SUMMARY
Malaria-specific antibody responses are short-lived in children, leaving them susceptible to repeated bouts of febrile malaria. The cellular and molecular mechanisms underlying this apparent immune deficiency are poorly understood. Recently, T follicular helper (Tfh) cells have been shown to play a critical role in generating long-lived antibody responses. We show that Malian children have resting PD-1+CXCR5+CD4+ Tfh cells in circulation that resemble germinal center Tfh cells phenotypically and functionally. Within this population PD-1+CXCR5+CXCR3− Tfh cells are superior to Th1-polarized PD-1+CXCR5+CXCR3+ Tfh cells in helping B cells. Longitudinally, we observed that malaria drives Th1 cytokine responses, and accordingly, the less functional Th1-polarized Tfh subset was preferentially activated and its activation did not correlate with antibody responses. These data provide insights into the Tfh cell biology underlying suboptimal antibody responses to malaria in children, and suggest that vaccine strategies that promote CXCR3− Tfh cell responses may improve malaria vaccine efficacy.
INTRODUCTION
The mosquito-borne Plasmodium falciparum parasite causes an estimated 200 million cases of malaria and 600,000 deaths each year, predominantly among African children (W.H.O., 2014). Several studies in malaria-endemic areas have demonstrated that children generally have short-lived antibody responses to P. falciparum infection, leaving them susceptible to repeated bouts of malaria (Portugal et al., 2013). Moreover, the most clinically advanced malaria vaccine candidate induces short-lived antibody responses (Alonso et al., 2005; Riley and Stewart, 2013) and confers only partial, short-term protection against malaria in African children (Rts, 2014). The mechanisms underlying short-lived antibody response to both natural malaria infection and candidate malaria vaccines, particularly in African children, are poorly understood—a critical knowledge gap that hinders the development of a highly effective malaria vaccine (Crompton et al., 2014; Langhorne et al., 2008).
In general, it is well-established that long-lived, high-affinity antibody responses, which are induced by many pathogens and vaccines after a single or few exposures (Amanna et al., 2007), depend on the generation of long-lived plasma cells (LLPCs) and memory B cells (MBCs) within germinal centers (GC) of secondary lymphoid organs (Tarlinton and Good-Jacobson, 2013). In the GC, follicular helper T (Tfh) cells, which express high levels of CXCR5 (Breitfeld et al., 2000; Schaerli et al., 2000) and the transcription factor Bcl6 (Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009), provide critical support for the differentiation of naïve B cells into isotype-switched, affinity-matured LLPCs and MBCs through their production of cytokines such as IL-4 and IL-21 and co-stimulatory molecules such as CD40L (Crotty, 2014). After providing help to B cells, GC Tfh cells may exit the GC, down-regulate Bcl6 and become memory CXCR5+CD4+ Tfh cells that recirculate in blood and then return to the GC upon antigen re-exposure (Hale et al., 2013; Kitano et al., 2011; Shulman et al., 2013), although it is not required that a Tfh cell progress through a GC Tfh state to become a memory Tfh cell (He et al., 2013). Studies in healthy adults have shown that circulating memory CXCR5+CD4+ Tfh cells resemble GC Tfh cells in their capacity to produce IL-21 and induce B cell differentiation (Chevalier et al., 2011; Ma and Deenick, 2014; Morita et al., 2011b). Although circulating Tfh cell subpopulations are diverse (Schmitt and Ueno, 2013), recent work in healthy adults identified circulating PD-1+CXCR3−CXCR5+ Tfh cells as the most closely related to bona fide GC Tfh cells by gene expression, cytokine profile and functional capacity (Locci et al., 2013). Whether these observations hold true in children is unknown—an important knowledge gap given that children are the primary target population for most vaccines, including candidate malaria vaccines. Furthermore, studies of Tfh cells in humans to date have been limited to healthy individuals following immunization (Bentebibel et al., 2013), or cross-sectional analyses of individuals with primary or acquired immunodeficiency (i.e., HIV) (Cubas et al., 2013), autoimmunity or various cancers (Ma and Deenick, 2014); whereas longitudinal studies of Tfh responses before, during and after an acute natural infection have not been published.
Despite the critical role of Tfh cells in humoral immunity, and the enormous disease burden of malaria worldwide, there are no published studies of Tfh cells in human malaria to date (Perez-Mazliah and Langhorne, 2014). Notably, in mouse models of malaria, immunotherapy targeting Tfh cells through blockade of PD-L1 and LAG-3 augmented Tfh cell and GC B cell frequencies, increased antibody levels and accelerated the clearance of blood-stage malaria parasites (Butler et al., 2011). Conversely, simultaneously activating OX40 and blocking PD-1 signaling revealed that excessive IFN-γ limits Tfh responses and humoral anti-Plasmodium immunity (Zander et al., 2015). Finally, it was recently reported that disruption of IL-21 signaling in mice affects T cell-B cell interactions and abrogates protective humoral immunity to malaria (Perez-Mazliah et al., 2015). Together, these reports identify pathways to potentially manipulate Tfh cells in humans to improve the efficacy of vaccines targeting malaria and other pathogens.
Here, we demonstrate that circulating memory PD-1+CXCR5+CD4+ Tfh cells in malaria-exposed children possess phenotypic and functional characteristics of GC Tfh cells. In these children, the PD-1+CXCR5+CXCR3− Tfh cell subset is superior to the Th1-polarized PD-1+CXCR5+CXCR3+ Tfh cell subset in providing B cell help. We show longitudinally that acute malaria drives a Th1 cytokine response, and accordingly, preferentially activates the less functional Th1-polarized PD1+CXCR5+CXCR3+ Tfh cell subset, consistent with a lack of correlation between Tfh cell responses and plasma cell/antibody responses to malaria in the same children.
RESULTS
Malian children maintain resting blood PD-1+CXCR5+CD4+ memory T cells that resemble GC Tfh cells in phenotype and IL-21 production capacity
Studies of healthy adults have shown that blood CXCR5+CD4+ T cells are the circulating counterparts of GC Tfh cells in secondary lymphoid tissue (Chevalier et al., 2011; He et al., 2013; Morita et al., 2011b), cells that support the development of high-affinity, long-lived antibody responses. Children exposed to intense malaria transmission tend to have short-lived P. falciparum-specific antibody responses and suffer repeated bouts of febrile malaria each year (Crompton et al., 2010; Portugal et al., 2013). To investigate Tfh cells in the context of pediatric malaria we took advantage of the sharply demarcated 6-month rainy season (intense malaria transmission) and 6-month dry season (negligible malaria transmission) in Mali to conduct a longitudinal analysis of circulating Tfh cells in children beginning at their healthy uninfected baseline at the end of the dry season and the same children during their first acute febrile malaria episode of the ensuing malaria season (Figure 1A).
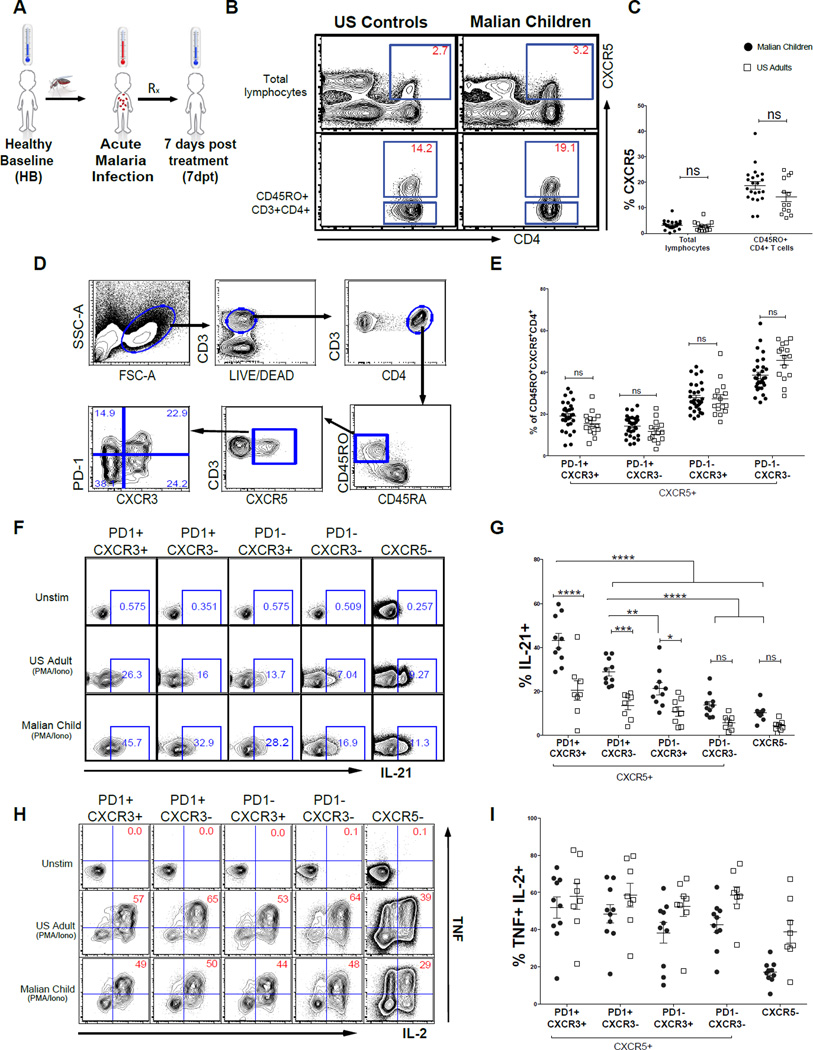
Figure 1. PD-1 expressing CXCR5+CD4+ T cells from Malian children have features of GC Tfh cells.
