Abstract
HIV-induced peripheral neuropathy is the most common neurological complication associated with HIV infection. In addition to virus-mediated injury of the peripheral nervous system (PNS), treatment of HIV infection with combination antiretroviral therapy (cART) may induce toxic neuropathy as a side effect. Anti-retroviral toxic neuropathy is clinically indistinguishable from the sensory neuropathy induced by HIV; in some patients, these two processes are likely superimposed. To study these inter-current PNS disease processes, we first established a simian immunodeficiency virus (SIV)/pigtailed macaque model in which over 90 percent of animals developed PNS changes closely resembling those seen in HIV-infected individuals with distal sensory neuropathy. To determine whether cART alters the progression of SIV-induced PNS damage, dorsal root ganglia and epidermal nerve fibers were evaluated in SIV-infected macaques following long-term suppressive cART. Although cART effectively suppressed SIV replication and reduced macrophage activation in the dorsal root ganglia, PGP 9.5 immunostaining and measurements of epidermal nerve fibers in the plantar surface of the feet of treated SIV-infected macaques clearly showed that cART did not normalize epidermal nerve fiber density. These findings illustrate that significant PNS damage persists in SIV-infected macaques on suppressive cART.
Keywords: Antiretroviral therapy, Epidermal nerve fibers, Human immunodeficiency virus (HIV), Macaque, Neuropathy, Simian immunodeficiency virus (SIV)
INTRODUCTION
Peripheral nervous system damage continues to be a clinically challenging aspect of the HIV/AIDS epidemic despite widespread use of suppressive combination antiretroviral therapy (cART). Peripheral neuropathy frequently develops in HIV-infected patients including those receiving antiretroviral therapy (1–4). The most common clinical manifestation of HIV-induced peripheral nervous system disease is distal sensory polyneuropathy, a debilitating syndrome characterized by gradual onset of bilateral pain and hypersensitivity most pronounced in the soles of the feet (2, 5, 6). Although HIV-induced peripheral neuropathy is not life threatening, the symptoms are often poorly responsive to conventional analgesics, and the relentless pain markedly impacts patients’ quality of life (1, 7–9).
The reported incidence of HIV neuropathy in cART-treated individuals varies from 13% to 52% depending on the cohort evaluated and the diagnostic criteria used in the study (2, 5, 10–12). Numerous reports have demonstrated that treatment of HIV infection with nucleoside reverse transcriptase inhibitors including zacitabine, stavudine, and didanosine, as well as certain protease inhibitors, may induce peripheral neuropathy as a neurotoxic side effect (13–17). The resulting clinical syndrome, known as anti-retroviral toxic neuropathy, is clinically indistinguishable from HIV-mediated peripheral neuropathy and, therefore, confounds clinically based studies of the pathogenesis of HIV-associated peripheral nervous system (PNS) disease.
The extensive use of cART in HIV-infected patients necessitates the development of animal models to determine the mechanisms underlying PNS damage and to dissect out the effects of HIV infection itself from the consequences of potentially neurotoxic antiretroviral drugs (3, 18, 19). To study the pathogenesis of HIV-induced PNS disease, our group developed a simian immunodeficiency virus (SIV)-infected macaque model that closely recapitulates key PNS alterations seen in HIV patients with distal sensory polyneuropathy. These changes include inflammatory cell infiltration, SIV replication within macrophages, and neuronal loss in the dorsal root ganglia (DRG), which house the cell bodies of peripheral sensory nerves (1, 20). Epidermal nerve fiber (ENF) loss also develops with SIV infection, resulting in a significant decline in intra-ENF density in SIV-infected macaques when compared to uninfected, age-matched animals (21). This striking loss of small sensory fibers in SIV-infected macaques parallels ENF decline in HIV-infected patients. Importantly, these findings also set the stage for examining neurotoxic damage caused by cART regimens in the SIV/macaque model. In the present study, we delivered cART therapy composed of 1 nucleoside reverse transcriptase inhibitor, 2 protease inhibitors, and an integrase inhibitor to pigtailed macaques beginning during the acute phase of SIV infection to investigate whether early administration of cART altered the pathogenesis of PNS disease.
Materials and Methods
Animal studies
Ten pigtailed macaques were inoculated intravenously simultaneously with the neurovirulent clone SIV/17E-Fr and the immunosuppressive swarm SIV/DeltaB670(22). This SIV/macaque model has been previously characterized in detail in studies of SIV central nervous system disease pathogenesis. SIV-infected animals uniformly progress to AIDS with comparable high plasma viral loads within 12 weeks post-inoculation (p.i.) (23, 24). Beginning 12 days after p.i., 5 of the 10 animals were treated daily with a 4-drug cART until necropsy. Treatment included the nucleoside reverse transcriptase inhibitor PMPA, an analog of tenofovir (Gilead, Foster City, CA, 30 mg/kg subcutaneously once daily); protease inhibitors saquinavir (Roche, Basel, Switzerland, 205 mg/kg orally twice daily) and atazanavir (Bristol-Myers Squibb, New York, NY, 270 mg/kg orally twice daily), and the integrase inhibitor L-870812 (Merck, Kenilworth, NJ, 10 mg/kg orally twice daily), as previously described (25, 26). The other 5 SIV-inoculated animals did not receive any treatment. Five additional age-matched uninfected, untreated pigtailed macaques served as mock-inoculated virus-negative controls. Blood samples were collected at 7, 10, 14, 21, 28 days p.i., and every 2 weeks thereafter until death. Procedures were performed on animals chemically restrained with ketamine hydrochloride. Untreated SIV-infected and uninfected macaques were killed 84 days p.i.; cART-treated SIV-infected macaques were killed approximately 170 days p.i. Additional SIV-infected macaques were killed at 7, 10, 14, 21 and 35 days p.i. (6 animals/time point). At death, animals were perfused with sterile saline to remove blood and circulating virus from tissues. Lumbar (L3–L6) DRG and footpad samples were harvested from all pigtailed macaques. These studies were performed in compliance with the recommendations in the National Research Council's Guide for the Care and Use of Laboratory Animals of the National Institutes of Health following a protocol approved by the Johns Hopkins University Institutional Animal Care and Use Committee (JHU IACUC, PR09M296).
