SUMMARY
Extrinsic cues from the niche are known to regulate adult stem cell self-renewal versus differentiation. Here we report that an aminopeptidase Slamdance (Sda) acts in the Drosophila testicular niche to maintain germline stem cells (GSCs) and regulate progenitor germ cell dedifferentiation. Mutations in sda lead to dramatic testicular niche deterioration and stem cell loss. Recombinant Sda has specific aminopeptidase activity in vitro, and the in vivo function of Sda requires an intact aminopeptidase domain. Sda is required for accumulation of mature DE-Cadherin and overexpression of DE-Cadherin rescues most sda mutant phenotypes, suggesting that DE-Cadherin is an important target of Sda. Finally, Sda is both necessary and sufficient to promote dedifferentiation during aging, and recovery from genetically manipulated depletion of GSCs. Together our results suggest that a niche factor promotes both stem cell maintenance and progenitor cell dedifferentiation.
Keywords: Germline stem cell, cyst stem cell, spermatogonia, niche, maintenance, dedifferentiation, Drosophila testis

INTRODUCTION
Adult stem cells give rise to many different cell types in the body, either continuously or in response to physiological signals or injuries. The ability of the stem cell system to maintain homeostasis in adult tissue relies on the maintenance of stem cell identity as well as regulation of progeny cell differentiatiation. Normal cellular differentiation from a limited number of adult stem cells often begins with a transit-amplification stage, during which progenitor cells undergo limited rounds of mitosis, followed by terminal differentiation. On the other hand, progenitor cells in multiple adult stem cell lineages have the plasticity to undergo a dedifferentiation process to replenish lost stem or progenitor cells during aging or upon injury (Barroca et al., 2009; Boyle et al., 2007; Brawley and Matunis, 2004; Cheng et al., 2008; Kai and Spradling, 2004; Lehoczky et al., 2011; Nakagawa et al., 2010; Rinkevich et al., 2011; Sheng et al., 2009; Wallenfang et al., 2006). Although misregulation of dedifferentiation has been implicated in tumorigenesis (Friedmann-Morvinski et al., 2012; Goldstein et al., 2010; Schwitalla et al., 2013), the molecular mechanisms governing dedifferentiation require further exploration.
The breakthrough discovery that terminally differentiated cells can be reprogrammed to become pluripotent cells [(Takahashi et al., 2007; Takahashi and Yamanaka, 2006; Yu et al., 2007), reviewed in (Yamanaka, 2012)] opened up new avenues for regenerative medicine. Since then, many studies have focused on understanding how intrinsic factors, such as transcriptional factors and chromatin regulators, govern cellular reprogramming [reviewed in (Apostolou and Hochedlinger, 2013; Jaenisch and Young, 2008)]. However, detailed analysis of reprogrammed cells also revealed genetic and epigenetic aberrations [reviewed in (Robinton and Daley, 2012)], raising concerns regarding medical applications. That said, many organs with short-lived cells, such as blood, skin, intestine, and testis, are maintained by continuous activity of adult stem cells. Reprogramming from the same adult stem cell lineage could provide a safer solution for tissue regeneration. The related question is how dedifferentiation is controlled in vivo and whether this process can be manipulated.
Drosophila germline stem cells (GSCs) have provided a model system to study cellular and molecular mechanisms that regulate adult stem cell maintenance and differentiation. In both female and male Drosophila GSC lineages, the differentiating daughter cells from asymmetric GSC divisions are displaced from the niche and undergo limited proliferation followed by meiosis and terminal differentiation (Clarke and Fuller, 2006; Fuller and Spradling, 2007). Previous studies have revealed that progenitor germ cells at the proliferative stage can undergo dedifferentiation to reoccupy the niche (Brawley and Matunis, 2004; Cheng et al., 2008; Kai and Spradling, 2004; Sheng et al., 2009; Sheng and Matunis, 2011) under physiological conditions, such as aging (Cheng et al., 2008; Wong and Jones, 2012), and during recovery from genetically manipulated depletion of GSCs (Brawley and Matunis, 2004; Kai and Spradling, 2004; Sheng and Matunis, 2011; Yadlapalli and Yamashita, 2013). To date, our understanding of the molecular mechanisms regulating dedifferentiation is limited. It has been reported that mis-expression of a dominant negative form of Drosophila E-cadherin homolog (DE-cadherin, E-cad) (Inaba et al., 2010) or suppressor of cytokine signaling 36E (Socs36E), an inhibitor of the JAK-STAT (Janus kinase and signal transducer and activator of transcription) signaling pathway (Sheng et al., 2009), in germ cells reduces spermatogonial dedifferentiation. In both cases, gene expression is manipulated in germ cells. Interestingly, live imaging has shown that dedifferentiating progenitor spermatogonial cells make initial contact with the male GSC niche (Sheng et al., 2009; Sheng and Matunis, 2011), but it is unclear whether and how cells in the niche regulate dedifferentiation (Sheng and Matunis, 2009).
Here we report in vivo evidence that an aminopeptidase, a niche-enriched factor, maintains GSCs and regulates dedifferentiation of progenitor germ cells under both physiological conditions and upon genetically manipulated depletion of stem cells. Our results provide an important advance toward understanding how a niche-specific peptidase influences stem cell self-renewal versus differentiation, as well as progenitor cell differentiation versus dedifferentiation, two critical decisions in an adult stem lineage.
RESULTS
Sda is required for maintaining stem cells and hub cells in the Drosophila testicular niche
In Drosophila testis, GSCs associate with two types of somatic cells: hub cells and cyst stem cells (CySCs) (Figure 1A). Through a RNA-seq screen (Z., Shi and C., Lim, unpublished data), we found that a gene termed slamdance (sda) is highly transcribed in a niche sample containing hub cells, GSCs and CySCs (Figure S1A). Mutations in the sda gene cause defects in Drosophila nervous system shown by increased seizure susceptibility, which were identified in a genetic screen for bang sensitive mutants (Zhang et al., 2002). To study the functions of Sda in the testicular niche, we obtained a strong loss-of-function allele (Zhang et al., 2002), sdaiso7.8, and used it in trans with a deficiency (Df) chromosome that uncovers the sda gene region. In the sda/Df (hereinafter called sda) mutant testicular niche, substantial changes were detected in hub cells, GSCs, and CySCs (Figures 1B-H, Figure S2). Compared to testes from 30-day-old (D30) wild-type (WT) males (Figures 1B-B’), hub cell number in sda mutant testes decreased (Figures 1C-C’, 1F), even though no hub cells were found to undergo cell death or transdifferentiation (SUPPLEMENTAL EXPERIMENTAL PROCEDURES). The lost hub cells from the first instar larvae (L1) to D30 male are approximately seven, which might be too few to detect during this ~ 40-day period of time due to technical limitations. In addition to hub cells, we found a significant decrease in the number of both GSCs (Figures 1B-C, 1G) and Zfh-1-positive early-stage cyst cells including CySCs (Issigonis et al., 2009; Leatherman and Dinardo, 2008) (Figures 1D-E, 1H).
