in MILIBS mutant-transfected cells (Fig. 7A, bottom; Video 2). Approximately 50% of the TIM-1-YFP expressed in Jurkat cells accumulated at the IS at later times of conjugate formation (Fig. 7B), showing protein polarization distinct from the non-polarized distribution of the MILIBS mutant. A fraction of hTIM-1 remained at the cell surface in ~60% of Jurkat cells that formed conjugates (Fig. 7C); in 30% of these cells, hTIM-1 clustered at the cell pole distal to the IS.
Figure 7. TIM-1 trafficking to the immune synapse.
A. Live cell imaging of Jurkat T-cells transfected with hTIM-1-YFP (top) or a MILIBS mutant (bottom) protein (green), conjugated with Raji B cells (blue) in the presence of SEE superantigen. Conjugates were monitored by time-lapse confocal microscopy (Video 1 and 2). Times indicated in minutes:seconds. B. Quantification of the amount of TIM-1 at the IS. Ratio of fluorescence signal intensity proximal to the Raji cell versus total in the transfected Jurkat T-cell are plotted. TIM-1 at cell conjugates with CD3 or LFA-1 at the IS (panel C) were quantified. Student’s t-test; *** P <0.001. C. TIM-1 colocalization with CD3 at the cSMAC. Confocal microscopy images of hTIM-1-transfected Jurkat T-cells conjugated with Raji B cells alone or with SEE. Jurkat cells were transfected with hTIM-1-YFP or a MILIBS mutant (right), then stained with CD3 and a red PE-labeled secondary antibody. Merged images are shown, as well as zoom images from 3D reconstruction of the Jurkat-Raji contact zone (bottom). Z-spacing for the 3D images were 7.0, 5.5, 3.4 and 6.7 μm (left to right). D. TIM-1 does not colocalize with LFA-1 at the IS. An LFA-1 mAb and a PE-labeled secondary antibody were used as in C. Representative images of 156 conjugates with TIM-1 at the IS (~70% of the total), from 5 independent experiments. Bar = (5 μm).
Confocal sections recorded early in cell conjugate formation in the presence of SEE showed hTIM-1-YFP colocalization with CD3 at vesicles moving toward the IS (Fig. 7C). Three-dimensional reconstruction of the IS surface showed consistent CD3 and hTIM-1 colocalization at the cSMAC (Fig. 7C), where CD3 and TCR complexes accumulate (34). The TIM-1 cluster was surrounded by LFA-1 (lymphocyte function-associated antigen 1) at the peripheral (p)SMAC (Fig. 7D). No hTIM-1-YFP protein was observed at Jurkat-Raji cell contacts in the absence of SEE (Fig. 7C), when the IS does not form and CD3 is not polarized. The MILIBS mutant was distributed over the cell surface (Fig. 7) and did not localize preferentially at the cSMAC or pSMAC. The polarized TIM-1 traffic during stable IS formation thus depended on its MILIBS motif.
Distinct trafficking of cell surface and intracellular TIM-1 to the immune synapse
After we showed hTIM-1 polarization during Jurkat-Raji conjugate formation in the presence of SEE, we studied the origin of the protein that trafficked to the IS. hTIM-1 in intracellular vesicles migrated to the IS (Figure 7A and Video 1), which indicates that the protein pool in endosomal compartments translocates to the IS. To track hTIM-1 traffic in intracellular vesicles in Jurkat cells during IS formation, we monitored hTIM-1 fused to a photoactivatable green fluorescent protein (PAGFP) in time-lapse experiments (Fig. 8A). The photoactivated intracellular hTIM-1-PAGFP pool translocated to the IS during Jurkat-Raji cell conjugate formation (Fig. 8A top and Video 3). In contrast, the photoactive cell surface protein did not move to the IS during of Jurkat-Raji cell conjugate formation (Fig. 8A bottom and Video 4). In contrast to the intracellular protein pool, cell surface TIM-1 thus does not appear to translocate to the IS, but remains on the surface and can accumulate at the so-called distal pole complex (DPC) (35). A mTIM-1 protein expressed preferentially on the Jurkat cell surface is reported to be confined at the DPC during IS formation (36). In some cases in which Raji cells conjugated with Jurkat cells at a site bearing surface TIM-1-YFP, we also observed protein exclusion from the IS (Supplementary Fig. 4).
Figure 8. Distinct trafficking of cell surface and endosomal TIM-1 to the immune synapse.