ENF Immunohistochemistry
To measure ENF density in the skin of the plantar footpad surface of the hind limb, 3-mm-diameter punch footpad samples were obtained at necropsy from the identical site on the metatarsal footpad between the third and fourth digits. Footpad skin sections were fixed for 12 to 24 hours in 2% paraformaldehyde/lysine/periodate fixative at 4°C, rinsed with 0.08 M Sorensen’s phosphate buffer, and then transferred to cryoprotective buffer (20% glycerol in 0.08 M Sorensen’s phosphate buffer) until processed as previously described (27). Cryoprotected samples were sectioned at a thickness of 50 µm on a sliding microtome and then immunostained for PGP9.5, a pan-axonal marker (1:10,000, ABD Serotec, Oxford, UK), as previously described (28, 29).
ENF Density Measurement
Epidermal nerve fiber density was measured using a modification of the method utilized by Kennedy et al (30) and McCarthy et al (28). Briefly, 15 adjacent, non-overlapping collapsed z-stack images were obtained for each PGP9.5-immunostained skin section. Serial z-stack images for each microscopic field were collected at 0.5-µm intervals using 400× magnification on a Zeiss microscope equipped with a z-motor. PGP9.5 immunoreactivity in the collapsed z-stack images was then measured by digital image analysis using iVision software (BioVision Technologies, Exton, PA) (23). Results were normalized to the thickness of each skin sample (the z distance of the stack) for each image to control for any variations in the thickness of immunostained skin sections.
ENF Length Measurement
Epidermal nerve fiber lengths from footpad biopsies were quantified with unbiased stereology methodology utilizing the space ball probe of Stereo Investigator software (MBF Bioscience, Williston, VT) (31–33). Briefly, 3 50-µm-thick PGP9.5-immunostained sections from 1 plantar footpad biopsy from each animal were measured. The area of interest was defined as the epidermal region extending from the epidermal/dermal junction to the stratum corneum and was drawn under a x2.50/0.075 Plan-Neofluor objective of a Zeiss Axiophot light microscope. The ENF length was measured under a x63/1.40 oil Plan-Neofluor objective. The radius of the hemisphere probe was 30 µm with a guard zone of 2 µm. Only nerve fibers within the epidermis and clearly crossing the epidermal/dermal junction were measured. The results were expressed as the length of PGP9.5-positive nerve fibers per cubic millimeter of epidermal tissue. All stereology measurements were obtained using DAT files of Stereo Investigator.
DRG Immunohistochemistry
Immunohistochemistry was performed on Streck-fixed, paraffin-embedded sections of DRG from SIV-infected and control animals. Tissue sections were deparaffinized in changes of Histo-Clear (National Diagnostics, Atlanta, GA), and then rehydrated in a gradient series of alcohol. After antigen retrieval in sodium citrate buffer for 8 minutes, sections were washed and then blocked against endogenous peroxidase followed by incubation in the appropriate antibody dilution (anti-CD68, 1:2000, clone KP1 or anti-glial fibrillary acidic protein [GFAP], 1:4,000, Z0334, DAKO, Carpinteria, CA) for 1 hour at room temperature. Sections were then incubated sequentially in biotinylated secondary multilink antibody and horseradish peroxidase labeled streptavidin (Biogenex, San Ramon, CA). The chromogen reaction was subsequently detected by incubating the sections in substrate 3,3’-diaminobenzidine. The washed and cleared tissue sections were then coverslipped with permount. The amount of immunostaining for the macrophage marker CD68 and the satellite cell activation marker GFAP was measured by digital image analysis, as described previously (23). Twenty non-overlapping adjacent fields at 200× magnification were captured with a Retiga 2000R digital camera mounted on a Nikon E600 microscope. Binarized images were then analyzed using iVision imaging software (BioVision Technologies). The mean total area occupied by immunopositive pixels in the DRG was then calculated for each animal.
Viral Load in DRG
To measure SIV RNA levels, quantitative RT-PCR was performed on RNA isolated from DRG. At postmortem, samples were snap frozen in liquid nitrogen for RNA isolation, as previously described (26). RNA was isolated from 25 mg of tissue using the RNA STAT-60 Kit (Tel-Test Inc., Friendswood, TX) and an RNeasy MiniKit (Qiagen, Valencia, CA). Quantitation of SIV RNA in extracted DRG tissue was done with SIV gag region primers and probe. Real time RT-PCR detected both cell-associated full-length viral transcripts and genomic RNA present in tissue-associated virions.
Statistical Analysis
All statistical inferences (p values) were calculated using non-parametric methods and GraphPad Prism software (Version 5.0d). When analysis was limited to 2 groups, comparisons were made using the Mann-Whitney test; the Kruskal-Wallis test was used to compare 3 or more groups. Dunn’s multiple comparison test utilizing an adjusted p value was then performed for pairwise comparisons. The Spearman correlation coefficient was used to analyze the relationships between continuous variables. In all analyses, statistical significance was defined as a p value of less than 0.05.
