Abstract
Carcinoids are neuroendocrine neoplasms that cause significant morbidity and mortality, and for which few effective therapies are available. Given the recent identification of the anti-cancer flavonoid chrysin, we sought to investigate its therapeutic potential in carcinoids. Here, we report chrysin’s ability to modulate the achaete-scute complex-like1 (ASCL1), a neuroendocrine-specific transcription factor highly implicated in the malignant phenotype of carcinoids and other neuroendocrine cancers. Moreover, we elucidate the role of ASCL1 in carcinoid growth and bioactivity. Treatment of two carcinoid cell lines (BON and H727) with varying chrysin concentrations suppressed cell proliferation, while reducing expression of ASCL1 and the neuroendocrine biomarker chromogranin A (CgA), demonstrated by Western blotting. Propidium iodide and PE AnnexinV/7-AAD staining and sorting following chrysin treatment revealed S/G2 phase arrest and apoptosis, respectively. This was corroborated by chrysin-induced cleavage of caspase-3 and PARP and activation of p21Waf1/Cip1. Furthermore, direct ASCL1 knockdown with an ASCL1-specific small interfering RNA inhibited CgA and synaptophysin expression as well as carcinoid proliferation, while also reducing cyclin B1 and D1, and increasing p21Waf1/Cip1 and p27Kip1 expression, suggesting an arrest of the cell-cycle. Collectively, these findings warrant the deliberation of targeted ASCL1 suppression by chrysin or other agents as a therapeutic approach for carcinoid management.
Introduction
Carcinoids comprise a rare heterogeneous subset of neuroendocrine tumors that originate from the diffuse enterochromaffin cells of the foregut, midgut, or hindgut endocrine system, and in rare instances the pancreas. These tumors occur most frequently in the small intestine, making up approximately 2% of malignant gastrointestinal tumors, followed by those of bronchopulmonary origin1. Carcinoids occur in approximately 5 in every 100,000 individuals, either sporadically or as part of familial neoplastic syndromes2, 3. Survival rates of these tumors are dependent on both location and disease progression. For patients with gastrointestinal carcinoids, 5-year survival rate for all sites is approximately 70%4. In patients with pulmonary carcinoids, survival rates depend on tumor type, with typical tumors conveying a 87% 5-year survival rate or greater, while atypical pulmonary carcinoids faring worse with survival rates of near 25%5. As expected, patients with metastatic disease have poorer survival rates than those with localized disease, and present complex challenges in clinical management. Carcinoid cancers can lead to significant morbidity due to the secretion of bioactive hormones, most commonly serotonin, which can cause flushing of the skin, abdominal cramping, bronchoconstriction, and right-sided heart failure. Carcinoid metastasis to the liver is common and second only to colorectal carcinoma. The carcinoid syndrome presents consequent to hepatic metastasis or due to carcinoids with bronchial involvement, which account for about 5-8% of cases6, 7. Although surgical resection offers the only potential cure for localized carcinoid disease, the majority of patients present with metastatic disease at the time of diagnosis rendering surgery ineffective6, 8. Unfortunately, no proven curative therapies are available for patients with advanced carcinoid tumors9, 10. While somatostatin analogues such as octreotide are effective in controlling symptoms, their long-term use can frequently lead to tachyphylaxis11. The morbidity and mortality of carcinoid tumors and lack of successful treatments emphasize the need to better elucidate molecular mechanisms that underlie carcinoid cell growth and progression.
Carcinoids, similar to other neuroendocrine tumors, overexpress a variety of biomarkers including a secreted acidic glycopeptide chromogranin A, synaptophysin, and seratonin. In addition, they highly express the achaete-scute complex-like1 (ASCL1), an evolutionarily conserved basic helix-loop-helix transcription factor that is tissue-specific to carcinoids and tumors of neuroendocrine origin12-20. ASCL1 serves as a key regulator in the normal development of neuroendocrine cells including parafollicular C-cells, adrenal medullary chromaffin cells, and pulmonary endocrine cells21-24. Expression of ASCL1 in carcinoids is markedly inhibited by targeted suppression of the oncogenic pathway PI3K-AKT or induction of Raf-1 and Notch1, thereby suppressing proliferation and the malignant phenotype25-28. Notch1 exerts tight control over ASCL1 as it has been shown to negatively regulate it’s expression during normal neuronal development and in other neuroendocrine cancers such as medullary thyroid cancer and small cell lung cancer29-36. Therefore, ASCL1 may play a critical role in carcinoid cell bioactivity. Furthermore, identifying novel agents that suppress ASCL1 activity may show promise against carcinoid tumorigenesis.
Accumulating reports on novel anti-cancer therapies have revealed that the compound chrysin (5,7-dihydroxyflavone), a naturally occurring flavonoid, exerts pro-apoptotic and cell-cycle modulatory effects in a variety of malignancies including melanoma, colorectal cancer, cervical cancer, esophageal cancer, gliomas, anaplastic thyroid cancer, and leukemia cell lines37-44. Chrysin, along with other flavones, has been described to possess potent anti-inflammatory, anti-oxidative, and antiproliferative effects45, 46. Notably, chrysin has been described for its ability to activate Notch1 and suppress cell growth in anaplastic thyroid cancer cell lines39. However, its effect has not yet been described in the context of carcinoids or neuroendocrine cancers. Given chrysin’s proven anti-cancer properties, and its ability to activate Notch1, a known suppressor of ASCL1 in neuroendocrine cancers, we sought to explore the potential of chrysin to modulate ASCL1 expression and exert a therapeutic effect on carcinoids. We also sought to investigate if direct suppression of ASCL1 could reduce carcinoid cell proliferation and the neuroendocrine phenotype.
In this study, we report for the first time that chrysin suppresses carcinoid cell proliferation and ASCL1 expression through the induction of apoptosis and cell-cycle arrest. We also show that specific gene silencing of ASCL1 arrests carcinoid cell growth, induces cell-cycle arrest markers p21Waf1/Cip1 and p27Kip1 while suppressing the production of neuroendocrine phenotypic markers.
Materials and Methods
Cell culture and chrysin treatment
Human GI carcinoid cancer cells (BON), were provided by Drs. B. Mark Evers and Courtney M. Townsend, Jr. (University of Texas Medical Branch, Galveston, TX, USA), and bronchopulmonary carcinoid (H727) cells were purchased from the American Type Culture Collection (Manassas, VA, USA). BON cells were maintained in DMEM/F-12 (Life Technologies, Grand Island, NY, USA) and H727 cells were maintained in RPMI/F-12 (Life Technologies, Grand Island, NY, USA), both as previously described. Both media were supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA), 100 IU/mL penicillin and 100μg/mL streptomycin (Life Technologies, Grand Island, NY, USA). Both cell lines were incubated in a humidified atmosphere of 5% CO2 at 37°C. Chrysin was purchased from MP Biomedicals (Solon, OH, USA) and dissolved in dimethyl sulfoxide (DMSO) at a 100mM stock concentration. Aliquots were stored at −80°C and freshly thawed prior to treatment. Cell treatments were conducted by plating cells at sub-confluency and allowing them to adhere overnight. The next day, cells were incubated in fresh medium containing chrysin (1-100μM) for up to 6 days. DMSO concentrations were equalized across all chrysin treatment groups (100μM DMSO).