A. Live cell imaging of Jurkat T cells transfected with hTIM-1 fused to a photoactivatable green fluorescent protein (hTIM-1-PAGFP), conjugated with Raji B cells in the presence of SEE (see Video 3 and 4). Fluorescence is shown pre- (−00:00) and at indicated times post-PAGFP activation for intracellular vesicles (top) and surface protein (bottom). Arrowheads mark the site of photoactivation (left). DIC/pseudocolored merged images are shown (calibration bar, right). B. Time-lapse confocal microscopy of conjugates formed by TIM-1-YFP transfected Jurkat T cells pretreated with PtdSer-liposomes and CMAC- and SEE-loaded Raji B cells (see also Video 5 and 6). Times indicate minutes:seconds. A, B. Representative images from at least 4 experiments are shown. Bar = (5 μm).
As high affinity TIM-1 ligands such as PtdSer enhanced the amount of cell surface protein (Fig. 2C, 6), we studied TIM-1 translocation to the IS in Jurkat cells treated with PtdSer-liposomes prior to conjugate formation with SEE-loaded Raji cells. In these conditions, most TIM-1-YFP was found on cell surfaces in Jurkat-Raji cell conjugates (Fig. 8B and Video 5, 6). Only a small fraction of TIM-1-YFP appeared to translocate to the IS in PtdSer-treated Jurkat cells (Fig. 8B, 1 min). In these experiments, we found that only 1 of 20 (5%) transfected Jurkat cells formed conjugates with TIM-1 at the IS. These results confirmed the finding that cell surface TIM-1 does not translocate to the IS, and further demonstrated the distinct sorting of intracellular and cell surface TIM-1 in T cells during IS formation.
Discussion
Several reports show TIM-1 association with components of the TCR complex such as membrane-bound CD3 protein and ZAP-70, a central protein kinase in TCR signaling (7, 21, 24). Here we found that TIM-1 colocalizes with CD3 in endosomes and that both translocate to the cSMAC; these results indicate that TIM-1 in the IS might participate with the TCR complex in Ag-driven T cell activation. Translocation of endosomal TIM-1 to the IS probably modulates signaling events and T cell activation (Fig. 9A, 9B). Indeed, Tyr residues in the TIM-1 cytoplasmic domain can be phosphorylated by the Src kinase Lck (21), a key component of the IS signaling layer (37). TIM-1 overexpression is reported to increase ZAP-70 phosphorylation in Jurkat cells and to enhance TCR signaling (24). Sorting of the protein to the cell membrane and to the DPC would sequester TIM-1 from the cSMAC and reduce its contribution to Ag-driven T cell activation (Fig. 9C); protein trafficking in and out the DPC is proposed to modulate T cell responses (35). In the case of mTIM-1 constructs that locate preferentially at the cell surface, TIM-1 translocation to the DPC is associated with ERM (ezrin, radixin, and moesin) proteins through a KRK motif in the cytoplasmic tail (36). This motif is not conserved in hTIM-1 (KKE), which could account for the low number of Jurkat cells with hTIM-1 protein at the DPC observed here. Association with ERM proteins might be facilitated by the extended TIM-1 conformation that accumulates on the cell surface (Supplementary Fig. 1A).
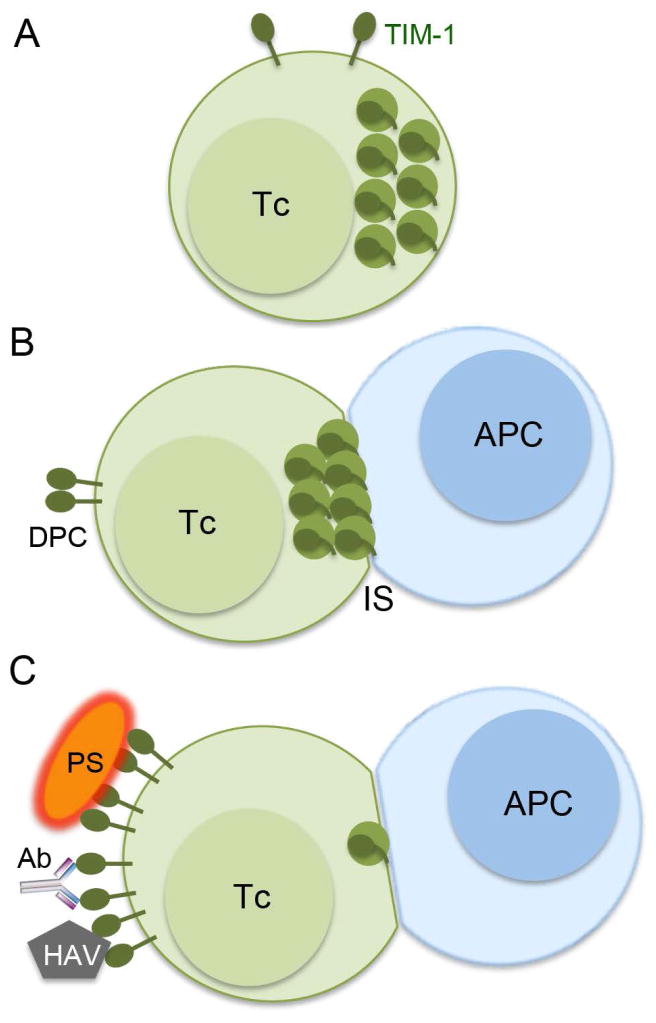
Figure 9. Subcellular TIM-1 location determines its trafficking to the immune synapse.