RESULTS
cART Reduces Plasma Viral Load
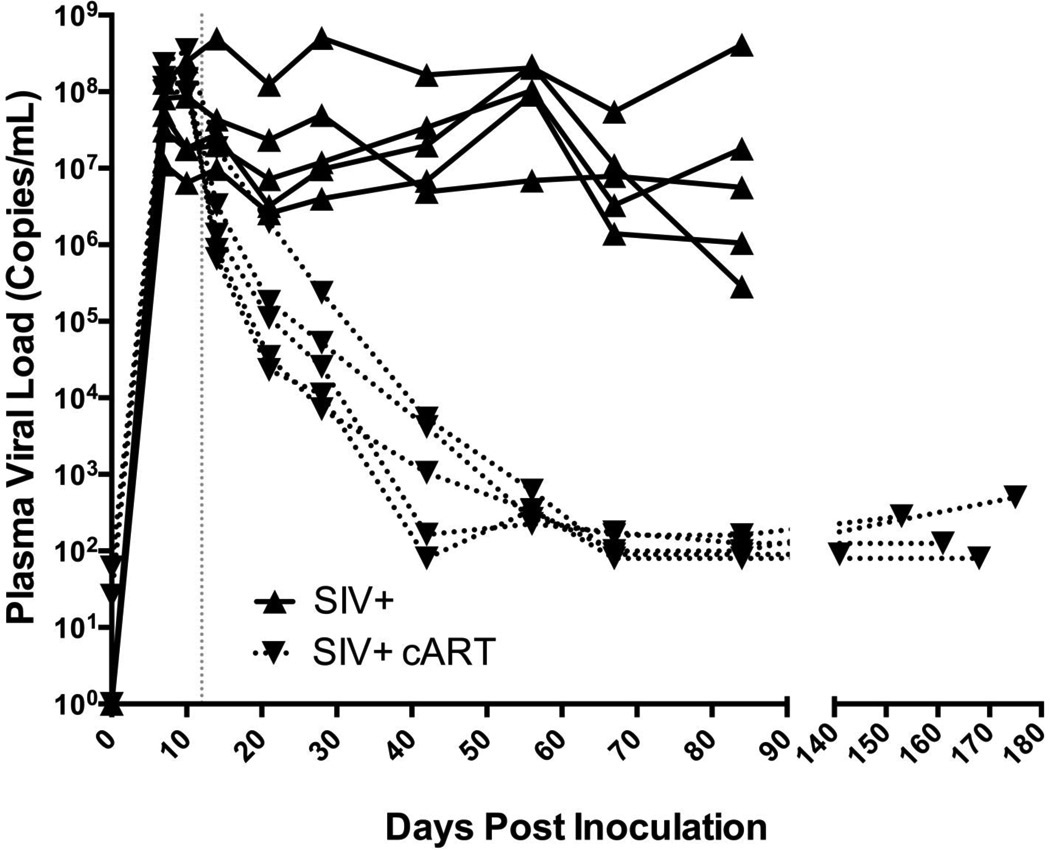
To compare plasma SIV RNA levels in cART-treated SIV-infected animals vs. untreated SIV-infected macaques (the 2 SIV-inoculated groups evaluated in this study), plasma viral loads were measured by SIV RNA quantitative RT-PCR in longitudinal samples obtained from acute through terminal time points. The plasma viral load of untreated SIV-infected macaques remained high throughout infection (median terminal PVL = 5.62 × 106 c/mL); in contrast, cART therapy reduced viral loads at all time points following initiation at 12 days p.i. By day 42, there was a marked viral load reduction in all treated animals with 2 animals having viral loads at the limit of detection (100 c/mL). By day 70, viral replication had been suppressed to undetectable levels in all treated animals and remained controlled until the time of death between 140 and 180 days p.i. (Fig. 1).
Figure 1.
Simian Immunodeficiency Virus (SIV) plasma viral load over time in untreated vs. combination antiretroviral therapy (cART)-treated SIV-infected animals. Longitudinal plasma SIV RNA levels among untreated macaques (SIV+, solid line) show a markedly higher SIV set point as compared to those treated with cART (SIV+ cART, dashed line). Following initiation of cART at day 12 post-inoculation plasma SIV RNA levels showed continual decline and by day 70 SIV RNA levels were undetectable (limit of detection = 100 c/mL) in all cART-treated animals.
Altered Epidermal Nerve Fiber Density and Length Despite cART
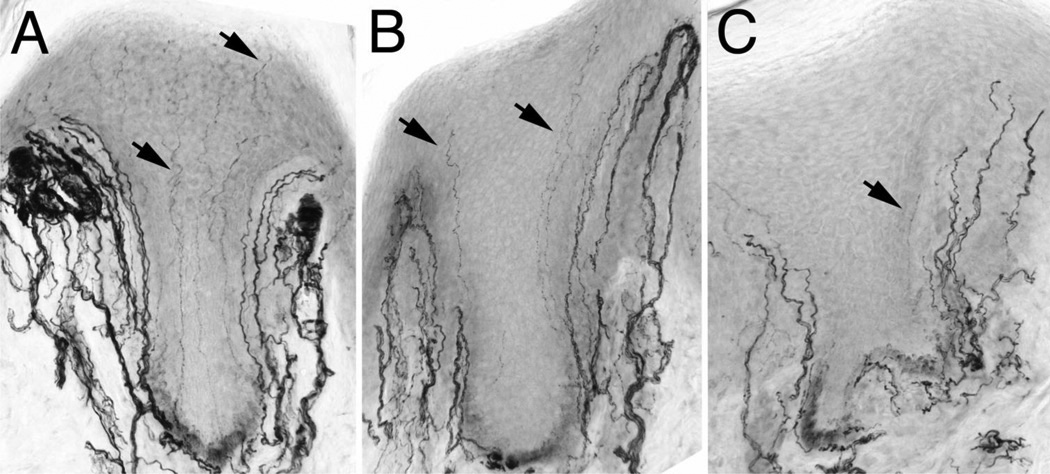
Skin biopsy is a valuable tool to diagnose small fiber neuropathies through the characterization and measurement of small unmyelinated C fiber loss, often the first clinical sign of PNS damage (34, 35). Despite the value of cART therapy in the suppression of systemic viral replication, cART has been implicated in toxic neuropathy (13, 17). Previous studies have shown significant declines in footpad ENF density among SIV-infected macaques paralleling ENF loss reported in HIV-infected individuals (21). In the present study, footpad skin biopsy was utilized to determine whether suppressive cART, initiated during acute infection, altered SIV-induced damage to sensory nerve fibers in the skin. Visual inspection of PGP9.5 immunostained fibers, a pan neuronal marker, showed a marked reduction in ENF density among SIV-infected macaques as compared to uninfected macaques (Fig. 2A, B). Interestingly, this decline was more pronounced in the cART-treated SIV-infected macaques, despite long-term suppression of systemic viral replication, with the epidermis relatively devoid of small, unmyelinated nerve fibers (Fig. 2C).
Figure 2.
PGP9.5 immunostaining of epidermal nerve fibers (ENF) in uninfected, untreated Simian Immunodeficiency Virus (SIV)-infected, and combination antiretroviral therapy (cART)-treated SIV-infected macaques. (A) Representative image of the epidermis in the footpad of an uninfected macaque depicting an abundance of long ENF (arrows) throughout the entire epidermal area. (B) In contrast, the epidermis from an untreated SIV-infected macaque depicts a marked reduction in ENF density with few fibers reaching the stratum corneum. (C) Among cART-treated SIV-infected macaques, ENF density is reduced further, evidenced by very few nerve fibers crossing the basement membrane of the epidermis.