(A) Longitudinal study design in which blood was collected at the healthy uninfected baseline before the malaria season (HB), during the first acute malaria episode of the ensuing malaria season (acute) and 7 days post-malaria treatment (7dpt). (B and C) The percentage of CXCR5+ cells within total circulating lymphocytes and antigen-experienced CD4+ T cells in Malian children and U.S. adults. (D) Gating strategy for CD45RO+CD45RA−CXCR5+ CD4+ T helper cell subsets. (E) Distribution of memory CXCR5+ T cell subsets in Malian children and U.S. adults. (F) Intracellular IL-21 production across CXCR5+ subsets with and without PMA/ionomycin in representative Malian child and U.S. adult. (G) Percentage of IL-21 producing cells across CXCR5+ subsets following PMA/ionomycin stimulation in Malian children (n=10) and U.S. adults (n=8). (H) Intracellular IL-2 and TNF production across CXCR5+ subsets with and without PMA/ionomycin in representative Malian child and U.S. adult. (I) Percentage CXCR5+ subsets producing intracellular IL-2 and TNF following PMA/ionomycin stimulation in Malian children (n=10) and U.S. adults (n=10). P values determined by Student’s t test with Bonferroni corrections for multiple comparisons or by ANOVA with Sidak corrections for multiple comparisons where appropriate. ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05, ns=not significant.
We first asked whether children residing in a malaria-endemic area show evidence of a general deficiency in generating and maintaining resting memory CXCR5+CD4+ T cells by analyzing blood samples from healthy uninfected children (n=25) at the end of the 6-month dry season. Characteristics of these children are summarized in Table S1. In parallel, we analyzed blood from healthy U.S. adults (n=15) as a benchmark to compare with published studies. Consistent with previous observations (Breitfeld et al., 2000; He et al., 2013; Morita et al., 2011b), we found in both Malian children and U.S. adults that 15–20% of circulating antigen-experienced (CD45RO+) CD4+ T cells express CXCR5 (Figures 1B and 1C), indicating that the inefficient acquisition of humoral immunity to malaria in children is not due to a global deficiency in generating and maintaining antigen-experienced CXCR5+CD4+ T cells in blood.
More recent work in healthy adults demonstrated that within the blood CXCR5+CD4+ memory T cell population, the PD-1+CXCR3−CXCR5+ subset most closely resembles bona fide GC Tfh cells, both phenotypically and functionally (Locci et al., 2013). Therefore, we asked whether the PD-1+CXCR3−CXCR5+ T cell subset could be detected in the peripheral blood of healthy Malian children at the end of the dry season. Similar to observations in healthy adults (Locci et al., 2013), we found that the majority of antigen-experienced CXCR5+CD4+ T cells were PD-1− (Figure 1D). In comparing Malian children to U.S. adults, we found that the percentage of PD-1+CXCR3− CXCR5+ T cells as well as the overall distribution of CXCR5+CD4+ T cell subsets to be similar between the two groups (Figure 1E). Next, we determined the distribution of central memory (TCM) and effector memory (TEM) cells among the CXCR5+CD4+ T cell subsets in children at their healthy baseline before the malaria season. CD45RO+CD4+ T cells are generally categorized as TCM or TEM cells on the basis of differential CCR7 expression (Sallusto et al., 1999). TCM cells express CCR7 and home to secondary lymphoid tissue, while TEM cells do not express CCR7 and migrate to sites of inflammation. Consistent with prior studies (Breitfeld et al., 2000; Chevalier et al., 2011; Locci et al., 2013), we identified the majority of CD45RO+CXCR5+CD4+ T cells as TCM cells (Figure S1A). Also comparable with prior work (Locci et al., 2013), both the PD-1+CXCR3−CXCR5+ and PD-1+CXCR3+CXCR5+ subsets had a higher percentage of TEM compared to the PD-1− subsets (Figures S1A and S1B). Thus, these data indicate that children in malaria-endemic Mali are capable of generating and maintaining memory PD-1+CXCR3−CXCR5+ T cells.
Although circulating PD-1+CXCR3−CXCR5+ memory T cells are clearly detectable in Malian children, it remained possible that these cells lacked other phenotypic markers of GC Tfh cells or the capacity to produce IL-21, the canonical GC Tfh cytokine that drives B cell differentiation (Linterman et al., 2010; Zotos et al., 2010). Therefore, we compared blood PD-1+CXCR5+CD4+ T cells to other CXCR5+CD4+ T cell subsets and non-Tfh cells (CXCR5−) by their expression of cell surface proteins (SLAMF6, CD200, TIGIT, CD150, CD84) and transcription factors (Bcl6 and cMaf) that characterize GC Tfh cells (Crotty, 2014; Ma and Deenick, 2014). In Malian children, we found that a higher percentage of both PD-1+CXCR3+CXCR5+CD4+ and PD-1+CXCR3− CXCR5+CD4+ T cells expressed SLAMF6, CD200, TIGIT, CD150 and CD84 compared to their PD-1− counterparts (Figures S1C), indicating that the PD-1+CXCR5+CD4+ T cell subsets are more closely related to GC Tfh cells. A similar pattern was observed in healthy U.S. adults (Figure S1C), consistent with previous reports (Ma et al., 2012). Also in line with prior studies we found that the expression of Bcl6, an essential transcription factor for GC Tfh differentiation (Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009), was similar across the CXCR5+ and CXCR5− T cell subsets, consistent with the notion that Bcl6 is downregulated as GC Tfh cells exit secondary lymphoid tissue into the peripheral circulation (Chevalier et al., 2011; Kitano et al., 2011; Morita et al., 2011b; Rasheed et al., 2006; Shulman et al., 2013). However, the overall level of Bcl6 expression was higher in Malian children compared to U.S. adults. In contrast, expression of the transcription factor cMaf, which is associated with IL-4 production and Tfh cell differentiation (Kroenke et al., 2012), was lower in Malian children compared to U.S. adults, but among Malian children, cMaf expression was higher in the PD-1+CXCR5+CD4+ T cell subsets compared to other CXCR5+CD4+ T cell subsets (Figure S1C).
Next, we compared resting blood PD-1+CXCR5+CD4+ memory T cells to other CXCR5+CD4+ T cell subsets and non-Tfh cells for their capacity to produce IL-21. As expected, following a brief stimulation with PMA and ionomycin we detected a higher percentage of IL-21 producing cells among the PD-1+CXCR5+CD4+ T cell subsets compared to other CXCR5+CD4+ T cell subsets and non-Tfh cells in both Malian children and U.S. adults (Figures 1F and 1G). Interestingly, Malian children had a higher percentage of IL-21 producing cells across all CXCR5+CD4+ T cell subsets compared to U.S. adults (Figure 1G). Of note, intracellular TNF and IL-2 were detectable across all subsets following stimulation with PMA/ionomycin (Figures 1H and 1I) demonstrating that the CXCR5+CD4+ T cell subsets are generally able to produce cytokines.
Together these data indicate that children in malaria-endemic areas are capable of generating a circulating population of resting PD-1+CXCR5+CD4+ memory T cells that resemble GC Tfh cells in phenotype and IL-21 production capacity.