A. In T cells, TIM-1 is sorted to endosomal vesicles (medium green spheres). B. TIM-1 in endosomes traffics to the immune synapse (IS) during Ag presentation by the antigen-presenting cell (APC). TIM-1 at the IS contributes to Ag-driven T-cell (Tc) activation. Cell surface TIM-1 does not traffic to the IS and can cluster at the distal pole complex (DPC). C. TIM-1 ligands inhibit its traffic to the IS. High affinity TIM-1 ligands such as PtdSer (PS)-containing vesicles or enveloped viruses, high affinity Ab, or hepatitis A virus (HAV) increase cell surface TIM-1. During Ag presentation, only few TIM-1 molecules that remain in endosomes translocate to the IS.
The contribution of TIM-1 in the IS to T cell activation is thus dependent on its sorting to endosomal vesicles (Fig. 9), which here we link to the domain structure of TIM-1 and elsewhere is associated to clathrin-dependent TIM-1 endocytosis (28). Nonetheless, our data showed that mutation of the PtdSer-binding MILIBS motif drove TIM-1 to the cell surface; it was thus more important in protein sorting to endosomes than the cytoplasmic tail, whose deletion only moderately increased the amount of cell surface protein (Fig. 1). These data suggest that the TIM-1 endocytosis rate and its translocation to endosomes are determined mainly by protein conformation (10). A bent protein with its IgV domain near the membrane would enable association to other membrane proteins such as CD3 through the IgV (Supplementary Fig. 1A). Lateral association of TIM-1 with membrane proteins could drive membrane bending and facilitate its endocytosis (38). The particularly PtdSer-rich membranes in endosomal vesicles (39) also facilitate IgV binding to the endosomal membrane surface and TIM-1 accumulation in endosomes. Ligands that bind to the IgV domain and increase the amount of cell surface TIM-1 (Fig. 2C) could drive protein conformation toward the extended form, which would prevent its association with membrane proteins and thus, endocytosis. In addition, TIM-1-mediated signaling events during ligand recognition could promote TIM-1 traffic to and accumulation on the cell surface.
Here we report that in lymphoid cells, TIM-1 is located in several endosome types. It was also recently shown that TIM-1 colocalizes with the early endosome marker EEA1 in mouse T cells (20). In kidney cells, where TIM-1 promotes nur77 degradation, the protein reaches lysosomes (28). These data show the preferential location of TIM-1 in compartments of the endocytic pathway, although the amount of protein in each compartment varies with cell type. This cell-specific difference in TIM-1 subcellular location might be related to difference in function. Protein traffic to lysosomes is needed for nur77 degradation (28), whereas preferential TIM-1 location in endosomes suggests a role in sensing enveloped viruses (40, 41). In Jurkat T cells, TIM-1 colocalization with CD63 in LE indicates possible traffic to the IS (33), which we confirm here. TIM-1 translocation to the IS is similar to that described for CD63 and components of the endosomal sorting complex (33), which is driven by the reorientation of the microtubule organizing center toward the APC.
TIM-1 is in intraluminal structures at LE/MVB, which can back-fuse with the endosomal limiting membrane or be secreted as extracellular vesicles (42). Experiments with transfected and apoptotic 300.19 preB cells showed TIM-1-YFP attached to the apoptotic cells, which suggested TIM-1 release in extracellular vesicles. Western blot showed TIM-1 in purified cell culture vesicles (Supplementary Fig. 3). CD63-positive exosomes can be released during IS formation (33), and could bear TIM-1. TIM proteins might anchor extracellular vesicles to cells with surface PtdSer. The identification of TIM protein in exosomes and their role in cell-cell communication nonetheless needs further study.
