Human immunodeficiency virus (HIV) infection was associated with greater increases in focal carotid artery plaque over 7 years among both women and men, particularly among those with lower CD4+ counts. Increased plaque was observed even among HIV-infected individuals with persistent virologic suppression.
Keywords: HIV infection, cardiovascular disease, atherosclerosis, intima-media thickness, viral load
Abstract
Background. Individuals infected with human immunodeficiency virus (HIV) live longer as a result of effective treatment, but long-term consequences of infection, treatment, and immunological dysfunction are poorly understood.
Methods. We prospectively examined 1011 women (74% HIV-infected) in the Women's Interagency HIV Study and 811 men (65% HIV-infected) in the Multicenter AIDS Cohort Study who underwent repeated B-mode carotid artery ultrasound imaging in 2004–2013. Outcomes included changes in right common carotid artery intima-media thickness (CCA-IMT) and new focal carotid artery plaque formation (IMT >1.5 mm) over median 7 years. We assessed the association between HIV serostatus and progression of subclinical atherosclerosis, adjusting for demographic, behavioral, and cardiometabolic risk factors.
Results. Unadjusted mean CCA-IMT increased (725 to 752 µm in women, 757 to 790 µm in men), but CCA-IMT progression did not differ by HIV serostatus, either in combined or sex-specific analyses. Focal plaque prevalence increased from 8% to 15% in women and 25% to 34% in men over 7 years. HIV-infected individuals had 1.6-fold greater risk of new plaque formation compared with HIV-uninfected individuals (relative risk [RR] 1.61, 95% CI, 1.12–2.32), adjusting for cardiometabolic factors; the association was similar by sex. Increased plaque occurred even among persistently virologically suppressed HIV-infected individuals compared with uninfected individuals (RR 1.56, 95% CI, 1.07–2.27). HIV-infected individuals with baseline CD4+ ≥500 cells/µL had plaque risk not statistically different from uninfected individuals.
Conclusions. HIV infection is associated with greater increases in focal plaque among women and men, potentially mediated by factors associated with immunodeficiency or HIV replication at levels below current limits of detection.
Survival among individuals infected with human immunodeficiency virus (HIV) has improved dramatically since the widespread availability of potent antiretroviral therapy (ART) [1, 2]. However, at the same time, increased morbidity and mortality due to cardiovascular disease (CVD) has been reported [3–5]. Several factors may contribute to increased CVD risk among HIV-infected individuals, including high smoking rates [6]; pro-atherogenic dyslipidemia and other adverse effects of ART [7, 8]; or HIV infection itself through immune activation and inflammation [9, 10]. Also potentially influencing CVD risk are recent treatment guidelines recommending ART use for all HIV-infected individuals, regardless of CD4+ T-cell (CD4+) count [11]. This represents a profound departure from prior practices that used CD4+ count thresholds to guide ART initiation, largely due to improved toxicity profiles of modern therapies and data suggesting that sustained HIV suppression and maintenance of T-cell levels are optimal in preventing AIDS and non-AIDS morbidity.
Assessment of carotid artery intima-media thickness (cIMT) has been used to understand the relationship between HIV-related risk factors and subclinical atherosclerosis, both in cross-sectional [12, 13] and longitudinal studies [14, 15]. Longitudinal studies have shown mixed results, with some demonstrating increased progression of cIMT by HIV serostatus [16], and others finding no association [17, 18]. HIV-related factors such as inflammation [18, 19], nadir CD4+ count [15, 17], and use of protease inhibitors [14, 15] have been found to affect cIMT progression. However, many studies have been limited by small samples or study duration. Unanswered questions include whether blood vessel wall characteristics in different carotid artery regions may be differentially affected by HIV infection [19], whether unmeasured confounders explain reported differences between HIV-infected and uninfected study populations [20], and whether long-term HIV suppressive therapy has pro-atherogenic effects.
In a cross-sectional analysis of 2 large cohorts of HIV-infected and uninfected individuals, we previously identified an increased prevalence of subclinical carotid artery atherosclerosis among HIV-infected persons, particularly in those with CD4+ counts <200 cells/µL [21]. We now report on the association of HIV infection with progression of subclinical atherosclerosis, after following this same well-characterized population over 7 years.
METHODS
Study Setting, Selection, and Inclusion Criteria
Participants were from 2 longstanding prospective multicenter cohort studies of individuals with or at risk for HIV infection: the Women's Interagency HIV Study (WIHS) and the Multicenter AIDS Cohort Study (MACS) [22, 23]. Details on study design are in the Supplementary Appendix. Briefly, each study has semi-annual follow-up visits, during which participants undergo similar detailed examinations and structured interviews. WIHS/MACS participants were recruited to participate in a vascular disease substudy beginning in 2004. The primary exclusion criterion was a history of coronary heart disease. All individuals provided informed consent, and the studies were approved by each institutional review board (IRB).
Data Collection
Data for the current study were collected at a baseline visit occurring between 2004 and 2006, and at each substudy follow-up visit, occurring every 2–3 years through 2013. Participants underwent high-resolution B-mode carotid artery ultrasound to image 6 locations in the right carotid artery according to previous published procedures [24]: the near and far walls of the common carotid artery (CCA), carotid bifurcation, and internal carotid artery (ICA). A standardized protocol was used at all sites [21], and carotid artery outcome measures were obtained at a centralized reading center (University of Southern California). Coefficients of variation of repeated CCA-IMT measures have been published [21], and replicate image acquisition and interpretation studies were repeated with each follow-up visit wave to ensure consistency over time. Additional demographic, clinical, and laboratory variables were collected with standardized protocols at semi-annual core study visits.