Circulating PD-1+CXCR3−CXCR5+CD4+ T cells in Malian children are superior to PD-1+CXCR3+CXCR5+CD4+T cells in providing B cell help
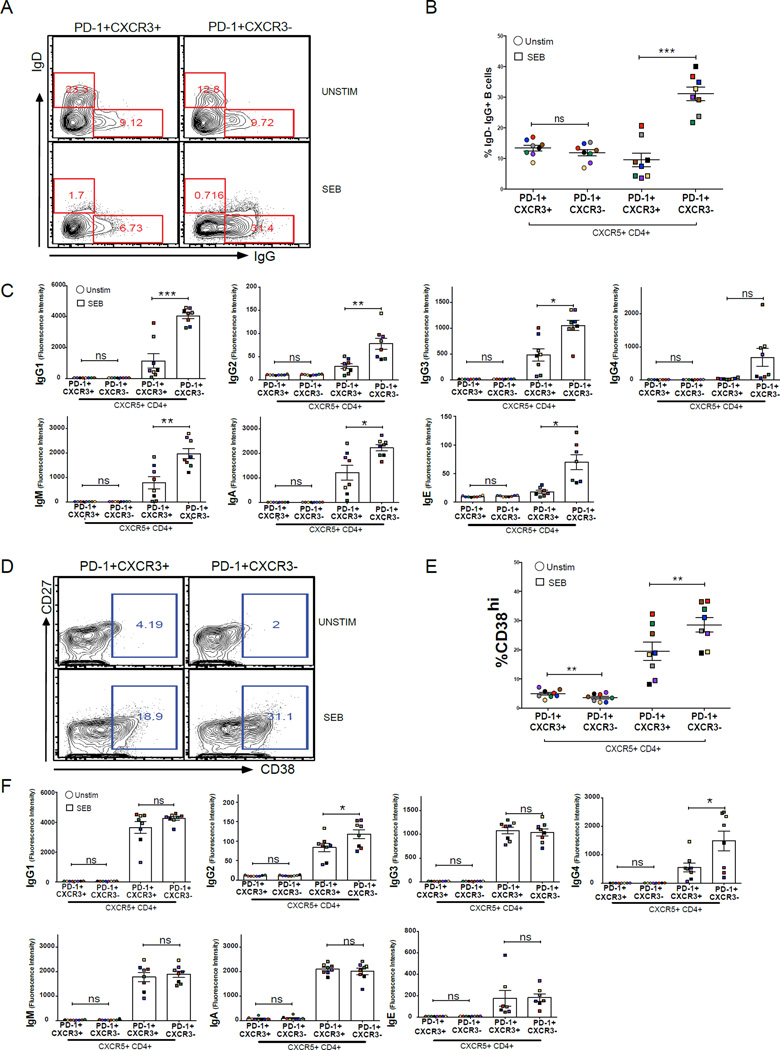
The data above indicate that the inefficient acquisition of malaria-specific antibodies in children cannot be explained by a global deficiency in generating circulating PD-1+CXCR5+CD4+ memory T cells; however, it remained possible that these cells do not function to provide B cell help in children during a malaria infection. As noted above, others have recently shown in healthy adults that among PD-1+CXCR5+CD4+ memory Tfh cells, the CXCR3− subset is superior in providing B cell help (Locci et al., 2013), a finding that we confirmed in healthy U.S. adults (Figure S2A–C). However, in children it is unknown if blood PD-1+CXCR5+CD4+ memory T cells can provide B cell help, and if so, whether CXCR3 expression discriminates a differential ability to help B cells. To determine their capacity to help B cells in Malian children, PD-1+CXCR3+CXCR5+CD4+ and PD-1+CXCR3−CXCR5+CD4+ T cells (hereafter referred to as CXCR3+ and CXCR3− Tfh cells, respectively) were FACS-sorted and cultured with autologous naïve B cells (CD19+CD21+CD27−) for 12 days with and without the superantigen SEB to mimic the interaction between antigen-specific T cells and antigen-presenting B cells (Morita et al., 2011b). After 12 days without SEB, we observed no difference in the percentages of IgD−IgG+ B cells in samples co-cultured with CXCR3+ or CXCR3− Tfh cells (Figures 2A and 2B). However, after 12 days in the presence of SEB there was a significantly higher percentage of IgD−IgG+ B cells in samples co-cultured with CXCR3− versus CXCR3+ Tfh cells (Figures 2A and 2B), suggesting that CXCR3− Tfh cells are more efficient than CXCR3+ Tfh cells in promoting class switching. Similarly, CXCR3− Tfh cells were more efficient than their CXCR3+ counterparts in inducing naïve B cells to produce IgG1–IgG3, IgM, IgA and IgE antibodies (Figure 2C). However, in the absence of SEB, naïve B cells produced little or no Ig (Figure 2C). Next, sorted CXCR3+ and CXCR3− Tfh cells were cultured with autologous MBCs (CD19+CD21+CD27+) for 6 days with and without SEB. After 6 days without SEB, we observed no significant difference in the percentage of CD38hi plasmablasts in samples cultured with CXCR3+ versus CXCR3− Tfh cells (Figures 2D and 2E), whereas 6 days with SEB yielded a significantly higher percentage of CD38hi plasmablasts in samples cultured with CXCR3− versus CXCR3+ Tfh cells (Figures 2D and 2E), suggesting that CXCR3− Tfh cells more efficiently induce MBCs to differentiate into plasmablasts. Likewise, CXCR3− Tfh cells were more efficient than their CXCR3+ counterparts in driving MBCs to produce IgG2 and IgG4 (Figure 2F), while in the absence of SEB, MBCs produced little or no Ig (Figure 2F). The failure of CXCR3+ Tfh cells to efficiently provide B cell help was not due to cell death as both SEB stimulated T cell subsets had similar percentages of viable cells after stimulation (data not shown).
Figure 2. PD-1+CXCR3−CXCR5+CD4+ Tfh cells are superior to PD-1+CXCR3+CXCR5+CD4+ Tfh cells in providing B cell help.
(A–C) PD-1+CXCR3− CXCR5+CD4+ and PD-1+CXCR3+CXCR5+CD4+ Tfh cells from Malian children (n=8) were FACS-sorted and cultured with autologous naïve B cells (CD19+CD21+CD27−) with and without SEB. After 12 days, surface expression of IgD and IgG on B cells and secreted Ig levels were measured. (A) Representative plots of IgD and IgG staining on B cells after 12-day co-culture. (B) Percentage of IgD−IgG+ B cells after 12-day co-culture. (C) Total IgG1-4, IgM, IgA and IgE levels in supernatants after 12-day co-culture. (D–F) The same FACS-sorted PD-1+CXCR3−CXCR5+CD4+ and PD-1+CXCR3+CXCR5+CD4+ Tfh subsets were cultured for 6 days with autologous MBCs (CD19+CD21+CD27+) with and without SEB. After 6 days, surface expression of CD27 and CD38 on B cells and secreted Ig levels were measured. (D) Representative plot of CD27 and CD38 staining on B cells after 6-day co-culture. (E) Percentage of B cells that were CD38hi plasmablasts after 6-day co-culture. (F) Total IgG1-4, IgM, IgA and IgE levels in supernatants after 6-day co-culture. Samples from the same subject are matched by color. P values were determined by paired Student’s t test with Bonferroni adjustments. ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05, ns=not significant.
Together, these data show that CXCR3− Tfh cells in Malian children exhibit superior Tfh cell functionality in helping B cells compared to their CXCR3+ counterparts.
CXCR3+ Tfh cells in Malian children are Th1-polarized
A clearer understanding of the differential capacity of blood Tfh cell subsets to provide B cell help could inform the development of vaccines that generate long-lived humoral immunity. For example, strategies that skew Tfh responses toward the more functional CXCR3− subset could potentially improve current malaria vaccine candidates such as RTS,S, which confer short-lived protection from malaria (Stanisic et al., 2013).
Prior work in healthy adults has shown that blood Tfh cells comprise functionally distinct subsets that share features of Th1, Th2 and Th17 cells, as defined by the differential expression of CXCR3 and CCR6 (Bentebibel et al., 2013; Morita et al., 2011b; Schmitt and Ueno, 2013). More specifically, it was shown in healthy adults that CXCR3− subsets are Th2- or Th17-polarized and provide efficient B cell help, while Th1-like CXCR3+ subsets are poor B cell helpers (Morita et al., 2011b). As noted earlier, more recent studies in healthy adults have demonstrated that PD-1 expression further distinguishes Tfh subsets and identifies the Th2-cell type PD-1+CXCR3− subset among quiescent blood Tfh cells as the most functional in terms of providing B cell help (He et al., 2013; Locci et al., 2013). However, it is unknown in children, particularly those residing in malaria-endemic areas, whether circulating PD-1+CXCR3− and PD-1+CXCR3+ subsets are similarly polarized along the Th1/Th2/Th17 axis.
