Abstract
Background Antibiotics are commonly given for the treatment of childhood diarrhoea, but are not indicated in most cases. Antibiotics modify the gastrointestinal microbiota, which may have unanticipated effects on the risk of subsequent diarrhoea.
Methods In a prospective observational cohort study, we assessed the effect of caregiver-reported antibiotic treatment for diarrhoea on the timing of a child’s next episode among 434 children followed from birth to 3 years of age in Vellore, India. We estimated median time differences and time ratios from inverse probability of exposure-weighted Kaplan-Meier curves for the time to next diarrhoea episode, comparing children who did and did not receive antibiotics for the previous episode.
Results Study children had more than five diarrhoea episodes on average in the first 3 years of life, and more than a quarter of all episodes were treated with antibiotics. Children who received antibiotics for their first diarrhoea episode had their second episode on average 8 weeks earlier (median time difference: −8, 95% confidence interval: −10, −3) than children who did not receive antibiotics. The effects of antibiotics on subsequent diarrhoea were greatest at earlier episodes and younger ages, and cefixime had a slightly larger effect compared with cotrimoxazole.
Conclusions Antibiotic treatment of diarrhoea was associated with reduced time to a subsequent diarrhoea episode, especially among younger infants. Whereas rational use of antibiotics has been advocated to reduce antimicrobial resistance in populations, we show that overuse of antibiotics may also have a direct adverse effect on individual patients.
Keywords: Diarrhoea, antibiotics, India, Kaplan-Meier survival curves, inverse probability weighting, time differences
Key Messages
Children who were treated with antibiotics for diarrhoea early in life experienced subsequent diarrhoea sooner than children who were not treated with antibiotics.
Exposure to antibiotics within the first 6 months of life was associated with the largest increases in diarrhoeal risk.
These effects may be due to antibiotic-induced modifications to the composition of microorganisms in the gut.
These results support recommendations for reduced and rational use of antibiotics.
Introduction
Diarrhoea is a universal and recurring disease during childhood, with the highest burden in low- and middle-income countries. In 2010, the global incidence of diarrhoea before age 5 years was estimated to be 2.7 episodes per child-year, which corresponded to approximately 1.7 billion total episodes and resulted in 700 000 deaths.1
Antibiotics are widely accessible and commonly used for the treatment of childhood diarrhoea in India. However, international and Indian organizations, including the World Health Organization, recommend against routine use of antibiotics to treat diarrhoea.2,3 Antibiotics are generally contraindicated for non-bloody diarrhoeas because they are ineffective against non-bacterial and resistant pathogens, and most episodes of diarrhoea are self-limiting.4,5 Despite these arguments, several healthcare facility-based studies in India have reported antibiotic prescription rates for acute childhood diarrhoea from 50% to 90%.6–9 In a nationwide community-based survey, 16% of children under 5 years who had diarrhoea in the 2 weeks preceding survey reported treatment with antibiotics, and another 30% reported treatment with unknown drugs that may have included antibiotics.10
Major concerns about inappropriate antibiotic use often focus on the development of pathogen resistance to antibiotics, but direct harm to patients is also possible and often overlooked.11 Specifically, antibiotics may disrupt the gastrointestinal (GI) microbiota—the complex community of microorganisms inhabiting the human GI tract—by causing a sharp reduction in the abundance and diversity of organisms.12,13 This disruption can be prolonged, and the recovery of the microbiota following antibiotic exposure is often incomplete.14,15 The microbiota is important for the development of the immune system,16,17 and may protect against diarrhoeal disease by occupying intestinal mucosal sites and inhibiting the attachment and growth of pathogens.18–20
Studies of the impact of antibiotics on diarrhoea most often focus on the incidence of antibiotic-associated diarrhoea (AAD) occurring within 8 weeks of antibiotic exposure,21,22 and often among hospitalized adults in developed countries.23 Longitudinal investigation of the effects of antibiotics on diarrhoeal risk has not been completed among children in the developing world. In a birth cohort of children from Vellore, India, we assessed the effect of antibiotic treatment for diarrhoea on the timing of a child’s next diarrhoea episode.