Several reports showed that TIM-1 costimulates T cells by triggering phosphorylation-dependent signaling events, which can lead to expression of distinct effector cytokine profiles (21, 23, 25, 26). Strong Th2 Ag-specific responses were observed in mice treated with the low affinity RMT1-10 mAb (26) and other mTIM-1-specific mAb (25). In contrast, the 3B3 mAb combined with foreign Ag enhanced T cell proliferation in mice (23, 26), which led to Th1-biased immune responses, distinct from those induced by low affinity TIM-1 Ab. The reported diversity in TIM-1-dependent cytokine expression might be related to protein sorting either to the IS or the cell surface/DPC during Ag presentation. Differential protein accumulation at the DPC affects cytokine expression in T cells (35). High affinity ligands, such as PtdSer or 3B3 mAb, increase the amount of cell surface TIM-1 (Fig. 2C), which hinders protein translocation to the IS and probably prevents cell costimulation by TIM-1 with the TCR (Fig. 9). In agreement with this model, Jurkat cell incubation with HAV increases cell surface TIM-1, inhibits TCR-dependent signaling events and shuts off Treg cell functions (7).
Here we show bipolar TIM-1 sorting during IS formation, determined by differences in its subcellular location (Fig. 9). TIM-1 in endosomes translocates to the IS, whereas the cell surface protein does not. The location of TIM-1 at the cSMAC of the IS or at the cell surface would have different effects on T cell activation. These findings define a mechanism of immune regulation by the TIM family that is linked to its intracellular trafficking. Agents that modulate TIM-1 recycling from the cell surface could be used to regulate T cell costimulation. Certain TIM ligands might thus prevent inflammatory reactions associated with TIM-1 and related proteins.
Materials and Methods
DNA and cells
Human and mouse TIM-1 cDNAs were cloned in-frame with the yellow (TM-1-YFP) or photoactivatable green (TIM-1-PAGFP) fluorescent proteins in the pEYFP-N1 (pEYFP/TIM-1) or pPAGFP-N1 (pPAGFP/TIM-1) vectors (Clontech) essentially as described (27). MILIBS mutants lacking the metal ion coordination Asn and Asp residues (ND/AA) or the PtdSer-binding Phe and Trp residues (FW/AA) have been described (10). The mTIM-1 SpHA mutant was prepared by cloning the cDNA encoding the mature protein in-frame with the IgK leader sequence and the influenza hemagglutinin (HA) epitope in the pDisplay vector (Invitrogen). The cDNA coding for the mTIM-1 signal peptide and IgV domain was cloned in-frame with the mature ICAM-1 coding sequence to generate the mT1(IgV)-IC1 fusion protein. mTIM-1 protein lacking the complete cytoplasmic domain was engineered by cloning the cDNA that encodes the signal peptide, IgV, mucin and transmembrane domains in-frame with the YFP protein (see Supplementary Fig. 1B).
Cell culture, transfections and stimulation
Jurkat E6-1 cells were cultured in DMEM (Gibco) with 10% fetal calf serum (FCS); 300.19 preB and Raji cells were cultured in RPMI with 10% FCS. Splenocytes were isolated from BALB/c mice by centrifugation on a Lympholyte gradient (Cedarlane), then cultured in RPMI with 10% FCS and activated (37°C, overnight) with phorbol myristate acetate (PMA, 50 ng/ml) or 1 μg/ml LPS (E. coli 055:B5, Sigma). Following separation of human peripheral blood mononuclear cells (PBMC) on Ficoll-Hypaque, naïve T cells were isolated using the CD4+ T cell isolation kit with CD45RO microbeads (Miltenyi Biotec). The negative column fraction contained >95% CD4+CD45RA+ cells as determined by flow cytometry (not shown). For CD3/CD28 stimulation, naïve CD4+ T cells were cultured for indicated times on plates coated with 1 μg/ml anti-CD3 (UCHT1, BD Biosciences) in DMEM with 10% FCS and 1 μg/ml soluble anti-CD28.
Both 300.19 preB cells and Jurkat cells (6.25 × 106 cell/ml) in RPMI were transfected by electroporation with ~15 μg of pEYFP/TIM-1 or pPAGFP/TIM-1 plasmids. Transfected 300.19 cells were treated with TIM-1 ligands in 24-well plates (5 × 105 cells/well). Ligands were added to wells at 6 h post-transfection and incubated overnight prior to flow cytometry. Liposome preparation has been described (10). Dead cells and debris were removed from transfected Jurkat cells on a Lympholyte gradient 2–3 h before co-culture with Raji cells for IS analysis.
Flow cytometry
For detection of hTIM-1 expression, freshly isolated CD4+ T cells were blocked with human IgG (10 μg/ml; Jackson ImmunoResearch) and stained for hTIM-1 with 1D12 mAb and phycoerythrin (PE)-conjugated secondary antibodies. For intracellular staining, we used the BD Cytofix/Cytoperm kit with Golgi Plug (BD Pharmingen). Analytical flow cytometry was carried out on a FACScan or Canto instrument (Becton Dickinson) and data were processed with FlowJo Software (Tree Star).