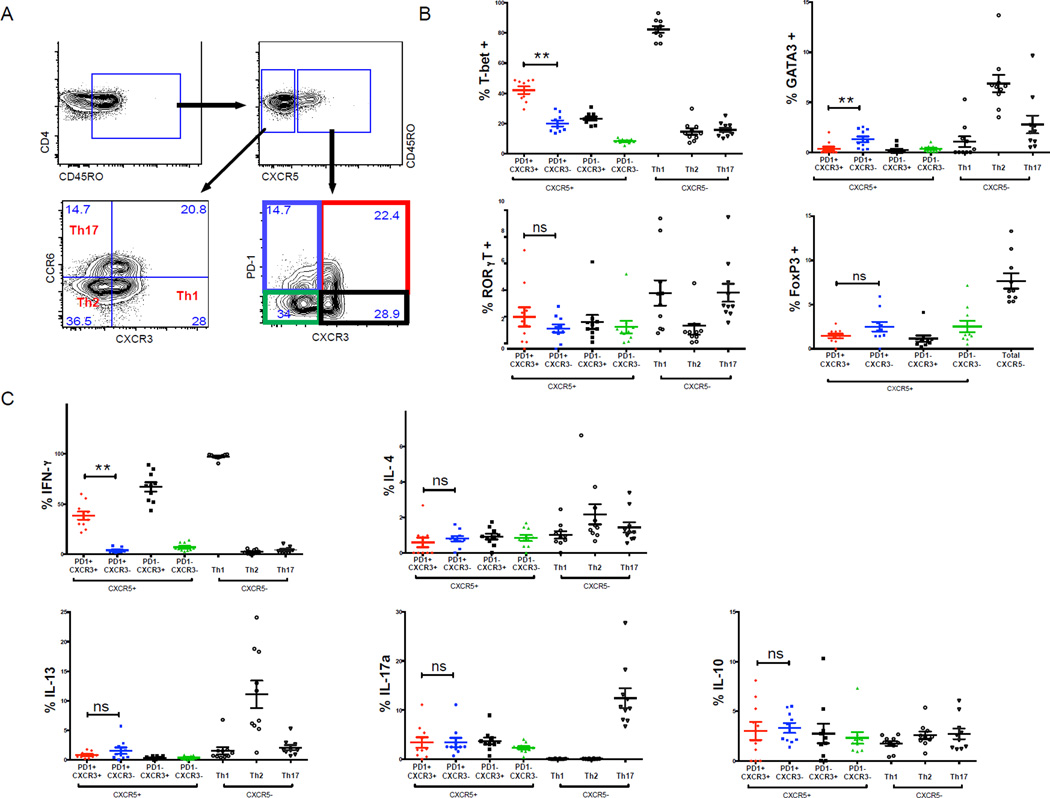
We first addressed this question in healthy uninfected children at the end of the dry season by quantifying the expression of the lineage-defining transcription factors T-bet, GATA3, RORγT and Foxp3 in antigen-experienced CXCR5+CD4+ Tfh subsets on the basis of PD-1 and CXCR3 expression (Figure 3A). In parallel, as a positive control within each subject, we quantified the expression of the same transcription factors in antigen-experienced CXCR5−CD4+ T cell subsets on the basis of CCR6 and CXCR3 expression in order to identify Th1, Th2 and Th17 cells (Figure 3A), as previously described (Morita et al., 2011a). As expected, the percentage of cells expressing T-bet and GATA3 were higher in the CXCR5− Th1 and Th2 subsets, respectively (Figure 3B). The percentage of cells expressing RORγT was higher in both the CXCR5− Th1 and Th17 subsets (Figure 3B). Among the CXCR5+ subsets, we found that a higher percentage of PD-1+CXCR3+ cells expressed T-bet, while a higher percentage of PD-1+CXCR3− cells expressed GATA3 (Figure 3B). The expression of RORγT and Foxp3 was similar across CXCR5+ subsets (Figure 3B).
Figure 3. PD-1+CXCR3+CXCR5+CD4+ Tfh cells are Th1-polarized.
(A) Gating strategy for the analysis of CXCR5+ and CXCR5− memory CD4+ T cell subsets. (B) Ex vivo expression of T-bet, GATA-3, RORγT and Foxp3 by CXCR5+ and CXCR5− CD4+ T cell subsets. (C) Intracellular production of indicated cytokines by CXCR5+ and CXCR5− CD4+ T cell subsets following PMA/ionomycin stimulation. Only PD1+CXCR3+ and PD1+CXCR3− were compared and p-values were determined by paired Student’s t test. ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05, ns=not significant.
We then measured cytokines corresponding to lineage-defining transcription factors after PMA/ionomycin stimulation. As expected, the CXCR5− Th1, Th2 and Th17 subsets expressed IFN-γ, IL-4/IL-13 and IL-17, respectively (Figure 3C). Predictably, PD-1+CXCR3+CXCR5+ cells expressed IFN-γ, while the PD-1+CXCR3−CXCR5+subset produced almost no IFN-γ (Figure 3C). Unexpectedly, PD-1+CXCR3−CXCR5+ cells, which had a higher percentage of GATA3-expressing cells, did not express higher levels of IL-4 or IL-13 compared to PD-1+CXCR3+CXCR5+cells (Figure 3C), possibly due to low expression of cMaf in Malian children (Figure S1C), a transcription factor essential for IL-4 production (Kroenke et al., 2012). We observed no difference in the percentage of cells producing IL-10 or IL-17 across the subsets (Figure 3C).
Together, these data indicate that in Malian children circulating PD-1+CXCR3+CXCR5+ Tfh cells are Th1-skewed while PD-1+CXCR3−CXCR5+ Tfh cells are not as clearly Th2-skewed as that reported in healthy adults.
Acute malaria drives a Th1 cytokine response and preferentially activates the less functional Th1-polarized CXCR3+ Tfh subset
Given that children in areas of intense P. falciparum transmission tend to have short-lived antibody responses to malaria, we hypothesized that acute malaria would preferentially activate the less functional Th1-polarized CXCR3+ Tfh subset. In general, circulating antigen-experienced CXCR5+CD4+ T cells in healthy individuals are considered to be resting memory Tfh cells and express moderate levels of PD-1 and low levels of the proliferation/activation markers Ki67, CD38, HLA-DR, and inducible T-cell costimulator (ICOS)—in contrast to their activated GC Tfh cell counterparts in secondary lymphoid tissue that express high levels of these markers (Crotty, 2014). Previous work showed that like other memory T helper cells, memory Tfh cells can be recalled during antigen re-exposure and preferentially become effector Tfh cells and GC Tfh cells upon reactivation (Hale et al., 2013). Therefore, to test the hypothesis that acute malaria preferentially activates the less functional Th1-polarzied CXCR3+ Tfh subset we analyzed thawed PBMCs collected from children at their healthy uninfected baseline at the end of the 6-month dry season, and from the same children during their first episode of febrile malaria during the ensuing 6-month malaria season—a prospective analysis made possible by the intense and sharply-demarcated malaria season at the study site (Tran et al., 2013). At the first malaria episode children were febrile or reported fever within 24 hours, were infected with P. falciparum (geometric mean: 29,991 asexual parasites/µl of blood) and had no other cause of fever discernible on physical examination. All subjects were treated with a standard 3-day course of artemether/lumefantrine. Characteristics of subjects are shown in Table S1.