Methods
We analysed data from a prospective observational cohort study on immune responses to cryptosporidiosis in 497 children followed from birth to 3 years of age from 2009 to 2013. The study population, enrolment strategy and data collection methods have been described previously.24 Briefly, baseline information on demography, socioeconomic indicators, health-seeking behaviour, environment, diet and characteristics of delivery were collected within 45 days of birth. Fieldworkers interviewed caregivers twice per week about any illnesses since the previous visit. Clinical characteristics of the diarrhoea episodes were recorded, including the number of stools per day, consistency and colour of stools, fever or vomiting, associated hospitalization and treatments given. Heights and weights were measured monthly at the study clinic, and breastfeeding histories (exclusive, non-exclusive, none) were collected every 2 weeks until breastfeeding was stopped completely.
Data and definitions
Diarrhoea was defined using the standard World Health Organization (WHO) definition as at least three loose or watery stools in a 24-h period.2 Duration of a diarrhoea episode was defined as the number of days from the first day of watery stools until the last day of watery stools inclusive. A new episode of diarrhoea was defined only after at least 48 h of normal bowel movements since the previous episode. Person-time at risk was defined as all days during follow-up excluding days with diarrhoea and 48 h after an episode of diarrhea, during which a new episode of diarrhoea could not be defined.
Severity of diarrhoea was assessed using the 20-point Vesikari scale.25 Episodes were classified as mild (1–5), moderate (6–10), severe (11–15) and very severe (16–20). Episodes were classified as acute if lasting 0–6 days or prolonged/persistent if lasting for 7 or more days.
The primary exposure was antibiotic treatment for diarrhoea based on caregiver report during the episode. A yes/no question was asked specifically about whether antibiotics were given and the name of the drug(s) was recorded if known (available for 64.0% of antibiotic reports). We also extracted antibiotic prescriptions from clinic records for all illnesses (most commonly respiratory, skin and ear infections) assessed at the study clinic.
Children were classified according to standard definitions as: underweight (weight-for-age z-score < −2 standard deviations (SD) from the 2006 WHO growth reference26); stunted (height-for-age z-score < −2 SD); and/or wasted (weight-for-height z- score < −2 SD).
Data analysis
We restricted this analysis to children who had at least one diarrhoea episode and therefore had the opportunity to be treated with antibiotics for diarrhoea. Because the proportion of missing data for baseline and diarrhoea severity-related covariates was 5% or less for all variables, we imputed the median values of variables for individuals and episodes with missing data (indicated in Table 1 footnote).
Table 1.
Demographic characteristics of 434 children with at least one diarrhoea episode in a birth cohort in Vellore, Tamil Nadu, India 2009–13
| 0 antibiotics reported for diarrhoea (n = 166) |
1 + antibiotics reported for diarrhoea (n = 268) |
|
|---|---|---|
| No. (%) children | No. (%) children | |
| Household characteristics | ||
| Socioeconomic statusa | ||
| Low | 114 (68.7) | 168 (62.7) |
| Medium | 50 (30.1) | 94 (35.1) |
| High | 2 (1.2) | 6 (2.2) |
| Maternal education | ||
| No formal education | 67 (40.4) | 93 (34.7) |
| Primary/middle school | 52 (31.3) | 97 (36.2) |
| Higher secondary school | 42 (25.3) | 69 (25.7) |
| College/polytechnic/professional school | 5 (3.0) | 9 (3.4) |
| Poor household hygieneb | 75 (45.2) | 149 (55.6) |
| Crowding | ||
| High (> 4 people/room) | 52 (31.3) | 78 (29.1) |
| Medium (3.1–4 people/room) | 63 (38.0) | 103 (38.4) |
| Low (≤ 3 people/room) | 51 (30.7) | 87 (32.5) |
| Child characteristics | ||
| Sex of child | ||
| Male | 87 (52.4) | 147 (54.9) |
| Female | 79 (47.6) | 121 (45.1) |
| Cesarean section | 29 (17.5) | 45 (16.8) |
| Low birthweightc | 33 (20.3) | 43 (16.3) |
| Preterm birthc | 19 (11.7) | 26 (9.9) |
| Baby kept in ICU at birth | 9 (5.4) | 23 (8.6) |
| Antibiotics at birthc | 3 (1.9) | 8 (3.1) |
| Age at first diarrhoea | ||
| <6 months | 103 (62.0) | 204 (76.1) |
| 6 month–1 year | 44 (26.5) | 52 (19.4) |
| >1 year | 19 (11.4) | 12 (4.5) |
| Age (months) at stopping exclusive breastfeeding (mean, SD) | 4.2 (2.0) | 3.8 (2.1) |
| Age (months) at stopping all breastfeeding (mean, SD) | 17.4 (8.7) | 16.2 (8.3) |
ICU, intensive care unit. SD, standard deviation
bPoor household hygiene was based a score of less than 12 on a scale developed from an assessment of water, food, and personal hygiene.46
cSeven missing values for low birthweight; 9 missing values for preterm birth; 13 missing values for antibiotics at birth.
