Salivary varicella zoster virus (VZV) DNA, a surrogate marker of active VZV infection, is diagnostically useful when VZV causes disease without rash. Salivary VZV DNA provides a noninvasive means of identifying enteric zoster in patients with unexplained abdominal pain.
Keywords: abdominal pain, bleeding peptic ulcer, enteric infections, enteric neurons, enteric nervous system
Abstract
Background. Varicella zoster virus (VZV) establishes latency in dorsal root, cranial nerve, and enteric ganglia and can reactivate to cause zoster. Serious gastrointestinal dysfunction can result from VZV reactivation in enteric neurons (enteric zoster), but an absence of rash makes diagnosis difficult. We thus determined whether detecting VZV DNA in saliva facilitates identification of enteric zoster.
Methods. Nested and real-time polymerase chain reaction were used to validate salivary VZV DNA as a surrogate marker of VZV reactivation and then to determine the utility of that marker for the identification of those individuals within a population defined by abdominal pain that might have enteric zoster.
Results. Salivary VZV DNA was detected in 0 of 20 healthy negative controls, 11 of 16 positive controls with zoster or varicella (P < .0001), 2 of 2 patients with zoster sine herpete (P < .01), 6 of 11 patients with unexplained abdominal pain (P < .001), and 0 of 8 patients with unrelated gastrointestinal disorders. Salivary VZV DNA disappeared after recovery in 9 of 9 tested subjects with zoster, 2 of 2 with zoster sine herpete, and 5 of 5 with abdominal pain. One patient with abdominal pain and salivary VZV DNA had perforated gastric ulcers, necessitating a wedge gastrectomy. VZV DNA (vaccine type) was found in the resected stomach; immediate early (ORF63p) and late (gE) VZV proteins were immunocytochemically detected in gastric epithelium. After recovery, VZV DNA and proteins were not detected in gastric biopsies or saliva.
Conclusions. Detection of salivary VZV DNA in patients with abdominal pain helps to identify putative enteric zoster for investigation and treatment.
Varicella zoster virus (VZV) is latent in neurons of the enteric nervous system (ENS) in most people who have experienced varicella or received the live attenuated varicella vaccine (vOka) [1]. Given the predilection of latent VZV in dorsal root ganglia and cranial nerve ganglia to reactivate and cause zoster, latent VZV is also likely to reactivate in the ENS to give rise to gastrointestinal (GI) dysfunction. VZV, however, is rarely suspected to cause GI disease. When patients present with the characteristic vesicular rash, varicella (chickenpox) or zoster (shingles) is suspected and thus readily diagnosed. In contrast, zoster sine herpete [2], meningitis [3], and enteric zoster [4] are difficult to diagnose because they can occur without cutaneous manifestations. Enteric zoster has been diagnosed, most often in immunocompromised patients, when abdominal pain accompanies or precedes the appearance of a zosteriform rash [4–6]. When enteric zoster causes abdominal pain and/or GI dysfunction without cutaneous manifestations [7, 8], however, enteric zoster may not be diagnosed until tissue is examined following surgery for GI obstruction or bleeding [4]. A noninvasive method is clearly needed to diagnose or suggest enteric zoster when a rash is absent.
The stress associated with space travel has been presumed to cause subclinical reactivations of herpesviruses because DNA from VZV [9], Epstein-Barr virus, and cytomegalovirus [10, 11] has been discovered in the saliva of asymptomatic astronauts during or after space travel. Since its discovery in astronauts, salivary VZV DNA has consistently been found in patients with clinical zoster [12] but not in healthy subjects [13]. These observations suggest that salivary VZV DNA is a biomarker of active VZV infection, whether or not there is a cutaneous accompaniment. The current investigation was undertaken to test the hypothesis that VZV DNA in saliva can be used to identify patients with gastroenterologically unexplained GI symptoms who are likely to have enteric zoster.
METHODS
Patients
Columbia University's Institutional Review Board approved this investigation. Saliva was obtained from 5 groups of subjects. The first group, healthy volunteers, was a negative control. The second group of patients with a characteristic rash clinically diagnosed as varicella or zoster served as a positive control; polymerase chain reaction (PCR) detected VZV DNA in cutaneous lesions. Saliva was collected during and after acute infection. Postrecovery saliva served as each patient's internal control. The third group consisted of patients with zoster sine herpete; saliva was collected both during the symptomatic illness and after recovery. The fourth group, candidates for enteric zoster, were referrals from a gastroenterologist for unexplained GI illness with moderate to severe abdominal pain; saliva was collected while symptoms were present and after recovery. Severe bleeding from perforated gastric ulcers in 1 patient necessitated surgical intervention. The affected bowel contained VZV DNA, transcripts, and protein. The fifth group consisted of patients with a long-standing GI disorder unrelated to VZV (see Supplementary materials for additional details).
Collection and Processing of Specimens
Saliva was collected with OriGene-Discover kits (Ottawa, Canada) or on swabs placed in the mouth for approximately 3 minutes. Swabs were subsequently soaked in sterile water and stored in buffer (50 mM Tris, pH 8.0, 1 mM ethylenediaminetetraacetic acid, 0.1% sodium azide) at 4°C. DNA was extracted with DNeasy Blood and Tissue Kit (Qiagen, Valencia, California).