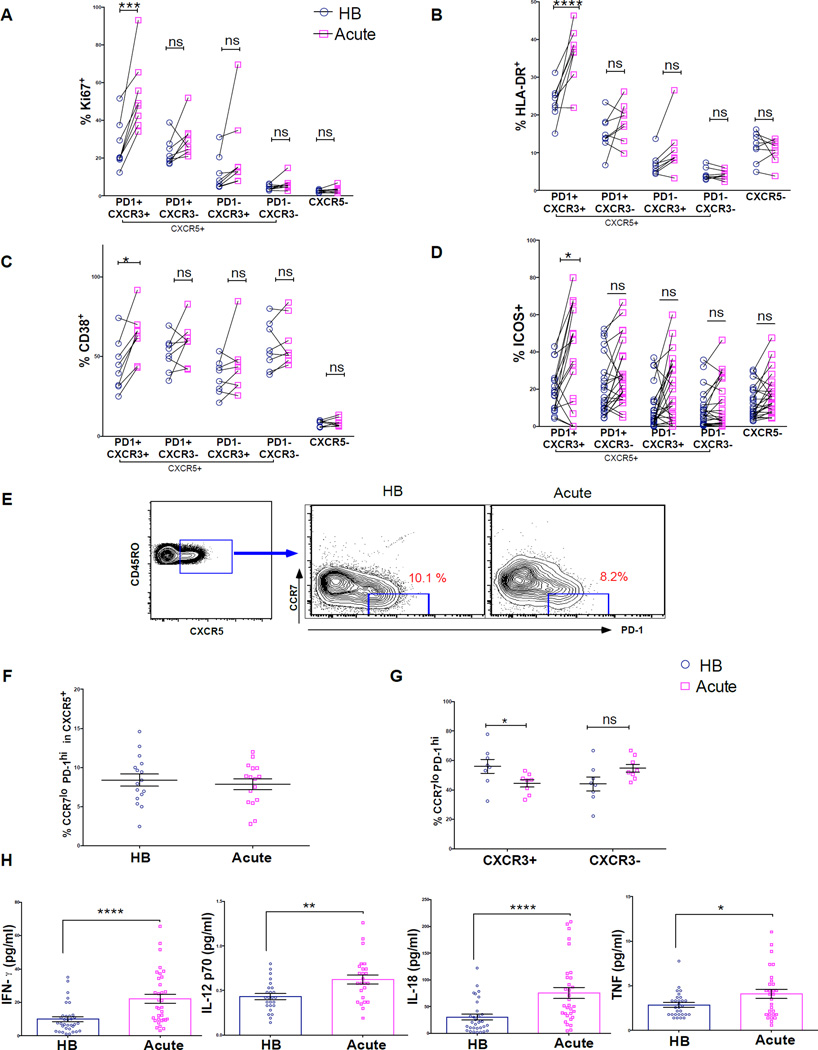
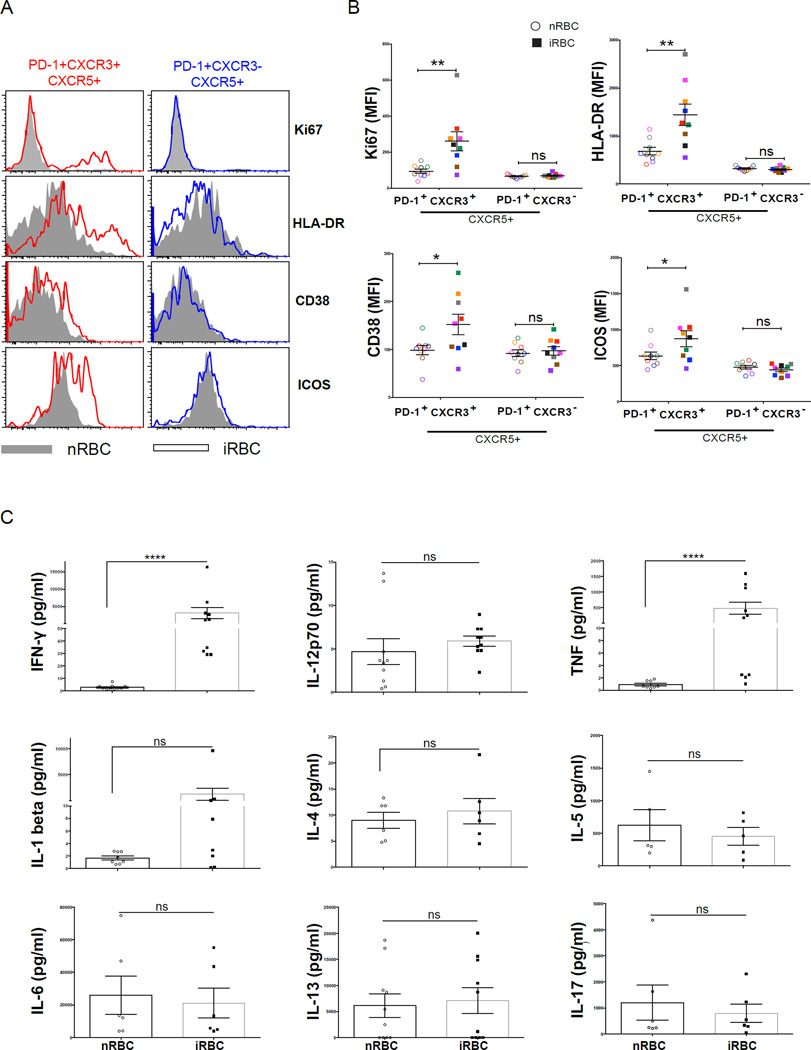
Consistent with our hypothesis, we found that only the less functional Th1-polarized CXCR3+ Tfh memory cells were activated and proliferated in response to acute malaria, as evidenced by a significant increase in the percentage of cells expressing Ki67, CD38 and HLA-DR (Figures 4A–4C). Of note, the majority of activated CXCR3+ Tfh cells were double positive for these markers (Figures S3A–C), indicating that this population is homogeneously activated. Besides the crucial role ICOS plays in Tfh development (Akiba et al., 2005) and function (Vogelzang et al., 2008), recent studies suggest that ICOS-expressing Tfh cells migrate toward B cells expressing ICOS ligand (ICOS-L) to initiate the formation of GCs (Xu et al., 2013). Interestingly, acute malaria was associated with a significant increase in ICOS expression only on CXCR3+ Tfh cells (Figure 4D).
Figure 4. Acute malaria induces Th1 cytokines and activates the less functional Th1-polarized CXCR3+ Tfh subset.
(A–D) Percentage of CXCR5+ subsets expressing the proliferation/activation markers Ki67, CD38, HLA-DR and the co-stimulatory molecule ICOS during acute febrile malaria (acute) compared to the healthy uninfected baseline (HB). (E) Representative FACS plot showing PD-1 and CCR7 expression within antigen-experienced CXCR5+CD4+ T cells in a Malian child at the HB and during acute malaria (F) Percentage CCR7loPD-1hi cells within CXCR5+CD4+ T cells at the HB and during acute malaria. (G) Percentage CCR7loPD-1hi cells within the CXCR3+ and CXCR3− Tfh subsets at the HB and during acute malaria. (H) Plasma cytokine levels at HB and acute. P values determined by paired-design ANOVA with Sidak corrections for multiple comparisons (A-D) or paired Student’s t test with Bonferroni corrections for multiple comparisons where appropriate (F–H). ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05, ns=not significant
Next, we asked if the increase in activation markers in CXCR3+ Tfh cells during acute malaria reflected the appearance of pre-GC Tfh effector cells (CCR7loPD-1hi) in circulation, as recently described in the context of influenza vaccination (He et al., 2013). Contrary to this hypothesis, acute malaria did not change the percentage of circulating CCR7loPD-1hi cells within the CXCR5+ subset (Figures 4E and 4F), and was associated with a decrease in circulating CCR7loPD-1hi cells within the CXCR3+ subset (Figure 4G), suggesting that the increase in activation/proliferation markers in the CXCR3+ population did not arise from newly generated pre-GC Tfh effector cells.
To gain insight into why the less functional Th1-polarized CXCR3+ Tfh subset is preferentially triggered during malaria we measured cytokines in plasma collected from the same children at their uninfected baseline before the malaria season and during acute malaria. We found that the Th1 cytokines IFN-γ, IL-12p70, IL-18 and TNF were significantly increased in plasma during acute malaria compared to baseline (Figure 4H), whereas the Th2 cytokines IL-4, IL-5 and IL-13 were not detectable at either time point (data not shown), consistent with the preferential activation of Th1-polarized CXCR3+ Tfh cells during malaria.
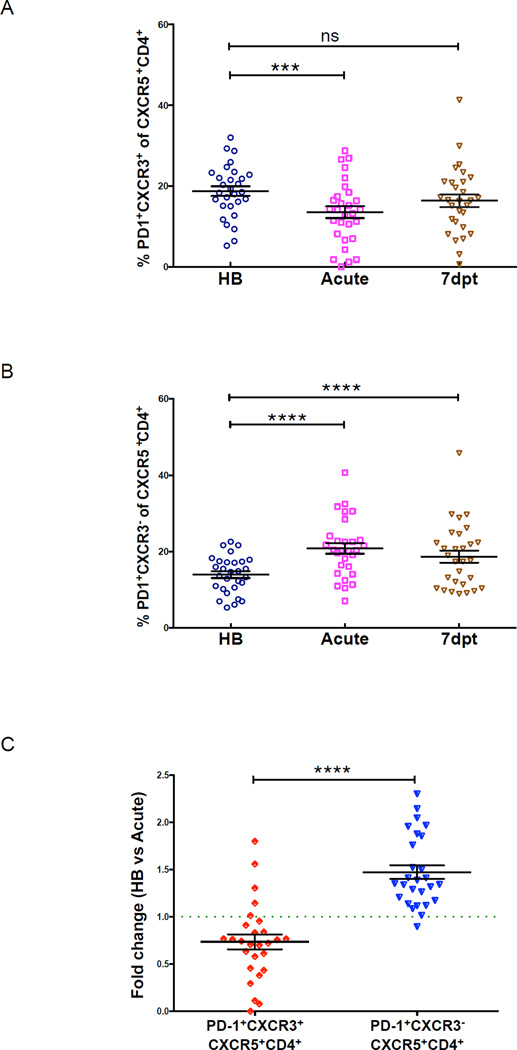
One possible outcome of activating blood Tfh memory cells is their migration to secondary lymphoid organs to provide B cell help in germinal centers. Indeed, studies in mice have demonstrated that an increase in activated blood Tfh cells correlates with increased frequencies of newly generated Tfh cell in secondary lymphoid organs (He et al., 2013). We tested this hypothesis indirectly by longitudinally tracking the kinetics of CXCR3+ and CXCR3− Tfh cells in children before, during and 7 days after treatment of an acute malaria episode. We found that CXCR3+ Tfh cells (the subset that proliferated in response to malaria) transiently decreased in blood during acute malaria (Figures 5A–C), suggesting that malaria-induced activation of CXCR3+ Tfh cells results in their migration from blood to secondary lymphoid tissues.