Logistic regression was used to calculate inverse probability of exposure weights stabilized by the marginal probability of exposure.27 Confounding variables for the exposure model were chosen by causal directed acyclic graph28 to account for baseline characteristics and indications for treatment. We were particularly concerned about confounding by diarrhoea episode severity, which was associated with higher antibiotic use rates and might also predict future diarrhoeal risk. We therefore included multiple characteristics of the diarrhoea episode to capture the multifaceted concept of illness severity. The final exposure model included episode number, socioeconomic status defined from the modified Kuppuswamy scale,29,30 maternal education, child sex, caesarean birth, low birthweight, preterm birth, hospitalization at birth, antibiotics given at birth and characteristics of the last diarrhoea episode: age, Vesikari score,25 duration, hospitalization, fever, dehydration, bloody diarrhoea, underweight, stunted, wasted, exclusive and any breastfeeding, zinc given, number of previous antibiotic courses for any illnesses, number of sick days between episodes, and other antibiotics given between episodes. Continuous variables were modelled flexibly with restricted quadratic splines,31 and covariate specifications were compared by Akaike’s information criterion. To remove extreme weight values,32 weights were censored at the 0.5th and 99.5th percentiles by resetting the value of weights greater than the 99.5th percentile and less than the 0.5th percentile to the value of the 99.5th and 0.5th percentile, respectively.
We estimated inverse probability-weighted Kaplan-Meier (KM) curves27,33 for the time to next diarrhoea episode, comparing children who did and did not receive antibiotics for the previous episode. The time scale33 was from 48 h after the previous diarrhoea episode to the incident day of the next episode. Children were censored at drop-out, death or the end of follow-up at 3 years of age. We assumed person-time during which children were temporarily unreachable was missing at random, and drop-out was non-informative given the small proportion of drop-outs (n = 50, 11.5% overall; n = 18, 4.1% between the first and second diarrhoea episode). We assessed each episode pair separately and then collapsed across episodes.
We calculated the time difference and time ratio at 50% diarrhoea-free survival, the median survival time, from the weighted KM curves. Confidence intervals were constructed by bootstrap34 with 200 resamples at the level of the individual to account for clustering of episodes within children.
We also estimated hazard ratios, comparing the same exposure groups using marginal structural Cox models33 with the same inverse probability weights. These models were estimated by pooled logistic regression with adjustment for time using a restricted quadratic spline.31 Correlation between outcomes from the same child was accounted for using generalized estimating equations with a robust variance estimator.
Effect measure modification
We assessed modification of the effect of antibiotics by age at exposure by stratification. We also considered the effect of specific antibiotics commonly given—trimethoprim/sulfamethoxazole (cotrimoxazole) and cephalosporins (97.4% of which were cefixime)by comparing children receiving each drug with children given no antibiotics.
Sensitivity analyses
To validate caregiver report of antibiotic treatment, we repeated analyses with alternative definitions of antibiotic exposure. First, we restricted the exposed group to only those children whose caregivers reported the name of a confirmed antibiotic in the free-response section of the questionnaire. Second, we considered children exposed if either their caregiver reported that antibiotics were given (by indicating yes/no) or if an antibiotic prescription was recorded in clinic records during the diarrhoea episode. Finally, we considered children exposed only if a confirmed antibiotic name was reported or if a prescription was recorded in the clinic records.