PCR Analysis of VZV DNA
Four methods were employed to distinguish wild-type (WT) from vaccine-type (vOka) VZV DNA: (1) Differences between circulating US/UK WT VZV and the Asian-derived (clade 2) vOka were used to screen samples [14]. PCR amplified, in gene 54, a 222-bp region that includes a BglI restriction site and, in gene 38, a 350-bp region that contains a PstI restriction site. The PstI restriction site is present in circulating Western strains but not in vOka. In contrast, vOka contains a BglI restriction site, which is present only in approximately 20% of Western strains. (2) To confirm vOka, a region containing a vOka-unique SmaI restriction site in open reading frame (ORF) 62 was amplified to yield a specific 268-bp amplicon [15–17]. Digestion of WT strains, which contain 2 SmaI restriction sites, produces 153-, 79-, and 36-bp fragment sets, whereas vOka, which contains 3 SmaI restriction sites, produces 112-, 79-, 41-, and 36-bp fragment sets. For final confirmation, the 268-bp amplicon was sequenced. WT VZV has a thymine at 106262, whereas vOka has a cytosine [18]. (3) Nested PCR was used to analyze ORFs 29, 31, 67, 40, or 68 in saliva where the abundance of VZV DNA is low [1]. The sensitivity of this assay, determined by serial dilution of plasmid-derived fragments, was 1–2 copies. (4) Real-time PCR was used to confirm nested PCR results. The single-nucleotide polymorphism (SNP) at 106262 of ORF62 was analyzed; sensitivity was 2–4 copies (see Supplementary materials for additional details).
Immunocytochemistry
Formalin-fixed, paraffin-embedded sections were exposed, after antigen retrieval at 100°C in citrate buffer (pH 6.0), to rabbit ORF62p antibodies [19] and monoclonal glycoprotein E (gE) antibodies (Virusys Corp, Taneytown, Maryland). Primary antibodies were omitted as a control. Alexa 488- and 594-conjugated secondary antibodies (against rabbit or mouse immunoglobulin G) (Molecular Probes, Eugene, Oregon) were used to visualize immunoreactivity. Nuclei were stained with bisbenzimide (Sigma-Aldrich, St Louis, Missouri) (see Supplementary materials for additional details).
RESULTS
Saliva from 54 individuals was studied. Salivary VZV DNA was not detected in any of the 20 individuals in group 1 (the negative controls), who manifested neither pain nor rash. Salivary VZV DNA was detected in 11 of 16 subjects in group 2 (the positive controls), with clinically diagnosed zoster (n = 15) or varicella (n = 1) (Figure 1A; 69%; P < .0001 vs control). PCR confirmation from skin samples verified the diagnosis in 12 of 12 (4 were not tested). Importantly, 9 of 9 convalescent (postrecovery) samples of saliva, obtained from subjects in whom VZV DNA had been detected during the acute illness, were clear of VZV DNA (Table 1). The presence of salivary VZV DNA was thus limited to the symptomatic phase of varicella or zoster. Neither of the 2 patients in group 3 (zoster sine herpete), manifested rash, GI symptoms, or abdominal pain; however, both had severe unilateral cutaneous pain and hyperesthesia in a dermatomal distribution. Salivary VZV DNA, collected while pain was present, was detected in 2 of 2 patients (P < .01 vs control; Figure 1B) and disappeared in each person after recovery (Table 2). The 11 subjects in group 4, possible enteric zoster, were referred by gastroenterologists because moderate to severe abdominal pain up to 4 months' duration could not be explained; none had a rash. Salivary VZV DNA was detected in 6 of 11 (Figure 1C; 55%; P < .001 vs control; Table 2, patients 3–8). Three patients recovered after treatment with valacyclovir, and 3 recovered without treatment. Importantly, salivary VZV DNA was no longer present in 5 of 5 samples obtained after abdominal pain disappeared. The 8 patients in group 5 had pain from a chronic GI disease unrelated to VZV. Five had gastroesophageal reflux, 2 had idiopathic gastroparesis, and 1 had chronic intestinal pseudo-obstruction. Salivary VZV DNA was detected in 0 of 8.
Figure 1.