Figure 1. Sda is required for maintaining stem cells and hub cells in the Drosophila testicular niche.
(A) A schematic diagram outlines the Drosophila testicular niche (the purple crescent outlines the testis apical tip). (B-E) Immunostaining of testes from D30 WT (B, B’, D) and sda/Df (sda) mutant (C, C’, E) males using anti-Vasa (germ cells), anti-Armadillo (Arm) (hub cells) and anti-Zfh-1 (CySCs and early-stage cyst cells); dots in (B-C) indicate GSCs, which are Vasa-positive cells adjacent to hub cells; arrows in (E) point to two Zfh-1-positive cells. Scale bar: 10μm. (F-H) Quantification of hub cells (F), GSCs (G), and Zfh-1-positive cells (H) in testes from WT and sda mutant males at different developmental stages (L1, L2 and L3: first, second and third instar larvae; D1, D15 and D30: 1-day-, 15-day- and 30-day-old adult males.). Error bar: 95% Confidence Interval (CI) of SEM (Standard Error of Mean, EXPERIMENTAL PROCEDURES). P value calculated by one-tailed t-test: not significant (n.s.) for L1 and <0.0001 for L2-D30 in (F); n.s. for L1, <0.01 for L2, <0.0001 for L3-D30 in (G); =0.01 for L3, <0.0001 for D1-D30 in (H).
Next, to determine whether loss of hub cells and both types of stem cells in the sda mutant occurred at the time of niche establishment (Le Bras and Van Doren, 2006) or later during homeostasis, we compared sda mutant and WT testes across distinct developmental stages, from L1 to D30 adult males. We found no significant difference between WT and sda mutant at early developmental stages (e.g., L1 for hub cells and GSCs and L3 for Zfh-1-positive cells, Figures 1F-H), suggesting maintenance rather than establishment defects. Noticeably, Sda promotes GSC expansion from the second instar larval (L2) to the third instar larval (L3) stage because GSCs increase 1.7-fold in WT testes and only 1.3-fold in sda mutant testes (Figure 1G).
Sda acts in hub cells to maintain stem cells and hub cells in the Drosophila testicular niche
To understand the cell type specificity of Sda in the Drosophila testicular niche, we examined its expression pattern using an enhancer trap line (Zhang et al., 2002). We found that sda is mainly expressed in hub cells (Figures 2A-A’), consistent with our RNA-seq results (Figure S1A). In addition, we determined that the endogenous function of Sda is required mainly in hub cells using two complementary assays. First, the sda RNAi transgene (UAS-ds sda) driven by a hub-specific upd-Gal4 driver (Boyle et al., 2007; Leatherman and Dinardo, 2010) recapitulated sda mutant phenotypes in the testicular niche (Figures 2B-D). Second, the sda mutant phenotypes were fully rescued by driving an HA-tagged full-length sda cDNA (HA-sdaFL) using the same upd-Gal4 driver (Figures 2E-F, 2I). In contrast, the use of a germline-specific nanos-Gal4 driver (Van Doren et al., 1998) or a cyst cell-specific c587-Gal4 driver (Manseau et al., 1997) to knock down sda did not lead to significant loss of GSCs, early cyst cells, or hub cells, compared to the knockdown experiments using the upd-Gal4 driver (Figure 2D). In addition, using the same upd-Gal4; UAS-ds sda (upd> ds sda) in a temperature shift assay (Eliazer et al., 2011) that specifically knocked down sda in adult flies was sufficient to recapitulate all sda mutant phenotypes in the testicular niche (Figure S3A). These results are consistent with the roles of Sda in maintaining but not establishing hub cells and stem cells (Figure 1F-H). In summary, these results demonstrate that Sda is required in hub cells to maintain normal testicular niche architecture in Drosophila.
Figure 2. Sda acts in hub cells to maintain stem cells and hub cells and such an activity requires a functional catalytic domain.
(A) Immunostaining with anti-Vasa and anti-LacZ using an sda enhancer trap line (Zhang et al., 2002). The LacZ staining is shown separately in (A’). (B-C) Testes from upd-Gal4 (B) and upd-Gal4; UAS-ds sda; UAS-dcr2 (C) males stained with anti-Vasa and anti-Arm; dots indicate GSCs. (D) Quantification of GSCs, Zfh-1-positive cells and hub cells in testes from nos-Gal4, c587-Gal4 and upd-Gal4 as controls and crossed to the UAS-ds sda; UAS-dcr2 background. P-value between any two genotypes except upd-Gal4; UAS-ds sda; UAS-dcr2 is n.s.; whereas P-value between any other genotype and upd-Gal4; UAS-ds sda; UAS-dcr2 < 0.01. (E) Structure of HA-sdaFL, HA-sdaΔCAT and HA-sdaE→A; TM: transmembrane domain. (F-H) Testes from upd-Gal4; UAS-HA-sdaFL; sda (F), upd-Gal4; UAS-HA-sdaΔCAT; sda (G), and upd-Gal4; UAS-HA-sdaE→A; sda (H) males stained with anti-Vasa, anti-Zfh-1 and anti-Arm; arrows point to Zfh-1-positive cells. Scale bar: 10μm. (I) Quantification of GSCs, Zfh-1-positive cells and hub cells. All quantification data were obtained using D15 males. For the two groups: (1) upd-Gal4 control and upd-Gal4; UAS-HA-sdaFL; sda; (2) upd-Gal4;; sda and upd-Gal4; UAS-HA-sdaΔCAT; sda and upd-Gal4; UAS-HA-sdaE→A; sda, P-value between any two genotypes within each group is n.s.; whereas P-value between any two genotypes from each group < 0.01. Error bar: 95% CI of SEM in (D) and (I), P-value calculated by one-tailed t-test.
Purified Sda exhibits aminopeptidase activity in vitro
Sda is predicted to be a type II membrane protein with a single transmembrane anchor and homology to mammalian zinc-dependent aminopeptidase N (APN) (Zhang et al., 2002). The identity and similarity between Sda and human APN is 33% and 51%, respectively, while their catalytic domains are 84% identical (31% identity and 50% similarity between Sda and mouse APN) (Zhang et al., 2002). We used the baculovirus system to express recombinant SdaΔN (extracellular domain in Figure 2E) in insect Sf9 cells, purified it to apparent homogeneity (Figure 3A), and evaluated it for aminopeptidase activity in parallel with human APN (hAPN1) as a control. SdaΔN displayed clear aminopeptidase activity that was dependent on both zinc and a critical Glu (E) in the AAMEN motif involved in substrate recognition (Luciani et al., 1998; Zhang et al., 2002) (Figures 2E, 3B-C). In addition, SdaΔN was robustly thermostable, monomeric by dynamic light scattering, and unstimulated by kosmotropic salts, suggesting it functions as a monomer (Figures S4A-B). Overall, the aminopeptidase activity of Sda is comparable to that of human APN, although it has considerably less catalytic activity but higher substrate selectivity in vitro.
