Abstract
The synthesis and incorporation into position 66 of green fluorescent protein (GFP) by in vitro protein translation of novel oxazole and thiazole based dipeptidomimetics are described. The compounds may be regarded as GFP chromophore analogues, and are strongly fluorescent. An α-amido-β-ketoester intermediate was obtained via bisacylation of a protected glycine. The intermediate underwent dehydrative cyclization to afford the 1,3-oxazole and was treated with Lawesson’s reagent to furnish the 1,3-thiazole. When these fluorophores were introduced into position 66 of GFP in place of Tyr66, the resulting GFP analogues exhibited fluorescence emission several-fold greater than wild-type GFP; the emission was also shifted to shorter wavelength. It may be noted that compared to the typical fluorophores formed in the natural and modified fluorescent proteins, the oxazole and thiazole fluorophores are completely stable and do not require activation by posttranslational modification to exhibit fluorescence.
Keywords: Oxazole, Thiazole, Dipeptidomimetics, Green fluorescent protein, Modified ribosome
Graphical abstract

Green fluorescent protein (GFP) is widely used as a fluorescent reporter in molecular and cell biology.1 Aequorea victoria GFP consists of 238 amino acid residues and has a 4-(p-hydroxybenzylidene)imidazolidin-5-one fluorophore, which forms by a posttranslational cyclization and oxidation of the polypeptide backbone, involving the Ser65-Tyr66-Gly67 residues (Figure 1).2 This results in an extended conjugated system capable of absorbing and emitting visible light. The maturation of the chromophore of GFP occurs spontaneously,3 which makes it an attractive molecular marker. The GFP chromophore has absorption peaks at 395 and 475 nm, usually assigned to the neutral and anionic forms of the chromophore, respectively, and an emission peak at 509 nm with a high fluorescence quantum yield (0.79).2 There have been extensive efforts to improve or alter the spectral properties of GFP. For example, Schultz and coworkers have replaced Tyr66 by phenylalanine analogues using site-directed mutagenesis.4 Numerous reports have described the study and spectral properties of novel fluorescent proteins5 and their chromophores.6 In comparison, there has been no report of the incorporation of a preformed fluorophore into a protein backbone by in vitro protein translation.
Figure 1.
Structures of fluorescent dipeptidomimetic analogues 1 and 2.
Although the ribosome has been optimized for the incorporation of α-L-amino acids, ribosomal modification can enable the incorporation of non α-amino acids.7 Recently, we have successfully incorporated D-amino acids8,9 as well as β-amino acids10,11 into DHFR and other proteins. This was achieved by reengineering the ribosomal peptidyltranferase center by modifying the 23S rRNA. In vitro protein translation using modified ribosomes is potentially very important, because in principle it could enable the synthesis of polypeptides or proteins having non α-amino acids which are difficult to obtain using any other strategy.
Peptidomimetics are small molecules which are designed to mimic a natural peptide or protein. They often produce very similar biological effects as the protein or peptide from which they are designed, or support some new effect. Peptidomimetics containing oxazoles and thiazoles are widespread among bioactive molecules. For example, microcin B17, a bacterial DNA gyrase inhibitor,12 and goadsporin, a promoter of secondary metabolism and morphogenesis in Streptomycetes,13 contain both oxazole and thiazole moieties. Thiostrepton, an inhibitor of bacterial ribosome function,14 contains multiple thiazole moieties. Considering the widespread appearance of oxazole and thiazole moieties in natural products, there has been significant interest in the use of such precursors in preparing bioactive molecules and peptidomimetic structures synthetically.15–19 Presently, we report the synthesis and incorporation of novel fluorescent oxazole and thiazole-based dipeptide analogues (1 and 2) which are also reminiscent of the fluorophores formed by naturally occurring fluorescent proteins (Figure 1). The structures of these dipeptide analogues are shown in Figure 1. Compounds 1 and 2 are both strongly fluorescent. The free amino acids both have λex in the range 296 – 302 nm but the λem of 2 is somewhat red-shifted compared to that of 1. This is summarized in Table 1.
Table 1.