Figure 5. PD-1+CXCR3+CXCR5+CD4+ T cells transiently decrease in circulation during acute malaria.
Percentage of circulating (A) PD-1+CXCR3+CXCR5+CD4+ and (B) PD-1+CXCR3−CXCR5+CD4+ T cells in Malian children at the healthy baseline (HB), during acute malaria (acute) and 7 days post-treatment (7dpt). (C) Fold change in percentage of circulating PD-1+CXCR3+CXCR5+CD4+ and PD-1+CXCR3−CXCR5+CD4+ T cells from HB to acute. P values determined by paired-design ANOVA with Tukey adjustments for multiple comparisons where appropriate. ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05, ns=not significant.
P. falciparum stimulation of PBMCs recapitulates acute malaria by inducing Th1 cytokines and preferentially activating CXCR3+ Tfh cells
To further test the hypothesis that acute malaria preferentially drives the expansion of memory CXCR3+ Tfh cells, rather than forming effector cells de novo with each infection, we stimulated PBMCs collected from uninfected children (before malaria season) with a prior history of malaria exposure with P. falciparum-infected red blood cells (iRBCs) for 5 days. Consistent with the ex vivo data obtained during acute malaria, only the CXCR3+ population showed an increase in the activation/proliferation markers Ki67, CD38, HLA-DR, and ICOS following in vitro stimulation with iRBCs (Figures 6A and 6B), suggesting that the preferential triggering of CXCR3+ Tfh cells during acute malaria is due to activation of pre-existing memory Tfh cells. Moreover, in response to iRBC stimulation the same PBMC samples secreted IFN-γ and TNF but not IL-4, IL-5 or IL-13 (Figure 6C), thus recapitulating the Th1-polarized response observed during acute malaria.
Figure 6. P. falciparum-infected RBCs drive a Th1 cytokine response and preferentially activate CXCR3+ Tfh cells.
PBMCs collected before the malaria season from healthy uninfected children with prior malaria exposure were stimulated in vitro with P. falciparum-infected red blood cells (iRBCs) or naïve red blood cells (nRBCs) for 5 days. (A) Representative plots showing the expression of Ki67, HLA-DR, CD38 and ICOS in the PD-1+CXCR3+ and PD-1+CXCR3− subsets following stimulation. (B) MFI of Ki67, HLA-DR, CD38 and ICOS in the PD-1+CXCR3+ and PD-1+CXCR3− subsets following stimulation. (C) Cytokine levels in supernatants of stimulated PBMCs. P values determined by paired-design ANOVA with Sidak corrections for multiple comparisons or paired Student’s t test with Bonferroni corrections for multiple comparisons. ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05, ns=not significant.
Tfh responses to acute malaria do not correlate with plasma cell or antibody responses
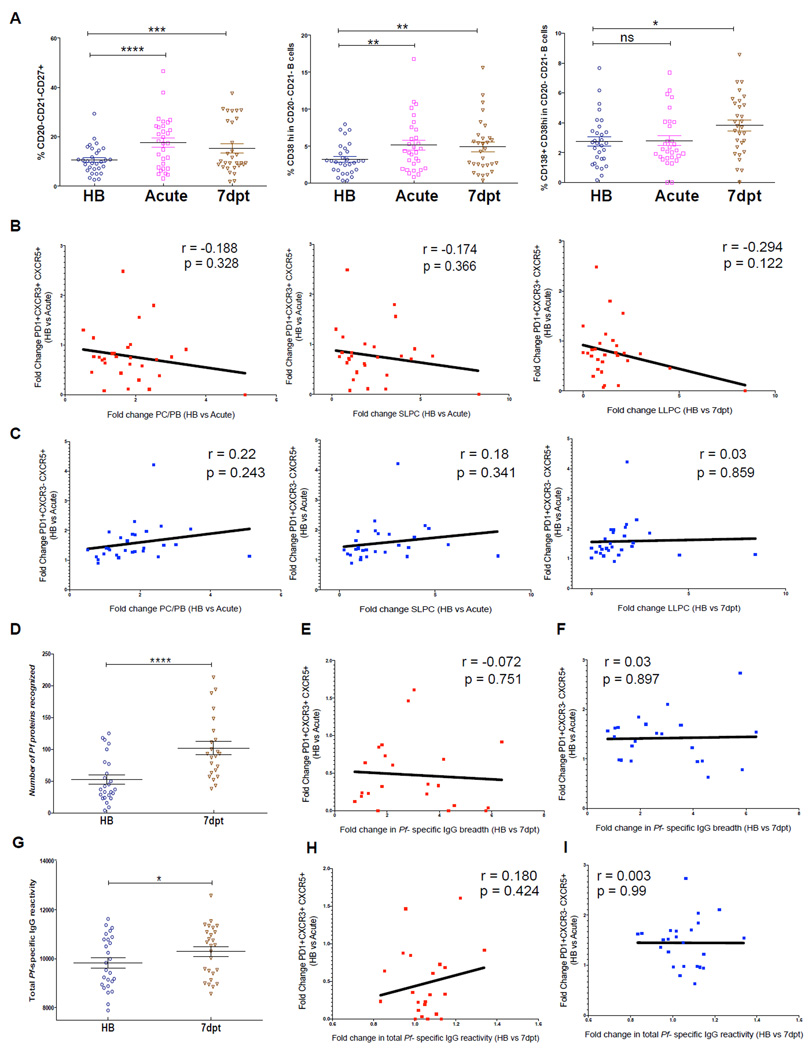
Others have recently shown in humans that circulating Tfh cell responses correlate with antibody responses to influenza vaccination (Bentebibel et al., 2013) and the development of broadly neutralizing antibodies against HIV (Locci et al., 2013). Because malaria preferentially activates the less functional CXCR3+ Tfh subset, we hypothesized that Tfh cell responses to malaria would not correlate with B cell and antibody responses induced by the same infection. We tested this hypothesis by examining longitudinally the relationship between Tfh cell responses to acute malaria and plasma cell (PC) and antibody responses to the same infection. We quantified total PCs, short-lived plasma cells (SLPCs) and long-lived plasma cells (LLPCs) (Figure S4) in the peripheral blood of children at baseline, during the first malaria episode of the ensuing malaria season and 7 days post-malaria treatment. Tfh subsets were enumerated in the same samples at baseline and during acute malaria. Compared to baseline, PCs and SLPCs increased during malaria, while an increase in LLPCs was not detected until day 7 post-malaria (Figure 7A). We observed no significant correlation between fold changes in CXCR3+ or CXCR3− Tfh cells from baseline to acute malaria and fold changes in PCs, SLPCs or LLPCs (Figures 7B and 7C).
Figure 7. Tfh cell responses to malaria do not correlate with plasma cell and antibody responses to the same infection.
(A) Circulating total PCs (CD20−CD21− CD27+) (left), SLPCs (CD20−CD21−CD27+CD38hi) (middle) and LLPCs (CD20−CD21−CD27+CD38hiCD138) (right) as a percentage of total CD19+ cells in children at healthy baseline (HB), during acute malaria (acute) and 7 days post-treatment (7dpt). (B) Fold change in CXCR3+ Tfh cells (HB to acute) versus fold change in total PCs (left) and SLPCs (middle) (HB to acute) and LLPCs (right) (HB to 7dpt). (C) Fold change in CXCR3− Tfh cells (HB to acute) versus fold change in total PCs (left) and SLPCs (middle) (HB to acute) and LLPCs (right) (HB to 7dpt). (D) Antibody breadth of IgG response to 1087 P. falciparum antigens at HB and at 7dpt. Fold change in (E) CXCR3+ or (F) CXCR3− Tfh cells from HB to acute versus fold change in breadth of IgG response from HB to 7dpt. (G) Total IgG reactivity to 1087 P. falciparum antigens during HB and 7dpt. Fold change in (H) CXCR3+ or (I) CXCR3− Tfh cells from HB to acute versus fold change in total IgG reactivity to 1087 P. falciparum antigens from HB to 7dpt. Student’s t test and Pearson correlation were used. ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05, ns=not significant.