Figure 3. Purified Sda exhibits aminopeptidase activity in vitro.
(A) SDS-PAGE analysis of truncated SDA (residues 126-1071, extracellular domain in Figure 2E) either with WT sequence (SDAΔN) or E→A mutation (EA) purified from baculovirus-infected Sf9 cells. (B) Real-time activity of SDAΔN and human aminopeptidase N (hAPN1) with L-Alanine-AMC (7-amino-4-methylcoumarin) at 25°C (1 read/min). Mutating a critical glutamate (E) or chelating zinc with 1, 10-phenanthroline inhibited proteolysis. (C) Substrate specificity of hAPN1 and SDAΔN relative to cleavage of L-Ala-AMC as the substrate.
An intact catalytic domain of Sda is required for its normal activity in vivo
To understand the in vivo function of Sda, the HA-tagged full-length sda cDNA (Figure 2E) was expressed using different cell type-specific Gal4 drivers in the testicular niche. We found that ectopic expression of HA-sdaFL in cyst cells using c587-Gal4 resulted in almost full rescue for the number of GSCs, early cyst cells and hub cells (Figure S3B). Ectopic expression of HA-sdaFL in germ cells using nos-Gal4 also resulted in partial rescue for GSC number (Figure S3B). We reasoned that these rescue results are due to the topology of Sda as a transmembrane protein and the prediction of its enzymatic domain to be extracellular. It is possible that as long as sda is expressed in any cell type in the testicular niche, its extracellular catalytic domain can still process the substrates which are likely present in extracellular matrix, which result in rescue to different degrees.
Next, to study whether Sda acts as an APN in vivo in the testicular niche, two mutated sda cDNAs encoding enzymatically inactive forms were generated: an HA-sdaΔCAT with a truncation at the predicted zinc-binding and APN domain; and an HA-sdaE→A with a point mutation in the essential E residue (Figure 2E), which is critical for substrate recognition (Luciani et al., 1998; Zhang et al., 2002) (Figure 3B) but not for protein stability (Figure S4A). Neither transgene could rescue the sda mutant phenotype when driven by upd-Gal4 (Figures 2G-I, Figure S3C-D), even though no significant difference could be detected at either transcript level (Figure S3E) or protein localization (Figures S3F-H), suggesting that an intact catalytic domain is required for Sda to maintain stem cells and hub cells in the Drosophila testicular niche.
Finally, by comparing testes with either loss-of-function or overexpression of sda with WT control in a degradomics analysis (Kleifeld et al., 2010), we demonstrated that Sda also has aminopeptidase activity in vivo (SUPPLEMENTAL EXPERIMENTAL PROCEDURES, Table S1). The limited identifiable Sda-mediated cleavage events are probably due to its restricted proteolytic activity in hub cells, which are under-represented using the entire testis sample.
Sda is required for accumulation of mature Drosophila E-Cadherin to maintain stem cells and hub cells in the testicular niche
To understand how Sda regulates different cell types in the testicular niche, we took a candidate gene approach by investigating whether sda interacts with cadherin molecules (Inaba et al., 2010) and JAK-STAT signaling pathway components (Sheng et al., 2009) known to act in the niche for stem cell maintenance and niche structure.
We found that removal of one copy of Cadherin genes made the sda loss-of-function phenotype more severe (Figure 4A). Furthermore, expression of either Drosophila E-Cad (DE-cadherin, E-cadherin homolog) (Figures 4B-C) or N-cad (DN-cadherin, N-cadherin homolog) in hub cells (Figures 4D) using upd-Gal4 or in all somatic gonadal cells including hub cells (Figures 4E) using tj-Gal4 (Tanentzapf et al., 2007) rescued completely of the loss of early cyst cell and hub cell phenotypes in sda mutant; whereas the rescue of GSC loss was substantial but not complete (Figures 4D-E). By contrast, expression of either E-Cad or N-Cad in germ cells did not rescue any of the cell loss phenotypes in the testicular niche (Figure 4F). Because Sda is predicted to be a peptidase and maturation of both E-Cad and N-Cad requires protein cleavage (Iwai et al., 1997; Oda and Tsukita, 1999), we examined how Sda may be required for expression and/or activity of Cadherins. Quantitative RT-PCR showed that the overall transcript level of E-Cad was comparable and even slightly higher in sda mutant testes than that in WT control (Figure 4G). However, immunoblotting showed that the mature E-Cad protein has an approximately 23% reduction in sda mutant testes compared to WT testes (Figure 4H). Noticeably, the actual E-Cad protein level change at the niche could be even more substantial since using the entire testes may underestimate this effect. Despite the change in E-Cad level, no significant change of E-Cad subcellular localization was detected in sda mutant testes compared to WT testes (Figure S5A-B). Finally, either using an NCadM12 allele whose product cannot be processed by peptidase (Iwai et al., 1997) or driving an E-Cad cDNA with the cleavage site mutated [i.e., unprocessable E-Cad (Oda and Tsukita, 1999)] resulted in phenotypes similar to those in sda mutant testes (Figure 4I).
Figure 4. Sda is required for accumulation of mature DE-Cadherin to maintain stem cells and hub cells in the Drosophila testicular niche.
(A) Quantification of GSCs, Zfh-1-positive cells and hub cells in testes from E-Cad, N-Cad/+, or sda/+, or E-Cad, N-Cad/+; sda/+ males. P-value between any two genotypes < 0.01. (B-C) Testes from upd-Gal4; sda (B) and upd-Gal4; UAS-E-Cad; sda (C) males stained with anti-Zfh-1, anti-Vasa and anti-Arm; dots indicate GSCs. Asterisk: hub area. Scale bar: 10μm. (D) Quantification of GSCs, Zfh-1-positive cells and hub cells in the testicular niche from upd-Gal4, or upd-Gal4; sda, or upd-Gal4; UAS-E-Cad; sda, or upd-Gal4; UAS-N-Cad; sda D15 males. For GSCs: P-value between any two genotypes < 0.01; for Zfh-1-positive cells and hub cells: P-value between any two genotypes except upd-Gal4; sda is n.s.; whereas P-value between any other genotype and upd-Gal4; sda < 0.01. (E) Quantification of GSCs, Zfh-1-positive cells and hub cells in the testicular niche from tj-Gal4, or tj-Gal4; sda, or tj-Gal4; UAS-E-Cad; sda, or tj-Gal4; UAS-N-Cad; sda D15 males. For GSCs: P-value between any two genotypes < 0.01; for Zfh-1-positive cells and hub cells: P-value between any two genotypes except tj-Gal4; sda is n.s.; whereas P-value between any other genotype and tj-Gal4; sda < 0.01. (F) Quantification of GSCs, Zfh-1-positive cells and hub cells in the testicular niche from nos-Gal4 or nos-Gal4; sda or nos-Gal4; UAS-E-Cad; sda or nos-Gal4; UAS-N-Cad; sda D15 males. For GSCs, Zfh-1-positive cells and hub cells: P-value between any two genotypes except nos-Gal4 is n.s.; whereas P-value between any other genotype and nos-Gal4 < 0.01. (G) Quantitative RT-PCR to measure E-Cad transcript levels in WT and sda mutant testes based on three independent experiments. (H) Immunoblot to measure mature E-Cad protein (~150 KD) levels in WT and sda mutant testes, the 200 KD unprocessed E-Cad is undetectable; CP190 (~190KD) is used as a loading control. (I) Quantification of GSCs, Zfh-1-positive cells and hub cells in testes from sda, or WT, or CadNM12 (N-Cadunprocessable/+), or nos-Gal4; dNc (nos>E-Cadunprocessable), or upd-Gal4; dNc (upd>E-Cadunprocessable) males. For GSCs, P-value between any two genotypes except WT and N-Cadunprocessable/+ is n.s.; P-value between any other genotype and WT < 0.01, and P-value between any other genotype and N-Cadunprocessable/+ < 0.05. For Zfh-1-positive cells: P-value between any two genotypes except WT and upd>E-Cadunprocessable is n.s.; P-value between any other genotype and WT or upd>E-Cadunprocessable < 0.01. For hub cells, P-value between any two genotypes except WT and N-Cadunprocessable/+ is n.s.; P-value between any other genotype and WT or Cadunprocessable/+ < 0.05. Error bar: 95% CI of SEM in (A), (D-F) and (I), P-value calculated by one-tailed t-test.