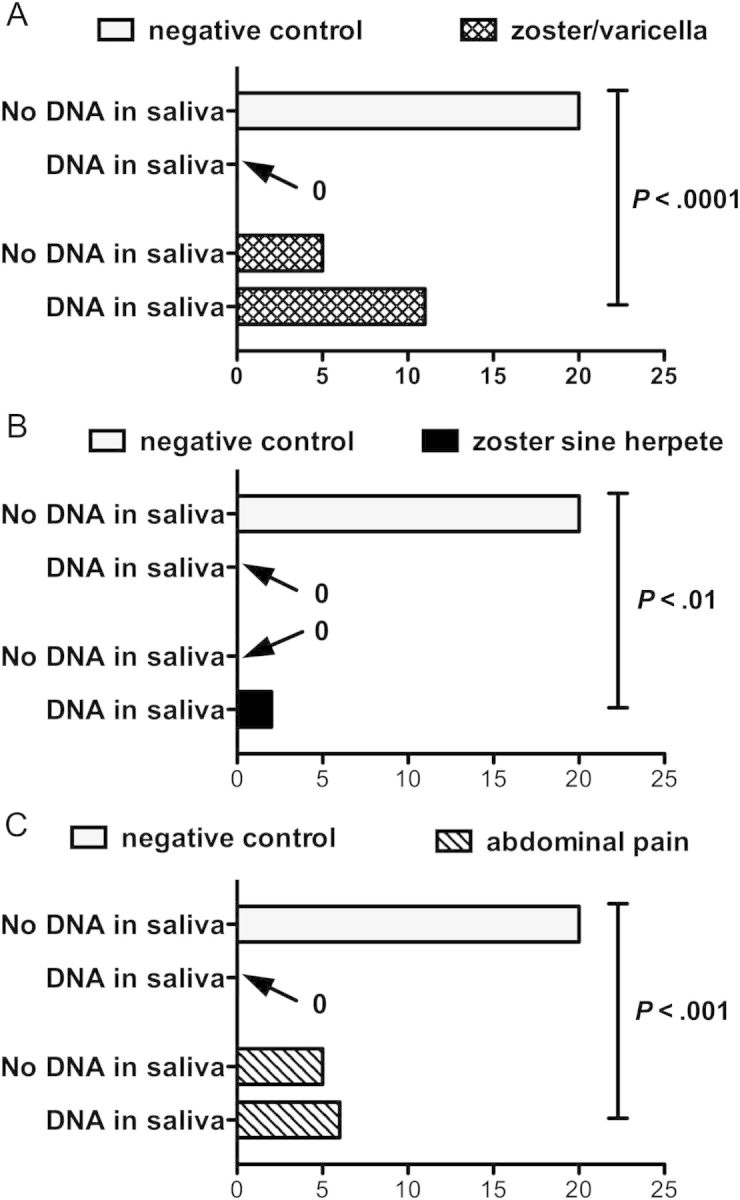
Frequency of salivary varicella zoster virus (VZV) DNA in saliva of negative control (healthy) subjects, positive control (zoster/varicella) subjects, patients with zoster sine herpete, and patients with unexplained abdominal pain (without rash). A χ2 analysis of the overall distribution (zoster/varicella and zoster sine herpete groups combined) was significantly different from chance (P < .0001). A, The difference in frequency between the detection of salivary VZV DNA in the positive control population is significantly greater than that in the negative control population. Fisher exact test. B, The difference in frequency between the detection of salivary VZV DNA in patients with zoster sine herpete is significantly greater than that in the negative control population. Fisher exact test. C, The difference in frequency between the detection of salivary VZV DNA in the group of patients with unexplained abdominal pain is significantly greater than that in the negative control population. Fisher exact test.
Table 1.
Varicella Zoster Virus DNA in Saliva: Otherwise Healthy Patients With Varicella or Zoster
| Patient Information |
VZV DNA Skin | VZV DNA Acute Saliva | VZV DNA Convalescent Saliva | ||
|---|---|---|---|---|---|
| Sex | Age, y | Description | |||
| Female | 6 | Rash left arm, axilla | Positive (vOka) | Negative | Not tested |
| Female | 17 | Lymphoma; unilateral rash | Positive | Negative | Not tested |
| Male | 23 | Rash on left back and shoulder | Not tested | Positive | Not tested |
| Female | 35 | Painful, faint rash on left thorax | Positive | Positive | Negative |
| Female | 40 | Unilateral rash on face | Positive (WT) | Positive | Not tested |
| Female | 43 | Itchy rash on left back | Positive | Negative | Negative |
| Female | 43 | Painful rash on left chest | Positive | Positive | Negative |
| Male | 47 | Painful rash on left cheek near eye; severe headache | Positive | Positive | Negative |
| Malea | 50 | Nurse with varicella; generalized vesicular rash, fever | Positive (WT) | Positive (WT) | Not tested |
| Femaleb | 50 | Painful rash left neck | Positive (WT) | Negative | Not tested |
| Female | 55 | Painful red maculopapular rash on left back and shoulder | Not tested; | Positive | Negative |
| Maleb | 60 | Painful rash left arm | Positive (WT) | Negative | Not tested |
| Male | 60 | Rash left axilla and back | Positive | Positive | Negative |
| Female | 61 | No skin rash, but vesicles in larynx | Not tested | Positive | Negative |
| Female | 71 | 5 small vesicles left wrist | Positive (WT) | Positive (WT) | Negative |
| Male | 88 | Rash on left forehead | Not tested | Positive | Negative |
Rows in italics refer to a cluster of subjects working in a varicella zoster laboratory.
Abbreviations: vOka, live attenuated strain of varicella zoster virus; VZV, varicella zoster virus; WT, wild type.
a Varicella.
b Asian immigrants to United States, VZV clade 2 [20].
Table 2.
Varicella Zoster Virus DNA in Saliva: Patients Without Skin Rash
| Patient Information |
VZV DNA Acute Saliva | VZV DNA Convalescent Saliva | ||
|---|---|---|---|---|
| Sex | Age | Description | ||
| Male | 16 | Severe pain, left chest, torso zoster sine herpete | Positive | Negative |
| Male | 72 | Severe pain right chest, back, shoulder zoster sine herpete | Positive | Negative |
| Male | 6 | Severe abdominal pain-response to valacyclovir | Positive (WT) | Not tested |
| Malea | 16 | Vaccinated—abdominal pain, GI bleeding, and perforated gastric ulcer | Positive (vOka) | Negative |
| Male | 27 | Severe abdominal pain | Positive | Negative |
| Female | 30 | Severe abdominal pain; Helicobacter pylori negative; response to valacyclovir | Positive | Negative |
| Female | 55 | Severe abdominal pain | Positive | Negative |
| Female | 60 | Severe abdominal pain; H. pylori negative; response to valacyclovir | Positive | Negative |
Patients noted in italics work in a VZV laboratory.