We then asked whether Tfh responses to malaria correlate with the breadth or magnitude of P. falciparum-specific IgG responses. By protein microarray (Crompton et al., 2010) we measured plasma IgG to 1087 P. falciparum antigens at baseline and 7 days post-malaria treatment. As expected, the breadth of P. falciparum-specific antibody responses (the number of antigens to which IgG reactivity exceeded 2 SD above the negative control) increased 7 days post-malaria compared to baseline (Figure 7D), but there was no correlation between fold changes in CXCR3+ or CXCR3− Tfh cells and fold changes in antibody breadth (Figures 7E and 7F). We also observed a significant increase in the overall magnitude of IgG reactivity to P. falciparum antigens 7 days post-malaria compared to baseline (Figure 7G), but again there was no correlation between fold changes in CXCR3+ or CXCR3− Tfh cells and the magnitude of antibody responses to malaria (Figures 7H and 7I).
Collectively, the lack of correlation between Tfh cell responses and B cell and antibody responses to malaria is consistent with the observation that acute malaria preferentially activates the less functional CXCR3+ Tfh cell subset.
DISCUSSION
Tfh cells are essential for generating high-affinity, long-lived antibody responses (Crotty, 2014; Ma and Deenick, 2014), and therefore there is considerable interest in understanding how Tfh memory cells are generated and maintained in humans. This is particularly true for infections like malaria, HIV and TB that impose enormous disease burdens worldwide and for which there are no highly effective licensed vaccines (Rappuoli and Aderem, 2011). In the case of malaria, antibody responses to natural P. falciparum infections in children tend to be short-lived, leaving children susceptible to repeated bouts of febrile malaria (Portugal et al., 2013). Here we studied the nature of Tfh responses to malaria in children that underlies the inefficient acquisition of protective, long-lived antibodies. More generally, we sought to determine if recently published findings on blood Tfh cells in adults hold true in children, since children are the key target population for most licensed and candidate vaccines, including candidate malaria vaccines.
In this study we show in children that circulating memory PD-1+CXCR5+CD4+ Tfh cells resemble GC Tfh cells phenotypically and functionally. We demonstrate that the CXCR3− Tfh cell subset is superior to the Th1-polarized CXCR3+ Tfh cell subset in providing B cell help in vitro, consistent with recent findings in healthy adults (Locci et al., 2013). We then show longitudinally that acute malaria preferentially activates the less functional Th1-polarized CXCR3+ Tfh subset, in concert with a malaria-driven Th1 cytokine response; whereas the highly functional CXCR3− Tfh subset does not appear to be triggered by malaria. Since only the CXCR3+ Tfh subset showed evidence of proliferating during acute malaria, the decrease in CXCR3+ Tfh cells during malaria may represent egress from circulation to secondary lymphoid tissues. Accordingly, we observed no correlation between Tfh responses to malaria and the B cell and malaria-specific antibody response to the same infection. These data offer a functional link between Tfh cells and suboptimal antibody responses to malaria in children, and are consistent with the hypothesis that malaria induces short-lived, low-affinity antibody responses that arise in large part from extra-follicular, T cell-independent reactions (Tarlinton and Good-Jacobson, 2013). It will be of interest in future studies to test the hypothesis that the gradual transition from non-protective to protective antibody responses from childhood to adulthood is due to contraction of CXCR3+ Tfh cells and expansion of the more functional CXCR3− Tfh cells.
Acute malaria was not associated with an increase in circulating pre-GC Tfh effector cells (CCR7loPD-1hi), as recently described in influenza vaccinees (He et al., 2013). This result, together with the finding that CXCR3+ Tfh cells from children with prior malaria exposure were activated by P. falciparum in vitro, suggests that the preferential activation of CXCR3+ Tfh cells during acute malaria is due to the expansion of pre-existing memory Tfh cells, rather than the induction of newly generated pre-GC Tfh effector cells. Elucidating the precise conditions favoring the expansion of pre-GC Tfh effector cells in some models and not others requires further investigation.
Unexpectedly, we found that CXCR3− Tfh cells in African children were not as clearly Th2-polarized as CXCR3− Tfh cells in healthy adults (He et al., 2013; Kroenke et al., 2012; Locci et al., 2013). In line with this observation we found that cMaf expression, which is required for IL-4 production (Kroenke et al., 2012), is lower in Tfh cells of Malian children compared to healthy adults. These potentially important differences between malaria-exposed and non-exposed children and adults merit further investigation and may have implications for vaccine strategies.
Previous studies in mice and humans have shown that acute Plasmodium blood-stage infection drives the early activation of Th1 cells that in mouse models are essential for early control of parasite replication and host survival through the activation of effector cells such as macrophages (Perez-Mazliah and Langhorne, 2015). The findings of our study suggest that Plasmodium-induced Th1 cell activation manifests within the circulating Tfh cell compartment as activation of the less functional CXCR3+ Th1 cell-type subset, and that the survival advantage resulting from early Th1-mediated control of parasite replication may come at the expense of high-affinity, long-lived antibody responses. The notion that heterogeneity in GC Tfh cell phenotypes is partly driven by environmental cues is reminiscent of chronic LCMV infection in mice in which intense Th1 polarization drives GC Tfh cells to express T-bet and IFN-γ (Johnston et al., 2009; Yusuf et al., 2010). It is also possible that malaria induces early Tfh cells that fail to mature into GC Tfh cells, a phenomenon that appears to occur during Salmonella infections that are characterized by high bacterial loads and excessive Th1 responses (Cunningham et al., 2007). Consistent with this, recent work has shown that excessive IFN-γ sharply limits Tfh activity during experimental malaria (Zander et al., 2015). It will be of interest to further test these hypotheses in mouse models of malaria, and to elucidate the lineage, antigen specificity and in vivo function of Tfh subsets during Plasmodium infection.
The most clinically advanced malaria vaccine candidate, RTS,S, targets the circumsporozoite protein (CSP) on the surface of sporozoites—the parasite stage transmitted from mosquitos to humans (Regules et al., 2011). RTS, S induces relatively short-lived CSP-specific antibody responses (Alonso et al., 2005; Riley and Stewart, 2013) and confers only partial, short-term protection from malaria in children (Rts, 2014). In light of our findings here, it is noteworthy that RTS,S induces Th1-skewed CS-specific CD4+ T cells in children residing in malaria-endemic areas (White et al., 2013). This raises the possibility that RTS,S activates the less functional CXCR3+ Th1 cell-type Tfh cells resulting in short-lived antibody responses. Going forward it will be critical in malaria vaccine trials to assess the phenotype and function of circulating Tfh cells induced by vaccination and how these parameters relate to antibody responses and clinical outcomes.
In summary, we demonstrate that circulating memory CXCR3+ Th1 cell-type Tfh cells in children exhibit impaired B cell help and are preferentially activated during acute malaria. These data provide important insights into the cellular and molecular basis of suboptimal antibody responses to malaria in children and establish a link between the quality of Tfh cell responses and the outcome of B cell germinal center responses during a natural infection. These findings also suggest that vaccine strategies that promote CXCR3− Tfh cell responses may improve malaria vaccine efficacy in children.
EXPERIMENTAL PROCEDURES
Human samples
The Ethics Committee of the Faculty of Medicine, Pharmacy and Dentistry at the University of Sciences, Technique and Technology of Bamako, and the Institutional Review Board of NIAID/NIH approved this study. Informed consent was obtained from parents or guardians of participating children. The field study, described in detail in Supplemental Experimental Procedures and elsewhere (Tran et al., 2013), was conducted in Mali where intense P. falciparum transmission occurs from June through December each year. Blood samples were obtained from children at their healthy uninfected baseline before the malaria season, as well as during and 7 days after treatment of their first acute malaria episode of the ensuing 6-month malaria season. Detection of P. falciparum infection and processing of blood samples is described in Supplemental Experimental Procedures.
Flow cytometry and cell sorting
See Supplemental Experimental Procedures for detailed descriptions of cell sorting and surface and intracellular staining of cells. Antibodies used for cell staining and sorting are described in Table S2.
Co-culture of B and T cell subsets
PBMCs were FACS-sorted into PD-1+CXCR3+CXCR5+CD4+ T cells, PD-1+CXCR3−CXCR5+CD4+ T cells, CD4−CD19+CD21+CD27− naïve B cells and CD4− CD19+CD21+CD27+ MBCs. Each T cell subset (2.5 × 104) was co-cultured with naïve B cells (2.5 × 104) for 12 days or MBCs (2.5 × 104) for 6 days in complete medium with staphylococcal enterotoxin B (SEB) (1.5µg/ml; Sigma-Aldrich). After co-culture, B cell number, phenotype and Ig levels in supernatants were assessed. See Supplemental Experimental Procedures for details.
PBMC stimulation with P. falciparum-infected RBC lysate and cytokine measurement
PBMCs were cultured with naïve red blood cells (nRBCs) or P. falciparum-infected red blood cells (iRBCs). After 5 days in culture PBMCs were analyzed by flow cytometry. Cytokines in plasma and supernatants of stimulated PBMCs were quantified by Luminex. See Supplemental Experimental Procedures for details.