It is known that E-Cadherin is synthesized as 230 kDa precursor that undergoes cleavage by proteases associated with the secretory pathway to remove its N terminal pro-protein to yield a matured 150 kDa functional protein (Oda et al., 1994). However, the anti-E-Cadherin used for immunoblotting could not reliably recognize the unprocessed form. Furin, a member of the pro-protein convertase family and a Ca++-dependent serine endopeptidase, has been shown to efficiently cleave the mammalian E-cadherin precursor, which is necessary for proper folding of its extracellular domain required for adhesion (Posthaus et al., 1998). It was speculated that furin-like enzyme may perform similar functions in processing Drosophila E-cadherin but Sda is distinct from furin. Together, these data suggest that cadherin molecules are important downstream targets of Sda, although we cannot exclude the possibility that they are indirect targets of Sda (Table S1).
Sda is both necessary and sufficient to promote spermatogonial dedifferentiation during homeostasis
During aging, as hub cell number decreases (Wallenfang et al., 2006), GSCs are maintained (Cheng et al., 2008) or slightly decreased (Boyle et al., 2007) due to self-renewal (Sheng and Matunis, 2011; Yamashita et al., 2003) of existing GSCs and spermatogonial dedifferentiation (Cheng et al., 2008; Sheng and Matunis, 2011). To understand the cellular mechanisms underlying GSC loss in the sda mutant testes (Figure 1, Figure S2), we evaluated the mitotic activity of GSCs using an anti-PH3 (Lim and Fuller, 2012) immunostaining for M-phase GSCs and a pulse EdU incorporation assay for S-phase GSCs (Insco et al., 2009). The percentage of PH3-positive GSCs for sda mutant (2.97%, n=101) was higher than that of WT testes (1.50%, n=334), as was the percentage of EdU-positive GSCs [sda mutant: 21% (n=125); WT: 17% (n=526)]. Therefore, in sda mutant testes, the number of GSCs decreased even with increased GSC mitotic activity.
We next investigated whether spermatogonial cells fail to undergo dedifferentiation in sda mutant testes. It has been shown that dedifferentiated spermatogonial cells tend to have misoriented centrosomes (Cheng et al., 2008). Indeed, we found that GSCs with misoriented centrosomes significantly decrease in sda mutant testes (Figures 5A-C), suggesting potential spermatogonial dedifferentiation defects. In addition to misoriented centrosomes, another cellular feature of dedifferentiated spermatogonial cells is the transient disintegrating fusome remnant (Brawley and Matunis, 2004; Cheng et al., 2008; Inaba et al., 2010; Sheng et al., 2009). Consistent with dedifferentiation defects, we found that GSCs with disintegrating fusomes are significantly reduced in sda mutant testes compared to WT control (Figures 5D-E, 5G). To confirm this, we also used Pavarotti-GFP (Pav-GFP) transgene to label midbody/ring canal (Minestrini et al., 2002). Cells with multiple ring canals (Brawley and Matunis, 2004; Cheng et al., 2008) were scored and compared between WT testes and sda mutant testes. GSCs with multiple ring canals have ~ 2-fold reduction in sda mutant (4/73 total GSCs) than that in WT control (14/120 total GSCs). Quantification of ring canal remnants using Pav-GFP in all germ cells (Sheng et al., 2009) between WT testes (46/26 testes= 1.8 per testis) and sda mutant (20/20 testes= 1.0 per testis) testes showed similar difference. Together, these data suggest that Sda is necessary for spermatogonial dedifferentiation during aging.
Figure 5. Sda is both necessary and sufficient to promote spermatogonial dedifferentiation during homeostasis.
(A-B) Centrosomes (anti-γ-Tubulin) are misoriented in D30 WT (A, yellow double-arrowed line), but always show proper orientation in sda mutant testes (B, white double-arrowed line). (C) Percentage of GSCs with misoriented centrosomes in D30 WT and sda mutant testes. (D) Germ cell cysts with multiple spectrosomes (arrows, anti-α-Spectrin), or cysts with degenerating fusomes (circled by dotted lines) in D30 WT testes. (E) Only GSC-GB pairs connected by a single spectrosome were detected in sda testes (arrowheads, also in D). (F) Overexpression of sda using upd-Gal4; UAS-HA-sdaFL led to increased spermatogonial cysts with degenerating fusomes (circled by dotted lines) adjacent to the hub. Asterisk: hub area. (G) Quantification of percentage of GSC with disintegrating fusomes; P-value calculated using Fisher’s Exact Test. Scale bar: 10μm.
In a complementary assay, we asked whether overexpression of Sda is sufficient to promote spermatogonial dedifferentiation. Indeed, overexpression of full-length sda in hub cells (upd> sdaFL) at wild-type background led to ~4-fold more GSCs with disintegrating fusome remnants compared to that in the control testes (either upd-Gal4 or UAS-sdaFL by itself), suggesting that Sda is sufficient to promote spermatogonial dedifferentiation (Figures 5F-G). In contrast, this effect was undetectable in the sda mutant background (upd> sdaFL; sda), suggesting that it depends on the level of Sda. Furthermore, we found that Sda overexpression in promoting spermatogonial dedifferentiation could be detected in males as early as 1-day-old, but becomes more obvious in older males (Figure S6), probably due to an increase in percentage of dedifferentiation-derived GSCs when flies age (Cheng et al., 2008; Sheng and Matunis, 2011; Wong and Jones, 2012). However, despite elevated spermatogonial dedifferentiation, overexpression of sda did not result in an overall increase of GSC number [10.8±0.9s.d. GSCs in upd>sdaFL testes (n=65); 10.5±1.7s.d. GSCs in WT testes (n=33), P> 0.1], suggesting that dedifferentiated spermatogonial cells may outcompete existing GSCs at the niche, as reported in the Drosophila female GSC niche (Jin et al., 2008).