Rows in italics refer to a cluster of subjects working in a varicella zoster laboratory.
Abbreviations: GI, gastrointestinal; vOka, live attenuated strain of varicella zoster virus; VZV, varicella zoster virus; WT, wild type.
a Patient in case report.
CASE HISTORY
The case of 1 patient from group 4, which is particularly noteworthy because it demonstrates that vOka can cause enteric zoster, has been reported to the Worldwide Adverse Experience System and to the US Food and Drug Administration. This subject was a 16-year-old African American male with a history of asthma and obesity who suddenly experienced severe epigastric abdominal pain, lost consciousness, and was hospitalized. An emergency laparotomy was undertaken after abdominal radiography revealed free air under the diaphragm. Multiple necrotic ulcers were found in the gastric fundus. A wedge gastrectomy was performed to remove the affected stomach along with an adjoining necrotic portion of greater omentum. Antibodies to neither Helicobacter pylori nor herpes simplex virus were detected in serum, and the gastrin level was normal. Immunoglobulin levels and numbers of circulating CD4, CD8, and natural killer cells were also normal. Abdominal pain and a fever persisted after surgery. Respiratory failure requiring intubation occurred and aspiration pneumonia was identified. Systemic bacterial infection was suspected; nevertheless, despite multiple cultures, no pathogens were identified. Medications administered during the patient's month-long hospitalization included vancomycin, ciprofloxacin, metronidazole, and fluconazole. At no time did the patient manifest a rash.
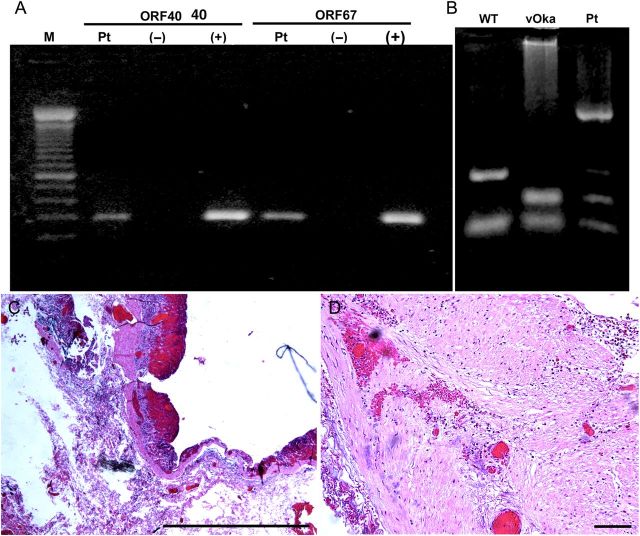
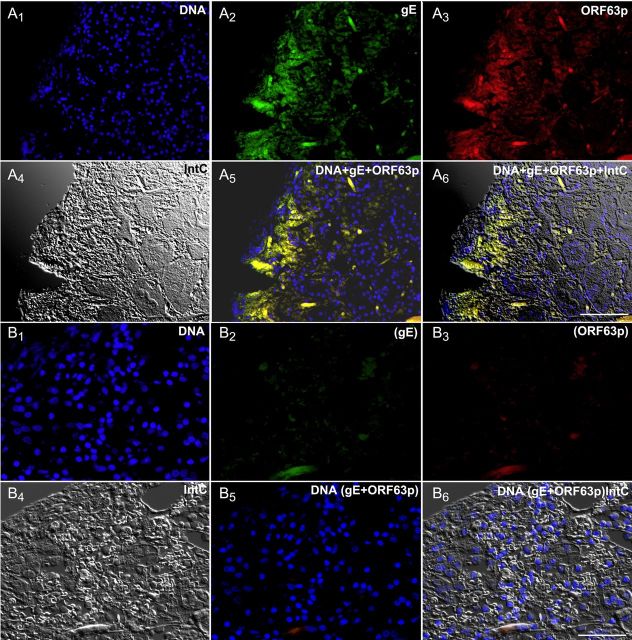
The patient's saliva, analyzed 2 weeks after the onset of illness, was found to contain DNA encoding VZV ORFs 40 and 67 (Figure 2A). VZV DNA was then found to be present in the resected stomach (Figure 2B). Sma1 restriction analysis and sequencing of position 106262 of ORF62 identified the viral DNA vOka-derived [17]. Gastric mucosal ulceration, hemorrhage, and inflammation were found microscopically (Figure 2C). Intact neurons were not encountered in the expected region of the myenteric plexus (Figure 2D), despite examination of multiple sections and immunostaining with antibodies to the neuronal marker PGP9.5 (data not shown). A mononuclear cell infiltrate and areas of hemorrhage were present between muscle bundles. Many ORF63p- and gE-immunoreactive cells were present in the epithelium of gastric glands and lamina propria adjacent to mucosal ulcers. ORF63p and gE immunoreactivities were coexpressed (Figure 3A1–A6). No immunoreactivity was found when antibodies to gE or ORF63p were omitted (Figure 3B1–B6). The patient recovered without antiviral therapy. At the time of discharge, VZV DNA could no longer be detected in saliva and the patient was free of abdominal pain; nevertheless, difficulty swallowing, diagnosed clinically as achalasia, remained. Further diagnostic testing was refused. Saliva was free of VZV DNA 3 weeks after discharge, when the gastric mucosa was found endoscopically to be normal and mucosal biopsies contained no detectable VZV DNA; moreover, immunocytochemical examination of biopsied tissue revealed no immunoreactivities of gE or ORF63p (Figure 4A1–A6).