Main Outcome Measures
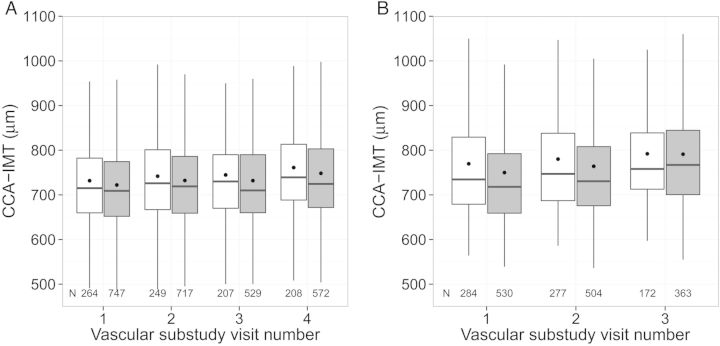
To assess progression of subclinical atherosclerosis, we examined 2 carotid artery ultrasound outcome measures. The first was individual change in cIMT from baseline at the far wall of the right CCA (CCA-IMT) over time. The far wall yields the least variability in measurement. Mean CCA-IMT at each visit was assessed from standardized ultrasound images by automated computerized edge detection [25]. Participants contributed up to 3 additional CCA-IMT measures after baseline for change assessment (Figure 1).
Figure 1.
Distribution of right common carotid artery intima-media thickness (CCA-IMT) by human immunodeficiency virus (HIV) serostatus, among (A) Women's Interagency HIV Study (WIHS), and (B) Multicenter AIDS Cohort Study (MACS) participants. Average years between visits: 2.5 (WIHS), 3 (MACS). White represents HIV-uninfected, grey represents HIV-infected, box represents interquartile range, dot represents mean CCA-IMT, middle line represents median CCA-IMT, vertical lines represent the interquartile range*1.5.
The second outcome was new focal plaque formation, defined as a dichotomous increase in the number of focal plaques measured over the six carotid artery locations between the baseline and final vascular study visit. We defined a plaque, or lesion, as an area with localized IMT >1.5 mm [26]. Because plaques were only assessed at 2 visits, sample size for this outcome (N = 1313) was smaller than for CCA-IMT change (N = 1822).
HIV Exposure and Other Variables
HIV infection was ascertained using serologic testing (enzyme-linked immunosorbent assay [ELISA]) and confirmed using Western blot. Self-reported variables included age, race/ethnicity, income, education, history of injection drug use, and current crack/cocaine and alcohol use. Hepatitis C virus (HCV) infection status was based on antibody or viral RNA testing. Cardiometabolic risk factors included body mass index (BMI), systolic blood pressure, total and high-density lipoprotein cholesterol, and the following self-reported variables: current cigarette smoking, current use of antihypertensive and lipid-lowering medications, and history of diabetes mellitus. Cardiometabolic risk factors were based on values recorded at the core study visit closest to the baseline vascular study visit.
We defined persistent virologic suppression as consistent plasma HIV RNA (viral load) levels <80 copies/mL (WIHS) or <50 copies/mL (MACS) during the period between the first and last vascular substudy visits (up to 16 measurements per participant). Persons continuously receiving ART with consistently suppressed viral loads were considered “persistently suppressed.” We allowed for 1 virologic “blip” during the period as long as it was <500 copies/mL, based on evidence that blips ≥500 copies/mL are associated with virologic rebound [27]. Participants were allowed to miss no more than 1 core visit to be eligible for being categorized as “persistently virologically suppressed.”
Other HIV clinical characteristics included baseline and nadir CD4+ count, cumulative duration of ART use at baseline, specific classes of ART drugs (protease inhibitors; nucleoside and non-nucleoside reverse transcriptase inhibitors), and history of AIDS-defining illness.
Statistical Methods
Within each cohort, we compared characteristics between HIV-infected and uninfected participants. For the CCA-IMT change outcome, we developed linear mixed models of the repeated CCA-IMT assessments and used time-by-covariate interaction terms to assess whether each covariate's impact on CCA-IMT varied over time. We serially adjusted for (1) demographic characteristics, (2) behavioral characteristics and HCV infection, and (3) cardiometabolic risk factors in nested models. Similarly, we used Poisson regression to assess new focal plaque formation by HIV serostatus. In sensitivity analyses, we examined new focal plaque formation limited to persons without any measured plaque at the first visit, to assess the incidence of atherosclerosis among persons with a healthier baseline vascular phenotype.
All models were first developed within each cohort. After comparing directionality of associations, we combined cohorts, because results were qualitatively similar. We confirmed the validity of combining cohorts by assessing cohort-covariate interaction terms. No terms were statistically significant.
Further analyses examined longitudinal change in subclinical atherosclerosis in key HIV-infected subsets. The first examined whether ART-treated and persistently suppressed HIV-infected participants had decreased progression of carotid artery disease, vs comparison groups of: (1) all other HIV-infected participants and, (2) HIV-uninfected participants. In a sensitivity analysis, we created an alternate definition of persistent HIV suppression that did not require consistent ART use, which would also include elite controllers. A second analysis examined HIV-infected groups defined by CD4+ count at entry into the vascular substudy. The comparisons of interest were the HIV-infected group with CD4+ counts in the normal range (≥500 cells/µL), vs (1) HIV-infected individuals with lower CD4+ counts, and (2) HIV-uninfected participants. Finally, among HIV-infected participants, we assessed the impact on carotid artery ultrasound parameters of cumulative duration of ART use (overall and by drug class), history of clinical AIDS, and nadir CD4+ count.
Analyses were conducted using SAS 9.3 and R 3.0.2. We determined statistical significance by a 2-sided P-value <.05. We used multiple imputation (5 datasets) based on multivariate sequential regression to account for the 7% of participants with missing data for a few covariates [28].
RESULTS
Participant Characteristics
There were 1011 women (74% HIV-infected) and 811 men (65% HIV-infected) who met study inclusion criteria and had at least 2 vascular substudy visits. Median follow-up time (years) was 6.5 for women and 7 for men, corresponding to 4 and 3 vascular assessments. Baseline characteristics are shown in Table 1. Median age (years) was 40 in women and 49 in men. Women were more likely to be of black race or Hispanic ethnicity than men (89% vs 35%). At baseline, women were more likely to report current cigarette smoking (47% vs 30%) and have higher BMI (median 28.2 vs 25.4 kg/m2) than men. Men had higher systolic blood pressures (124 vs 116 mmHg) and total cholesterol levels (194 vs 172 mg/dL) and were more likely to be current users of anti-hypertensive (23% vs 18%) or lipid-lowering medications (24% vs 5%). HIV-infected and uninfected participants were generally similar, although HIV-infected participants were more likely to have previously injected drugs and be co-infected with HCV. The majority of HIV-infected individuals reported using ART at baseline (67% among women; 76% among men).