Sda is required for the testicular niche to promote spermatogonial dedifferentiation during tissue regeneration
In addition to aging, it has been shown that spermatogonial cells undergo robust dedifferentiation when GSCs are depleted by genetic manipulations (Sheng and Matunis, 2011; Yadlapalli and Yamashita, 2013). To genetically deplete GSCs, we used a similar heat shock regime as reported previously (Sheng et al., 2009) to transiently overexpress a differentiation factor encoded by bag-of-marbles (bam) (Figure 6A). With this treatment, GSCs differentiate and leave the niche in both hs-bam control and hs-bam; sda mutant testes (Figures 6B, 6D), leaving ~0.15 GSC per testis (Figure 6F) and ~85.3% of testes with zero GSC (Figure 6G). However, upon subsequent recovery, 58.7% of hs-bam control testes regained GSCs (Figures 6C, 6G) through spermatogonial dedifferentiation, leading to an average of 7.2 GSCs per testis (Figure 6F). In contrast, 95.7% hs-bam; sda mutant testes had zero GSC after the same recovery time, suggesting that spermatogonial cells failed to dedifferentiate (Figures 6E-G). Furthermore, we found that only full-length sdaFL, but not the sdaΔCAT or the sdaE→A, could rescue the dedifferentiation defect in hs-bam; sda mutant males upon genetic depletion of GSCs (Figures 6F-G), suggesting that a functional catalytic domain of Sda is required for promoting spermatogonial dedifferentiation during tissue regeneration.
Figure 6. Sda is required for the testicular niche to promote spermatogonial dedifferentiation during tissue regeneration.
(A) Heat shock regime [modified from (Sheng et al., 2009)]. (B-C) Testes from hs-bam control males after heat shock treatment before recovery (B) and after recovery (C). (D-E) Testes from hs-bam; sda males after heat shock treatment before recovery (D) and after recovery (E). (F-G) Quantification of recovery efficiency, presented as the average number of GSCs (F) and the percentage of testes containing at least one GSC (G) in males with the corresponding genotype. Error bar: 95% CI of SEM in (F) and (G), P-value calculated by one-tailed t-test.
Sda promotes spermatogonial dedifferentiation independent of E-Cad and JAK-STAT signaling
Although both hub cells and Zfh-1-positive cells were fully rescued by overexpression of cadherin molecules (Figures 4D-E), the GSC number was only recovered to ~84% of that in WT control (P< 10−3 compared to control). Analysis of disintegrating fusome remnants indicated that the dedifferentiation defects in sda mutant testes were not rescued by overexpression of E-Cad in hub cells (Figure S5C-D). These data also suggest that spermatogonial dedifferentiation defects are separable from hub cell- and CySC-loss phenotypes, indicating that Sda may have multiple substrates for different functions in vivo, such as promoting spermatogonial dedifferentiation or maintaining hub cells and CySCs, respectively.
To explore whether spermatogonial dedifferentiation defects in sda mutant testes result from compromised JAK-STAT signaling in germ cells, we examined the expression pattern of Stat92E in sda mutant testes as a read-out of active JAK-STAT signaling. Similar to GSCs in control testes (Figures S7A-A’), GSCs in sda mutant testes are enriched with Stat92E immunostaining signal (Figures S7B-C’), suggesting that the JAK-STAT signaling is properly received by GSCs in sda mutant testes. Furthermore, overexpression of the JAK-STAT ligand Upd in hub cells (Boyle et al., 2007; Toledano et al., 2012) (upd-Gal4; UAS-upd) was insufficient to rescue GSC loss in sda mutant testes (upd-Gal4; sdaE→A; sda in Figure S7D), suggesting that either Sda promotes dedifferentiation independent of the JAK-STAT signaling pathway or Sda acts downstream of Upd.
DISCUSSION
In this study we demonstrate that an aminopeptidase encoded by the sda gene has important roles in the Drosophila testicular niche. Aminopeptidase is a class of enzymes that have never been shown to act in any stem cell system previously. Sda protein has typical aminopeptidase activity and substrate specificity in vitro; and its aminopeptidase domain is required for in vivo function in the Drosophila testicular niche. Within the niche, Sda acts specifically in hub cells to maintain niche structure, including hub cells, CySCs and GSCs. Maintenance of GSCs by Sda is through Cadherin-dependent retention of existing GSCs and Cadherin-independent dedifferentiation of spermatogonial cells. Furthermore, Sda is both necessary and sufficient to promote spermatogonial dedifferentiation, under both physiological conditions such as aging and during tissue regeneration upon genetic depletion of existing GSCs (Figure 7). Currently we cannot exclude the possibility that the mechanisms contributing to dedifferentiation under these two conditions are different. Interestingly, Sda also showed specific expression pattern in the terminal filament cells, an important component of female GSC niche (Figures S1B-B’), suggesting that it may act as a niche-specific factor in multiple stem cell systems.
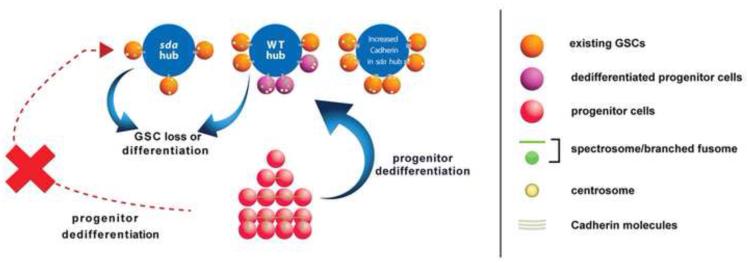
Figure 7. A schematic diagram outlines activities of Sda in the Drosophila testicular niche to maintain GSCs.
In WT testicular niche, GSC number is maintained by both self-renewal of existing GSCs and dedifferentiation of progenitor germ cells, including gonialblasts and spermatogonial cells. In sda mutant niche, progenitor cells fail to undergo dedifferentiation, therefore all retained GSCs are from existing GSCs that are established during embryogenesis (Le Bras and Van Doren, 2006). In sda mutant niche with overexpression of Cadherin molecules such as E-Cad or N-Cad, existing GSCs have increased adhesion to hub cells and are therefore lost less frequently, consistent with published work (Boyle et al., 2007; Inaba et al., 2010; Yamashita et al., 2003). Dedifferentiated GSCs can be recognized by misoriented centrosomes or transient disintegrating fusome remnants.