To assess the impact of long episode duration contributing to shorter time between episodes, we repeated the main analyses excluding all episode pairs where the first episode lasted for more than 7 days (n = 194, 8.6% total; n = 42, 9.8% among first episodes).
To assess whether antibiotics were associated with the severity of subsequent diarrhoea when another episode occurred, we estimated the effects of antibiotic treatment for the previous episode on the severity and duration of the next episode. In models weighted for the same covariates as in the above analyses, we used inverse probability-weighted linear regression with the Vesikari score and number of days with diarrhoea as continuous outcomes. We also estimated the adjusted relative risk for a severe (Vesikari ≥ 11) and prolonged/persistent (≥ 7 days) next episode using inverse probability-weighted log-binomial regression.
Last, we compared the results from the main study with two earlier cohorts of children from the same study area.35,36 The earlier cohorts lacked complete records of antibiotics given to treat non-diarrhoeal illnesses, and the type of antibiotics given for diarrhoea were unknown. In addition, the most recent earlier study was a smaller quasi-experimental study, in which children were followed once-weekly for only 2 years and enrolled after birth if still exclusively breastfed.36 Despite these limitations, we present the results from these cohorts for completeness.
Results
Almost all children in the birth cohort (434 of 497, 87.3%) had at least one diarrhoea episode and were included in the analysis. Of these, 412, 393 and 384 children completed the first, second and third study year of follow-up respectively (drop-out rate of 11.5%). Six children died during follow-up; two deaths were associated with diarrhoea. Most children were of low socioeconomic status (n = 282, 65%, Table 1) and approximately half had poor household hygiene (n = 210, 48.4%). By 6 months of age, most children had stopped exclusive breastfeeding (n = 370, 85.3%) and had their first episode of diarrhoea (n = 307, 70.7%). Children who received antibiotics were slightly more likely to be from households with poor hygiene. These children stopped all breastfeeding on average 1 month earlier, and had their first diarrhoea episode at younger ages (Table 1).
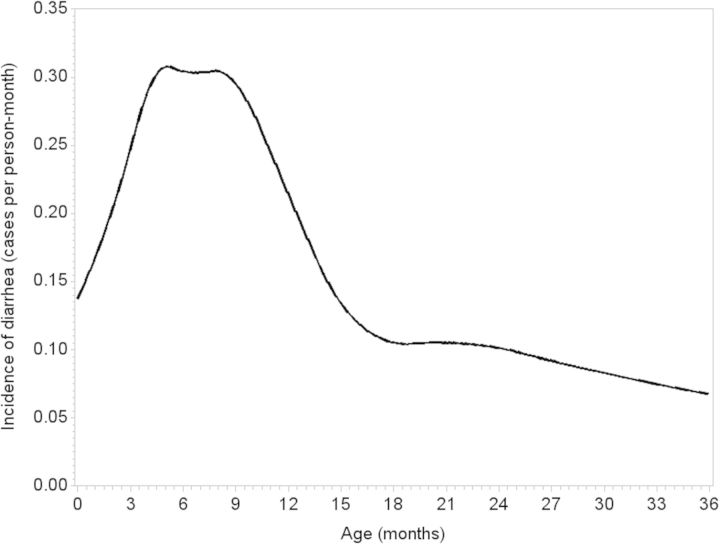
The total accumulated follow-up was 1013.3 person-years, including 981.8 diarrhoea-free person-years included as person-time at risk in analyses. Incidence of diarrhoea was highest around 6 months of age, with an incidence of 32.4 episodes per 100 person-months among children between 5 and 7 months of age (Figure 1).
Figure 1.
Incidence of diarrhoea by age (using restricted quadratic splines31) among 434 children in a birth cohort in Vellore, Tamil Nadu, India 2009–13.