Figure 2.
Varicella zoster virus (VZV) infection was detected in saliva and resected stomach from a patient with abdominal pain, bleeding, and perforated gastric ulcer. A, DNA from saliva amplified with polymerase chain reaction. DNA encoding open reading frame (ORF) 40 and ORF67 was amplified in the patient's saliva (Pt) and in the positive control DNA prepared from a plasmid (+) but not in the negative control (−). B, The genotype of the viral DNA from resected stomach is that of vOka. DNA was digested with the Pst1 restriction endonuclease. C, Low-power micrograph showing the ulcerated mucosa of the patient's resected stomach. This is the region of the stomach from which DNA was extracted. Hematoxylin and eosin (H&E) stain; scale bar = 100 µm. D, Muscularis of the stomach. The myenteric plexus is not evident. H&E stain; scale bar = 100 µm. Abbreviations: M, size marker; Pt, DNA extracted from the resected stomach of the patient; vOka, DNA extracted from cells infected with vOka; WT, DNA extracted from cells infected with wild-type VZV (Dumas).
Figure 3.
The immunoreactivity of the varicella zoster virus proteins glycoprotein E (gE) and open reading frame (ORF) 63p are present and can be detected immunocytochemically in the mucosa of the resected stomach. A1–6, The same field is shown in all 6 panels, illuminated to demonstrate the fluorescence of DNA (A1), gE (A2), and ORF63 (A3), as well as an interference contrast image of the mucosa (A4), the merged image of DNA + gE + ORF63 (A5), and the merged fluorescence and interference contrast images (A6). Note that the immunofluorescence of gE and ORF63 are coincident in the mucosa and are found in epithelial cells, underlying stromal cells of the lamina propria, and some mucosal blood vessels (linear structures). B, Control immunoreactivity. The primary antibodies to gE and ORF63p were omitted, but the sections were processed as in A. B1–6. Again, a single field is illustrated in all 6 panels, illuminated to demonstrate the fluorescence of DNA (B1), gE (B2), ORF63 (B3), an interference contrast image (B4), the merged image of DNA + gE + ORF63 (B5), and the merged fluorescence and interference contrast images (B6). No green or red fluorescence can be detected. Scale bars = 50 µm.
Figure 4.
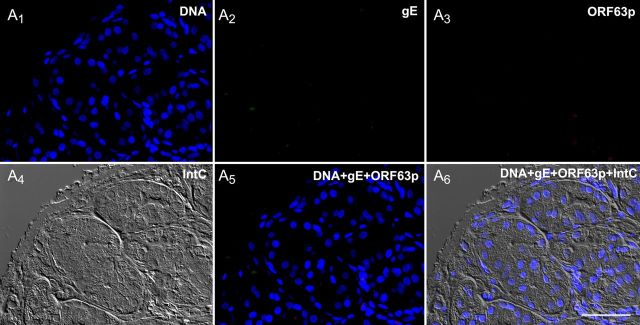
An endoscopic biopsy of the gastric mucosa obtained after the recovery of the patient following gastric surgery. The mucosa is now normal and no immunoreactivity of glycoprotein E (gE) or open reading frame (ORF) 63p can be detected. A1–6. The same field is illustrated in all 6 panels, illuminated to demonstrate the fluorescence of DNA (A1), gE (A2), ORF63 (A3), an interference contrast image (A4), the merged image of DNA + gE + ORF63 (A5), and the merged fluorescence and interference contrast images (A6). The structure of the mucosa has been repaired. No ulceration, inflammation, or varicella zoster virus immunoreactivity can any longer be detected. Scale bar = 50 µm.
DISCUSSION
The current study supports the utility of salivary VZV DNA as an indicator of enteric zoster. We first confirmed that our methods did not detect VZV DNA in the saliva of healthy, nonstressed, asymptomatic adults (0/20). In contrast, we detected VZV DNA in the saliva of 11 of 16 subjects clinically diagnosed with zoster or varicella. The difference in salivary VZV DNA between the positive and negative control populations was highly significant (P < .0001). VZV also disappeared from saliva upon recovery in 9 of 9 positive control subjects from whom convalescent samples were obtained. Salivary VZV DNA has been reported in a numerically greater, but not significantly different, proportion of cases of varicella [21] than found in our study, predominantly of zoster. VZV DNA may be more readily detected in vesicles than in saliva; nevertheless, when skin lesions are absent, salivary VZV DNA may be required to confirm VZV infection [22]. Salivary VZV DNA and its disappearance after recovery, for example, provided our only laboratory confirmation of zoster sine herpete.