Fluorescence properties of dipeptidomimetics 1 and 2 in MeOH
| Compound | λex,max (nm) | λem, max (nm) | ε (M−1cm−1) | ΦF20 |
|---|---|---|---|---|
| 1 | 296 | 391 | 26800 | 0.59 |
| 2 | 300 | 420 | 11000 | 0.20 |
The synthesis of the pdCpA derivatives of 1 and 2 is shown in Scheme 1. The synthetic strategy started with fully protected glycine. Commercially available glycine methyl ester hydrochloride was treated with benzophenone imine to obtain 3 in 81% yield.21 Condensation of 3 with p-methoxybenzoyl chloride in the presence of 1 M NaHMDS in THF, and subsequent hydrolysis of the imine with conc. HCl afforded 4 as a crude product. This was condensed directly with 5 to obtain α-amido-β-ketoester intermediate 6 in 67% overall yield.22 Cyclodehydration of the α-amido-β-ketoester using triphenylphosphine in the presence of iodine and triethylamine in dichloromethane at room temperature afforded oxazole 7 in 78% yield.23 The corresponding thiazole 8 was obtained by treating the α-amido-β-ketoester with Lawesson’s reagent.22 Subsequent removal of the Fmoc group with piperidine followed by treatment with pentenoyloxy succinimide24 yielded the pentenoyl protected compounds 9 and 10 in 42% and 45% yields, respectively. Saponification of 9 and 10 afforded the free acids, which were then activated as cyanomethyl esters 11 and 12 in 37% and 38% yields, respectively.25 The cyanomethyl esters were then used for the acylation of the dinucleotide pdCpA.26 A mixture of the cyanomethyl ester and tris(tetrabutylammonium) salt of pdCpA in 9:1 DMF-Et3N was sonicated for 4 h and purified by reversed phase HPLC using a semi-preparative C18 column, which provided the corresponding pdCpA derivatives 13 and 14 in 60% yields.
Scheme 1.
Scheme employed for synthesizing the pdCpA derivatives of 1 and 2.
The activated pdCpA derivatives were ligated to an abbreviated tRNACUA-COH transcript using T4 RNA ligase (Supplementary Content, Figure S1), affording the respective misacylated suppressor tRNA transcripts. The N-pentenoyl protecting group was removed by treatment with aqueous iodine.24 Removal of the protecting group was done immediately prior to the use of misacylated tRNAs in protein synthesis. The ligation of each pdCpA derivative to the abbreviated tRNACUA-COH was realized with 100% efficiency, as judged by analysis on a polyacrylamide gel.
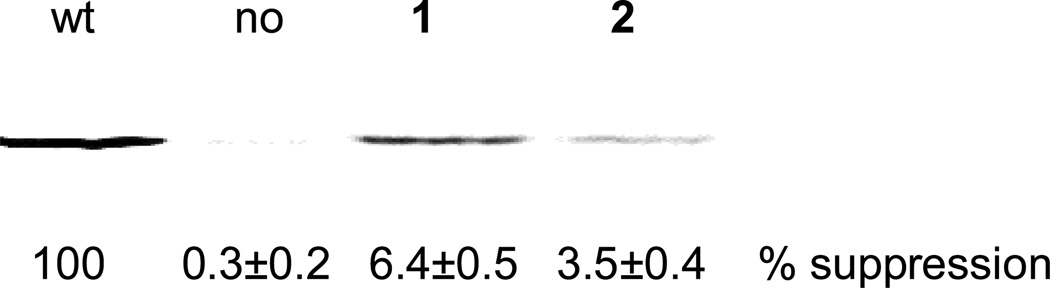
The deprotected tRNAs were used in a cell-free coupled transcription-translation system containing an S-30 fraction prepared from Escherichia coli, programmed with a GFP analogue construct having a TAG codon at position 66 (pETGFP66 plasmid). The introduction of what may be regarded as “dipeptide analogues” into a single position of GFP reflected our analysis of the structure of that region of GFP, and the consequent belief that the substitution would be well tolerated. The S-30 system contained the modified ribosomes, the latter of which had altered peptidyltransferase centers8–11 which enabled them to recognize dipeptides and dipeptide analogues in addition to α-L-amino acids.27 These modified ribosomes were selected by the use of a dipeptidylpuromycin derivative. Recognition of the dipeptidomimetic analogues by these modified ribosomes is presumably due to the fact that the distance between the amine and carboxylate groups in the dipeptidomimetics is similar to the distance between the free amine and carboxylate groups of a dipeptide. The suppression efficiencies were expressed relative to the wild-type GFP synthesis from the wild-type mRNA. As a negative control, wild-type GFP synthesis from the modified mRNA in the presence of nonacylated tRNACUA was measured for each experiment. The amounts of GFP produced were quantified with a phosphoimager, which monitored the incorporation of 35S-methionine into proteins. S-30 preparations having the modified ribosomes from clone 010326R627 produced full length GFP in ~6.5% yield relative to wild-type GFP synthesis in case of 1, while in case of 2 the suppression yield was ~3.5% relative to wild-type GFP synthesis (Figure 2). It may be noted that 1 has previously been incorporated into position 10 of E. coli DHFR in 14–15% yield.27 An elevated concentration (0.6 – 1.0 µg/µL) of the activated suppressor tRNACUA was essential for successful translation, suggesting diminished binding of such species to one or more factors essential for protein synthesis. This concentration was quite high compared to the suppressor tRNACUA concentration employed for the expression of α-amino acids (0.1 – 0.2 µg/µL). At lower concentrations of the aminoacyl-tRNACUA minimal suppression was observed.