A total of 2295 diarrhoea episodes were reported, of which 658 (28.9%) were treated with antibiotics. We excluded 16 diarrhoea episodes (0.7%) due to missing antibiotic treatment information. More than half of children (n = 268, 61.8%) reported at least one antibiotic course for diarrhoea, and 154 (35.5%) reported two or more antibiotic courses for diarrhoea in the first 3 years of life. Antibiotic treatment of diarrhoea was associated with older age at the time of the episode and increased episode severity and duration (Table 2). The most common antibiotic given was cotrimoxazole, accounting for 50.3% of caregiver-reported antibiotics and 57.8% of antibiotics prescribed at the study clinic for diarrhoea. Cefixime accounted for another 24.6% of caregiver-reported antibiotics and 34.5% of antibiotic prescriptions at the clinic. All other antibiotics, such as fluoroquinolones, penicillins and macrolides, were reported for less than 5% of cases.
Table 2.
Characteristics of diarrhoea episodes and their association with antibiotic treatment among 434 children in a birth cohort in Vellore, Tamil Nadu, India 2009–13
| No. (%) total episodes (n = 2279a) | No. (%) episodes treated with antibiotics (n = 658) | Crude ORb (95% CI) | |
|---|---|---|---|
| Age at episode | |||
| < 6 months | 589 (25.8) | 110 (16.7) | 1. |
| 6 mo.–1 yr. | 701 (30.8) | 213 (32.4) | 1.90 (1.46, 2.47) |
| 1–2 years | 596 (26.2) | 209 (31.8) | 2.35 (1.80, 3.07) |
| 2–3 years | 393 (17.2) | 126 (19.1) | 2.05 (1.53, 2.76) |
| Severityc | |||
| Mild | 1125 (49.4) | 235 (35.7) | 1. |
| Moderate | 900 (39.5) | 302 (45.9) | 1.91 (1.57, 2.33) |
| Severe | 221 (9.7) | 104 (15.8) | 3.37 (2.49, 4.55) |
| Very severe | 33 (1.4) | 17 (2.6) | 4.02 (2.00, 8.08) |
| Durationd | |||
| Acute | 2011 (88.2) | 549 (83.4) | 1. |
| Prolonged | 234 (10.3) | 93 (14.1) | 1.76 (1.33, 2.32) |
| Persistent | 34 (1.5) | 16 (2.4) | 2.37 (1.20, 4.67) |
| Bloody stools | |||
| No | 2231 (97.9) | 634 (96.4) | 1. |
| Yes | 48 (2.1) | 24 (3.7) | 2.52 (1.42, 4.47) |
| Fevere | |||
| No | 1990 (87.3) | 518 (78.7) | 1. |
| Yes | 289 (12.7) | 140 (21.3) | 2.67 (2.08, 3.43) |
| Dehydration | |||
| No | 1652 (72.5) | 410 (62.3) | 1. |
| Yes | 627 (27.5) | 248 (37.7) | 1.98 (1.63, 2.41) |
| Hospitalization | |||
| No | 2219 (97.4) | 623 (94.7) | 1. |
| Yes | 60 (2.6) | 35 (5.3) | 3.59 (2.13, 6.04) |
aExcludes 16 episodes for which antibiotic treatment was unknown.
bOdds ratio for antibiotic treatment of diarrhoea episode by diarrhoea characteristics.
cSeverity based on the Vesikari score. Mild: 1–5; moderate: 6–10; severe: 11–15; very severe: 16–20.
dDuration in days. Acute: 1–6 days; prolonged: 7–13 days; persistent: ≥ 14 days.
eCaregiver-reported.
Effect on diarrhoea incidence
Of 434 children experiencing a first diarrhoea episode, we excluded 3 children with missing antibiotic treatment and 1 child who dropped out on the first day following their first episode. Among children who had a second diarrhoea episode (n = 375, 87.2%), the median time to second diarrhoea episode was 10 weeks [interquartile range (IQR): 3, 20]. The crude difference in median time to second diarrhoea episode among children who were treated with antibiotics for their first episode (n = 84) compared with children who were not treated (n = 289) was 2 weeks (median time difference (MTD): −2, 95% confidence interval (CI): −8, 3). The crude hazard ratio from a Cox proportional hazards model was 1.15 (95% CI: 0.77, 1.72).