We used persistent abdominal pain, which was unexplained by a gastroenterological workup, to screen subjects for possible enteric zoster. VZV DNA was present in the saliva of 6 of 11 (55%) subjects, which differed significantly from the negative control population (0/20; P < .0001). Salivary VZV DNA was no longer detectable in any of 5 individuals after pain disappeared, either in apparent response to valacyclovir (3/5) or spontaneously (2/5). These observations are consistent with the hypothesis that salivary VZV DNA helps detect enteric zoster and monitor its therapy. In fact, given the nonspecific nature of unexplained abdominal pain, the proportion of subjects with salivary VZV DNA is surprisingly large. No salivary VZV DNA was detected in subjects with gastroesophageal reflux or other chronic GI disorders, suggesting that the stress of GI dysfunction is not, by itself, sufficient to cause VZV DNA to appear in saliva.
We conclude that salivary VZV DNA is a surrogate marker of active VZV infection, which can, in the presence of abdominal pain, suggest enteric zoster. The internal nature of enteric zoster, in which cutaneous manifestations are absent, would otherwise necessitate an invasive procedure to obtain tissue for diagnosis. VZV reactivation as a cause of disease without rash was, until recently, thought to be rare or nonexistent. It is now clear that this is not the case [2, 23]; meningitis, myelitis, and zoster sine herpete are examples [24]. VZV establishes latency in nodose [25], celiac [25], and enteric neurons [26], which project to the gut but not to the skin. Subsets of each innervate the mucosa [27]; therefore, reactivation of VZV in these enteric-projecting neurons would be expected, because of axonal transport, to deliver infectious VZV to the bowel and the mucosal epithelium [27]. VZV-induced mucosal damage would be expected to cause GI ulceration, bleeding, and pain. Even if a reactivated enteric VZV infection were to be confined to enteric ganglia, the resulting neuronal dysfunction would disturb GI motility/secretion, leading to intestinal distension and abdominal pain.
Saliva, in which DNA is abundant, can be obtained easily and noninvasively. The appearance of VZV DNA in the saliva of highly stressed individuals, such as astronauts [9] or hospitalized children in intensive care [28], occurred in the absence of overt disease and was, therefore, due to subclinical reactivation of VZV. Moreover, the appearance of VZV DNA in saliva of patients with obvious zoster supports the idea that salivary VZV DNA accurately reflects the presence of active VZV infection [12, 22]. A genomic study showed that in patients with varicella, saliva and skin contained identical VZV clades, suggesting that salivary VZV DNA was derived from the rash-causing virus [21]. If salivary VZV DNA is due to a subclinical reactivation that boosts immunity, its detection may clinically be inconsequential. In contrast, if salivary VZV DNA reveals that a symptomatic condition is due to VZV, as in patients with unexplained abdominal pain and GI dysfunction, its detection may allow antiviral treatment to be initiated in time to provide relief.
The nearly catastrophic case of a 16-year-old boy illustrates the utility of testing saliva to detect enteric zoster. The patient experienced not just abdominal pain, but also gastric ulceration and perforation, necessitating a wedge gastrectomy. The discovery of salivary VZV DNA was, in fact, what caused the resected gastric tissue to be analyzed for VZV. That analysis subsequently revealed VZV DNA in the stomach, identified the DNA as vOka-derived, and revealed that the mucosal epithelium and lamina propria along ulcer borders contained gE and ORF63p immunoreactivities. Because the individual had previously received 2 doses of varicella vaccine, it is likely that vOka reactivated from latency in enteric neurons. The absence of ganglia in the resected stomach is consistent with this idea and suggests that the neurons in which VZV reactivated did not survive. The patient was left with a residual swallowing problem. In an in vitro guinea pig model, latently infected enteric neurons die 48–72 hours after VZV reactivation [29]. The immunocytochemical detection of both early and late proteins indicated that VZV was proliferating in gastric mucosa at the time of resection and thus was probably the source of the VZV DNA in saliva. After recovery, when bleeding and pain had disappeared, VZV could no longer be found in mucosal biopsies, and salivary VZV DNA also disappeared. Unfortunately, the family did not agree to further testing to identify an underlying immunological deficit, such as natural killer cell function [30–32], that might explain the reactivation of vOka or the severity of enteric zoster.
The vOka strain has previously been reported to cause latency and zoster, albeit at a lower frequency than that of WT VZV [33]. Millions of doses of varicella vaccine have been distributed since its approval in 1995; nevertheless, gastric ulcer has not previously been reported as a complication of vaccination and must therefore be rare. In contrast, zoster and postherpetic neuralgia are commonly observed in patients with gastric ulcer disease [34]; this correlation is consistent with the idea that enteric reactivations of VZV may occur together with, or predispose to, VZV reactivations in dorsal root ganglia or cranial nerve ganglia.
Zoster in 2 subjects, who experienced childhood varicella in China, was due to a clade 2 VZV (WT, not vOka), which is common in Asia but rare in the West. Unexpectedly, 6 of 16 (38%) subjects with zoster in the current study worked in a laboratory where VZV is investigated. The incidence of zoster encountered in this population (100 per 1000 person-years) is very high. The incidence of zoster in the placebo group of a recent study of fingolimod was 6 per 1000 person-years, and that in patients treated with fingolimod was 11 per 1000 person-years [35]. The chance of experiencing zoster has recently been estimated to be about 30% over a lifetime [36]. Because exposure to VZV causes varicella rather than zoster in susceptible individuals, the apparent cluster of zoster in a VZV laboratory is probably due to awareness and availability of diagnostic tools. Zoster may thus be more common than currently supposed and might be diagnosed more frequently if the index of suspicion were higher. The relative ease of detection of salivary VZV DNA may therefore increase identification of treatable patients. It is also possible that asymptomatic reactivation of VZV in the GI tract and occurrences of enteric zoster boost immunity to VZV, thereby facilitating long-term protection against subsequent illness [37, 38].