To detect mTIM-1, we used the mTIM-1 3B3 mAb and an Alexa647-conjugated secondary antibody (Invitrogen), or a biotin-RMT1-4 mAb and streptavidin-Pacific Blue (BioLegend). In mouse splenocytes, anti-mouse CD3e-FITC antibody or CD19-APC antibody (Coulter) was used to identify CD3+ or CD19+ cells. Cell surface and total mTIM-1 were determined by flow cytometry of untreated or permeabilized cells, respectively. Cells fixed with 2% paraformaldehyde (PFA) at 4°C were permeabilized by 0.1% saponin treatment (room temperature, 5 min), and incubated with primary antibodies in phosphate-buffered saline (PBS) with 0.6% goat serum (4°C, 1 h). Flow cytometry was carried out with Cytomix 500FC and in XL-MCL cytometers. Cell samples were analyzed with CxP software.
Fluorescence confocal microscopy
To assess TIM-1-YFP colocalization with markers of intracellular compartments, we used 300.19 and Jurkat cells plated on poly-L-lysine (50 μg/ml)-coated slides. Cell were fixed with 4% PFA, permeabilized with 0.1% saponin, blocked with 2% goat serum in PBS, and stained with antibodies to subcellular compartment markers, followed by Alexa647-secondary antibodies (Invitrogen). We used the following primary antibodies: mTIM-1 3B3 mAb, hTIM-1 1D12 and 2E4 mAb (eBiosciences), hTfR mAb (Zymed), mTfR polyclonal Ab (Abcam), EEA1 polyclonal Ab (Abcam), hCD63 TEA 3/18 mAb (kindly provided by Dr. F. Sánchez-Madrid), Golgi matrix protein 130 kDa (GM130, BD) and KDEL mAb (Enzo). LysoTracker Red DND-99 was used at 100 nM (Invitrogen).
TIM-1 colocalization with CD3 and LFA-1 at the IS was detected in cell conjugates of hTIM-1-YFP-transfected Jurkat and Raji cells preloaded with CellTracker Blue CMAC (10 μM; Invitrogen) alone or with SEE (1 μg/ml). To prepare cell conjugates, equal numbers of Jurkat and Raji cells were mixed and plated on poly-L-lysine-coated coverslips; the reaction was terminated with 4% PFA at 5, 15, 30, 45 or 60 min. For immunostaining, we used CD3 OKT3 mAb (Thermo Scientific) and LFA1 TS2.4 mAb, followed by Alexa647-labeled secondary Ab.
For live cell imaging of IS formation, we used hTIM-1-YFP-transfected Jurkat cells adhered to poly-L-lysine-coated 8-well chambered coverslips (Ibidi GmbH). SEE-preloaded Raji cells were added to chambers and interaction with Jurkat cells tracked by time-lapse confocal microscopy (elapsed time 30 s). In experiments with liposome-treated transfected Jurkat cells, the cells were incubated overnight with liposomes (250 μM) prepared with an equimolar mixture of PtdSer and phosphatidylcholine, prior to Raji cell addition. In photoactivation experiments, Raji cells were preloaded with CellTrace Calcein Red-Orange (2 μM; Invitrogen) and SEE (1 μg/ml) and plated with pPAGFP/hTIM-1-transfected Jurkat cells.
mTIM-1-YFP-transfected and untransfected apoptotic 300.19 cells were co-incubated (37°C, 4 h) and conjugates analyzed. Apoptotic 300.19 cells were obtained by overnight treatment with 50 μM etoposide in 24-well plates (106 cells/ml). Apoptotic cells were labeled with 7AAD (eBioscience).
For fluorescence microscopy, we used a Leica SP5 confocal scanning laser microscope equipped with an x63/1.4 NA oil immersion objective. TIM-1-YFP was excited with a 514 nm line and fluorescence detected with a 525–586 nm bandwidth, whereas a 633 nm line and a 665–758 nm bandwidth were used for Alexa647. TIM-1-PAGFP was photoactivated with 20 pulses of a 405 nm diode laser (100% output), and images were acquired every 5 s by excitation at 488 nm. Fluorescence emission was detected using a 498–565 nm bandwidth.
Image analysis, 3D reconstructions of confocal sections and Mander’s overlap coefficients were obtained using ImageJ software (NIH). The 3D reconstructions shown in Figure 7 contained assembled confocal sections separated 0.4 μm in the z-axis.