Immunoblot Analyses
Total cell lysates were prepared and analyzed for protein expression by Western blotting. Treated cells were first washed with 1× PBS, scraped and collected into sterile tubes, centrifuged to pellet form, and then lysed in sample buffer (50mM Tris, 0.15 M NaCl, 0.5% Na/deoxycholate, 0.1% SDS, 1% Nonidet P-40, 0.1% protease inhibitor cocktail and 0.6mM phenylmethanesulfonylfluoride or phenylmethylsulfonyl fluoride). A bicinchoninic acid (BCA) assay (Pierce, Rockford, IL, USA) was used to determine the quantity of total cellular protein in each sample. Subsequently, these samples were denatured and resolved on 7.5%, 10%, or 12% SDS-PAGE gels (Bio-Rad Laboratories, Hercules, CA, USA). Proteins were then transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA). Protein bound membranes were blocked for at least 30 minutes in a milk solution (1× PBS, 5% dry milk, 0.05% Tween-20), and then incubated overnight at 4°C in their respective primary antibodies. Each antibody was diluted as follows: 1:2000 for mammalian achaete-scute complex-like1 (ASCL1) (BD Pharmingen, San Diego, CA, USA), 1:3000 for chromogranin A (Zymed Laboratories, San Francisco, CA, USA), 1:10,000 for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Trevigen, Gaithersburg, MD, USA), and 1:1000 for synaptophysin, p27Kip1 (Santa Cruz Biotechnology, Dallas, TX, USA), p21Waf1/Cip1, cyclin D1, phosphorylated cdc2 (tyrosine 15), total cdc2, cleaved poly-ADP-ribosyl polymerase, total caspase-3, cleaved caspase-3 and β-actin (Cell Signaling Technology, Beverly, MA, USA). Following incubation in primary antibody, membranes were washed 3 × 5 minutes in PBS-T wash buffer (1× Phosphate Buffered Saline, 0.05% Tween 20). Blots were then incubated in either anti-rabbit or anti-mouse secondary antibodies (Pierce, Rockford, IL, USA), which were conjugated to horseradish peroxidase depending on the source of the primary antibody. Membranes were washed for 3 × 5 minutes or 3 × 10 minutes in PBS-T wash buffer. Supersignal West Pico, Femto, Dura (Pierce, Rockford, IL, USA), or Immunstar (Bio-Rad Laboratories, Hercules, CA, USA) kits were then used to develop the membranes according to the manufacturers’ instructions. Chrysin treatments lasted for 2 days and ASCL1-siRNA treatments lasted for 2, 4 or 6 days before proteins were harvested. All immunoblots were repeated to ensure reproducibility and we show the most representative findings.
Quantitative real-time PCR of ASCL1 and NOTCH1 message levels
Following treatment with chrysin (1-100μM) for 2 days, RNA was isolated using an RNeasy Mini-kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s directions and quantified using NanoDrop instrumentation (Thermo Scientific, Waltham, MA USA). Using 2μg of RNA per sample, cDNA was synthesized with the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, USA). ASCL1 sequence primers were generated using a random primer labeling kit (New England Biolabs Inc., Beverly, MA, USA). The Notch1 primer sequences were as follows- forward: 5’-GTCAACGCCGTAGATGACCT-3’ and reverse: 5’-TTGTTAGCCCCGTTCTTCAG-3’. GAPDH was used as a housekeeping control to which ASCL1 and NOTCH1 messages were normalized. The GAPDH primer sequences were as follows- forward: 5’-ACCTGCCAAATATGATGAC-3’ and reverse: 5’-ACCTGGTGCTCAGTGTAG-3’). Quantitative real-time PCR reactions were performed using MyiQ Thermal Cycler (Bio-Rad Laboratories Hercules, CA, USA). Each reaction was plated in triplicate. Assay RNA expression levels were calculated using the comparative cycle threshold method (ΔCt) method, as described in the Real-Time PCR Applications Guide (Bio-Rad Laboratories Hercules, CA, USA). Once normalized to GAPDH, relative ASCL1 expression for each treatment group was plotted as a mean ± SEM.
Cell-cycle distribution analysis by propidium iodide staining
Following chrysin treatment for 2 days (0-100μM), BON cells were trypsinized, pelleted and resuspended in chilled 1× PBS, twice. Next, cell pellets were resuspended in chilled 95% ethanol and fixed at −20°C for at least 2 hours. Fixed cells were then pelleted and washed in 1× PBS twice, and resuspended in a solution containing 33μg/ml of propidium iodide solution (Sigma-Aldrich, St. Louis, MO, USA), 1mg/ml RNase A (Life Technologies, Grand Island, NY, USA), and 0.1% Triton ×100 (Sigma-Aldrich, St. Louis, MO, USA) in 1× PBS. Cells were then stored in the dark at 4°C overnight and then quantitatively sorted based on their flourescent signal using the FACSCalibur™ (BD Biosciences, San Jose, CA, USA) fluorescent-activated cell sorting (FACS) instrumentation. ModFit™ (Verity SoftwareHouse, Topsham, ME, USA) software was used to interpret quantitative outputs and determine the proportion of cells in each sample in the G1, S and G2 phase of the cell-cycle. Data from three biological replicates of this experiment were averaged and graphed as a mean ± SEM.
Detection of apoptosis by PE Annexin V/7-AAD staining
FACS was also used to determine the proportion of cells entering apoptosis following chrysin treatment using a PE Annexin V and 7-AAD Apoptosis Detection Kit I (BD Pharmingen, San Jose, CA, USA). Cells were harvested using Cellstripper (Cellgro, Manassas, VA, USA), a nonenzymatic cell dissociation solution, and floating cells were harvested as well. Cells were pelleted and resuspended in 1× PBS twice and then resuspended in binding buffer (10mM HEPES/NaOH, pH=7.4, 140mM NaCl, and 2.5mM CaCl2) at 1×106 cells/ml. Next, cell suspensions were incubated in PE Annexin V and 7-AAD (7 Aminoactinomycin D) fluorescein solutions for 15 minutes in the dark at room temperature and sorted using the FACSCalibur™. Cell distributions were determined using FlowJo v10.0.8 software (TreeStar, Inc., Ashland, OR, USA). Three biological replicates of this experiment are presented as a mean ± SEM.
ASCL1 gene silencing
Transient knockdown of ASCL1 expression was performed in BON and H727 cell lines. A pool of four gene-specific small interfering RNA (siRNA) sequences against ASCL1 (catalog no. sc-37692, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and a nonspecific-siRNA (catalog no. sc-37007, Santa Cruz Biotechnology, Inc.) as a negative control were dissolved in media containing Lipofectamine 2000® (Life Technologies, Grand Island, NY, USA) as per manufacturer instructions, and added dropwise to plated cells. Cells were treated with concentrations of siRNA ranging from 0nM to 40nM. The third treatment group included cells treated with Lipofectamine 2000® alone, serving as a control. Cells were allowed to incubate overnight and media was replaced the following day. Cells were harvested for Western blotting or assessed for viability by assay 2, 4 or 6 days after transfection.
Cell proliferation assay
Carcinoid cell viability was determined using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich, St. Louis, MO, USA) rapid colorimetric assay. BON and H727 cells were plated in 24-well plates and allowed to adhere overnight. Cells were then either treated with varying doses of chrysin for 2 days (0-100μM), or transfected with either the ASCL1-siRNA or a nonspecific control vector. Each treatment group was plated in quadruplicate. In order to determine cell viability for a given day, media in each well receiving treatment was replaced with 250μL of serum-free media containing 0.5mg/mL MTT. Plates were then incubated at 37°C for 3.5 hours followed by the addition of 750μL of Dimethyl Sulfoxide (DMSO) (Fischer Scientific, Pittsburgh, PA, USA). The optical densities of each well were measured at 540nm using a spectrophotometer (μQuant, Bio-Tek Instruments, Winooski, VT, USA). MTT readings were taken 2, 4 and 6 days following chrysin treatment or ASCL1 gene silencing. Quadruplicate optical densities were averaged for each treatment group and presented ± SEM.
Statistical Analysis
A two tailed Student’s T-test was used to determine statistical significance using Microsoft Excel® for Mac 2011 (Microsoft, Redmond, WA, USA). A p<0.05 was the applied cutoff to qualify as significant.