Still to be determined is whether the presence of VZV DNA in the saliva of patients with abdominal pain is, by itself, sufficient to make a diagnosis of enteric zoster. Severe stress alone may be associated with the appearance of VZV DNA in saliva [9]; conceivably, therefore, the stress of an underlying GI illness might reactivate VZV in the gut or elsewhere. Salivary VZV DNA, however, was not found in patients with chronic GI disorders unrelated to VZV. The observations, moreover, that salivary VZV DNA disappeared in apparent response to valacyclovir in 3 patients and in all patients after their unexplained abdominal pain relented is consistent with the supposition that the abdominal pain resulted from enteric zoster. Although zoster commonly occurs in patients with inflammatory bowel disease, this comorbidity is probably due to the underlying immunological defect and/or immunosuppressive therapy [39]. Endoscopic or surgical confirmation of enteric zoster and its course will be needed in the future; however, the current work suggests that salivary VZV DNA can be used to select patients for treatment and appropriate investigation. Further studies are also needed to determine whether episodes of enteric zoster act as an unsuspected initiator of idiopathic GI disorders, such as postinfectious irritable bowel syndrome, gastroparesis, and intestinal pseudo-obstruction.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank Kara Gross Margolis, MD, Anne Pierog, MD, Christina Gagliardo, MD, and Sruti Nadimpali, MD, for help with patients, and Yan Zhou, Sharon Steinberg, Alexander Diacou, and Dong Wu, PhD, for their laboratory assistance.
Financial support. This work was supported by the National Institutes of Health (grant number DK093094).
Potential conflicts of interest. A. A. G. has received institutional support through the following companies: consulting honorarium from Pfizer, review board activities for GlaxoSmithKline, and a laboratory diagnostic service contract from Merck for safety of VZV vaccines. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Chen J, Gershon AA, Li Z, Cowles RA, Gershon MD. Varicella zoster virus (VZV) infects and establishes latency in enteric neurons. J Neurovirol 2011; 17:578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilden DH, Wright R, Schneck S, Gwaltney JM, Mahalingam R. Zoster sine herpete, a clinical variant. Ann Neurol 1994; 35:530–3. [DOI] [PubMed] [Google Scholar]
- 3.Pahud BA, Glaser CA, Dekker CL, Arvin AM, Schmid DS. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis 2011; 203:316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edelman DA, Antaki F, Basson MD, Salwen WA, Gruber SA, Losanoff JE. Ogilvie syndrome and herpes zoster: case report and review of the literature. J Emerg Med 2009; 39:696–700. [DOI] [PubMed] [Google Scholar]
- 5.Pui JC, Furth EE, Minda J, Montone KT. Demonstration of varicella-zoster virus infection in the muscularis propria and myenteric plexi of the colon in an HIV-positive patient with herpes zoster and small bowel pseudo-obstruction (Ogilvie's syndrome). Am J Gastroenterol 2001; 96:1627–30. [DOI] [PubMed] [Google Scholar]
- 6.Milligan KL, Jain AK, Garrett JS, Knutsen AP. Gastric ulcers due to varicella-zoster reactivation. Pediatrics 2012; 130:e1377–81. [DOI] [PubMed] [Google Scholar]
- 7.Nomdedeu JF, Nomdedeu J, Martino R, et al. Ogilvie's syndrome from disseminated varicella-zoster infection and infarcted celiac ganglia. J Clin Gastroenterol 1995; 20:157–9. [DOI] [PubMed] [Google Scholar]
- 8.Scholl S, Hocke M, Hoffken K, Sayer HG. Acute abdomen by varicella zoster virus induced gastritis after autologous peripheral blood stem cell transplantation in a patient with non-Hodgkin's lymphoma. Acta Haematol 2006; 116:58–61. [DOI] [PubMed] [Google Scholar]
- 9.Mehta SK, Cohrs RJ, Forghani B, Zerbe G, Gilden DH, Pierson DL. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol 2004; 72:174–9. [DOI] [PubMed] [Google Scholar]
- 10.Mehta SK, Stowe RP, Feiveson AH, Tyring SK, Pierson DL. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J Infect Dis 2000; 182:1761–4. [DOI] [PubMed] [Google Scholar]
- 11.Stowe RP, Pierson DL, Barrett AD. Elevated stress hormone levels relate to Epstein-Barr virus reactivation in astronauts. Psychosom Med 2001; 63:891–5. [DOI] [PubMed] [Google Scholar]
- 12.Mehta SK, Tyring SK, Gilden DH, et al. Varicella-zoster virus in the saliva of patients with herpes zoster. J Infect Dis 2008; 197:654–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birlea M, Cohrs RJ, Bos N, Mehta SK, Pierson DL, Gilden D. Search for varicella zoster virus DNA in saliva of healthy individuals aged 20–59 years. J Med Virol 2014; 86:360–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaRussa P, Lungu O, Hardy I, Gershon A, Steinberg S, Silverstein S. Restriction fragment length polymorphism of polymerase chain reaction products from vaccine and wild-type varicella-zoster virus isolates. J Virol 1992; 66:1016–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loparev VN, Argaw T, Krause PR, Takayama M, Schmid DS. Improved identification and differentiation of varicella-zoster virus (VZV) wild-type strains and an attenuated varicella vaccine strain using a VZV open reading frame 62-based PCR. J Clin Microbiol 2000; 38:3156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Argaw T, Cohen J, Klutch M, et al. Nucleotide sequences that distinguish Oka vaccine from parental Oka and other varicella-zoster virus isolates. J Infect Dis 2000; 181:1153–7. [DOI] [PubMed] [Google Scholar]