Figure 2.
Autoradiogram of a 15% SDS-polyacrylamide gel depicting the incorporation of dipeptidomimetic analogues 1 and 2 into position 66 of GFP. Translation of protein from a wild-type (lane 1) and modified GFP (lanes 2-4) mRNA (UAG codon in position 66) by the use of an S-30 system prepared from ribosomal clone 010326R6 in the presence of different suppressor tRNAs. Lane 1, wild-type GFP expression; lane 2, modified GFP mRNA in the presence of an abbreviated suppressor tRNACUA-COH; lane 3, tRNACUA activated with 1; lane 4, tRNACUA activated with 2. Phosphorimager analysis was performed using an Amersham Biosciences Storm 820 equipped with ImageQuant version 5.2 software from Molecular Dynamics.
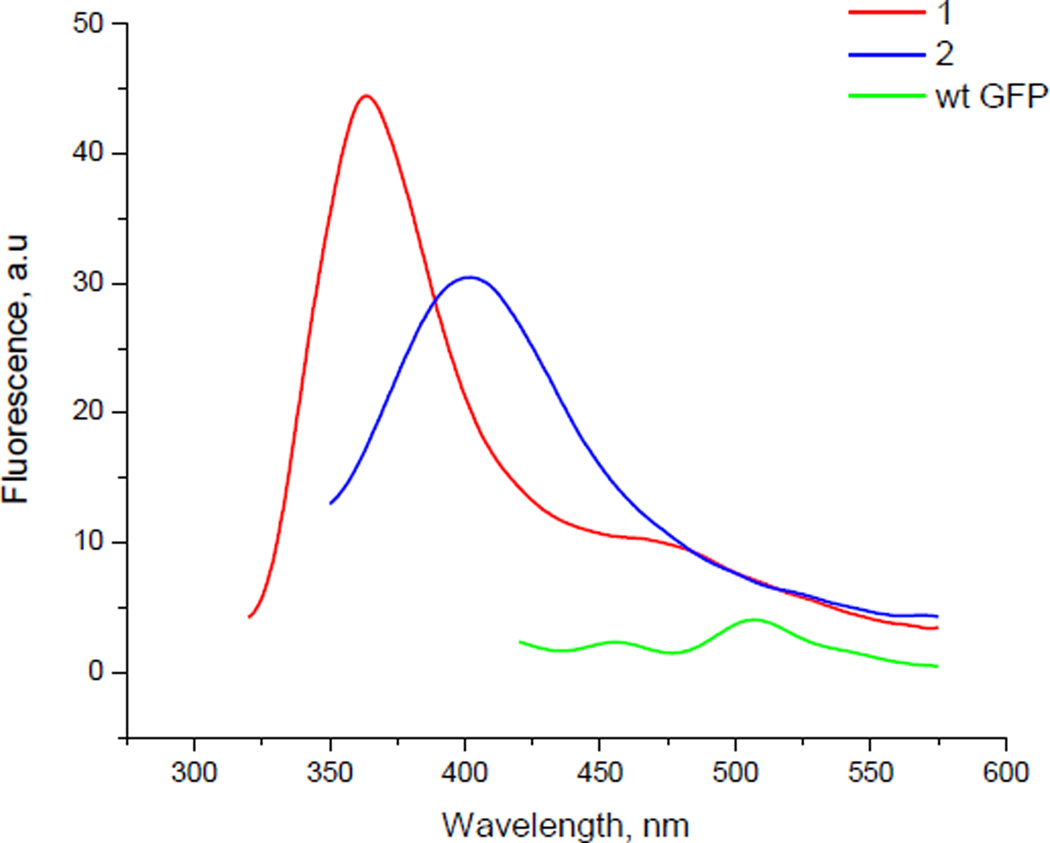
The GFP analogues having 1 and 2 at position 66 were prepared at larger scale and purified to permit study of the fluorescence intensity of 1 and 2 in the protein. The plasmid pETGFP66 was designed such that the translated protein had a hexahistidine moiety at its N-terminal and could be purified via Ni-NTA agarose chromatography.28 The purified proteins containing 1 and 2 were excited at 305 and 302 nm, respectively. The GFP analogue containing 1 exhibited a fluorescence emission maximum at ~375 nm whereas the protein carrying 2 had an emission maximum at ~403 nm (Figure 3). In addition, the fluorescence intensities of both the GFP analogues were compared with wild-type GFP. The fluorescence intensities of the modified GFP analogues were significantly greater than wild-type GFP at the same protein concentration.29 We suggest the descriptor “artificial fluorescent protein” for this new type of fluorescent species. As may be noted in Table 1, the two fluorophores have somewhat different fluorescence emission properties. We anticipate that a more extensive library of such fluorophores would enable the synthesis of artificial fluorescent proteins having diverse excitation and emission wavelengths, analogous to the diverse species based on the natural fluorescent proteins.
Figure 3.
Fluorescence emission spectra of three different GFP samples. Red trace is of GFP having dipeptidomimetic analogue 1 at position 66; excitation at 305 nm. Blue trace is of GFP having dipeptidomimetic analogue 2 at position 66; excitation was at 302 nm. Green trace is of wild-type GFP; sample excitation was at 395 nm. The fluorescence emission spectra were recorded at ~20 nM GFP concentration in 25 mM Tris-HCl, pH 7.4, containing 0.5 M NaCl.