Results
Chrysin dose dependently suppresses BON and H727 cell proliferation as well as ASCL1 expression
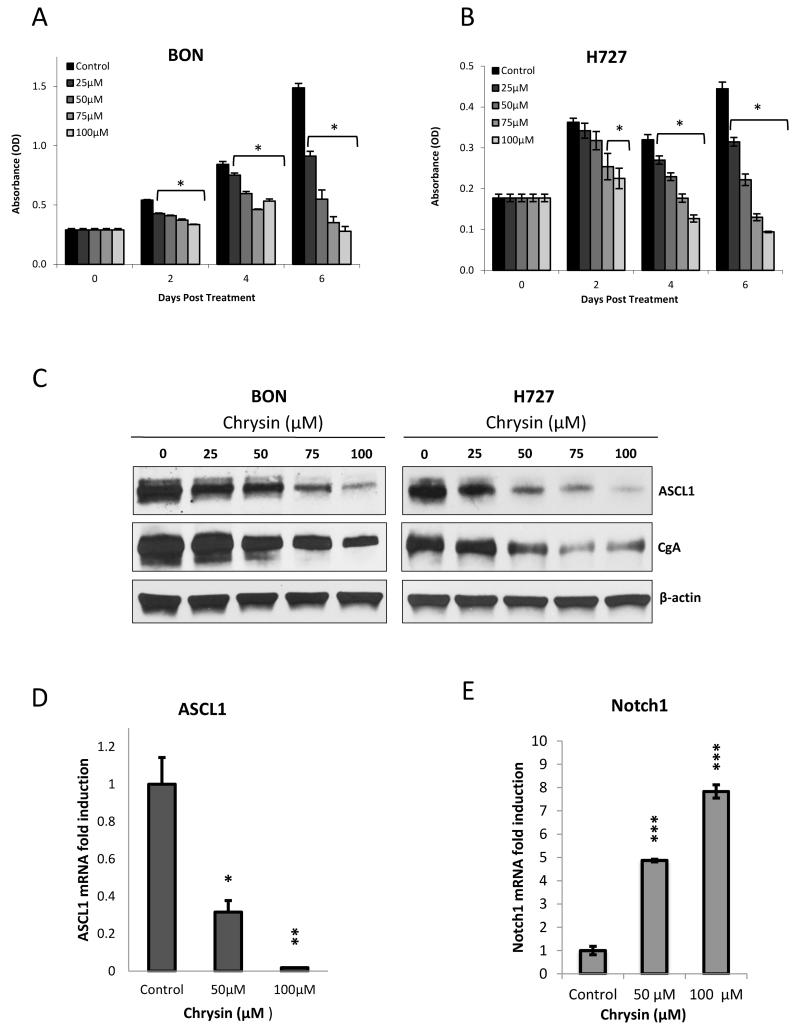
In order to determine the therapeutic range of chrysin, we conducted a cell viability assay following treatment with chrysin dosages ranging from 0 to 100μM. BON and H727 cells were allowed to grow in treatment over a 6 day period, with treatment replenished every two days to ensure drug potency. Figure 1 shows that chrysin treatment caused a dose dependent reduction in BON (A) and H727 (B) cell proliferation over a 6 day period. In BON cells, the shortest time point of 2 days was sufficient to achieve a significant reduction in cell proliferation with the lowest tested dose of 25μM chrysin (p<0.0001). H727 sensitivity to chrysin treatment became statistically significant with 75μM of chrysin treatment by as early as the 2nd day of treatment (p=0.03). By the 6th day of treatment, BON cells treated with 100μM of chrysin experienced 81.3% suppression in proliferation (Figure 1A) and identically treated H727 cells exhibited a 78.9% suppression in proliferation compared to controls (Figure 1B). Parallel treated BON and H727 cells were immunoblotted for protein levels of ASCL1 and CgA. Increasing concentrations of chrysin dose dependently inhibited ASCL1 expression as well as CgA levels in both cell lines. Chrysin’s ability to activate Notch1 signaling, a negative regulator of ASCL1, led us to compare the effect of drug treatment on Notch1 with its effect on ASCL1 expression. BON cells treated with 50μM and 100μM of chrysin exhibited steadily reduced levels of ASCL1 message as expected (p=0.01 and p=0.002, respectively), while Notch1 message increased 7.8 fold following 100μM chrysin administration (p<0.001) (Figure 1D and E).
Figure 1. Chrysin exerts an antiproliferative effect on BON and H727 carcinoid cells while inhibiting ASCL1 and CgA.
BON GI (A) carcinoid and H727 (B) pulmonary carcinoid cell lines were treated with chrysin doses ranging from 0-100μM over a 6 day period. A 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay performed every 2 days demonstrated that chrysin dose dependently reduced cell proliferation in both cell lines. Chrysin also suppressed ASCL1 protein expression along with the neuroendocrine marker CgA in both cell lines following 2 days of treatment, as demonstrated by Western blotting (C). Finally, chrysin inhibited ASCL1 transcription (D) while activating Notch1 transcription (E) in BON cells, shown by quantitative real-time polymerase chain reaction. Both occurred to statistically significant levels (D and E) (all graphs displayed as ± SEM) (*p<0.05, **p<0.01, ***p<0.001).
Chrysin treatment induces S/G2 phase cell-cycle arrest
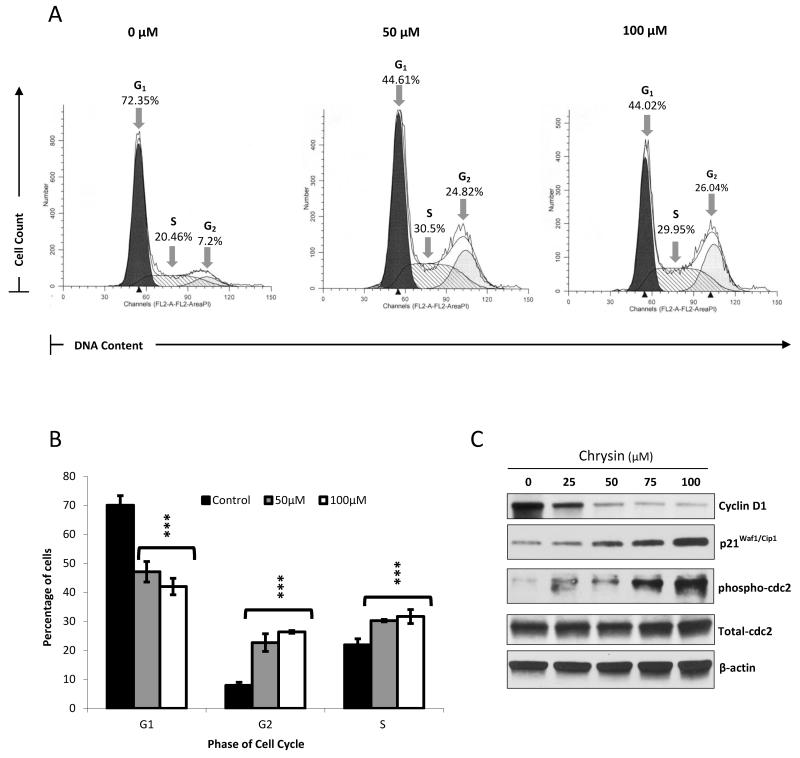
Chrysin has been described as a profound inhibitor of cell-cycle transit in a variety of cancer types including melanoma, esophageal, colorectal and gliomas41-44. In carcinoids, ASCL1 suppression has also been strongly associated with an arrest in cell-cycle progression47-50. Therefore, we aimed to determine the effect of chrysin treatment on carcinoid cell-cycle kinetics. Flow cytometry analysis of propidium iodide stained cells revealed a profound accumulation of S and G2 phase arrested cells with 50μM and 100μM of chrysin treatment accompanied by a dramatic reduction in G1 phase cells (p<0.001) (Figure 2A and B). Figure 2A shows an independent experimental replicate of chrysin’s effect on cell-cycle phase populations, while the average is shown in Figure 2B. We next explored the effect of chrysin treatment on the expression of the cyclin family protein cyclin D1, which is required for cell-cycle transit, and demonstrated a dose dependent reduction in its expression in BON cells51. Western analysis also revealed that chrysin caused a dose dependent induction in p21Waf1/Cip1. In the currently accepted model, p21Waf1/Cip1 binds and inhibits the activity of cyclin dependent kinase 2 complexes, which are necessary regulators of cell-cycle progression52, 53. Finally, we observed that chrysin administration increased the phosphorylation of the cell division cycle protein 2 homolog (cdc2) at tyrosine 15, while total levels of cdc2 stayed constant. Because entry into mitosis requires cdc2 dephosphorylation at Tyr15 and Thr14, the observed increase in phospho-cdc2 indicates a halt in carcinoid cell division54-56. Altogether, these data signify the occurrence of cell-cycle arrest in response to chrysin treatment.
Figure 2. Chrysin causes S/G2 phase arrest in BON cells.
Propidium iodide staining was performed following treatment of BON cells with 0μM, 50μM and 100μM chrysin over a 2 day period. The percentage of cells in the G1, S and G2 phases of the cell cycle in each treated sample was then determined using fluorescent activated cell sorting on the BD FACSCalibur™ platform followed by analysis using the ModFit™ software. Treatment with 50μM and 100μM of chrysin led to an accumulation of cells in the S phase and G2 phase, shown here in one experimental replicate (A). Data from three biological replicates of this experiment were averaged and graphed ± SEM (B) (***p<0.001). Increasing chrysin doses resulted in a decrease in cyclin D1 expression, and an increase in p21Waf1/Cip1 and phosphorylated cdc2, as indicated by Western blotting, thus corroborating the induction of cell-cycle arrest (C).