Table 1.
Study Population Characteristics, by Cohort and Human Immunodeficiency Virus Serostatus
| Characteristic | Women's Interagency HIV Study (WIHS) |
Multicenter AIDS Cohort Study (MACS) |
||
|---|---|---|---|---|
| HIV+ (N = 747) % or Median (IQR) | HIV− (N = 264) % or Median (IQR) | HIV+ (N = 530) % or Median (IQR) | HIV− (N = 284) % or Median (IQR) | |
| Demographic characteristics | ||||
| Age, years (median, IQR) | 41 (35–47) | 40 (33–45) | 48 (44–53) | 52 (46–58) |
| Race/ethnicity | ||||
| Black (non-Hispanic) | 59.8 | 67.8 | 30.2 | 21.1 |
| Hispanic | 28.3 | 25.0 | 3.8 | 5.3 |
| White (non-Hispanic) | 9.2 | 5.3 | 60.8 | 71.5 |
| Other | 2.7 | 1.9 | 5.3 | 2.1 |
| Income | ||||
| <$30 000 per year | 83.5 | 84.9 | 48.5 | 35.9 |
| $30 000+ per year | 16.5 | 15.1 | 51.5 | 64.1 |
| Education (at study entry) | ||||
| Did not complete high school | 40.7 | 35.6 | 7.6 | 3.5 |
| Completed high school | 30.3 | 33.3 | 13.6 | 11.6 |
| Some college or completed college | 27.4 | 30.3 | 54.0 | 45.8 |
| Attended/complete graduate school | 1.6 | 0.8 | 24.9 | 39.1 |
| Behavior-related characteristics | ||||
| History of injection drug use | 27.8 | 22.4 | 8.5 | 2.8 |
| Current crack/cocaine use | 6.4 | 13.6 | 14.4 | 7.8 |
| Current alcohol use | ||||
| Abstainer | 54.1 | 39.4 | 20.3 | 11.6 |
| Light (<3 drinks/week, WIHS; 1–3, MACS) | 34.5 | 39.8 | 54.8 | 51.1 |
| Moderate (3–13, WIHS; 4–13 MACS) | 9.2 | 13.3 | 19.8 | 29.2 |
| Heavier (14+ drinks/week) | 2.1 | 7.6 | 5.1 | 8.1 |
| History of hepatitis C infection | 31.7 | 21.2 | 17.4 | 9.2 |
| Metabolic risk factors | ||||
| Current smoker | 44.7 | 55.3 | 34.2 | 23.2 |
| Body mass index, kg/m2 (median, IQR) | 27.5 (24.0–31.8) | 30.3 (25.3–36.8) | 24.9 (22.6–27.7) | 26.0 (23.9–28.8) |
| Systolic blood pressure, mmHg (median, IQR) | 115 (106–127) | 116 (107–128) | 123 (115–131) | 124 (116–132) |
| Total cholesterol, mg/dL (median, IQR) | 172 (148–199) | 177 (154–203) | 190 (162–219) | 198 (175–228) |
| HDL cholesterol, mg/dL (median, IQR) | 45 (36–56) | 52 (43–65) | 42 (36–52) | 49 (41–56) |
| Current use of anti-hypertensive medications | 19.4 | 14.4 | 21.5 | 26.1 |
| Current use of lipid lowering medications | 6.0 | 1.9 | 26.2 | 19.1 |
| History of diabetes | 19.7 | 23.5 | 9.1 | 7.8 |
| HIV-specific characteristics | ||||
| Baseline CD4+ T-cell count, cells/mm3 (median, IQR) | 441 (274–658) | 513 (359–698) | ||
| Baseline HIV-1 viral load, copies/mL (median, IQR) | 270 (80–9300) | 40 (40–1683) | ||
| Undetectable baseline HIV-1 viral load | 42.6 | 60.4 | ||
| History of clinical AIDS | 35.7 | 12.8 | ||
| Potent ART use in past 6 mo | 67.3 | 75.8 | ||
| Cumulative exposure of potent ARTa, years (median, IQR) | 4 (2.5–6.5) | 5.7 (3.4–7.7) | ||
| of PIs, years (median, IQR) | 2.5 (0.5–5) | 4.1 (0.5–6.8) | ||
| of NNRTIs, years (median, IQR) | 1.5 (0–3) | 2.1 (0.3–4.7) | ||
| of NRTIs, years (median, IQR) | 6 (3–9) | 7.7 (5.0–10.8) | ||
| Nadir CD4+ T-cell count before ART usea, cells/mm3 (median, IQR) | 280 (162–409) | 280 (156–393) | ||
All characteristics assessed at baseline unless otherwise noted.
Abbreviations: ART, antiretroviral therapy; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
a Among those using ART at baseline.
Predictors of CCA-IMT Increase: HIV Serostatus and CVD Risk Factors
HIV infection was not associated with the magnitude of CCA-IMT increase over time (Figure 1). Multivariable models adjusting for demographic, behavioral, and cardiometabolic risk factors showed no significant difference in CCA-IMT change by HIV serostatus (adjusted β = 0.2 µm/year higher in HIV-infected persons, 95% confidence interval [CI], −.6–1.0). Statistically significant predictors of CCA-IMT increase in multivariable analyses included black race, Hispanic ethnicity, and crack/cocaine use (Supplementary Appendix Table 1). Use of antihypertensive medications was associated with a decrease in CCA-IMT.