In summary, oxazole and thiazole based dipeptidomimetic analogues have been synthesized. Oxazole 1 was prepared more conveniently than was possible by an earlier reported route,27 and the new route also permitted the preparation of the respective thiazole 2. The compounds are structurally analogous to dipeptides and to the GFP chromophore and exhibit strong fluorescence. When these analogues were incorporated into position 66 of GFP by the use of activated suppressor tRNACUAs the resulting GFP analogue containing 1 had ~10–15-fold greater fluorescence intensity compared to wild-type GFP at the same protein concentration, while the GFP analogue containing 2 had ~7–10-fold greater fluorescence intensity. Thus these fluorophores are sensitive to their environment, a property which should be useful for many applications.
Supplementary Material
Acknowledgement
This work was supported by the National Institutes of Health Research Grant GM103861, awarded by the National Institute of General Medical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Chudakov DM, Matz MV, Lukyanov S, Lukyanov KA. Physiol. Rev. 2010;90:1103. doi: 10.1152/physrev.00038.2009. [DOI] [PubMed] [Google Scholar]
- 2.Tsien RY. Annu. Rev. Biochem. 1998;67:509. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 3.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Science. 1994;263:802. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Xie J, Deniz AA, Schultz PG. J. Org. Chem. 2003;68:174. doi: 10.1021/jo026570u. [DOI] [PubMed] [Google Scholar]
- 5.Tsien RY. Angew. Chem. Int. Ed. 2009;48:5612. doi: 10.1002/anie.200901916. [DOI] [PubMed] [Google Scholar]
- 6.Ivashkin PE, Iampol'skii IV, Luk'ianov KA. Bioorg. Khim. 2009;35:726. doi: 10.1134/s1068162009060028. [DOI] [PubMed] [Google Scholar]
- 7.Liu CC, Schultz PG. Annu Rev. Biochem. 2010;79:413. doi: 10.1146/annurev.biochem.052308.105824. [DOI] [PubMed] [Google Scholar]
- 8.Dedkova LM, Fahmi NE, Golovine SY, Hecht SM. J. Am. Chem. Soc. 2003;125:6616. doi: 10.1021/ja035141q. [DOI] [PubMed] [Google Scholar]
- 9.Dedkova LM, Fahmi NE, Golovine SY, Hecht SM. Biochemistry. 2006;45:15541. doi: 10.1021/bi060986a. [DOI] [PubMed] [Google Scholar]
- 10.Dedkova LM, Fahmi NE, Paul R, del Rosario M, Zhang L, Chen S, Feder G, Hecht SM. Biochemistry. 2012;51:401. doi: 10.1021/bi2016124. [DOI] [PubMed] [Google Scholar]