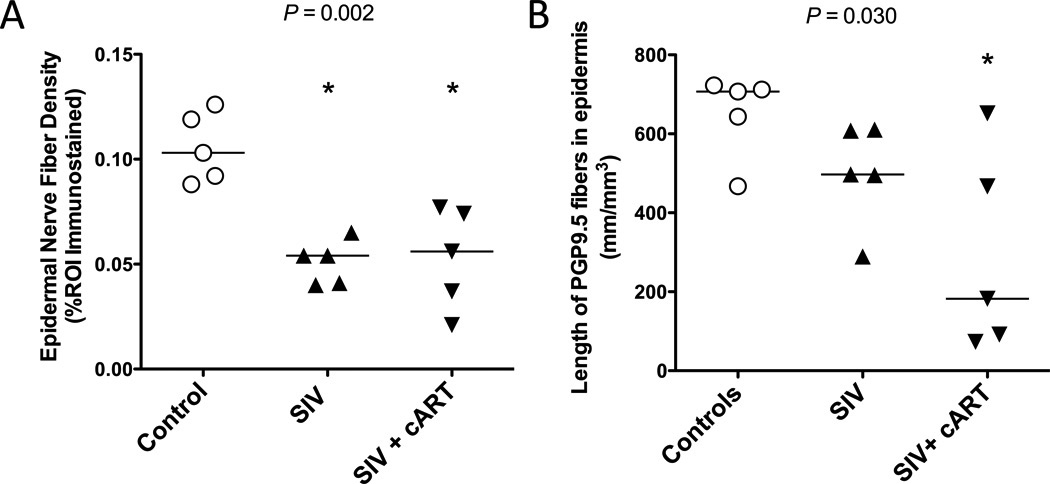
Two different methods were utilized to measure the extent of sensory nerve fiber loss in the skin among untreated and cART-treated SIV-infected macaques. The first involved measuring the area of the epidermis occupied by PGP9.5-immunostained unmyelinated nerve fibers. Utilizing this method, both untreated and cART-treated SIV-infected macaques showed a significant decline in ENF density as compared to uninfected controls (Pcontrol v SIV+ = 0.017; Pcontrol v SIV+ cART = 0.033); however, ENF density did not differ between the untreated and treated SIV-infected groups (P SIV+ v SIV+ cART > 0.99; Poverall = 0.002) (Fig. 3A).
Figure 3.
Epidermal nerve fiber (ENF) density and length are reduced in untreated and combination antiretroviral therapy (cART)-treated Simian Immunodeficiency Virus (SIV)-infected macaques. (A) Measurement of ENF density demonstrates a significantly reduced density in both untreated (triangles) (p = 0.017, Dunn*) and treated (upside-down triangles) SIV-infected macaques (p = 0.033, Dunn*) vs. controls (open circles) (Overall p = 0.002, Kruskal-Wallis). (B) While ENF length tended to be lower in both untreated and treated SIV-infected animals compared to controls (Overall p = 0.030, Kruskal-Wallis), this difference was only significant between uninfected control and cART-treated SIV-infected macaques (p = 0.033, Dunn*). Asterisks in figure denote significant pairwise comparison with the control group. *p value adjusted for multiple comparisons.
The second method of ENF quantification measured the length of PGP9.5 immunostained ENF using design-based stereology. Although SIV-infected macaques showed a decreased nerve fiber length compared to uninfected controls, the finding was not significant (Pcontrol v SIV+ = 0.41). In contrast, the cART-treated SIV-infected macaques had a significantly lower ENF length compared to control animals (Pcontrol v SIV+ cART = 0.033). Moreover, median ENF length for cART-treated animals was lower than that of SIV-infected untreated animals; however, group comparisons did not show a significant difference (P SIV+ v SIV+ cART = 0.87; Poverall = 0.030) (Fig. 3B).
cART Reduces SIV Replication and Macrophage Activation in the DRG
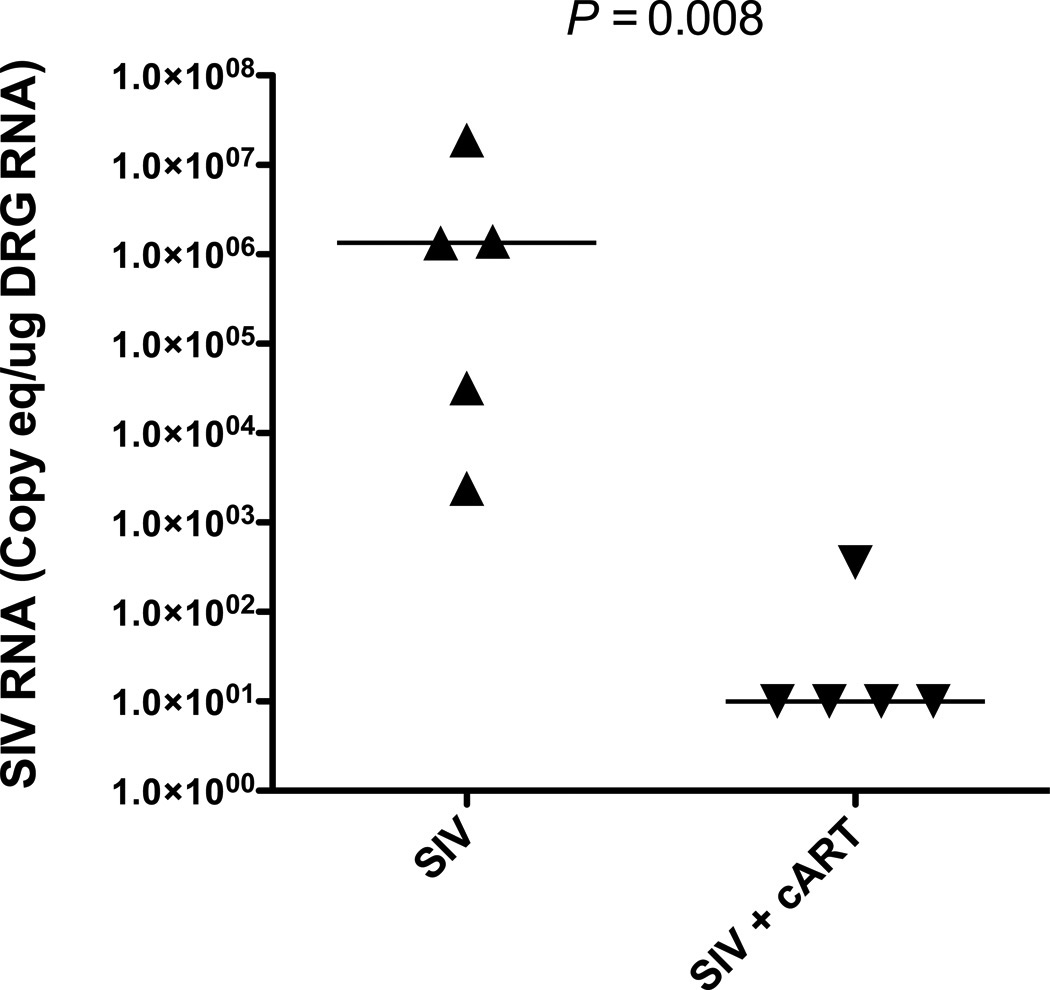
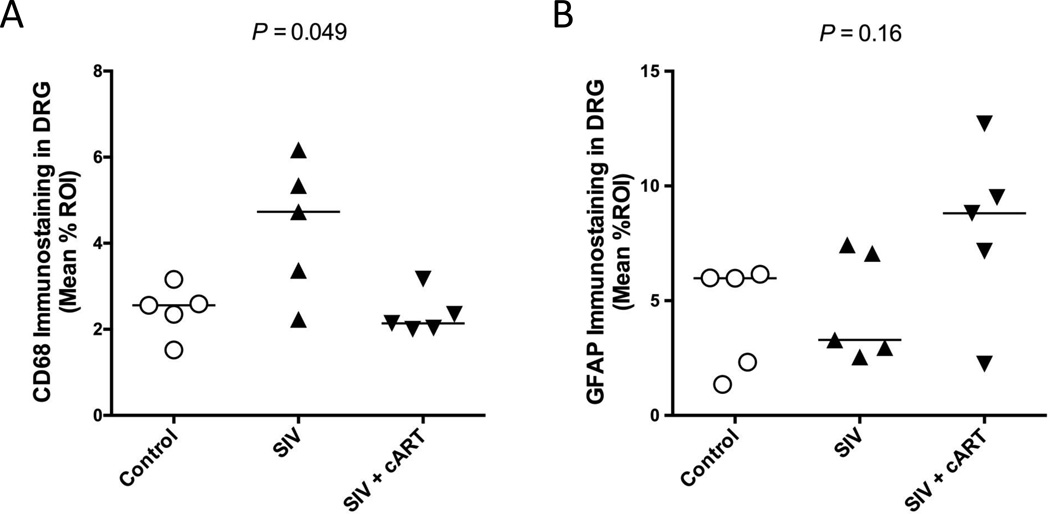