Chrysin treatment induces apoptosis in BON carcinoid cell line
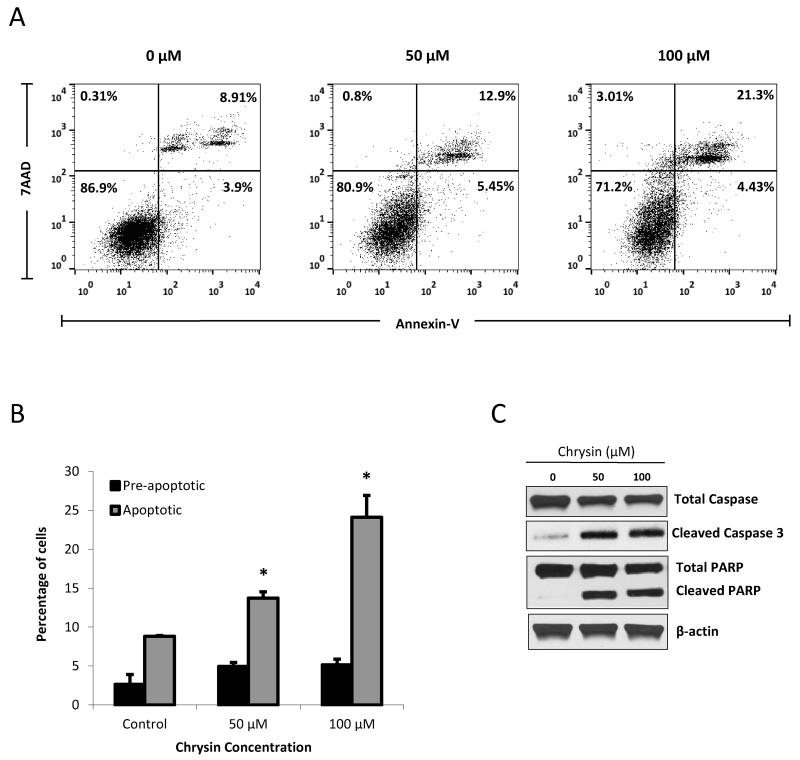
It has been shown that chrysin exerts its antiproliferative effect in a variety of cancers in part by inducing apoptosis37-40. While this has not yet been described in carcinoids, it has been observed that ASCL1 suppression in carcinoids occurs concurrently with activation of the apoptotic cascade27, 57-59. Given chrysin’s ability to suppress ASCL1, we sought to explore the mechanism of growth inhibition achieved by chrysin treatment. Figure 3A shows cell distribution from one experimental replicate of PE Annexin V/7 AAD stained cells treated with chrysin. We sorted and quantified the population of cells in each treatment group undergoing apoptosis (upper right quadrant), those in the preapoptotic phase (lower right quadrant), those undergoing necrosis (upper left quadrant), and those that were still viable in the face of chrysin treatment (lower left quadrant). Staining and sorting was repeated thrice and averaged in Figure 3B. We demonstrate that while BON cells receiving no treatment comprised 8.82% apoptotic cells, chrysin treatments of 50μM and 100μM significantly increased apoptotic cell populations to 13.7% (p=0.02) and 24.1% (p=0.03), respectively (Figure 3B). Expectedly, the populations of viable cells steadily decreased with increasing chrysin treatment, as shown in the representative replicate Figure 3A. Interestingly, the effect on pre-apoptotic cell populations did not change significantly. In order to verify the induction of apoptosis by chrysin treatment, we investigated its effect on the the terminal steps of the apoptotic cascade, which include PARP and caspase-3 protein cleavage. Figure 3C shows a profound induction in immunoprobed levels of cleaved PARP and caspase-3 with 50μM and 100μM of chrysin treatment, while total levels of caspase-3 were steadily reduced with increasing drug concentrations.
Figure 3. Chrysin treatment induces apoptosis in BON cells.
Following 2 days of chrysin treatment (0μM, 50μM and 100μM), BON cells were stained with PE Annexin V/7-AAD and quantified by phosphatidylserine exposure. Fluorescent activated cell sorting using the BD FACSCalibur™ instrumentation revealed an increase in apoptotic populations following chrysin treatment of 50μM and 100μM, shown here in one experimental replicate (A). In each pane, the lower left quadrant represents viable cells (Annexin V-negative/7-AAD-negative); the lower right quadrant represents pre-apoptotic cells; the upper right quadrant indicates late apoptotic cells (Annexin V-positive/7-AAD-positive); the upper left quadrant represents cells that were positive for 7-AAD only. Data from three independent experiments were averaged and graphed ± SEM (B) (*p<0.05). Induction of apoptosis following chrysin treatment was confirmed by cleavage of caspase-3 and the terminal apoptotic marker PARP, shown by Western blotting (C).
ASCL1 silencing suppresses carcinoid cell proliferation and reduces bioactive hormone production
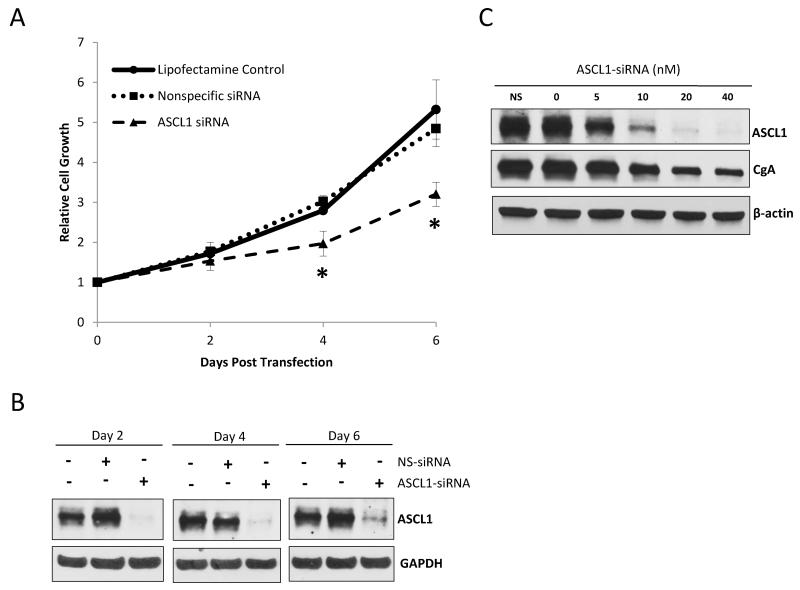
Chrysin’s ability to suppress ASCL1 expression while inducing apoptosis and cell-cycle arrest led us to investigate the role of ASCL1 on carcinoid tumor biology. To do this, we directly silenced ASCL1 using a specific siRNA, and assessed the subsequent effect on carcinoid cell proliferation and neuroendocrine marker expression. BON cells were treated with either an ASCL1-specific siRNA, the equivalent amount of a nonspecific-siRNA as a negative control, or an equivalent concentration of Lipofectamine 2000® without any siRNA. The concentration of siRNA required to achieve complete suppression of ASCL1 was 40nM. Following 2, 4 and 6 days after the given treatment, the resultant cell viability from each group was determined by MTT assay. On each day, parallel treated cells were prepared and assessed for ASCL1 expression by Western blotting. On day 2 following treatment, cells transfected with the ASCL1-siRNA did not significantly differ in cell viability relative to treatment groups given the nonspecific-siRNA or Lipofectamine 2000® alone (Figure 4A). At day 2, ASCL1 gene expression was completely silenced in the ASCL1-siRNA treated group while still present in both controls (Figure 4B). By the 4th day following treatment, ASCL1 silencing was sustained among the ASCL1-siRNA treated cells, and notably, cell proliferation was significantly reduced (p=0.02) to 70.4% of the untransfected treated cells receiving Lipofectamine 2000® alone. Furthermore, the ASCL1-silenced cells only grew 1.9 fold relative to their density on the day of transfection, while the nonspecific-siRNA treated cells and the lipofectamine treated cells grew 3.0 and 2.8 fold respectively. By the 6th day following siRNA silencing, ASCL1 still appeared to be suppressed relative to controls. The effect of ASCL1 depletion on cell proliferation was most profound by this time point, increasing to only 60.2% of those receiving no siRNA treatment. ASCL1 silencing caused BON cells to only grow by 3.2 fold while treatment with the nonspecific-siRNA or Lipofectamine 2000® alone allowed cells to proliferate 4.8 and 5.3 fold, respectively. Comparing the growth of the ASCL1-silenced treatment group to the proliferative fold increase of the Lipofectamine 2000® treated cells 6 days following transfection generated a p-value of 0.02 (Figure 4A).