- 17.Quinlivan ML, Jensen NJ, Radford KW, Schmid DS. Novel genetic variation identified at fixed loci in ORF62 of the Oka varicella vaccine and in a case of vaccine-associated herpes zoster. J Clin Microbiol 2012; 50:1583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker SP, Quinlivan M, Taha Y, Breuer J. Genotyping of varicella-zoster virus and the discrimination of Oka vaccine strains by TaqMan real-time PCR. J Clin Microbiol 2006; 44:3911–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lungu O, Annunziato P, Gershon A, et al. Reactivated and latent varicella-zoster virus in human dorsal root ganglia. Proc Natl Acad Sci U S A 1995; 92:10980–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breuer J, Grose C, Norberg P, Tipples G, Schmid DS. A proposal for a common nomenclature for viral clades that form the species varicella-zoster virus: summary of VZV Nomenclature Meeting 2008, Barts and the London School of Medicine and Dentistry, 24–25 July 2008. J Gen Virol 2010; 91:821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinlivan M, Sengupta N, Papaevangelou V, et al. Use of oral fluid to examine the molecular epidemiology of varicella zoster virus in the United Kingdom and continental europe. J Infect Dis 2013; 207:588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung J, Harpaz R, Baughman AL, et al. Evaluation of laboratory methods for diagnosis of varicella. Clin Infect Dis 2010; 51:23–32. [DOI] [PubMed] [Google Scholar]
- 23.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Neurological disease produced by varicella zoster virus reactivation without rash. Curr Top Microbiol Immunol 2010; 342:243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilden D, Mahalingam R, Nagel MA, Pugazhenthi S, Cohrs RJ. Review: the neurobiology of varicella zoster virus infection. Neuropathol Appl Neurobiol 2011; 37:441–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilden DH, Gesser R, Smith J, et al. Presence of VZV and HSV-1 DNA in human nodose and celiac ganglia. Virus Genes 2001; 23:145–7. [DOI] [PubMed] [Google Scholar]
- 26.Gershon AA, Chen J, Davis L, et al. Latency of varicella zoster virus in dorsal root, cranial, and enteric ganglia in vaccinated children. Trans Am Clin Climatol Assoc 2012; 123:17–33; discussion 33-5. [PMC free article] [PubMed] [Google Scholar]
- 27.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol 2014; 817:39–71. [DOI] [PubMed] [Google Scholar]
- 28.Papaevangelou V, Quinlivan M, Lockwood J, et al. Subclinical VZV reactivation in immunocompetent children hospitalized in the ICU associated with prolonged fever duration. Clin Microbiol Infect 2013; 19:E245–51. [DOI] [PubMed] [Google Scholar]
- 29.Gershon AA, Chen J, Gershon MD. A model of lytic, latent, and reactivating varicella-zoster virus infections in isolated enteric neurons. J Infect Dis 2008; 197(suppl 2):S61–5. [DOI] [PubMed] [Google Scholar]
- 30.Banovic T, Yanilla M, Simmons R, et al. Disseminated varicella infection caused by varicella vaccine strain in a child with low invariant natural killer T cells and diminished CD1d expression. J Infect Dis 2011; 204:1893–901. [DOI] [PubMed] [Google Scholar]
- 31.Levy O, Orange JS, Hibberd P, et al. Disseminated varicella infection due to vaccine (Oka) strain varicella-zoster virus in a patient with a novel deficiency in natural killer cells. J Infect Dis 2003; 188:948–53. [DOI] [PubMed] [Google Scholar]
- 32.Ornstein BW, Hill EB, Geurs TL, French AR. Natural killer cell functional defects in pediatric patients with severe and recurrent herpesvirus infections. J Infect Dis 2013; 207:458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gershon AA, Gershon MD. Pathogenesis and current approaches to control of varicella-zoster virus infections. Clin Microbiol Rev 2013; 26:728–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen JY, Cheng TJ, Chang CY, et al. Increased incidence of herpes zoster in adult patients with peptic ulcer disease: a population-based cohort study. Int J Epidemiol 2013; 42:1873–81. [DOI] [PubMed] [Google Scholar]
- 35.Arvin AM, Wolinsky JS, Kappos L, et al. Varicella-zoster virus infections in patients treated with fingolimod: risk assessment and consensus recommendations for management. JAMA Neurol 2015; 72:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–84. [DOI] [PubMed] [Google Scholar]
- 37.Luby J, Ramirez-Ronda C, Rinner S, Hull A, Vergne-Marini P. A longitudinal study of varicella zoster virus infections in renal transplant recipients. J Infect Dis 1977; 135:659–63. [DOI] [PubMed] [Google Scholar]
- 38.Hope-Simpson RE. The nature of herpes zoster: a long term study and a new hypothesis. Proc R Soc Med 1965; 58:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beutner KR, Friedman DJ, Forszpaniak C, Andersen PL, Wood MJ. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother 1995; 39:1546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.