HIV-induced damage to the cell bodies of sensory neurons in the DRG has been proposed as 1 factor contributing to HIV neuropathy, and likely results due to a combination of effects from neurotoxic viral proteins and inflammatory mediators. (4, 36). Previous studies have shown that both viral replication and elevated immune responses are evident in the DRG of SIV-infected macaques (21). To determine if viral replication in the DRG was suppressed with cART, SIV RNA was quantitated by quantitative RT-PCR. cART treatment effectively controlled SIV replication in the DRG, with 4 out of 5 treated animals exhibiting undetectable SIV RNA levels in the DRG. In comparison, all untreated infected macaques had detectable viral loads in the DRG with median levels of SIV RNA 5 logs higher than cART-treated macaques (p = 0.008) (Fig. 4). To determine if there was a concomitant dampening of immune responses in the DRG of treated macaques, DRG sections were immunostained separately for the macrophage marker CD68 and the satellite glial cell marker GFAP followed by quantitative image analysis. Macrophage activation (CD68 immunostaining) was reduced by cART treatment to levels found in uninfected control animals; although CD68 appeared elevated in untreated SIV-infected macaques, differences in macrophage activation between untreated and cART-treated SIV-infected macaques did not reach statistical significance (PSIV+ v SIV+ cART = 0.064; Poverall = 0.049) (Fig. 5A). In contrast, satellite cell activation in both treated and untreated SIV-infected macaques did not significantly differ from uninfected control animals (Poverall = 0.16) (Fig. 5B).
Figure 4.
Simian Immunodeficiency Virus (SIV) viral replication is suppressed in dorsal root ganglia (DRG) of combination antiretroviral therapy (cART)-treated macaques. cART treatment effectively controlled SIV viral replication in DRG of treated, Simian Immunodeficiency Virus (SIV)-infected macaques (upside-down triangles), evidenced by SIV RNA levels at the limit of detection (100 c/mL) in 4 out of 5 animals. SIV viral loads were significantly elevated in untreated macaques (triangles) (p = 0.008, Mann-Whitney), with median levels of SIV RNA 5 logs higher than in treated animals.
Figure 5.
Alterations in CD68 and glial fibrillary acidic protein (GFAP) expression in dorsal root ganglia (DRG) of untreated and combination antiretroviral therapy (cART)-treated Simian Immunodeficiency Virus (SIV)-infected macaques. (A) In cART-treated animals (upside-down triangles), macrophage activation, depicted by CD68 immunostaining, was equivalent to that in controls (open circles). Although, macrophage activation appears higher in untreated, SIV-infected animals (triangles), a significant difference was not reached (p = 0.064, Dunn*; Overall p = 0.049, Kruskal-Wallis). (B) Overall, satellite cell activation, represented by GFAP immunostaining, varied across groups; however, no significant differences were found between control and both treated and untreated, infected groups (Overall p = 0.16, Kruskal-Wallis). *p value adjusted for multiple comparisons.