Figure 4. ASCL1 suppression inhibits BON cell proliferation and CgA expression.
Treatment with an ASCL1-specific siRNA resulted in an inhibition in BON cell proliferation over a 6 day period. A MTT cell viability assay was performed every 2 days, and optical densities were normalized to values starting from the day of transfection. No effect was observed on cell proliferation among cells treated with the nonspecific-siRNA (NS-siRNA) relative to cells treated with Lipofectamine 2000® alone (no siRNA). Data is graphed ± SEM (*p<0.05) (A). Cell lysates were harvested every 2 days and Western blotting was performed to assess the degree of ASCL1 knockdown. Protein expression of ASCL1 was continuously suppressed over the 6 day period while still present in cells treated with the NS-siRNA or Lipofectamine 2000® alone (B). BON cells were treated with ASCL1-siRNA at concentrations ranging from 5nM to 40nM to achieve a steady reduction in ASCL1 expression. Western blotting revealed that CgA levels directly correlated with ASCL1 levels as they were progressively diminished alongside incremental ASCL1 depletion. Once again, the NS-siRNA treated group (NS) did not experience any change in ASCL1 or CgA levels (C).
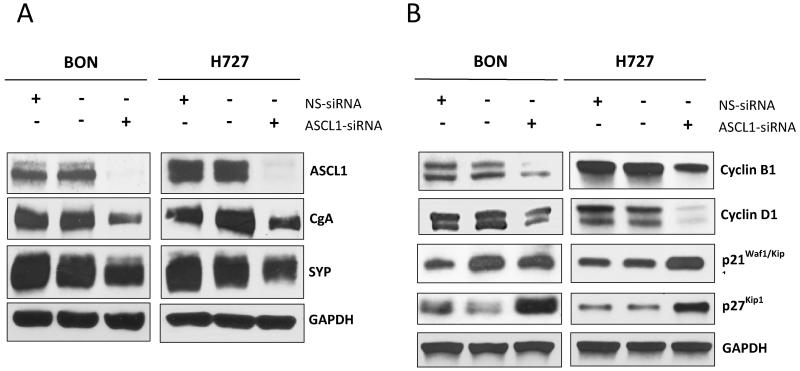
It has been shown that inhibition of ASCL1 by antisense oligonucleotides leads to a reduction in neuroendocrine markers in small cell lung cancer and medullary thyroid cancer12, 29. Given this relationship between ASCL1 expression and neuroendocrine hormones, we were led to investigate the effect of ASCL1 silencing on expression of the acidic glycopeptide CgA. We were able to demonstrate that steadily decreasing levels of ASCL1 expression, achieved by increasing concentrations of ASCL1-siRNA from 0nM to 40nM, caused a proportional reduction in CgA levels (Figure 4C). ASCL1-siRNA treatments lasted for 2 days. Importantly, no effect on ASCL1 or CgA expression were observed in the nonspecific-siRNA treated cells relative to cells receiving Lipofectamine 2000® alone. Along with CgA, carcinoids are known to co-secrete the presynaptic integral membrane protein synaptophysin (SYP)60-62. Together, SYP and CgA are important prognositic indicators for patients with carcinoid malignancies63, 64. In order to investigate the role of ASCL1 expression on SYP, we subjected both BON cells and the H727 pulmonary carcinoid cell line to either 40nM of ASCL1-silencing siRNA, the same concentration of a no-target siRNA treatment, or the equivalent concentration of Lipofectamine 2000® alone for 2 days before proteins were harvested and Western blotting was performed. Figure 5A shows that basal levels of SYP were abundantly present in both cell lines. However, ASCL1 knockdown resulted in a reduction in the expression of SYP along with CgA in both BON and H727 cells. Those cells receiving the nonspecific targeted siRNA exhibited no effect on ASCL1, SYP or CgA relative to cells receiving no siRNA, but only Lipofectamine 2000®.
Figure 5. ASCL1 suppression reduces neuroendocrine marker production and induces cell-cycle arrest in BON and H727 cells.
Treatment of BON and H727 cells with an ASCL1-specific siRNA reduced chromogranin A (CgA) and synaptophysin (SYP) levels, shown by Western blotting. Cells receiving the nonspecific-siRNA (NS-siRNA), exhibited no visible change in ASCL1, CgA or SYP levels relative to cells treated with Lipofectamine 2000® alone (no siRNA) (A). Concurrent with ASCL1 suppression following ASCL1-siRNA treatment, BON and H727 cell lines exhibited a reduction in cyclin B1 and D1 expression, and an increase in p21Waf1/Cip1 and p27Kip1 expression, as demonstrated by Western blotting, therefore suggesting the occurrence of cell-cycle arrest. Cells receiving the NS-siRNA experienced no effect on levels of any of these markers (B).
ASCL1 knockdown induces cell-cycle arrest in BON and H727 cell lines
Following our observation that ASCL1 is critical for both cell proliferation (Figure 4) and production of NE markers in two carcinoid cell lines (Figure 5A), we next sought to elucidate the role that this transcription factor plays in promoting cell survival. Following silencing of ASCL1 with 40nM of ASCL1-siRNA, BON and H727 cells were examined for their expression levels of p21Waf1/Cip1, p27Kip1, cyclin B1 and cyclin D1 using Western blot analysis. Determining levels of these proteins would allow us to assess the effect of ASCL1 suppression on cell-cycle kinetics in both BON and H727. In both cell lines, ASCL1 suppression caused an elevation in levels of p21Waf1/Cip1 as well as p27Kip1, both of which behave as tumor suppressors by binding cyclin dependent kinase complexes and enforcing restriction points during the cell-cycle52, 53, 65. In addition, ASCL1 silencing led to the inhibition in expression of cyclins B1 and D1 relative to both controls, further suggesting the induction of cell-cycle arrest (Figure 5B). Together, cyclin B1 and D1 are necessary for activating cyclin dependent kinases and allowing for cell-cycle progression51, 66. Collectively, these results indicate that ASCL1 may regulate carcinoid cell growth through inducing cell-cycle arrest. Hence, targeted inhibition of ASCL1 may contribute to the anti-cancer effects of chrysin and other such agents in carcinoids.
Discussion
The evolutionarily conserved ASCL1 is a basic helix-loop-helix transcription factor fundamental to early neurogenesis in Drosophila melanogaster and mammals67, 68. Within its neurogenic role, ASCL1 has been implicated in normal development of adrenal chromaffin and pulmonary endocrine cells, parafollicular C-cells and enteric neurons21-24. Several studies also describe the role of ASCL1 in accelerating the differentiation of endocrine cells in the developing stomach and gut, underscoring its importance during GI organogenesis35, 36. Tight control of ASCL1’s temporal expression is thus necessary for normal neuroendocrine development. On this basis, ASCL1’s link to human diseases of neuroendocrine origin has also been explored. Constitutive expression of ASCL1 has been shown to be an intrinsic feature of neuroendocrine cancers, such as pheochromocytomas, small cell lung cancer, retinoblastoma, medullary thyroid cancer, and carcinoids12, 13, 16, 18-20. Despite their difference in organ of origin, these cancers share the neuroendocrine phenotype, which likely reflects their neuroendocrine precursors. ASCL1 expression has been described as a key determinant of neuroendocrine characteristics that define these malignancies16, 18. Non-neuroendocrine cancers lack detectible ASCL1, and moreover, silencing of ASCL1 effectively represses neuroendocrine marker production in cancers arising from this origin such as small cell lung cancer12. Therefore we hypothesized that inhibiting ASCL1 could suppress the carcinoid neuroendocrine phenotype and serve as a potential therapy for these cancers.
Repressing ASCL1 can be achieved by activation of Notch1, which has been shown to negatively regulate ASCL1 in both normal neural and neuroendocrine development and in neuroendocrine tumorigenesis28-35. This knowledge led us to investigate the ability of the Notch1 activating drug chrysin to reduce ASCL1 expression and thereby inhibit neuroendocrine phenotype in carcinoids. Notably, the anti-cancer agent chrysin, a biologically active flavonoid, has been shown to activate Notch1 expression in non-neuroendocrine tumors such as anaplastic thyroid cancer, and concomitantly inhibit their proliferation39. Taken together, we surmised that chrysin would block ASCL1 expression in carcinoids while inhibiting cell growth. Finally, we sought to demonstrate if direct ASCL1 silencing would diminish carcinoid cell viability and neuroendocrine marker production.