Studies in recent years have demonstrated a dynamic crosstalk between stem cells and their surrounding microenvironment termed as “niche” (Losick et al., 2011). Even though the niche was first characterized in Drosophila gonads, similar structure and function have been identified in mammals [reviewed in (Hsu and Fuchs, 2012; Lander et al., 2012; Morrison and Spradling, 2008)]. Many studies focus on understanding how extrinsic cues from the niche and intrinsic factors in stem cells cooperate to determine and maintain stem cell identity and activity. However, the molecular mechanisms governing the dedifferentiation process remain largely unclear. Here our results provide an important advance in understanding how a niche-specific peptidase influences the decision of progenitor cells to differentiate versus dedifferentiate, suggesting that the dedifferentiation process in vivo is not an entirely cell-autonomous decision, but rather, requires external cues from the stem cell niche. The advantage of regulation from the niche on dedifferentiation in vivo may provide a spatial control to avoid ectopic reprogramming of progenitor cells, which could lead to tissue hyperplasia and diseases. Indeed, it has been shown mis-regulation of dedifferentiation may lead to cancers (Friedmann-Morvinski et al., 2012; Schwitalla et al., 2013). Therefore understanding how molecules from niche cells regulate dedifferentiation may shed light on various disease states such as cancer.
On the other hand, guided dedifferentiation may provide another way for tissue regeneration. For example, it has been shown that dedifferentiated cells could be functional in vivo for tissue/organ maintenance and repair in multiple systems from different organisms (Brawley and Matunis, 2004; Cheng et al., 2008; Jopling et al., 2010; Kai and Spradling, 2004; Rawlins et al., 2009; Sheng et al., 2009; Sheng and Matunis, 2011; Tata et al., 2013; van Es et al., 2012). Spermatogonial cells in mice can also undergo dedifferentiation to become germinal stem cells in a process that shares many cellular commonalities with those in Drosophila (Barroca et al., 2009; Nakagawa et al., 2010). It would be interesting to investigate whether dedifferentiation in Drosophila and mice share similar molecular mechanism. Understanding how niche cells regulate progenitor cell dedifferentiation may assist in the application of dedifferentiated cells from the same lineage to repopulate the endogenous niche and function like bona fide stem cells (Jopling et al., 2011), which should provide a powerful solution for tissue regeneration.
EXPERIMENTAL PROCEDURES
Fly strains and husbandry
Fly stocks were raised using standard Bloomington medium at 25°C or 29°C as noted. The following fly stocks were used: y,w (as wild-type or WT), sdaP (Bloomington Stock Center BL-10344) as the enhancer trap line, sdaiso7.8 as a strong loss-of-function allele (Zhang et al., 2002), Df(3R)ED6235 that uncovers the sda gene region (BL-9878), UAS-sda dsRNA (Vienna Drosophila Research Center GD11680 and (Mummery-Widmer et al., 2009), both lines showed similar knockdown phenotypes including germline stem cell and hub architecture changes, when driven by either tj-Gal4 or upd-Gal4 driver.], UAS-Dicer2 (Vienna Drosophila Research Center #V60008), UAS-DE-Cadherin (Sanson et al., 1996), UAS-DN-Cadherin (Iwai et al., 1997), NCadM12 (BL-229) as an unprocessable DN-Cadherin mutation, dNc as an unprocessable DE-cadherin mutation (Oda and Tsukita, 1999), UAS-upd (Terry et al., 2006), UAS-GFP.nls (BL-4776), UAS-Gal4 (BL 5939), hs-bam (BL-24636), and Pav-GFP (from Y. Yamashita, University of Michigan, Ann Arbor, MI, USA). The Gal4 drivers are upd-GAL4 (Leatherman and Dinardo, 2010), c587-gal4 (from A. Spradling, Carnegie Institution Department of Embryology, Baltimore, MD, USA), tj-Gal4 (from M. Van Doren, Johns Hopkins University, Baltimore, MD, USA) and nos-gal4 (Van Doren et al., 1998).
Temperature shift assay to knock down sda in adult flies
Flies with the UAS-sda dsRNA; UAS-dicer2 transgenes were paired with different Gal4 drivers and raised using standard Bloomington medium at 18°C. For the negative control, flies were kept at 18°C throughout development until adulthood so that there is low or no knockdown of sda. For the positive control, flies were shifted to 29°C at L1 stage to maximize the knockdown effect. To specifically knock down sda in adult flies, newly eclosed males were shifted to 29 °C for 15 days before analyzing the phenotypes.
Heat shock regime
Newly eclosed males with noted genotypes were collected and aged for 20 days with female flies in an 18°C incubator. Before heat shock, males were transferred to bottles that had been air-dried for 24 hours. Bottles were submerged with all air area underneath water in a circulating 37°C water bath for 30 minutes at approximately 9 AM and 4 PM daily for 5 days, for a total of ten times of heat shock. Flies were placed in a 29°C incubator between heat shock treatments and returned to 18°C after the final heat shock. Flies were then allowed for a 7-day recovery at 18°C.
Immunostaining
Immunofluorescence staining was performed as described previously (Cheng et al., 2008). The primary antibodies used were as follows: mouse anti-γ tubulin (1:100; Sigma T9026); mouse anti-Armadillo (1:100; DSHB N2 7A1 clone); mouse anti-α Spectrin (1:20; DSHB 3A9 clone); EdU (Invitrogen C10350); Lysotracker (according to manufacturer recommendation, Invitrogen L7528); rabbit anti-Ser10-phosphorylated Histone H3 (1:200; Upstate 07-424); rat anti-Vasa (1:40; a gift from Dr. Allan Spradling, Carnegie Institution for Science); rabbit anti-Zfh-1 (1:4000; a gift from Dr. Ruth Lehmann, Skirball Institute of Biomolecular Medicine); rabbit anti-STAT [1:1000, a gift from Denise Montell (Silver et al., 2005)]; rabbit anti-active Caspase-3 (1:500, BD 559565), and mouse anti-β Galactosidase (1:5000; Promega z3781). Images were taken using a Zeiss Apotome microscope with a 63x oil immersion objective and processed using Adobe Photoshop software.
Isolation of total RNA and quantitative reverse transcription PCR (qRT-PCR)
Total RNA was isolated from y,w (WT) and sda/Df adult testes using TRIzol reagent (Invitrogen, #15596-018) according to the manufacturer’s instructions. Yield and quality of RNA were determined with a NanoDrop spectrometer (NanoDrop Technology, San Diego, CA, USA). Reverse transcription was performed using the RevertAid First Strand cDNA Synthesis Kit (Fermentas, #K1621). Transcript levels were measured using Taqman universal PCR master mix (ABI, #4324018), E-Cad (shotgun) primers (ABI, #4351372), and normalized to rpl 32 (ABI, #4331182).