Antibody profiling
As described in Supplemental Experimental Procedures and elsewhere (Davies et al., 2005), protein microarrays (Antigen Discovery Inc., Irvine, CA) containing 1087 sequence-verified P. falciparum polypeptides (RTS 100 Escherichia coli HY kits; Roche) were used to profile P. falciparum-specific IgG responses in plasma.
Statistical analysis
Continuous data were compared using the paired or unpaired Student's T-test and ANOVA. Bonferroni adjustments (T-tests) and Sidak adjustments (ANOVA) were applied to correct for multiple comparisons where appropriate. Correlations were calculated with Pearson correlation coefficient and their significance was determined using Fisher’s Z-test. All analyses were performed in Prism 6.0e (GraphPad Software) or R 3.1.2 (R-Core-Team, 2013). See Supplemental Experimental Procedures for a detailed description of protein microarray data analysis.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the residents of Kalifabougou, Mali for participating in this study. The study was funded by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. For assistance with images we thank Ethan Tyler (Medical Arts Design Section, National Institutes of Health). Protein microarray experiments were funded by NIAID grants U19AI089686 and R01AI095916 (P.L.F.). P.L.F. has an equity interest in Antigen Discovery, Inc., which is developing products related to the protein microarray platform used in this study. In addition, P.L.F serves on the advisory board of ADI and receives compensation for these services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
N.O. contributed to the study design, performed, and/or contributed critically to all experiments, analyzed the data, and co-wrote the manuscript. N.O. was assisted by S.P., T.M.T., T.B.Y. and S.L. in some experiments. T.M.T. and J.S. contributed to data analysis. A.J. performed protein microarray experiments and was supervised by P.L.F. O.K.D., K.K., A.O., B.T. and P.D.C. designed, organized and conducted the field studies in Mali that generated the clinical data and blood samples. P.D.C. drove the study design, analyzed the data and wrote the manuscript with N.O. All authors read and approved the manuscript.
The terms of this arrangement have been reviewed and approved by the University of California in accordance with its conflict of interest policies. No other author declares a conflict of interest.
REFERENCES
- Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, Ma J, Tezuka K, Yagita H, Okumura K. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. Journal of immunology. 2005;175:2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Aide P, Sigauque B, Milman J, Mandomando I, Bassat Q, et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366:2012–2018. doi: 10.1016/S0140-6736(05)67669-6. [DOI] [PubMed] [Google Scholar]
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. 2013;5:176ra132. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2011;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D, Sallusto F, Tangye SG, Mackay CR. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol. 2011;186:5556–5568. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- Crompton PD, Kayala MA, Traore B, Kayentao K, Ongoiba A, Weiss GE, Molina DM, Burk CR, Waisberg M, Jasinskas A, et al. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci U S A. 2010;107:6958–6963. doi: 10.1073/pnas.1001323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton PD, Moebius J, Portugal S, Waisberg M, Hart G, Garver LS, Miller LH, Barillas C, Pierce SK. Malaria Immunity in Man and Mosquito: Insights into Unsolved Mysteries of a Deadly Infectious Disease. Annual Review of Immunology. 2014;32 doi: 10.1146/annurev-immunol-032713-120220. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. T Follicular Helper Cell Differentiation, Function, and Roles in Disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G, Jr, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med. 2013;19:494–499. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AF, Gaspal F, Serre K, Mohr E, Henderson IR, Scott-Tucker A, Kenny SM, Khan M, Toellner KM, Lane PJ, MacLennan IC. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J Immunol. 2007;178:6200–6207. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- Davies DH, Liang X, Hernandez JE, Randall A, Hirst S, Mu Y, Romero KM, Nguyen TT, Kalantari-Dehaghi M, Crotty S, et al. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc Natl Acad Sci U S A. 2005;102:547–552. doi: 10.1073/pnas.0408782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale JS, Youngblood B, Latner DR, Mohammed AU, Ye L, Akondy RS, Wu T, Iyer SS, Ahmed R. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38:805–817. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, Sun X, Vandenberg K, Rockman S, Ding Y, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano M, Moriyama S, Ando Y, Hikida M, Mori Y, Kurosaki T, Okada T. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34:961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, Crotty S. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol. 2012;188:3734–3744. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, et al. Human circulating PD-1+CXCR3−CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Deenick EK. Human T follicular helper (Tfh) cells and disease. Immunology and cell biology. 2014;92:64–71. doi: 10.1038/icb.2013.55. [DOI] [PubMed] [Google Scholar]
- Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp Med. 2012;209:1241–1253. doi: 10.1084/jem.20120994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R, Schmitt N, Bentebibel S-E, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, et al. Human Blood CXCR5+CD4+ T Cells Are Counterparts of T Follicular Cells and Contain Specific Subsets that Differentially Support Antibody Secretion. Immunity. 2011a;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011b;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Mazliah D, Langhorne J. CD4 T-cell subsets in Malaria: TH1/TH2 revisited. Frontiers in Immunology. 2014;5:671. doi: 10.3389/fimmu.2014.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Mazliah D, Langhorne J. CD4 T-cell subsets in Malaria: TH1/TH2 revisited. Frontiers in Immunology. 2015;5 doi: 10.3389/fimmu.2014.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Mazliah D, Ng DH, Freitas do Rosario AP, McLaughlin S, Mastelic-Gavillet B, Sodenkamp J, Kushinga G, Langhorne J. Disruption of IL-21 signaling affects T cell-B cell interactions and abrogates protective humoral immunity to malaria. PLoS Pathog. 2015;11:e1004715. doi: 10.1371/journal.ppat.1004715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal S, Pierce SK, Crompton PD. Young lives lost as B cells falter: what we are learning about antibody responses in malaria. J Immunol. 2013;190:3039–3046. doi: 10.4049/jimmunol.1203067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R-Core-Team. R: A Language and Environment for Statistical Computing. 2013 [Google Scholar]
- Rappuoli R, Aderem A. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature. 2011;473:463–469. doi: 10.1038/nature10124. [DOI] [PubMed] [Google Scholar]
- Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- Regules JA, Cummings JF, Ockenhouse CF. The RTS,S vaccine candidate for malaria. Expert Rev Vaccines. 2011;10:589–599. doi: 10.1586/erv.11.57. [DOI] [PubMed] [Google Scholar]
- Riley EM, Stewart VA. Immune mechanisms in malaria: new insights in vaccine development. Nat Med. 2013;19:168–178. doi: 10.1038/nm.3083. [DOI] [PubMed] [Google Scholar]
- Rts, S.C.T.P. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 2014;11:e1001685. doi: 10.1371/journal.pmed.1001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt N, Ueno H. Blood Tfh cells come with colors. Immunity. 2013;39:629–630. doi: 10.1016/j.immuni.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman Z, Gitlin AD, Targ S, Jankovic M, Pasqual G, Nussenzweig MC, Victora GD. T follicular helper cell dynamics in germinal centers. Science. 2013;341:673–677. doi: 10.1126/science.1241680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisic DI, Barry AE, Good MF. Escaping the immune system: How the malaria parasite makes vaccine development a challenge. Trends Parasitol. 2013;29:612–622. doi: 10.1016/j.pt.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Tarlinton D, Good-Jacobson K. Diversity among memory B cells: origin, consequences, and utility. Science. 2013;341:1205–1211. doi: 10.1126/science.1241146. [DOI] [PubMed] [Google Scholar]
- Tran TM, Li S, Doumbo S, Doumtabe D, Huang CY, Dia S, Bathily A, Sangala J, Kone Y, Traore A, et al. An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin Infect Dis. 2013;57:40–47. doi: 10.1093/cid/cit174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- W.H.O. WHO Press; 2014. World Malaria Report, 2014. [Google Scholar]
- White MT, Bejon P, Olotu A, Griffin JT, Riley EM, Kester KE, Ockenhouse CF, Ghani AC. The relationship between RTS,S vaccine-induced antibodies, CD4(+) T cell responses and protection against Plasmodium falciparum infection. PLoS One. 2013;8:e61395. doi: 10.1371/journal.pone.0061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Li X, Liu D, Li J, Zhang X, Chen X, Hou S, Peng L, Xu C, Liu W, et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496:523–527. doi: 10.1038/nature12058. [DOI] [PubMed] [Google Scholar]
- Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, Barnett B, Crotty S. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150) J Immunol. 2010;185:190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander RA, Obeng-Adjei N, Guthmiller JJ, Kulu DI, Li J, Ongoiba A, Traore B, Crompton PD, Butler NS. PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity. Cell host & microbe. 2015 doi: 10.1016/j.chom.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotos D, Coquet JM, Zhang Y, Light A, D'Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.