In this study we show that chrysin exerts an antiproliferative effect on both BON and H727 carcinoid cell lines while concurrently inhibiting ASCL1 transcription and translation. We also demonstrate that NOTCH1 gene transcription is steadily induced with increasing doses of chrysin. This corroborates previous findings that Notch1 activation, in this case by chrysin administration, drives ASCL1 suppression in a neuroendocrine context. Given ASCL1’s inextricable link to the neuroendocrine phenotype, we expected to see a reduction in expression of neuroendocrine marker expression. Indeed, chrysin treatment resulted in a dose dependent decrease in the neuroendocrine marker CgA alongside ASCL1 inhibition.
Accruing evidence has suggested that chrysin’s antiproliferative effect is due to its ability to both halt cell-cycle transit and induce apoptosis37-44. Though this has not been demonstrated in carcinoids or any neuroendocrine derived cancers, it has been repeatedly reported that carcinoid cell growth inhibition via apoptosis or cell-cycle arrest is invariably accompanied by a reduction in ASCL1 expression27, 47-50, 57-59, 69. Having established chrysin’s ability to suppress ASCL1 in two carcinoid cell lines, we expected an effect on cell-cycle kinetics and the apoptotic cascade in BON cells following exposure to chrysin. Our results show that chrysin impedes cell-cycle transition during the S/G2 phase while activating p21Waf1/Cip1 and reducing cyclin D1 expression. Furthermore, chrysin activated the apoptotic cascade indicated by a cleavage of caspase-3 and PARP and an increase in apoptotic gated cells following PE Annexin/7-AAD staining. The observed G2 phase arrest during cell-cycle progression has also been observed in esophageal adenocarcinoma and colorectal carcinoma cell lines subjected to chrysin treatment, alongside an increase in p21Waf1/Cip1 expression as well70. Notably, the identical effect on p21Waf1/Cip1 expression has also been observed in a melanoma cell line, wherein chrysin administration increased p21Waf1/Cip1 promoter activity and also initiated apoptosis41. Chrysin’s ability to induce p21Waf1/Cip1 may partly underlie its growth suppressive effects, given that this enzyme inhibitor gene functions as a suppressor of cyclin dependent kinase 2, an essential protein during cell-cycle transition52, 53. Furthermore, chrysin’s ability to reduce cyclin D1 expression is important as this gene promotes oncogenicity and is overamplified in a variety of cancers71-74. Chrysin also caused an increase in the phosphorylation of cdc2 at Tyr15. During the cell-cycle, cdc2 dephosphorylation at Tyr15 and Thr14 is required for mitotic transit to occur54-56. Hence, chrysin’s ability to increase cdc2 phosphorylation at Tyr15 strongly indicates a drug-induced cell-cycle arrest.
We next investigated the effect of direct ASCL1 inhibition using siRNA gene silencing on BON cell proliferation and neuroendocrine marker expression. Given that ASCL1 depletion using antisense oligonucleotides erased the neuroendocrine phenotype in small cell lung cancer, we expected that blocking this gene’s expression in BON cells would reduce neuroendocrine marker levels12. Furthermore, since Notch1-induced ASCL1 suppression has been shown to reduce cell proliferation in carcinoids, we anticipated that direct ASCL1 silencing would reduce BON carcinoid cell proliferation as well28. Indeed, we observed a significant reduction in relative cell growth following prolonged ASCL1 silencing. ASCL1 knockdown also suppressed CgA and SYP expression. As neurosecreted markers, both CgA and SYP are strong indicators of the neuroendocrine phenotype, together serving useful both diagnostically and prognostically 60-62. We were able to show that the degree of CgA reduction was proportional to the level of ASCL1 silencing, implying that ASCL1 tightly regulates CgA expression. Findings from this study also suggest that ASCL1 affects BON cell-cycle progression. Because p21Waf1/Cip1 is a known inhibitor of cyclin dependent kinase activity and responds to the tumor suppressor p53, its induction following ASCL1 suppression signifies the occurrence of cell-cycle arrest. This along with the observed induction in the tumor suppressor p27Kip1 and reduction in cyclins B1 and D1 collectively suggest that the growth inhibitory effect from silencing ASCL1 may be due to an alteration in cell-cycle kinetics.
In summary, this report illustrates that chrysin suppresses ASCL1 gene expression and reduces BON and H727 cell proliferation through initiating apoptosis and cell-cycle arrest. Moreover, our findings affirm past reports that ASCL1 expression is an intrinsic feature of the neuroendocrine phenotype, and that when suppressed, can alter expression of bioactive hormones CgA and SYP while arresting cell-cycle progression. Chrysin’s ability to illicit antiproliferative effects in BON and H727 may be rooted in its ability to diminish ASCL1, but may also involve parallel downstream events. This may be true as direct ASCL1 suppression was unable to achieve a growth inhibitory effect commensurate to that of chrysin, implying that while ASCL1 may be central to chrysin’s downstream targets, other pathways may be involved as well. Some investigators have taken to exploring more specific derivatives of chrysin in order to enhance its potency while preserving the properties of its parent drug75. To this vein, understanding downstream intermediaries specific to neuroendocrine cancers like ASCL1 may also offer ways to enhance the observed therapeutic effect. These studies justify future investigations into the role of ASCL1 and validate its candidacy as a potential therapeutic target for carcinoids.
Acknowledgments
Grant Support: This research was supported under the NIH National Research Service Award T32 GM07215 (Y.S), the Howard Hughes Medical Institute Medical Research Fellows Program (Y.S.), a research scholarship from the American College of Surgeons (B.Z.D.), a NIH grant R01 CA121115 (H.C.), the American Cancer Society MEN2 Thyroid Cancer Professorship 120319-RPM-11-080-01-TBG and Research Scholar Award RSGM TBE-121413 (H.C.), and the Layton F. Rikkers, MD, Chair in Surgical Leadership Professorship (H.C.).
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest exist.