Predictors of New Focal Plaque Formation: HIV Serostatus and CVD Risk Factors
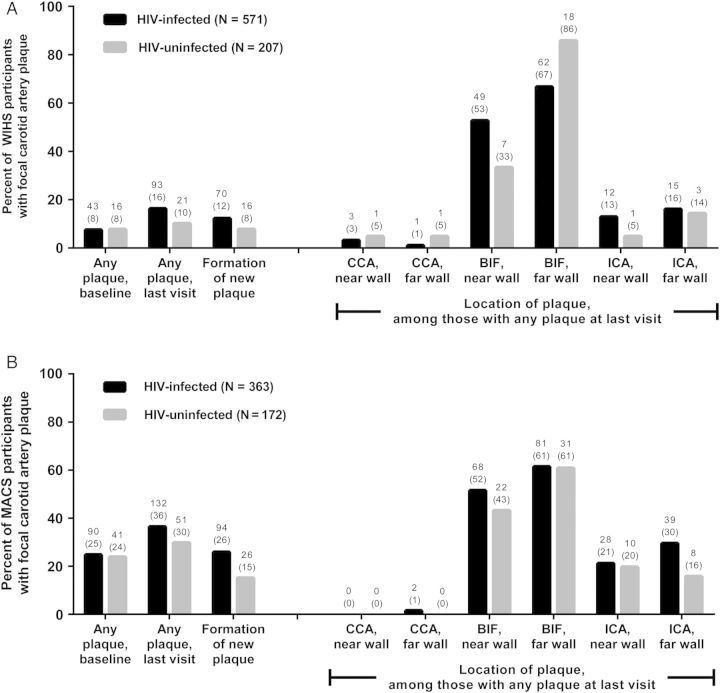
Figure 2 shows the presence of focal carotid artery plaques at the baseline and last vascular substudy visits by HIV serostatus. Plaques were more common among the older men (24% with at least 1 plaque at baseline and 34% at the last visit) than the younger women (8% and 15%). The older men were also more likely to have an increase over time in number of plaques (22% vs 11%). Focal plaque was more commonly observed at the carotid artery bifurcation compared with the ICA, and least commonly observed at the CCA.
Figure 2.
Distribution of focal carotid artery plaque by human immunodeficiency virus (HIV) serostatus, among (A) Women's Interagency HIV Study (WIHS), and (B) Multicenter AIDS Cohort Study (MACS) participants. Labels represent N (%). Abbreviations: BIF, carotid artery bifurcation; CCA, common carotid artery; ICA, internal carotid artery.
HIV infection was associated with an unadjusted 66% increase in new focal plaque formation (relative risk [RR] 1.66, 95% CI, 1.18–2.34) (Table 2). After adjusting for demographic, behavioral, and cardiometabolic risk factors, this association remained significant (adjusted RR [aRR] 1.61, 95% CI, 1.12–2.32). Similar associations were observed among males (aRR 1.65, 95% CI, 1.03–2.66) and females (aRR 1.53, 95% CI, .85–2.76). A sensitivity analysis among participants without preexisting lesions showed similar results: a 59% increase in new focal plaque formation among HIV-infected individuals relative to uninfected individuals (95% CI, 1.04–2.43). Current smoking was associated with a 42% increase in focal plaque formation (95% CI, 1.03–1.96) (Table 3). Other associated factors in fully adjusted analyses included use of antihypertensive medications, higher total cholesterol, and older age.
Table 2.
Association Between Human Immunodeficiency Virus Serostatus and Formation of New Focal Carotid Artery Plaque, Overall and by Cohort
| Women's Interagency HIV Study (WIHS) |
Multicenter AIDS Cohort Study (MACS) |
Combined |
||||
|---|---|---|---|---|---|---|
| Risk Ratio (95% CI) | P Value | Risk Ratio (95% CI) | P Value | Risk Ratio (95% CI) | P Value | |
| Among all eligible participants (N events / total) | 86 / 778 | 120 / 535 | 206 / 1313 | |||
| Model 1: HIV infection only | 1.59 (.92, 2.73) | .096 | 1.71 (1.11, 2.64) | .015 | 1.66 (1.18, 2.34) | .003 |
| Model 2: Model 1 + adjusted for demographic characteristicsa | 1.51 (.87, 2.63) | .141 | 1.74 (1.11, 2.73) | .016 | 1.73 (1.23, 2.45) | .002 |
| Model 3: Model 2 + adjusted for behavioral characteristics and HCV infectionb | 1.67 (.95, 2.93) | .074 | 1.80 (1.14, 2.84) | .012 | 1.78 (1.25, 2.53) | .001 |
| Model 4: Model 3 + adjusted for cardiometabolic risk factorsc | 1.53 (.85, 2.76) | .155 | 1.65 (1.03, 2.66) | .038 | 1.61 (1.12, 2.32) | .010 |
| Among eligible participants without preexisting focal plaque at baseline (N events / total) | 75 / 719 | 84 / 404 | 159 / 1123 | |||
| Model 1: HIV infection only | 1.90 (1.02, 3.52) | .042 | 1.64 (.98, 2.74) | .057 | 1.74 (1.18, 2.58) | .006 |
| Model 2: Model 1 + adjusted for demographic characteristicsa | 1.86 (1.00, 3.49) | .052 | 1.62 (.96, 2.74) | .073 | 1.79 (1.21, 2.67) | .004 |
| Model 3: Model 2 + adjusted for behavioral characteristics and HCV infectionb | 2.03 (1.07, 3.84) | .030 | 1.60 (.93, 2.73) | .088 | 1.84 (1.23, 2.76) | .003 |
| Model 4: Model 3 + adjusted for cardiometabolic risk factorsc | 1.59 (.81, 3.11) | .179 | 1.52 (.86, 2.66) | .147 | 1.59 (1.04, 2.43) | .032 |
Abbreviations: CI, confidence interval; HCV, hepatitis C virus infection; HDL, high-density lipoprotein; HIV, human immunodeficiency virus.
a Demographic characteristics: age, race/ethnicity, education, income, study site.
b Behavioral characteristics: history of injection drug use, crack/cocaine use, current smoking, current alcohol use, history of HCV.
c Cardiometabolic risk factors: body mass index, systolic blood pressure, total and HDL cholesterol, use of anti-hypertension or anti-cholesterol medications, history of diabetes.