- 11.Maini R, Nguyen D, Chen S, Dedkova LM, Roy Chowdhury SR, Alcala-Torano R, Hecht SM. Bioorg. Med. Chem. 2013;21:1088. doi: 10.1016/j.bmc.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Vizan JL, Hernandez-Chico C, del Castillo I, Moreno F. EMBO J. 1991;10:467. doi: 10.1002/j.1460-2075.1991.tb07969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igarashi Y, Kan Y, Fujii K, Fujita T, Harada K, Naoki H, Tabata H, Onaka H, Furubai T. J. Antibiot. (Tokyo) 2001;54:1045. doi: 10.7164/antibiotics.54.1045. [DOI] [PubMed] [Google Scholar]
- 14.Bagley MC, Dale JW, Merritt EA, Xiong X. Chem. Rev. 2005;105:685. doi: 10.1021/cr0300441. [DOI] [PubMed] [Google Scholar]
- 15.Wipf P, Miller CP. J. Am. Chem. Soc. 1992;114:10975. [Google Scholar]
- 16.Phillips AJ, Uto Y, Wipf P, Reno MJ, Williams DR. Org. Lett. 2000;2:1165. doi: 10.1021/ol005777b. [DOI] [PubMed] [Google Scholar]
- 17.Bertram A, Pattenden G. Heterocycles. 2002;58:521. [Google Scholar]
- 18.Singh EK, Ramsey DM, McAlpine SR. Org. Lett. 2012;14:1198. doi: 10.1021/ol203290n. [DOI] [PubMed] [Google Scholar]
- 19.Wahyudi H, Tantisantisom W, Liu X, Ramsay DM, Singh EK, McAlpine SR. J. Org. Chem. 2012;77:10596. doi: 10.1021/jo3017499. [DOI] [PubMed] [Google Scholar]
- 20.Lakowicz JR. Principles of Fluorescence Spectroscopy. 3rd ed. New York: Springer; 2006. [Google Scholar]
- 21.O’Donnell MJ, Polt RL. J. Org. Chem. 1982;47:2663. [Google Scholar]
- 22.Sanz-Cervera JF, Blasco R, Piera J, Cynamon M, Ibáñez I, Murguía M, Fustero S. J. Org. Chem. 2009;74:8988. doi: 10.1021/jo9016265. [DOI] [PubMed] [Google Scholar]
- 23.Wipf P, Miller CP. J. Org. Chem. 1993;58:3604. [Google Scholar]
- 24.Lodder M, Golovine S, Laikhter AL, Karginov VA, Hecht SM. J. Org. Chem. 1998;63:794. doi: 10.1021/jo971692l. [DOI] [PubMed] [Google Scholar]
- 25.Robertson SA, Ellman JA, Schultz PG. J. Am. Chem. Soc. 1991;113:2722. [Google Scholar]
- 26.Robertson SA, Noren CJ, Anthony_Cahill SJ, Griffith MC, Schultz PG. Nucleic Acids Res. 1989;17:9649. doi: 10.1093/nar/17.23.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maini R, Dedkova LM, Paul R, Madathil MM, Chowdhury SR, Chen S, Hecht SM. J. Am. Chem. Soc. doi: 10.1021/jacs.5b03135. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janknecht R, de Martynoff G, Lou J, Hipskind RA, Nordheim A, Stunnenberg HG. Proc. Natl. Acad. U. S. A. 1991;88:8972. doi: 10.1073/pnas.88.20.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The protein concentrations were calculated by standard BSA assay. The intensities of Coomassie Brilliant Blue staining of wild-type or GFP analogue samples were compared with a BSA standard of known concentration in an SDS-PAGE experiment.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.