References
- 1.Oberg K. Genetics and molecular pathology of neuroendocrine gastrointestinal and pancreatic tumors (gastroenteropancreatic neuroendocrine tumors) Curr Opin Endocrinol Diabetes Obes. 2009;16(1):72–8. doi: 10.1097/med.0b013e328320d845. [DOI] [PubMed] [Google Scholar]
- 2.Sippel RS, Chen H. Carcinoid tumors. Surg Oncol Clin N Am. 2006;15(3):463–78. doi: 10.1016/j.soc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Calender A. Genetics of neuroendocrine tumors. Rev Prat. 2002;52(3):256–61. [PubMed] [Google Scholar]
- 4.Maggard MA, O’Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg. 2004;240(1):117–22. doi: 10.1097/01.sla.0000129342.67174.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas CF, Tazelaar HD, Jett JR. Typical and atypical pulmonary carcinoids : outcome in patients presenting with regional lymph node involvement. Chest. 2001;119(4):1143–50. doi: 10.1378/chest.119.4.1143. [DOI] [PubMed] [Google Scholar]
- 6.Maroun J, Kocha W, Kvols L, Bjarnason G, Chen E, Germond C, et al. Guidelines for the diagnosis and management of carcinoid tumours. Part 1: the gastrointestinal tract. A statement from a Canadian National Carcinoid Expert Group. Curr Oncol. 2006;13(2):67–76. doi: 10.3390/curroncol13020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasieka JL. Carcinoid tumors. Surg Clin North Am. 2009;89(5):1123–37. doi: 10.1016/j.suc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Hardacre J, Uzar A, Cameron J, Choti M. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg. 1998;187(1):88–92. doi: 10.1016/s1072-7515(98)00099-4. discussion 92-3. [DOI] [PubMed] [Google Scholar]
- 9.Zuetenhorst JM, Taal BG. Metastatic carcinoid tumors: a clinical review. Oncologist. 2005;10(2):123–31. doi: 10.1634/theoncologist.10-2-123. [DOI] [PubMed] [Google Scholar]
- 10.Modlin IM, Latich I, Kidd M, Zikusoka M, Eick G. Therapeutic options for gastrointestinal carcinoids. Clin Gastroenterol Hepatol. 2006;4(5):526–47. doi: 10.1016/j.cgh.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 11.de Herder WW, Hofland LJ, van der Lely AJ, Lamberts SW. Somatostatin receptors in gastroentero-pancreatic neuroendocrine tumours. Endocr Relat Cancer. 2003;10(4):451–8. doi: 10.1677/erc.0.0100451. [DOI] [PubMed] [Google Scholar]
- 12.Borges M, Linnoila RI, van de Velde HJ, Chen H, Nelkin BD, Mabry M, et al. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature. 1997;386(6627):852–5. doi: 10.1038/386852a0. [DOI] [PubMed] [Google Scholar]
- 13.Nakakura E, Sriuranpong V, Kunnimalaiyaan M, Hsiao E, Schuebel K, Borges M, et al. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J Clin Endocrinol Metab. 2005;90(7):4350–6. doi: 10.1210/jc.2005-0540. [DOI] [PubMed] [Google Scholar]
- 14.Sippel R, Carpenter J, Kunnimalaiyaan M, Chen H. The role of human achaete-scute homolog-1 in medullary thyroid cancer cells. Surgery. 2003;134(6):866–71. doi: 10.1016/s0039-6060(03)00418-5. discussion 871-3. [DOI] [PubMed] [Google Scholar]
- 15.Jiang S, Kameya T, Asamura H, Umezawa A, Sato Y, Shinada J, et al. hASH1 expression is closely correlated with endocrine phenotype and differentiation extent in pulmonary neuroendocrine tumors. Mod Pathol. 2004;17(2):222–9. doi: 10.1038/modpathol.3800038. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Biel MA, Borges MW, Thiagalingam A, Nelkin BD, Baylin SB, et al. Tissue-specific expression of human achaete-scute homologue-1 in neuroendocrine tumors: transcriptional regulation by dual inhibitory regions. Cell Growth Differ. 1997;8(6):677–86. [PubMed] [Google Scholar]
- 17.Shida T, Furuya M, Kishimoto T, Nikaido T, Tanizawa T, Koda K, et al. The expression of NeuroD and mASH1 in the gastroenteropancreatic neuroendocrine tumors. Mod Pathol. 2008;21(11):1363–70. doi: 10.1038/modpathol.2008.121. [DOI] [PubMed] [Google Scholar]
- 18.Ball DW, Azzoli CG, Baylin SB, Chi D, Dou S, Donis-Keller H, et al. Identification of a human achaete-scute homolog highly expressed in neuroendocrine tumors. Proc Natl Acad Sci U S A. 1993;90(12):5648–52. doi: 10.1073/pnas.90.12.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Udelsman R, Zeiger M, Ball D. Human achaete-scute homolog-1 is highly expressed in a subset of neuroendocrine tumors. Oncol Rep. 1997;4(4):775–8. doi: 10.3892/or.4.4.775. [DOI] [PubMed] [Google Scholar]
- 20.Carney ME, O’Reilly RC, Sholevar B, Buiakova OI, Lowry LD, Keane WM, et al. Expression of the human Achaete-scute 1 gene in olfactory neuroblastoma (esthesioneuroblastoma) J Neurooncol. 1995;26(1):35–43. doi: 10.1007/BF01054767. [DOI] [PubMed] [Google Scholar]
- 21.Guillemot F, Joyner AL. Dynamic expression of the murine Achaete-Scute homologue Mash-1 in the developing nervous system. Mech Dev. 1993;42(3):171–85. doi: 10.1016/0925-4773(93)90006-j. [DOI] [PubMed] [Google Scholar]
- 22.Guillemot F, Nagy A, Auerbach A, Rossant J, Joyner AL. Essential role of Mash-2 in extraembryonic development. Nature. 1994;371(6495):333–6. doi: 10.1038/371333a0. [DOI] [PubMed] [Google Scholar]
- 23.Lo L, Guillemot F, Joyner AL, Anderson DJ. MASH-1: a marker and a mutation for mammalian neural crest development. Perspect Dev Neurobiol. 1994;2(2):191–201. [PubMed] [Google Scholar]
- 24.Lanigan TM, DeRaad SK, Russo AF. Requirement of the MASH-1 transcription factor for neuroendocrine differentiation of thyroid C cells. J Neurobiol. 1998;34(2):126–34. [PubMed] [Google Scholar]
- 25.Sippel RS, Carpenter JE, Kunnimalaiyaan M, Lagerholm S, Chen H. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2003;285(2):G245–54. doi: 10.1152/ajpgi.00420.2002. [DOI] [PubMed] [Google Scholar]
- 26.Kunnimalaiyaan M, Traeger K, Chen H. Conservation of the Notch1 signaling pathway in gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2005;289(4):G636–42. doi: 10.1152/ajpgi.00146.2005. [DOI] [PubMed] [Google Scholar]
- 27.Pitt SC, Chen H, Kunnimalaiyaan M. Inhibition of phosphatidylinositol 3-kinase/Akt signaling suppresses tumor cell proliferation and neuroendocrine marker expression in GI carcinoid tumors. Ann Surg Oncol. 2009;16(10):2936–42. doi: 10.1245/s10434-009-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakakura EK, Sriuranpong VR, Kunnimalaiyaan M, Hsiao EC, Schuebel KE, Borges MW, et al. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J Clin Endocrinol Metab. 2005;90(7):4350–6. doi: 10.1210/jc.2005-0540. [DOI] [PubMed] [Google Scholar]
- 29.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Overexpression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J Biol Chem. 2006;281(52):39819–30. doi: 10.1074/jbc.M603578200. [DOI] [PubMed] [Google Scholar]
- 30.Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci. 2005;8(6):709–15. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- 31.Maillard I, Pear WS. Notch and cancer: best to avoid the ups and downs. Cancer Cell. 2003;3(3):203–5. doi: 10.1016/s1535-6108(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 32.Sriuranpong V, Borges MW, Strock CL, Nakakura EK, Watkins DN, Blaumueller CM, et al. Notch signaling induces rapid degradation of achaete-scute homolog 1. Mol Cell Biol. 2002;22(9):3129–39. doi: 10.1128/MCB.22.9.3129-3139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sriuranpong V, Borges MW, Ravi RK, Arnold DR, Nelkin BD, Baylin SB, et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res. 2001;61(7):3200–5. [PubMed] [Google Scholar]
- 34.de la Pompa JL, Wakeham A, Correia KM, Samper E, Brown S, Aguilera RJ, et al. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development. 1997;124(6):1139–48. doi: 10.1242/dev.124.6.1139. [DOI] [PubMed] [Google Scholar]
- 35.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24(1):36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 36.Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, et al. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400(6747):877–81. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 37.Monasterio A, Urdaci MC, Pinchuk IV, López-Moratalla N, Martínez-Irujo JJ. Flavonoids induce apoptosis in human leukemia U937 cells through caspase- and caspase-calpain-dependent pathways. Nutr Cancer. 2004;50(1):90–100. doi: 10.1207/s15327914nc5001_12. [DOI] [PubMed] [Google Scholar]
- 38.Woo KJ, Jeong YJ, Park JW, Kwon TK. Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells. Biochem Biophys Res Commun. 2004;325(4):1215–22. doi: 10.1016/j.bbrc.2004.09.225. [DOI] [PubMed] [Google Scholar]