Immunoelectron microscopy
Colocalization of mTIM-1 and markers of subcellular compartments was studied in 300.19 cells by electron microscopy (EM) using double immunogold labeling. Transfected cells were fixed with 4% PFA and 0.1% glutaraldehyde (GA) in 60 mM PIPES, 25 mM HEPES, 10 mM EGTA and 2 mM MgCl2 (0.1 M PHEM buffer pH 6.9, 30 min), then incubated overnight in 1% PFA in the same buffer. Samples were resuspended in 12% gelatin in PBS and infiltrated with 2.3 M sucrose (overnight, 4°C) for cryo-protection. Frozen samples were trimmed at −90°C in a cryo-ultramicrotome (UC6/FC6, Leica Microsystems) and ~70 nm ultrathin cryo-sections were prepared at −110°C with a cryo-Immuno diamond knife (DiATOME). Sections were picked up in a drop containing 2% methylcellulose and 2.3 M sucrose (v/v 1:1), warmed to room temperature and transferred to Formvar film-coated, carbon-stabilized palladium-copper grids (Agar Scientific).
For immunogold labeling, grids were blocked with 1.5% BSA and 0.1% Fish Skin Gelatin (FSG) and then with mTIM-1 RMT1-4 mAb (25 μg/ml) in PBS with 1.5% BSA. After PBS washing, grids were incubated (20 min) with a rabbit anti-rat linker (Rockland), washed five times with PBS, and incubated (20 min) with 5 nm colloidal gold-labeled protein-A in PBS with 1.5% BSA. Sections were further fixed with 1% GA in PBS (5 min), washed 5 times with PBS, quenched with 50 mM glycine and blocked with 1.5% BSA and 0.1% FSG in PBS (15 min). Sections were incubated with antibodies to protein markers of subcellular compartments (see confocal microscopy section) or Lamp1 polyclonal Ab (Abcam). After washing with PBS, sections were incubated (20 min) with donkey anti-mouse conjugated to 12 nm colloidal gold (Jackson ImmunoResearch) for Lamp1 labeling, and with protein-A 10 nm colloidal gold for remaining markers. Sections were fixed again, washed, and embedded in a thin film of 2% methylcellulose containing 0.3% uranyl acetate. For imaging, samples were analyzed in a Biotwin CM120 Philips electron microscope.
Extracellular vesicles and exosome isolation
Extracellular vesicles were prepared by differential centrifugation of cell cultures of TIM-1-YFP-transfected 300.19 cells (2 × 107), in media depleted of exosomes by ultracentrifugation (100,000 g, 3 h). At 24 h post-transfection, cell cultures were harvested and cells sedimented (750 g, 15 min). Dead cells and debris were removed from supernatants by centrifugation (10,000 g, 45 min). Supernatants were filtered through 0.22 μm membranes, then extracellular vesicles and exosomes were pelleted by ultracentrifugation (100,000 g, 2 h). Cells and exosomes were resuspended in lysis buffer (20 mM Tris pH 8, 150 mM NaCl, 1% NP40, 10% glicerol and protease inhibitors), and TIM-1-YFP in samples was identified in Western blot with an anti-GFP mAb (Roche).
Supplementary Material
Synopsis of Content.
The T-cell costimulatory molecule TIM-1 sorts mainly to endosomes in lymphoid cells. At difference from the cell surface protein, endosomal TIM-1 translocates to the immune synapse (IS), where it can contribute to antigen-dependent T-cell costimulation. TIM-1 ligands increase the amount of cell surface protein, preventing its traffic to the IS. The bipolar sorting of TIM-1 observed during IS formation is determined by differences in its subcellular location, and probably modulates antigen-driven immune responses.
Acknowledgments
We thank I Merida and F Sánchez-Madrid for materials, S Gutiérrez for confocal services, the CNB cytometry unit, and C Mark for editorial assistance. ME received a FPI fellowship from the Spanish Ministry of Sciences. PRN is supported by the Ramón y Cajal program (RYC-2011-08664). This research was supported by a grant from the US National Institutes of Health (P01AI054456 to JMC, RDK and GGK).