To quantify expression of different HA-tagged sda transgenes, flies with following genotypes upd-Gal4; UAS-HA-sdaFL or upd-Gal4; UAS-HA-sdaE→A or upd-Gal4; UAS-HA-sdaΔCAT were aged to D30 at 25°C. 40 pairs of testis were dissected for each genotype. Three replicates were generated. Total RNA were purified out using Picopure RNA isolation kit (Invitrogen, #KIT0204) according to the manufacturer’s instructions. Reverse transcription was performed using oligo dT( Invitrogen, #18418-012) and Superscript III(Invitrogen, # 18080044), following the manufacturer’s instruction. Transcript levels were measured using SYBR Green PCR master mix (Invitrogen, # 4385612) and normalized to Fas III. The following primers are used to perform qRT-PCR.
FasIII Forward: GTACGGCAGATCCATCAACTAC
FasIII Reverse: GCTCCGAAGTACGTGAATCC
HA-sda Forward: CCTATCCATATGACGTTCCAGATT
HA-sda Reverse: CCGATGGAGGTCCATTGAAA
Isolation of total protein from testes and immunoblotting
Adult testes isolated from y,w (WT) and sda/Df males were lysed in 2X Laemmli sample buffer (Bio-Rad, #161-0737). Samples were applied on 7% SDA-PAGE (Invitrogen, #EA0355BOX) for electrophoresis and transferred to Hybond ECL membrane (GE, #RPN2020D) according to the Novex SDS PAGE system manufacturer’s instruction (Invitrogen # E10002, LA0041, and NP0006). For analysis of the E-Cad levels, rat α-E-cad (DCAD1, 1:1000, a gift from Dr. Tadashi Uemura) signal was normalized to rabbit α-CP190 (1:2000, a gift from Victor Corces) signal. HRP conjugated secondary antibodies (Jackson Immunoresearch) were used at 1:2000 and detected using the Amersham ECL-plus kit (GE #RPN2232). Relative levels of E-Cad and CP190 were determined using ImageJ software.
Calculating mitotic index
We used EdU (Invitrogen C10350) incorporation assay and anti-phosphorylated histone 3 (H3S10P, Upstate 07-424) immunostaining to compute mitotic index as EdU- or PH3-positive GSCs/total GSCs. Since GSC mitosis is sensitive to CO2 anesthetization, we dissected testes within 5 minutes of anesthetization followed by immediate fixation. The mitotic index results using both anti-PH3 immunostaining and EdU incorporation were based on two independent experiments, respectively.
Calculation of 95% confidence interval (CI)
95% CI = Mean ± (1.96 × SEM); SEM: standard error of Mean.
P-value calculation and explanation
We used student t-test and Fisher exact test for P-value calculation. Student t-test was used when the experimental data is on continuous scale. Fisher exact test was used when a null hypothesis was tested and the experimental data had only two possibilities (e.g. equal vs. unequal).
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Allan Spradling, Mark Van Doren, Haiqing Zhao, Rejji Kuruvilla, Yukiko Yamashita and Chen lab members for their critical comments, Michelle Rozo and Antonia Thomas for helping characterizing sda expression pattern and mutant phenotype, Jayachandran N. Kizhakkedathu and UBC Vancouver for provision of the HPG-ALD polymer, Franz Jehle and Bettina Mayer for technical assistance. This work has been supported by the 49th Mallinckrodt Scholar Award from the Edward Mallinckrodt, Jr. Foundation, the David and Lucile Packard Foundation, the R01HD065816 from NICHD, NIH and the Johns Hopkins University start-up funding for X.C, the Howard Hughes Medical Institute and the David and Lucile Packard Foundation for S.U. and S.G., Deutsche Forschungsgemeinschaft (SCHI 871/2 and SCHI 871/5, SCHI 871/6, GR 1748/6, and INST 39/900-1) and the DFG SFB850 (Project B8), a starting grant of the European Research Council (Programme “Ideas” Call identifier: ERC-2011-StG 282111-ProteaSys), and the Excellence Initiative of the German Federal and State Governments (EXC 294, BIOSS) for O.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Conceptualization, C.L. and X.C.; Methodology, C.L., S.G., M.B., L.F., O.S., S.U. and X.C.; Investigation, C.L., S.G., M.B., L.F., O.S., S.U. and X.C.; Writing – Original Draft, C.L., S.G., M. B. and X.C.; Funding Acquisition, O.S., S.U. and X.C.; Supervision, O.S., S.U. and X.C.
REFERENCES
- Apostolou E, Hochedlinger K. Chromatin dynamics during cellular reprogramming. Nature. 2013;502:462–471. doi: 10.1038/nature12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroca V, Lassalle B, Coureuil M, Louis JP, Le Page F, Testart J, Allemand I, Riou L, Fouchet P. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol. 2009;11:190–196. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- Boyle M, Wong C, Rocha M, Jones DL. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell. 2007;1:470–478. doi: 10.1016/j.stem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- Cheng J, Turkel N, Hemati N, Fuller MT, Hunt AJ, Yamashita YM. Centrosome misorientation reduces stem cell division during ageing. Nature. 2008;456:599–604. doi: 10.1038/nature07386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Eliazer S, Shalaby NA, Buszczak M. Loss of lysine-specific demethylase 1 nonautonomously causes stem cell tumors in the Drosophila ovary. Proc Natl Acad Sci U S A. 2011;108:7064–7069. doi: 10.1073/pnas.1015874108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann-Morvinski D, Bushong EA, Ke E, Soda Y, Marumoto T, Singer O, Ellisman MH, Verma IM. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science. 2012;338:1080–1084. doi: 10.1126/science.1226929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568–571. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Fuchs E. A family business: stem cell progeny join the niche to regulate homeostasis. Nat Rev Mol Cell Biol. 2012;13:103–114. doi: 10.1038/nrm3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba M, Yuan H, Salzmann V, Fuller MT, Yamashita YM. E-cadherin is required for centrosome and spindle orientation in Drosophila male germline stem cells. PLoS One. 2010;5:e12473. doi: 10.1371/journal.pone.0012473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insco ML, Leon A, Tam CH, McKearin DM, Fuller MT. Accumulation of a differentiation regulator specifies transit amplifying division number in an adult stem cell lineage. Proc Natl Acad Sci U S A. 2009;106:22311–22316. doi: 10.1073/pnas.0912454106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issigonis M, Tulina N, de Cuevas M, Brawley C, Sandler L, Matunis E. JAK-STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science. 2009;326:153–156. doi: 10.1126/science.1176817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai Y, Usui T, Hirano S, Steward R, Takeichi M, Uemura T. Axon patterning requires DN-cadherin, a novel neuronal adhesion receptor, in the Drosophila embryonic CNS. Neuron. 1997;19:77–89. doi: 10.1016/s0896-6273(00)80349-9. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Kirilly D, Weng C, Kawase E, Song X, Smith S, Schwartz J, Xie T. Differentiation-defective stem cells outcompete normal stem cells for niche occupancy in the Drosophila ovary. Cell Stem Cell. 2008;2:39–49. doi: 10.1016/j.stem.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol. 2011;12:79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- Jopling C, Sleep E, Raya M, Marti M, Raya A, Izpisua Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- Kleifeld O, Doucet A, auf dem Keller U, Prudova A, Schilling O, Kainthan RK, Starr AE, Foster LJ, Kizhakkedathu JN, Overall CM. Isotopic labeling of terminal amines in complex samples identifies protein N-termini and protease cleavage products. Nat Biotechnol. 2010;28:281–288. doi: 10.1038/nbt.1611. [DOI] [PubMed] [Google Scholar]