Changes in ENF Density Develop During Acute SIV Infection
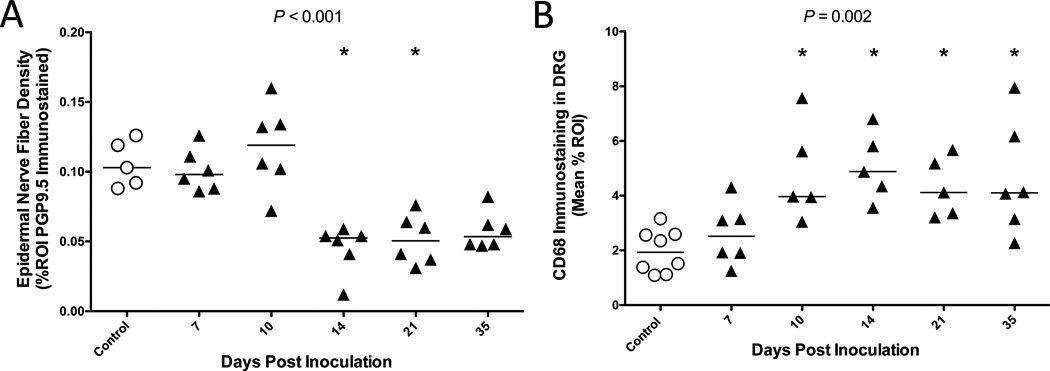
One explanation for the high rates of HIV peripheral neuropathy in patients treated with cART is that HIV infection predisposes individuals to antiretroviral-related toxicity (13, 37). To determine whether SIV induces damage to sensory neurons during the early stages of infection, prior to viral suppression by cART, we measured ENF density of 5 untreated SIV-infected groups of animals killed at progressive time points beginning at day 7 p.i. (Fig. 6A). Epidermal nerve fiber density was maintained at control levels through day 10; however, a significant decline in ENF density was evident in animals killed at day 14 (Pcontrol v d14 = 0.014), and persisted in animals killed at days 21 and 35 (Poverall < 0.001). To investigate whether increased macrophage activation in the DRG coincided with ENF loss during early infection, we also measured CD68 immunostaining in DRG of these animals. There was a progressive increase in CD68 expression in the DRG following infection, which reached significance by day 10 p.i. (Pcontrol v d10 = 0.003), demonstrating that macrophage activation in the DRG preceded detectable ENF loss. Elevated CD68 expression was then sustained through day 35 p.i. (Poverall = 0.002) (Fig. 6B). Furthermore, the degree of macrophage activation was inversely correlated with ENF density (p = 0.017, r = −0.41, data not shown), suggesting an association between the extent of inflammation in the somatosensory ganglia and ENF decline.
Figure 6.
Changes in epidermal nerve fiber (ENF) density and CD68 expression in dorsal root ganglia (DRG) during the acute phase of Simian Immunodeficiency Virus (SIV) infection. (A) Through 10 days post-infection (PI), ENF density in untreated, SIV-infected macaques (triangles) remains the same as that seen in control animals (open circles). However, by day 14 PI, ENF density significantly declined vs. control animals (p = 0.014, Dunn*), and remains low through day 35 PI (Overall p < 0.001, Kruskal-Wallis). (B) Macrophage activation, represented by CD68 immunostaining, progressively increased following SIV-infection and was found to be significantly elevated beyond control by day 10 PI (P = 0.003, Dunn*), with levels sustained through day 35 PI (Overall p = 0.002, Kruskal-Wallis). Asterisk in figure denotes significant pairwise comparison with the control group. *p value adjusted for multiple comparisons.
DISCUSSION
In previous studies, we established a SIV/macaque model to study HIV-induced damage to the PNS independent of cART. Major findings included abundant viral replication in macrophages and pronounced cellular immune responses in the DRG, as well as loss of epidermal nerve fibers. These findings closely resemble reports of PNS alterations in HIV-infected individuals. (21, 38). In this report, cART was administered to SIV-infected macaques beginning during acute infection to determine whether early initiation of cART alters SIV-induced PNS damage. In the DRG, cART therapy significantly reduced SIV RNA levels and dampened cellular immune responses, evidenced by lack of enhanced satellite glial cell or macrophage activation (compared to control animal levels). Nevertheless, despite reducing viral replication and inflammatory responses in the DRG, footpad ENF loss persisted. These findings set the stage to examine neurotoxic damage induced by drugs included in cART regimens and the possible contribution of ongoing low-level systemic immune activation using the SIV/macaque model.
Although cART has been invaluable in decreasing HIV-associated morbidity and mortality, cART drugs have been implicated in a wide range of antiretroviral toxicities and may play a role in the persistent high prevalence of HIV peripheral neuropathy despite control of systemic infection. Several different hypotheses have been put forward to explain this phenomenon. It is well known that both HIV infection and anti-retroviral drugs can independently cause mitochondrial dysfunction, which subsequently leads to sensory neuropathies (13, 39, 40). However, one theory proposes an additive scenario, where pre-existing HIV-induced mitochondrial damage renders infected individuals more susceptible to clinically relevant toxicity when exposed to neurotoxic cART drugs (37). Recently, Lehmann et al demonstrated that both HIV and SIV induced mitochondrial damage in distal nerve segments and hypothesized that subsequent mitochondrial dysfunction may play a role in distal degeneration of sensory nerve fibers. In addition, they also found that mitochondrial damage, as measured by mitochondrial permeability transition, was more pronounced in cART-treated SIV-infected macaques than in untreated SIV-infected macaques (41). Another study demonstrated rapid development of neuropathy in simian retrovirus-2-infected animals treated with nucleoside reverse transcriptase inhibitors whereas no neuropathy occurred in uninfected macaques treated with comparatively high levels of nucleoside reverse transcriptase inhibitors (42). Taken together, these findings suggest that retroviral infection and cART therapy may act synergistically to contribute to mitochondrial dysfunction, ultimately leading to distal sensory neuropathy.
Our observation of a more robust decline in ENF length among cART-treated SIV-infected macaques as compared to untreated, infected animals supports the notion of additive antiretroviral toxicity. Although cART treatment effectively controlled systemic viral replication throughout the greater part of SIV-infection in our model, SIV still induced PNS damage very early in infection. Prior to the initiation of cART at day 12 p.i., macrophage activation in the DRG was already significantly elevated by day 10 p.i. Moreover, significant ENF loss was apparent by day 14 p.i. in untreated SIV-infected macaques – a time point when cART is unlikely to have achieved therapeutic effect. This early SIV-induced damage may render the PNS more susceptible to neurotoxic effects of the antiretroviral drugs. In addition, it is possible that residual SIV replication (below the level of detection of 100 copies/mL) persisted systemically and/or locally within the DRG of cART-treated animals throughout therapy, leading to state of sustained, low-grade immune activation that was not evident by anti-CD68 or -GFAP immunostaining. This would allow for a substantial window of additive SIV/immune-mediated and antiretroviral-induced neurotoxicity. To address this issue, we plan to implement more sensitive techniques for measuring both SIV RNA and immune activation in future studies of cART-treated macaques.