Table 3.
Association Between non-Human Immunodeficiency Virus Risk Factors and Formation of New Focal Carotid Artery Plaque, Overall and by Cohort
| Women's Interagency HIV Study (WIHS) (N = 778) |
Multicenter AIDS Cohort Study (MACS) (N = 535) |
Combined (N = 1313) |
||||
|---|---|---|---|---|---|---|
| Risk Ratio (95% CI) | P Value | Risk Ratio (95% CI) | P Value | Risk Ratio (95% CI) | P Value | |
| Demographics | ||||||
| Age (per year) | 1.05 (1.02, 1.08) | <.001 | 1.02 (.98, 1.05) | .38 | 1.04 (1.01, 1.06) | .002 |
| Race/ethnicity (ref. = white or other) | ||||||
| Black | 0.97 (.45, 2.09) | .93 | 1.05 (.62, 1.80) | .85 | 1.00 (.66, 1.52) | .99 |
| Hispanic | 0.76 (.33, 1.75) | .52 | 0.64 (.28, 1.46) | .29 | 0.73 (.43, 1.23) | .23 |
| Income (ref. = income <$30 000/year) | ||||||
| $30 000/year or more | 0.84 (.40, 1.75) | .64 | 0.73 (.47, 1.13) | .162 | 0.75 (.52, 1.09) | .136 |
| Education (ref. = did not complete high school) | ||||||
| Completed high school | 0.74 (.43, 1.29) | .29 | 1.15 (.46, 2.93) | .76 | 0.86 (.55, 1.35) | .52 |
| At least some college | 0.85 (.49, 1.47) | .57 | 1.03 (.43, 2.45) | .95 | 0.89 (.58, 1.36) | .60 |
| Behavior-related characteristics | ||||||
| History of injection drug use | 0.78 (.38, 1.59) | .49 | 1.31 (.66, 2.60) | .45 | 0.96 (.60, 1.53) | .85 |
| Current crack/cocaine use | 1.07 (.48, 2.39) | .86 | 0.86 (.46, 1.60) | .63 | 0.98 (.61, 1.59) | .94 |
| Current alcohol use | ||||||
| Abstainer | ||||||
| Light (<3 drinks/week, WIHS; 1–3, MACS) | 1.21 (.75, 1.95) | .43 | 0.96 (.56, 1.60) | .84 | 1.00 (.70, 1.41) | .99 |
| Moderate (3–13, WIHS; 4–13 MACS) | 0.71 (.27, 1.91) | .50 | 1.47 (.81, 2.67) | .21 | 1.26 (.79, 2.00) | .32 |
| Heavier (14+ drinks/week) | 1.34 (.45, 4.00) | .59 | 1.64 (.67, 3.98) | .27 | 1.45 (.74, 2.82) | .27 |
| History of hepatitis C infection | 1.16 (.59, 2.27) | .67 | 1.16 (.65, 2.08) | .61 | 1.14 (.75, 1.75) | .54 |
| Cardiometabolic risk factors | ||||||
| Current smoker | 1.83 (1.12, 2.98) | .016 | 1.11 (.71, 1.74) | .65 | 1.42 (1.03, 1.96) | .030 |
| Body mass index (per kg/m2) | 0.96 (.93, 1.00) | .029 | 0.98 (.93, 1.02) | .35 | 0.97 (.94, 1.00) | .027 |
| Systolic blood pressure (per 10 mm Hg) | 1.00 (.88, 1.14) | .94 | 1.08 (.93, 1.25) | .31 | 1.04 (.95, 1.14) | .42 |
| Use of anti-hypertension medications | 1.70 (.97, 2.96) | .062 | 1.39 (.87, 2.20) | .164 | 1.44 (1.02, 2.03) | .040 |
| Total cholesterol (per 10 mg/dL) | 1.05 (1.00, 1.11) | .071 | 1.04 (1.00, 1.07) | .025 | 1.04 (1.01, 1.07) | .003 |
| HDL-cholesterol (per 10 mg/dL) | 0.97 (.84, 1.12) | .69 | 0.91 (.79, 1.05) | .200 | 0.94 (.85, 1.04) | .21 |
| Use of anti-cholesterol medications | 1.54 (.75, 3.14) | .24 | 1.15 (.72, 1.82) | .56 | 1.23 (.84, 1.82) | .29 |
| History of diabetes | 1.67 (1.01, 2.76) | .047 | 1.19 (.65, 2.15) | .58 | 1.41 (.98, 2.03) | .066 |
Models are adjusted for all covariates listed, plus HIV infection and study site.
Abbreviations: CI, confidence interval; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; ref., reference group.
HIV-infected Participants With Persistent Virologic Suppression
We identified 199 HIV-infected participants who were receiving ART and persistently virologically suppressed (16% of HIV-infected women, N = 92; 29% of HIV-infected men, N = 107) (Table 4 and Supplementary Appendix Table 2). Among this selected group, increased risk of new focal plaque formation compared with the HIV-uninfected group remained (aRR 1.77, 95% CI, 1.13–2.77). Additional analyses using an alternate definition of persistent virologic suppression or limited to individuals without preexisting lesions did not alter this finding. No associations were found between persistent virologic suppression and CCA-IMT progression, compared with uninfected individuals.
Table 4.