- Lander AD, Kimble J, Clevers H, Fuchs E, Montarras D, Buckingham M, Calof AL, Trumpp A, Oskarsson T. What does the concept of the stem cell niche really mean today? BMC Biol. 2012;10:19. doi: 10.1186/1741-7007-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bras S, Van Doren M. Development of the male germline stem cell niche in Drosophila. Dev Biol. 2006;294:92–103. doi: 10.1016/j.ydbio.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman JL, Dinardo S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat Cell Biol. 2010;12:806–811. doi: 10.1038/ncb2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehoczky JA, Robert B, Tabin CJ. Mouse digit tip regeneration is mediated by fate-restricted progenitor cells. Proc Natl Acad Sci U S A. 2011;108:20609–20614. doi: 10.1073/pnas.1118017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JG, Fuller MT. Somatic cell lineage is required for differentiation and not maintenance of germline stem cells in Drosophila testes. Proc Natl Acad Sci U S A. 2012;109:18477–18481. doi: 10.1073/pnas.1215516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick VP, Morris LX, Fox DT, Spradling A. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell. 2011;21:159–171. doi: 10.1016/j.devcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani N, Marie-Claire C, Ruffet E, Beaumont A, Roques BP, Fournie-Zaluski MC. Characterization of Glu350 as a critical residue involved in the N-terminal amine binding site of aminopeptidase N (EC 3.4.11.2): insights into its mechanism of action. Biochemistry. 1998;37:686–692. doi: 10.1021/bi971705p. [DOI] [PubMed] [Google Scholar]
- Manseau L, Baradaran A, Brower D, Budhu A, Elefant F, Phan H, Philp AV, Yang M, Glover D, Kaiser K, et al. GAL4 enhancer traps expressed in the embryo, larval brain, imaginal discs, and ovary of Drosophila. Dev Dyn. 1997;209:310–322. doi: 10.1002/(SICI)1097-0177(199707)209:3<310::AID-AJA6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Minestrini G, Mathe E, Glover DM. Domains of the Pavarotti kinesin-like protein that direct its subcellular distribution: effects of mislocalisation on the tubulin and actin cytoskeleton during Drosophila oogenesis. J Cell Sci. 2002;115:725–736. doi: 10.1242/jcs.115.4.725. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery-Widmer JL, Yamazaki M, Stoeger T, Novatchkova M, Bhalerao S, Chen D, Dietzl G, Dickson BJ, Knoblich JA. Genome-wide analysis of Notch signalling in Drosophila by transgenic RNAi. Nature. 2009;458:987–992. doi: 10.1038/nature07936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H, Tsukita S. Nonchordate classic cadherins have a structurally and functionally unique domain that is absent from chordate classic cadherins. Dev Biol. 1999;216:406–422. doi: 10.1006/dbio.1999.9494. [DOI] [PubMed] [Google Scholar]
- Oda H, Uemura T, Harada Y, Iwai Y, Takeichi M. A Drosophila homolog of cadherin associated with armadillo and essential for embryonic cell-cell adhesion. Dev Biol. 1994;165:716–726. doi: 10.1006/dbio.1994.1287. [DOI] [PubMed] [Google Scholar]
- Posthaus H, Dubois CM, Laprise MH, Grondin F, Suter MM, Muller E. Proprotein cleavage of E-cadherin by furin in baculovirus over-expression system: potential role of other convertases in mammalian cells. FEBS Lett. 1998;438:306–310. doi: 10.1016/s0014-5793(98)01330-1. [DOI] [PubMed] [Google Scholar]
- Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich Y, Lindau P, Ueno H, Longaker MT, Weissman IL. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature. 2011;476:409–413. doi: 10.1038/nature10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson B, White P, Vincent JP. Uncoupling cadherin-based adhesion from wingless signalling in Drosophila. Nature. 1996;383:627–630. doi: 10.1038/383627a0. [DOI] [PubMed] [Google Scholar]
- Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Goktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Sheng XR, Brawley CM, Matunis EL. Dedifferentiating spermatogonia outcompete somatic stem cells for niche occupancy in the Drosophila testis. Cell Stem Cell. 2009;5:191–203. doi: 10.1016/j.stem.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng XR, Matunis E. Live imaging of the Drosophila spermatogonial stem cell niche reveals novel mechanisms regulating germline stem cell output. Development. 2011;138:3367–3376. doi: 10.1242/dev.065797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng XR, Matunis EL. Make room for dedifferentiation. Fly (Austin) 2009;3:283–285. doi: 10.4161/fly.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver DL, Geisbrecht ER, Montell DJ. Requirement for JAK/STAT signaling throughout border cell migration in Drosophila. Development. 2005;132:3483–3492. doi: 10.1242/dev.01910. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tanentzapf G, Devenport D, Godt D, Brown NH. Integrin-dependent anchoring of a stem-cell niche. Nat Cell Biol. 2007;9:1413–1418. doi: 10.1038/ncb1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry NA, Tulina N, Matunis E, DiNardo S. Novel regulators revealed by profiling Drosophila testis stem cells within their niche. Dev Biol. 2006;294:246–257. doi: 10.1016/j.ydbio.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Toledano H, D'Alterio C, Czech B, Levine E, Jones DL. The let-7-Imp axis regulates ageing of the Drosophila testis stem-cell niche. Nature. 2012;485:605–610. doi: 10.1038/nature11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doren M, Williamson AL, Lehmann R. Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr Biol. 1998;8:243–246. doi: 10.1016/s0960-9822(98)70091-0. [DOI] [PubMed] [Google Scholar]
- van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenfang MR, Nayak R, DiNardo S. Dynamics of the male germline stem cell population during aging of Drosophila melanogaster. Aging Cell. 2006;5:297–304. doi: 10.1111/j.1474-9726.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- Wong C, Jones DL. Efficiency of spermatogonial dedifferentiation during aging. PLoS One. 2012;7:e33635. doi: 10.1371/journal.pone.0033635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadlapalli S, Yamashita YM. Chromosome-specific nonrandom sister chromatid segregation during stem-cell division. Nature. 2013;498:251–254. doi: 10.1038/nature12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012;10:678–684. doi: 10.1016/j.stem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhang H, Tan J, Reynolds E, Kuebler D, Faulhaber S, Tanouye M. The Drosophila slamdance gene: a mutation in an aminopeptidase can cause seizure, paralysis and neuronal failure. Genetics. 2002;162:1283–1299. doi: 10.1093/genetics/162.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.