The hypothesis that there is ongoing or sustained damage induced by cART is strengthened by the failure of ENF to regenerate to control levels following suppression of viral replication and inflammation in the DRG. Previous studies have shown that SIV infection delayed ENF regeneration and sprouting at every time point as compared to uninfected macaques in an excisional axotomy model (43). Nevertheless, complete reinnervation of axotomy sites at the densely innervated back areas occurred by 56 days post-axotomy despite SIV infection. In our study, at the distal leg sites there was no indication of regeneration of nerve fibers into the degenerated regions of epidermis in the cART-treated animals although virus replication had been suppressed for at least 70 days, a sufficient amount of time for reinnervation to begin. Another possible explanation for the marked ENF length decline is that cART may impair ENF regeneration in addition to inducing primary nerve damage. To gain a better understanding of ENF regeneration in the presence of cART therapy, the excisional axotomy SIV/macaque model could be utilized with subsequent characterization of regeneration including nerve morphometry as well as Schwann cell density and distribution. In summary, the SIV/macaque model of HIV-induced PNS damage offers the opportunity to dissect and define the complex interactions between viral-induced damage and PNS alterations attributable to anti-retroviral therapeutics.
ACKNOWLEDGMENTS
The authors would like to thank Ming Li, Rachel Weinberg, and Elizabeth Engle for their excellent technical assistance.
These studies were supported by NIH NS055651, NIH MH070306, NIH NS077869, NIH NS089482 NIH ORIP P40 OD013117, NIH ORIP T32 OD011089, and NIH R25MH08066108.
REFERENCES
- 1.McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–555. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- 2.Brinley FJ, Jr, Pardo CA, Verma A. Human immunodeficiency virus and the peripheral nervous system workshop. Arch Neurol. 2001;58:1561–1566. doi: 10.1001/archneur.58.10.1561. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy JM, Hoke A, Zhu Y, et al. Peripheral neuropathy in lentivirus infection: evidence of inflammation and axonal injury. AIDS. 2004;18:1241–1250. doi: 10.1097/00002030-200406180-00002. [DOI] [PubMed] [Google Scholar]
- 4.Keswani SC, Polley M, Pardo CA, et al. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann Neurol. 2003;54:287–296. doi: 10.1002/ana.10645. [DOI] [PubMed] [Google Scholar]
- 5.Cornblath DR, McArthur JC. Predominantly sensory neuropathy in patients with AIDS and AIDS-related complex. Neurology. 1988;38:794–796. doi: 10.1212/wnl.38.5.794. [DOI] [PubMed] [Google Scholar]
- 6.Kaku M, Simpson DM. HIV neuropathy. Curr Opin HIV AIDS. 2014;9:521–526. doi: 10.1097/COH.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 7.Simpson DM, Tagliati M. Neurologic manifestations of HIV infection. Ann Intern Med. 1994;121:769–785. doi: 10.7326/0003-4819-121-10-199411150-00008. [DOI] [PubMed] [Google Scholar]
- 8.Krashin DL, Merrill JO, Trescot AM. Opioids in the management of HIV-related pain. Pain Physician. 2012;15:157–168. [PubMed] [Google Scholar]
- 9.Ellis RJ, Rosario D, Clifford DB, et al. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67:552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgello S, Estanislao L, Simpson D, et al. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: the Manhattan HIV Brain Bank. Arch Neurol. 2004;61:546–551. doi: 10.1001/archneur.61.4.546. [DOI] [PubMed] [Google Scholar]
- 11.Schifitto G, McDermott MP, McArthur JC, et al. Incidence of and risk factors for HIV-associated distal sensory polyneuropathy. Neurology. 2002;58:1764–1768. doi: 10.1212/wnl.58.12.1764. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh S, Chandran A, Jansen JP. Epidemiology of HIV-related neuropathy: a systematic literature review. AIDS Res Hum Retroviruses. 2011;28:36–48. doi: 10.1089/AID.2011.0116. [DOI] [PubMed] [Google Scholar]
- 13.Cherry CL, McArthur JC, Hoy JF, et al. Nucleoside analogues and neuropathy in the era of HAART. J Clin Virol. 2003;26:195–207. doi: 10.1016/s1386-6532(02)00118-x. [DOI] [PubMed] [Google Scholar]
- 14.Cherry CL, Duncan AJ, Mackie KF, et al. A report on the effect of commencing enfuvirtide on peripheral neuropathy. AIDS Res Hum Retroviruses. 2008;24:1027–1030. doi: 10.1089/aid.2007.0300. [DOI] [PubMed] [Google Scholar]
- 15.Cherry CL, Skolasky RL, Lal L, et al. Antiretroviral use and other risks for HIV-associated neuropathies in an international cohort. Neurology. 2006;66:867–873. doi: 10.1212/01.wnl.0000203336.12114.09. [DOI] [PubMed] [Google Scholar]
- 16.Pettersen JA, Jones G, Worthington C, et al. Sensory neuropathy in human immunodeficiency virus/acquired immunodeficiency syndrome patients: protease inhibitor-mediated neurotoxicity. Ann Neurol. 2006;59:816–824. doi: 10.1002/ana.20816. [DOI] [PubMed] [Google Scholar]
- 17.Stavros K, Simpson DM. Understanding the etiology and management of HIV-associated peripheral neuropathy. Curr HIV/AIDS Rep. 2014;11:195–201. doi: 10.1007/s11904-014-0211-2. [DOI] [PubMed] [Google Scholar]
- 18.Clements JE, Mankowski JL, Gama L, et al. The accelerated simian immunodeficiency virus macaque model of human immunodeficiency virus-associated neurological disease: from mechanism to treatment. J Neurovirol. 2008;14:309–317. doi: 10.1080/13550280802132832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y, Jones G, Tsutsui S, et al. Lentivirus infection causes neuroinflammation and neuronal injury in dorsal root ganglia: pathogenic effects of STAT-1 and inducible nitric oxide synthase. J Immunol. 2005;175:1118–1126. doi: 10.4049/jimmunol.175.2.1118. [DOI] [PubMed] [Google Scholar]