References
- 1.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Manzanet R, DeKruyff R, Kuchroo VK, Umetsu DT. The costimulatory role of TIM molecules. Immunoll Rev. 2009;229:259–270. doi: 10.1111/j.1600-065X.2009.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kane LP. T Cell Ig and Mucin Domain Proteins and Immunity. J Immunol. 2010;184(6):2743–2749. doi: 10.4049/jimmunol.0902937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 5.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD, Hirashima M, Uede T, Takaoka A, Yagita H, Jinushi M. Tumor-infiltrating DCs suppress nucleic acid–mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13:832–842. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manangeeswaran M, Jacques J, Tami C, Konduru K, Amharref N, Perrella O, Casasnovas JM, Umetsu DT, DeKruyff RH, Freeman GJ, Perrella A, Kaplan GG. Binding of Hepatitis A Virus to Its Cellular Receptor 1 Inhibits T-Regulatory Cell Functions in Humans. Gastroenterology. 2012;142:1516–1525. e1513. doi: 10.1053/j.gastro.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McIntire JJ, Umetsu SE, Akbari O, Potter M, Kuchroo VK, Barsh GS, Freeman GJ, Umetsu DT, DeKruyff RH. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol. 2001;2:1109–1116. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- 9.McIntire JJ, Umetsu SE, Macaubas C, Hoyte EG, Cinnioglu C, Cavalli-Sforza LL, Barsh GS, Hallmayer JF, Underhill PA, Risch NJ, Freeman GJ, DeKruyff RH, Umetsu DT. Hepatitis A virus link to atopic disease. Nature. 2003;425(6958):576–576. doi: 10.1038/425576a. [DOI] [PubMed] [Google Scholar]
- 10.Santiago C, Ballesteros A, Martinez-Munoz L, Mellado M, Kaplan GG, Freeman GJ, Casasnovas JM. Structures of T Cell Immunoglobulin Mucin Protein 4 Show a Metal-Ion-Dependent Ligand Binding Site where Phosphatidylserine Binds. Immunity. 2007;27:941–951. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeKruyff RH, Bu X, Ballesteros A, Santiago C, Chim Y-LE, Lee H-H, Karisola P, Pichavant M, Kaplan GG, Umetsu DT, Freeman GJ, Casasnovas JM. T Cell/Transmembrane, Ig, and Mucin-3 Allelic Variants Differentially Recognize Phosphatidylserine and Mediate Phagocytosis of Apoptotic Cells. J Immunol. 2010;184:1918–1930. doi: 10.4049/jimmunol.0903059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 13.Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, et al. TIM-1 and TIM-4 Glycoproteins Bind Phosphatidylserine and Mediate Uptake of Apoptotic Cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama M, Akiba H, Takeda K, Kojima Y, Hashiguchi M, Azuma M, Yagita H, Okumura K. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 2009;113(16):3821–3830. doi: 10.1182/blood-2008-10-185884. [DOI] [PubMed] [Google Scholar]
- 16.Wong K, Valdez PA, Tan C, Yeh S, Hongo J-A, Ouyang W. Phosphatidylserine receptor Tim-4 is essential for the maintenance of the homeostatic state of resident peritoneal macrophages. Proc Natl Acad Sci USA. 2010;107:8712–8717. doi: 10.1073/pnas.0910929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Manzanet R, Sanjuan MA, Wu HY, Quintana FJ, Xiao S, Anderson AC, Weiner HL, Green DR, Kuchroo VK. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proc Natl Acad Sci USA. 2010;107:8706–8711. doi: 10.1073/pnas.0910359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, Chalasani G, Sayegh MH, Najafian N, Rothstein DM. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan G, Totsuka A, Thompson P, Akatsuka T, Moritsugu Y, Feinstone SM. Identification of a surface glycoprotein on african green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 1996;15:4282–4296. [PMC free article] [PubMed] [Google Scholar]
- 20.Angiari S, Donnarumma T, Rossi B, Dusi S, Pietronigro E, Zenaro E, DellaBianca V, Toffali L, Piacentino G, Budui S, Rennert P, Xiao S, Laudanna C, Casasnovas JM, Kuchroo VK, et al. TIM-1 Glycoprotein Binds the Adhesion Receptor P-Selectin and Mediates T Cell Trafficking during Inflammation and Autoimmunity. Immunity. 2014;40:542–553. doi: 10.1016/j.immuni.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Souza AJ, Oriss TB, O’Malley KJ, Ray A, Kane LP. T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation. Proc Natl Acad Sci USA. 2005;102:17113–17118. doi: 10.1073/pnas.0508643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyers JH, Chakravarti S, Schlesinger D, Illes Z, Waldner H, Umetsu SE, Kenny J, Zheng XX, Umetsu DT, DeKruyff RH, Strom TB, Kuchroo VK. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6:455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 23.Umetsu SE, Lee WL, McIntire JJ, Downey L, Sanjanwala B, Akbari O, Berry GJ, Nagumo H, Freeman GJ, Umetsu DT, DeKruyff RH. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol. 2005;6:447–454. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- 24.Binne LL, Scott ML, Rennert PD. Human TIM- 1 Associates with the TCR Complex and Up-Regulates T Cell Activation Signals. J Immunol. 2007;178:4342–4350. doi: 10.4049/jimmunol.178.7.4342. [DOI] [PubMed] [Google Scholar]