Association Between Human Immunodeficiency Virus-related Characteristics, Including Persistent Virologic Suppression, and Formation of New Focal Carotid Artery Plaque, Overall and by Cohort
| HIV-related Characteristic | Women's Interagency HIV Study (WIHS) |
Multicenter AIDS Cohort Study (MACS) |
Combined |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Risk Ratio (95% CI) | P Value | N | Risk Ratio (95% CI) | P Value | N | Risk Ratio (95% CI) | P Value | |
| Among all participants | |||||||||
| Persistent virologic suppression | |||||||||
| HIV-uninfected | 207 | 1.00 | 172 | 1.00 | 379 | 1.00 | |||
| Persistently suppresseda | 92 | 1.53 (.67, 3.52) | .31 | 107 | 1.95 (1.12, 3.40) | .018 | 199 | 1.77 (1.13, 2.77) | .013 |
| Not persistently suppressed | 479 | 1.53 (.84, 2.79) | .161 | 256 | 1.51 (.91, 2.50) | .107 | 735 | 1.56 (1.07, 2.27) | .022 |
| Baseline CD4+ T-cell count | .001b | .026b | <.001b | ||||||
| HIV-uninfected | 207 | 1.00 | 172 | 1.00 | 379 | 1.00 | |||
| ≥500 cells/µL | 240 | 1.02 (.50, 2.06) | .96 | 204 | 1.43 (.85, 2.41) | .174 | 444 | 1.28 (.85, 1.94) | .23 |
| 350–499 cells/µL | 134 | 1.71 (.81, 3.62) | .163 | 72 | 1.67 (.89, 3.11) | .108 | 206 | 1.65 (1.03, 2.64) | .039 |
| 200–349 cells/µL | 130 | 1.96 (.91, 4.18) | .084 | 52 | 1.89 (.96, 3.72) | .066 | 182 | 1.96 (1.20, 3.21) | .007 |
| <200 cells/uL | 67 | 3.01 (1.34, 6.78) | .008 | 30 | 2.16 (.95, 4.92) | .065 | 97 | 2.57 (1.48, 4.46) | <.001 |
| Baseline HIV-1 viral load | |||||||||
| HIV-uninfected | 207 | 1.00 | 172 | 1.00 | 379 | 1.00 | |||
| Undetectable | 259 | 1.28 (.65, 2.53) | .48 | 238 | 1.60 (.97, 2.64) | .068 | 497 | 1.46 (.98, 2.18) | .061 |
| Detectable | 312 | 1.70 (.87, 3.30) | .120 | 120 | 1.53 (.85, 2.76) | .154 | 432 | 1.69 (1.10, 2.59) | .017 |
| Among HIV-infected | |||||||||
| Duration of ART use (per year) | 0.99 (.89, 1.10) | .81 | 1.07 (.99, 1.15) | .078 | 1.04 (.98, 1.10) | .21 | |||
| History of clinical AIDS | 198 | 0.92 (.54, 1.58) | .77 | 46 | 1.31 (.73, 2.34) | .37 | 244 | 1.03 (.70, 1.51) | .90 |
| Among HIV-infected on ART | |||||||||
| Nadir CD4+ T-cell count before ART | .69b | .47b | .44b | ||||||
| ≥500 cells/µL | 38 | 1.00 | 32 | 1.00 | 70 | 1.00 | |||
| 350–499 cells/µL | 78 | 1.29 (.37, 4.44) | .69 | 54 | 0.55 (.17, 1.73) | .30 | 132 | 0.85 (.39, 1.85) | .68 |
| 200–349 cells/µL | 120 | 1.05 (.32, 3.41) | .94 | 103 | 0.98 (.38, 2.50) | .96 | 223 | 0.90 (.45, 1.82) | .78 |
| <200 cells/µL | 154 | 0.96 (.28, 3.22) | .94 | 108 | 1.30 (.52, 3.24) | .58 | 262 | 1.13 (.56, 2.29) | .73 |
| History of ART use | |||||||||
| Duration PI use (per year) | 0.92 (.78, 1.08) | .30 | 1.12 (1.01, 1.25) | .039 | 1.04 (.96, 1.13) | .34 | |||
| Duration NNRTI use (per year) | 0.97 (.80, 1.16) | .71 | 1.05 (.95, 1.17) | .35 | 0.99 (.90, 1.08) | .76 | |||
| Duration NRTI use (per year) | 0.99 (.86, 1.13) | .84 | 0.97 (.88, 1.06) | .51 | 1.00 (.93, 1.07) | .94 | |||
All analyses control for age, race/ethnicity, education, income, study site, history of injection drug use, crack/cocaine use, current smoking, current alcohol use, history of hepatitis C infection, body mass index, systolic blood pressure, total and HDL cholesterol, use of anti-hypertension medications, use of anti-cholesterol medications, history of diabetes. All models (except persistent suppression model) also control for history of AIDS. Counts are prior to multiple imputation.
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
a Persistently suppressed viral load (<50 copies/mL for MACS, <80 copies/mL for WIHS) and consistently on ART throughout study period.
b P for trend.
HIV-infected Participants With Higher CD4+ Counts
HIV-infected participants with CD4+ counts ≥500 cells/µL at baseline had a risk of new focal plaque formation that was not statistically different from HIV-uninfected participants (aRR 1.28, 95% CI, .85–1.94). HIV-infected participants with the lowest CD4+ count at baseline (ie, <200 cells/µL) had the greatest risk (aRR 2.57, 95% CI, 1.48–4.46) compared with HIV-uninfected participants, with a gradient of decreasing risk by increasing categories of CD4+ count (Table 4).
Other HIV-related Predictors
Our analyses did not find any association of nadir CD4+ count, history of AIDS, and duration of ART use with new focal plaque formation. We found a slightly increased risk of plaque formation with greater exposure to protease inhibitors among men (aRR 1.12 per year of cumulative use at baseline, 95% CI, 1.01–1.25) but not among women. No other associations between other ART classes and focal plaque formation were found nor were any HIV-related parameters associated with increased CCA-IMT progression (Supplementary Appendix Table 3).
DISCUSSION
Using noninvasive carotid B-mode ultrasonography, we demonstrated that HIV-infected women and men had a 61% greater risk of new focal carotid artery plaque formation over seven years compared with uninfected controls, regardless of baseline vascular phenotype and after controlling for cardiometabolic risk factors. The HIV-associated risk was higher than that associated with smoking. Furthermore, the elevated risk persisted among ART-treated individuals with persistent HIV viral suppression, suggesting that sustained suppression of circulating HIV RNA to below detectable limits does not eliminate excess CVD risk in the treated HIV-infected population. However, HIV-infected individuals with high CD4+ counts were not significantly different from HIV-uninfected individuals in changes in carotid artery measures over time.