- 20.Pardo CA, McArthur JC, Griffin JW. HIV neuropathy: insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Syst. 2001;6:21–27. doi: 10.1046/j.1529-8027.2001.006001021.x. [DOI] [PubMed] [Google Scholar]
- 21.Laast VA, Shim B, Johanek LM, et al. Macrophage-mediated dorsal root ganglion damage precedes altered nerve conduction in SIV-infected macaques. Am J Pathol. 2011;179:2337–2345. doi: 10.1016/j.ajpath.2011.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zink MC, Suryanarayana K, Mankowski JL, et al. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol. 1999;73:10480–10488. doi: 10.1128/jvi.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mankowski JL, Clements JE, Zink MC. Searching for clues: tracking the pathogenesis of human immunodeficiency virus central nervous system disease by use of an accelerated, consistent simian immunodeficiency virus macaque model. J Infect Dis. 2002;186(Suppl 2):S199–S208. doi: 10.1086/344938. [DOI] [PubMed] [Google Scholar]
- 24.Zink MC, Laast VA, Helke KL, et al. From mice to macaques--animal models of HIV nervous system disease. Curr HIV Res. 2006;4:293–305. doi: 10.2174/157016206777709410. [DOI] [PubMed] [Google Scholar]
- 25.Dinoso JB, Rabi SA, Blankson JN, et al. A simian immunodeficiency virus-infected macaque model to study viral reservoirs that persist during highly active antiretroviral therapy. J Virol. 2009;83:9247–9257. doi: 10.1128/JVI.00840-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zink M, Brice A, Kelly K, et al. Simian immunodeficiency virus-infected macaques treated with highly active antiretroviral therapy have reduced central nervous system viral replication and inflammation but persistence of viral DNA. J Infect Dis. 2010;202:161–170. doi: 10.1086/653213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holland NR, Stocks A, Hauer P, et al. Intraepidermal nerve fiber density in patients with painful sensory neuropathy. Neurology. 1997;48:708–711. doi: 10.1212/wnl.48.3.708. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy BG, Hsieh ST, Stocks A, et al. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45:1848–1855. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]
- 29.Nolano M, Provitera V, Crisci C, et al. Quantification of myelinated endings and mechanoreceptors in human digital skin. Ann Neurol. 2003;54:197–205. doi: 10.1002/ana.10615. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology. 1996;47:1042–1048. doi: 10.1212/wnl.47.4.1042. [DOI] [PubMed] [Google Scholar]
- 31.Ebenezer GJ, McArthur JC, Polydefkis M, et al. SIV-induced impairment of neurovascular repair: a potential role for VEGF. J Neurovirol. 2012;18:222–230. doi: 10.1007/s13365-012-0102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebenezer GJ, O'Donnell R, Hauer P, et al. Impaired neurovascular repair in subjects with diabetes following experimental intracutaneous axotomy. Brain. 2011;134:1853–1863. doi: 10.1093/brain/awr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mouton PR, Gokhale AM, Ward NL, et al. Stereological length estimation using spherical probes. J Micros. 2002;206:54–64. doi: 10.1046/j.1365-2818.2002.01006.x. [DOI] [PubMed] [Google Scholar]
- 34.Lauria G, Lombardi R. Skin biopsy in painful and immune-mediated neuropathies. J Periph Nerv Sys. 2012;17(Suppl 3):38–45. doi: 10.1111/j.1529-8027.2012.00430.x. [DOI] [PubMed] [Google Scholar]
- 35.Herrmann DN. Noninvasive and minimally invasive detection and monitoring of peripheral neuropathies. Expert Rev Neurother. 2008;8:1807–1816. doi: 10.1586/14737175.8.12.1807. [DOI] [PubMed] [Google Scholar]
- 36.Moss PJ, Huang W, Dawes J, et al. Macrophage-sensory neuronal interaction in HIV-1 gp120-induced neurotoxicity. Br J Anaesth. 2015;114:499–508. doi: 10.1093/bja/aeu311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cherry CL, Wesselingh SL. Nucleoside analogues and HIV: the combined cost to mitochondria. J Antimicrob Chemother. 2003;51:1091–1093. doi: 10.1093/jac/dkg203. [DOI] [PubMed] [Google Scholar]
- 38.Laast VA, Pardo CA, Tarwater PM, et al. Pathogenesis of simian immunodeficiency virus-induced alterations in macaque trigeminal ganglia. J Neuropathol Exp Neurol. 2007;66:26–34. doi: 10.1097/nen.0b013e31802c398d. [DOI] [PubMed] [Google Scholar]
- 39.Perl A, Banki K. Genetic and metabolic control of the mitochondrial transmembrane potential and reactive oxygen intermediate production in HIV disease. Antioxid Redox Signal. 2000;2:551–573. doi: 10.1089/15230860050192323. [DOI] [PubMed] [Google Scholar]
- 40.Kakuda TN. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin Therapeutics. 2000;22:685–708. doi: 10.1016/S0149-2918(00)90004-3. [DOI] [PubMed] [Google Scholar]
- 41.Lehmann HC, Chen W, Borzan J, et al. Mitochondrial dysfunction in distal axons contributes to human immunodeficiency virus sensory neuropathy. Ann Neurol. 2011;69:100–110. doi: 10.1002/ana.22150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai CC, Follis KE, Yarnall M, et al. Toxicity and efficacy of 2',3'-dideoxycytidine in clinical trials of pigtailed macaques infected with simian retrovirus type 2. Antimicrob Agent Chemother. 1989;33:1908–1914. doi: 10.1128/aac.33.11.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebenezer GJ, Laast VA, Dearman B, et al. Altered cutaneous nerve regeneration in a simian immunodeficiency virus / macaque intracutaneous axotomy model. J Comp Neurol. 2009;514:272–283. doi: 10.1002/cne.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]