- 39.Yu XM, Phan T, Patel PN, Jaskula-Sztul R, Chen H. Chrysin activates Notch1 signaling and suppresses tumor growth of anaplastic thyroid carcinoma in vitro and in vivo. Cancer. 2013;119(4):774–81. doi: 10.1002/cncr.27742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khoo BY, Chua SL, Balaram P. Apoptotic effects of chrysin in human cancer cell lines. Int J Mol Sci. 2010;11(5):2188–99. doi: 10.3390/ijms11052188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pal-Bhadra M, Ramaiah MJ, Reddy TL, Krishnan A, Pushpavalli SN, Babu KS, et al. Plant HDAC inhibitor chrysin arrest cell growth and induce p21WAF1 by altering chromatin of STAT response element in A375 cells. BMC Cancer. 2012;12:180. doi: 10.1186/1471-2407-12-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weng MS, Ho YS, Lin JK. Chrysin induces G1 phase cell cycle arrest in C6 glioma cells through inducing p21Waf1/Cip1 expression: involvement of p38 mitogen-activated protein kinase. Biochem Pharmacol. 2005;69(12):1815–27. doi: 10.1016/j.bcp.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Q, Zhao XH, Wang ZJ. Cytotoxicity of flavones and flavonols to a human esophageal squamous cell carcinoma cell line (KYSE-510) by induction of G2/M arrest and apoptosis. Toxicol In Vitro. 2009;23(5):797–807. doi: 10.1016/j.tiv.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Wang W, VanAlstyne PC, Irons KA, Chen S, Stewart JW, Birt DF. Individual and interactive effects of apigenin analogs on G2/M cell-cycle arrest in human colon carcinoma cell lines. Nutr Cancer. 2004;48(1):106–14. doi: 10.1207/s15327914nc4801_14. [DOI] [PubMed] [Google Scholar]
- 45.Woodman OL, Chan ECh. Vascular and anti-oxidant actions of flavonols and flavones. Clin Exp Pharmacol Physiol. 2004;31(11):786–90. doi: 10.1111/j.1440-1681.2004.04072.x. [DOI] [PubMed] [Google Scholar]
- 46.Middleton E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52(4):673–751. [PubMed] [Google Scholar]
- 47.Cook MR, Pinchot SN, Jaskula-Sztul R, Luo J, Kunnimalaiyaan M, Chen H. Identification of a novel Raf-1 pathway activator that inhibits gastrointestinal carcinoid cell growth. Mol Cancer Ther. 2010;9(2):429–37. doi: 10.1158/1535-7163.MCT-09-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wyche TP, Dammalapati A, Cho H, Harrison AD, Kwon GS, Chen H, et al. Thiocoraline activates the Notch pathway in carcinoids and reduces tumor progression in vivo. Cancer Gene Ther. 2014;21(12):518–25. doi: 10.1038/cgt.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenblatt DY, Cayo M, Ning L, Jaskula-Sztul R, Haymart M, Kunnimalaiyaan M, et al. Suberoyl bishydroxamic acid inhibits cellular proliferation by inducing cell cycle arrest in carcinoid cancer cells. J Gastrointest Surg. 2007;11(11):1515–20. doi: 10.1007/s11605-007-0249-1. discussion 1520. [DOI] [PubMed] [Google Scholar]
- 50.Pinchot SN, Jaskula-Sztul R, Ning L, Peters NR, Cook MR, Kunnimalaiyaan M, et al. Identification and validation of Notch pathway activating compounds through a novel high-throughput screening method. Cancer. 2011;117(7):1386–98. doi: 10.1002/cncr.25652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lukas J, Bartkova J, Bartek J. Convergence of mitogenic signalling cascades from diverse classes of receptors at the cyclin D-cyclin-dependent kinase-pRb-controlled G1 checkpoint. Mol Cell Biol. 1996;16(12):6917–25. doi: 10.1128/mcb.16.12.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13(12):1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 53.Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, et al. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18(6):1571–83. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67(1):197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- 55.Atherton-Fessler S, Liu F, Gabrielli B, Lee MS, Peng CY, Piwnica-Worms H. Cell cycle regulation of the p34cdc2 inhibitory kinases. Mol Biol Cell. 1994;5(9):989–1001. doi: 10.1091/mbc.5.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Norbury C, Blow J, Nurse P. Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. EMBO J. 1991;10(11):3321–9. doi: 10.1002/j.1460-2075.1991.tb04896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen JY, Cook MR, Pinchot SN, Kunnimalaiyaan M, Chen H. MG-132 inhibits carcinoid growth and alters the neuroendocrine phenotype. J Surg Res. 2010;158(1):15–9. doi: 10.1016/j.jss.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pitt S, Chen H, Kunnimalaiyaan M. Phosphatidylinositol 3-kinase-Akt signaling in pulmonary carcinoid cells. J Am Coll Surg. 2009;209(1):82–8. doi: 10.1016/j.jamcollsurg.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Somnay Y, Simon K, Harrison AD, Kunnimalaiyaan S, Chen H, Kunnimalaiyaan M. Neuroendocrine phenotype alteration and growth suppression through apoptosis by MK-2206, an allosteric inhibitor of AKT, in carcinoid cell lines in vitro. Anticancer Drugs. 2013;24(1):66–72. doi: 10.1097/CAD.0b013e3283584f75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seregni E, Ferrari L, Bajetta E, Martinetti A, Bombardieri E. Clinical significance of blood chromogranin A measurement in neuroendocrine tumours. Ann Oncol. 2001;12(Suppl 2):S69–72. doi: 10.1093/annonc/12.suppl_2.s69. [DOI] [PubMed] [Google Scholar]
- 61.Tomassetti P, Migliori M, Simoni P, Casadei R, De Iasio R, Corinaldesi R, et al. Diagnostic value of plasma chromogranin A in neuroendocrine tumours. Eur J Gastroenterol Hepatol. 2001;13(1):55–8. doi: 10.1097/00042737-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 62.Chejfec G, Falkmer S, Grimelius L, Jacobsson B, Rodensjo M, Wiedenmann B, et al. Synaptophysin. A new marker for pancreatic neuroendocrine tumors. Am J Surg Pathol. 1987;11(4):241–7. [PubMed] [Google Scholar]
- 63.Chejfec G, Falkmer S, Grimelius L, Jacobsson B, Rodensjö M, Wiedenmann B, et al. Synaptophysin. A new marker for pancreatic neuroendocrine tumors. Am J Surg Pathol. 1987;11(4):241–7. [PubMed] [Google Scholar]
- 64.Seregni E, Ferrari L, Bajetta E, Martinetti A, Bombardieri E. Clinical significance of blood chromogranin A measurement in neuroendocrine tumours. Ann Oncol. 2001;12(Suppl 2):S69–72. doi: 10.1093/annonc/12.suppl_2.s69. [DOI] [PubMed] [Google Scholar]
- 65.Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, et al. p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol. 1999;154(2):313–23. doi: 10.1016/S0002-9440(10)65277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lorca T, Labbé JC, Devault A, Fesquet D, Capony JP, Cavadore JC, et al. Dephosphorylation of cdc2 on threonine 161 is required for cdc2 kinase inactivation and normal anaphase. EMBO J. 1992;11(7):2381–90. doi: 10.1002/j.1460-2075.1992.tb05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Campuzano S, Carramolino L, Cabrera CV, Ruíz-Gómez M, Villares R, Boronat A, et al. Molecular genetics of the achaete-scute gene complex of D. melanogaster. Cell. 1985;40(2):327–38. doi: 10.1016/0092-8674(85)90147-3. [DOI] [PubMed] [Google Scholar]
- 68.Sommer L, Shah N, Rao M, Anderson DJ. The cellular function of MASH1 in autonomic neurogenesis. Neuron. 1995;15(6):1245–58. doi: 10.1016/0896-6273(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 69.Greenblatt DY, Vaccaro AM, Jaskula-Sztul R, Ning L, Haymart M, Kunnimalaiyaan M, et al. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist. 2007;12(8):942–51. doi: 10.1634/theoncologist.12-8-942. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Q, Zhao XH, Wang ZJ. Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem Toxicol. 2008;46(6):2042–53. doi: 10.1016/j.fct.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 71.Zhou P, Jiang W, Zhang Y, Kahn S, Schieren I, Santella R, et al. Antisense to cyclin D1 inhibits growth and reverses the transformed phenotype of human esophageal cancer cells. Oncogene. 1995;11(3):571–80. [PubMed] [Google Scholar]
- 72.Arber N, Doki Y, Han E, Sgambato A, Zhou P, Kim N, et al. Antisense to cyclin D1 inhibits the growth and tumorigenicity of human colon cancer cells. Cancer Res. 1997;57(8):1569–74. [PubMed] [Google Scholar]
- 73.Russell A, Thompson MA, Hendley J, Trute L, Armes J, Germain D. Cyclin D1 and D3 associate with the SCF complex and are coordinately elevated in breast cancer. Oncogene. 1999;18(11):1983–91. doi: 10.1038/sj.onc.1202511. [DOI] [PubMed] [Google Scholar]
- 74.Lukas J, Müller H, Bartkova J, Spitkovsky D, Kjerulff AA, Jansen-Dürr P, et al. DNA tumor virus oncoproteins and retinoblastoma gene mutations share the ability to relieve the cell’s requirement for cyclin D1 function in G1. J Cell Biol. 1994;125(3):625–38. doi: 10.1083/jcb.125.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu H, Liu K, Huang Z, Park CM, Thimmegowda NR, Jang JH, et al. A chrysin derivative suppresses skin cancer growth by inhibiting cyclin-dependent kinases. J Biol Chem. 2013;288(36):25924–37. doi: 10.1074/jbc.M113.464669. [DOI] [PMC free article] [PubMed] [Google Scholar]