Our findings are consistent with a growing body of evidence linking HIV infection with CVD. To our knowledge, our study is the largest and longest examining whether HIV infection is associated with incidence and progression of subclinical carotid atherosclerosis, with >1800 study participants recruited across 10 US locations. By design, our comparison group of HIV-uninfected participants had similar sociodemographic and behavioral characteristics as participants with HIV infection. This is a major strength, given the well-known threats to validity when making comparisons of age-related disease outcomes that may stem from health behaviors (eg, drug use, smoking) in HIV-infected individuals that differ from those in the general population [20].
We did not find differences in CCA-IMT progression over the seven years by HIV serostatus, consistent with smaller studies [17, 18]. Moreover, prior studies that have measured several carotid artery segments have found that IMT in the internal carotid artery and carotid bifurcation have been more consistently associated with HIV infection than CCA-IMT [16, 19]. The increased rate of plaque progression in disease-prone segments of the carotid arterial tree that we found among HIV-infected participants corroborates these previous findings. Collectively, these findings add strong support to the hypothesis that development of atherosclerosis in HIV-infected individuals involves endothelial shear stress preferentially occurring at vascular branch points [19], which may represent a common pathway with atherosclerosis arising from other conditions such as rheumatoid arthritis [29].
Although HIV infection was the factor most strongly associated with formation of new focal plaque, elevated risk was seen with smoking and increased total cholesterol levels, both of which are amenable to modification [30]. Over one-third of our HIV-infected participants were current smokers. Thus, our findings, consistent with other studies among both HIV-infected individuals and the general population [15, 31], support recommendations promoting smoking cessation for HIV-infected individuals [32].
Use of antihypertensive medications appeared to be associated with decreased CCA-IMT progression but increased plaque. Although antihypertensive medication through its effects on blood pressure may reduce wall hypertrophy in the CCA [33], it may not reduce or correct turbulent blood flow, so it may appear as though such medications associate with plaque formation. Most of the plaque we detected was found in the bifurcation and ICA segments, which are affected more by turbulent flow, supporting this premise. Alternatively, antihypertensive medication could serve as a marker for longstanding hypertension that has led to plaque formation.
Our finding that participants who maintained HIV suppression still had an increased risk of new focal plaque formation suggests that vigilance with respect to the long-term adverse consequences of ART remains warranted for all HIV-infected individuals. This elevated risk may be due in part to low-level HIV replication and inflammation that persists despite reductions in viral load [10, 34, 35]. Future work should confirm our findings and further explore the extent to which these factors play a role in CVD development.
We found a slightly elevated association between exposure to protease inhibitors and new focal plaque formation among men but not women, consistent with our cross-sectional findings [21]. Concerns about protease inhibitor use initially arose based on data from the D:A:D Study, in which an elevated risk of myocardial infarction among protease inhibitor users was observed [8]. Given the small magnitude of our effect estimate (aRR 1.12 per year of cumulative use) and improvements in risk profiles of available ART drugs since this study was initiated, our finding should be interpreted with caution. The overwhelming benefits of ART should not be minimized, since participants with CD4+ counts in the normal range (≥500 cells/µL) had atherosclerotic risks similar to HIV-uninfected participants. In fact, CD4+ count was the single HIV-related factor conferring a linear, monotonic risk of plaque formation, supporting treatment guidelines that advocate early ART initiation, allowing for better CD4+ maintenance [11].
Our study has limitations. First, only the right carotid artery was examined; however, other studies have found wall thickness to be correlated bilaterally [36]. Second, the use of long-term cohort studies may induce selection bias, and some participants were not able to attend all vascular study visits. However, we do not have a strong basis to believe that inferences would change as a result (Supplementary Appendix). Furthermore, the women and men in our study have the same age distribution as the US HIV-infected population [22, 37], supporting the generalizability of our results. We carefully adjusted for many established confounders, including cardiometabolic risk factors but did not have comprehensive data on diet, physical activity, and other lifestyle factors and therefore cannot rule out the possibility of unmeasured confounding. We used carotid artery B-mode ultrasonography to examine structural measures of atherosclerosis; imaging techniques examining other structural or functional aspects of CVD, such as coronary computed tomographic angiography [38], provide additional information. Finally, our analysis does not examine clinical CVD outcomes such as myocardial infarction; although subclinical carotid artery disease has been strongly associated with such outcomes in non-HIV studies [39], this relationship has not yet been replicated in HIV-infected cohorts.
More HIV-infected individuals are initiating ART earlier in the course of their infection, with concomitant improvements in long-term virologic control [40]. Our data support earlier ART initiation, before CD4+ counts decline, which may mitigate HIV-associated increased CVD risks. Better understanding of these processes is necessary, both to prevent or delay CVD development and to improve treatment strategies in the growing and increasingly older HIV-infected population.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. Women's Interagency HIV Study (WIHS) (Principal Investigators): Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen), U01-AI-034993; Metropolitan Washington WIHS (Mary Young), U01-AI-034994; Connie Wofsy Women's HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Alexandra Levine and Marek Nowicki), U01-HD-032632 (WIHS I - WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute (NCI), the National Institute on Drug Abuse, and the National Institute on Mental Health. Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and other Communication Disorders (NIDCD), and the National Institutes of Health (NIH) Office of Research on Women's Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA).
Multicenter AIDS Cohort Study (MACS) (Principal Investigators): Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels), U01-AI35040; University of Pittsburgh (Charles Rinaldo), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson), UM1-AI35043. The MACS is funded primarily by the NIAID, with additional co-funding from the NCI. Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute, and the NIDCD. MACS data collection is also supported by UL1-TR000424 (JHU CTSA).
Other funding sources for this study include R01-HL-083760, R01-HL-095140, and R21-HL-120394 to R. C. K., R01-HL-095129 to W. S. P., and P30-AI-051519 to the Einstein-Montefiore Center for AIDS Research.