- 25.Sizing ID, Bailly V, McCoon P, Chang W, Rao S, Pablo L, Rennard R, Walsh M, Li Z, Zafari M, Dobles M, Tarilonte L, Miklasz S, Majeau G, Godbout K, et al. Epitope-Dependent Effect of Anti-Murine TIM-1 Monoclonal Antibodies on T Cell Activity and Lung Immune Responses. J Immunol. 2007;178:2249–2261. doi: 10.4049/jimmunol.178.4.2249. [DOI] [PubMed] [Google Scholar]
- 26.Xiao S, Najafian N, Reddy J, Albin M, Zhu C, Jensen E, Imitola J, Korn T, Anderson AC, Zhang Z, Gutierrez C, Moll T, Sobel RA, Umetsu DT, Yagita H, et al. Differential engagement of Tim-1 during activation can positively or negatively costimulate T cell expansion and effector function. J Exp Med. 2007;204:1691–1702. doi: 10.1084/jem.20062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santiago C, Ballesteros A, Tami C, Martinez-Munoz L, Kaplan GG, Casasnovas JM. Structures of T Cell Immunoglobulin Mucin Receptors 1 and 2 Reveal Mechanisms for Regulation of Immune Responses by the TIM Receptor Family. Immunity. 2007;26:299–310. doi: 10.1016/j.immuni.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balasubramanian S, Kota SK, Kuchroo VK, Humphreys BD, Strom TB. TIM Family Proteins Promote the Lysosomal Degradation of the Nuclear Receptor NUR77. Sci Signal. 2012;5:ra90. doi: 10.1126/scisignal.2003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pols MS, Klumperman J. Trafficking and function of the tetraspanin CD63. Exp Cell Res. 2009;315:1584–1592. doi: 10.1016/j.yexcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Spence MTZ, Carlsbad CA. Probes for organelles. In: Haugland RP, editor. The Handbook – a guide to fluorescence probes and labeling technologies 10th ed: Molecular Probes. 2005. pp. 580–583. [Google Scholar]
- 31.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10:623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 32.Das V, Nal B, Dujeancourt A, Thoulouze MI, Galli T, Roux P, Dautry-Varsat A, Alcover A. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity. 2004;20:577–588. doi: 10.1016/s1074-7613(04)00106-2. [DOI] [PubMed] [Google Scholar]
- 33.Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, González S, Sánchez-Cabo F, González MÁ, Bernad A, Sánchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Comm. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dustin ML, Depoil D. New insights into the T cell synapse from single molecule techniques. Nat Rev Immunol. 2011;11:672–684. doi: 10.1038/nri3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cullinan P, Sperling AI, Burkhardt JK. The distal pole complex: a novel membrane domain distal to the immunological synapse. Immunol Rev. 2002;189:111–122. doi: 10.1034/j.1600-065x.2002.18910.x. [DOI] [PubMed] [Google Scholar]
- 36.Lin J, Chen L, Kane LP. Murine Tim-1 is excluded from the immunological synapse. F1000Research. 2012;1:10. doi: 10.12688/f1000research.1-10.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dustin ML. T-cell activation through immunological synapses and kinapses. Immunol Rev. 2008;221:77–89. doi: 10.1111/j.1600-065X.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- 38.Stachowiak JC, Brodsky FM, Miller EA. A cost-benefit analysis of the physical mechanisms of membrane curvature. Nat Cell Biol. 2013;15:1019–1027. doi: 10.1038/ncb2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S. Membrane Phosphatidylserine Regulates Surface Charge and Protein Localization. Science. 2008;319:210–213. doi: 10.1126/science.1152066. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura N, Lill JR, Phung Q, Jiang Z, Bakalarski C, de Mazière A, Klumperman J, Schlatter M, Delamarre L, Mellman I. Endosomes are specialized platforms for bacterial sensing and NOD2 signalling. Nature. 2014;509:240–244. doi: 10.1038/nature13133. [DOI] [PubMed] [Google Scholar]
- 41.Jemielity S, Wang JJ, Chan YK, Ahmed AA, Li W, Monahan S, Bu X, Farzan M, Freeman GJ, Umetsu DT, DeKruyff RH, Choe H. TIM-family Proteins Promote Infection of Multiple Enveloped Viruses through Virion-associated Phosphatidylserine. PLoS Pathog. 2013;9:e1003232. doi: 10.1371/journal.ppat.1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bissig C, Gruenberg J. ALIX and the multivesicular endosome: ALIX in Wonderland. Trends Cell Biol. 2014;24:19–25. doi: 10.1016/j.tcb.2013.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.