Disclaimer. Data in this manuscript were collected by the WIHS and MACS. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the NIH.
Potential conflicts of interest. K. A. and L. P. J. have served as consultants for Bristol-Myers Squibb. F. J. P. has served as a consultant for Gilead Sciences, and has participated in speakers bureaus for Gilead Sciences, Janssen Pharmaceutica, Merck, and Bristol-Myers Squibb. P. C. T. reports board membership with AbbVie and Bristol-Myers Squibb, and has served as a consultant for Genentech. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853–60. [DOI] [PubMed] [Google Scholar]
- 2.Wada N, Jacobson LP, Cohen M, French A, Phair J, Munoz A. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984–2008. Am J Epidemiol 2013; 177:116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med 2006; 145:397–406. [DOI] [PubMed] [Google Scholar]
- 4.Berry SA, Fleishman JA, Moore RD, Gebo KA, HIV Research Network. Trends in reasons for hospitalization in a multisite United States cohort of persons living with HIV, 2001–2008. J Acquir Immune Defic Syndr 2012; 59:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browning KK, Wewers ME, Ferketich AK, Diaz P. Tobacco use and cessation in HIV-infected individuals. Clin Chest Med 2013; 34:181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan RC, Kingsley LA, Sharrett AR, et al. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis 2007; 45:1074–81. [DOI] [PubMed] [Google Scholar]
- 8.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–35. [DOI] [PubMed] [Google Scholar]
- 9.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr 2009; 51:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA 2012; 308:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf Accessed 12 March 2013.
- 12.Grunfeld C, Delaney JA, Wanke C, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS 2009; 23:1841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsue PY, Hunt PW, Schnell A, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS 2009; 23:1059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker JV, Henry WK, Patel P, et al. Progression of carotid intima-media thickness in a contemporary human immunodeficiency virus cohort. Clin Infect Dis 2011; 53:826–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volpe GE, Tang AM, Polak JF, Mangili A, Skinner SC, Wanke CA. Progression of carotid intima-media thickness and coronary artery calcium over 6 years in an HIV-infected cohort. J Acquir Immune Defic Syndr 2013; 64:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation 2004; 109:1603–8. [DOI] [PubMed] [Google Scholar]
- 17.Currier JS, Kendall MA, Henry WK, et al. Progression of carotid artery intima-media thickening in HIV-infected and uninfected adults. AIDS 2007; 21:1137–45. [DOI] [PubMed] [Google Scholar]
- 18.Hileman CO, Longenecker CT, Carman TL, McComsey GA. C-reactive protein predicts 96-week carotid intima media thickness progression in HIV-infected adults naive to antiretroviral therapy. J Acquir Immune Defic Syndr 2014; 65:340–4. [DOI] [PubMed] [Google Scholar]
- 19.Hsue PY, Scherzer R, Hunt PW, et al. Carotid intima-media thickness progression in HIV-infected adults occurs preferentially at the carotid bifurcation and is predicted by inflammation. J Am Heart Assoc 2012; 1:jah3-e000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Althoff KN, Gange SJ. A critical epidemiological review of cardiovascular disease risk in HIV-infected adults: the importance of the HIV-uninfected comparison group, confounding, and competing risks. HIV Med 2013; 14:191–2. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS 2008; 22:1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bacon MC, von Wyl V, Alden C, et al. The Women's Interagency HIV Study: An observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987; 126:310–8. [DOI] [PubMed] [Google Scholar]
- 24.Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001; 135:939–53. [DOI] [PubMed] [Google Scholar]
- 25.Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis 2001; 154:185–93. [DOI] [PubMed] [Google Scholar]
- 26.Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). In: An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis 2012; 34:290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grennan JT, Loutfy MR, Su D, et al. Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J Infect Dis 2012; 205:1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodol 2001; 27:85–95. [Google Scholar]
- 29.Kobayashi H, Giles JT, Polak JF, et al. Increased prevalence of carotid artery atherosclerosis in rheumatoid arthritis is artery-specific. J Rheumatol 2010; 37:730–9. [DOI] [PubMed] [Google Scholar]
- 30.Markus RA, Mack WJ, Azen SP, Hodis HN. Influence of lifestyle modification on atherosclerotic progression determined by ultrasonographic change in the common carotid intima-media thickness. Am J Clin Nutr 1997; 65:1000–4. [DOI] [PubMed] [Google Scholar]
- 31.Howard G, Burke GL, Szklo M, et al. Active and passive smoking are associated with increased carotid wall thickness. The Atherosclerosis Risk in Communities Study. Arch Intern Med 1994; 154:1277–82. [PubMed] [Google Scholar]
- 32.Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:1–10. [DOI] [PubMed] [Google Scholar]
- 33.Boutouyrie P, Bussy C, Hayoz D, et al. Local pulse pressure and regression of arterial wall hypertrophy during long-term antihypertensive treatment. Circulation 2000; 101:2601–6. [DOI] [PubMed] [Google Scholar]
- 34.Hatano H, Delwart EL, Norris PJ, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol 2009; 83:329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landay A, Golub ET, Desai S, et al. HIV RNA levels in plasma and cervical-vaginal lavage fluid in elite controllers and HAART recipients. AIDS 2014; 28:739–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howard G, Burke GL, Evans GW, et al. Relations of intimal-medial thickness among sites within the carotid artery as evaluated by B-mode ultrasound. ARIC Investigators. Atherosclerosis Risk in Communities. Stroke 1994; 25:1581–7. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. HIV Surveillance Report, 2013; vol. 25. Available at: http://www.cdc.gov/hiv/library/reports/surveillance/ Accessed 8 March 2015.
- 38.Post WS, Budoff M, Kingsley L, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med 2014; 160:458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med 1998; 128:262–9. [DOI] [PubMed] [Google Scholar]
- 40.Yehia BR, Fleishman JA, Metlay JP, Moore RD, Gebo KA. Sustained viral suppression in HIV-infected patients receiving antiretroviral therapy. JAMA 2012; 308:339–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.