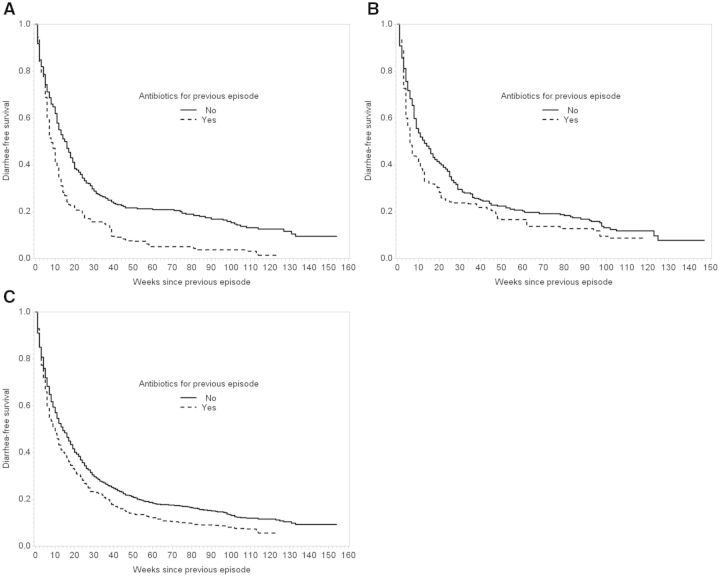
Figure 2A shows inverse probability of treatment-weighted Kaplan-Meier curves for time to second diarrhoea episode among children who were (n = 93) and were not (n = 337) treated with antibiotics for their first episode. Based on the weighted curves, children who received antibiotics for their first diarrhoea episode had their second episode on average 8 weeks earlier (MTD: −8, 95% CI: −10, −3) or twice as soon (median time ratio (MTR): 0.50, 95% CI: 0.38, 0.79) as children who did not receive antibiotics (Table 3). In a Cox proportional hazards model weighted for the same covariates, the adjusted hazard ratio was 1.38 (95% CI: 1.05, 1.82).
Figure 2.
Inverse probability of treatment-weighted Kaplan-Meier curves for time to next diarrhoea episode by antibiotic treatment for the previous diarrhoea episode among 430 children from Vellore, Tamil Nadu, 2009–13. A. Weighted diarrhoea-free survival from first to second episode. B. Weighted diarrhoea-free survival from second to third episode. C. Weighted diarrhoea-free survival for all episode pairs.
Table 3.
Estimated effect of antibiotic treatment for the previous diarrhoea episode on time to next episode by episode pair and age at first episode among 430 children in a birth cohort in Vellore, Tamil Nadu, India 2009–13
| Antibiotics for previous episode | No. of children | Median time difference (weeks; 95% CI)a | Median time ratio (95% CI)a | Hazard ratiob (95% CI)a | |
|---|---|---|---|---|---|
| Episode pair | |||||
| 1st to 2nd | No | 337 | 0. | 1. | 1. |
| Yes | 93 | −8 (−10, −3) | 0.50 (0.38, 0.79) | 1.38 (1.05, 1.82) | |
| 2nd to 3rd | No | 273 | 0. | 1. | 1. |
| Yes | 94 | −7 (−11, 1) | 0.46 (0.29, 1.10) | 1.53 (1.05, 2.23) | |
| 3rd to 4th | No | 234 | 0. | 1. | 1. |
| Yes | 75 | 1 (−11, 11) | 1.07 (0.37, 1.90) | 0.79 (0.54, 1.16) | |
| >4th | No | 762 | 0. | 1. | 1. |
| Yes | 393 | −2 (−7, 5) | 0.86 (0.57, 1.39) | 1.23 (0.94, 1.61) | |
| All | No | 1606 | 0. | 1. | 1. |
| Yes | 655 | −4 (−9, 0) | 0.71 (0.44, 0.96) | 1.35 (1.11, 1.64) | |
| Age at first episode | |||||
| < 6 months | No | 472 | 0. | 1. | 1. |
| Yes | 108 | −4 (−6, 0) | 0.60 (0.40, 1.00) | 1.72 (1.27, 2.32) | |
| 6–12 months | No | 491 | 0. | 1. | 1. |
| Yes | 212 | −4 (−9, 3) | 0.76 (0.53, 1.22) | 1.42 (1.14, 1.76) | |
| ≥ 12 months | No | 643 | 0. | 1. | 1. |
| Yes | 335 | −2 (−10, 6) | 0.91 (0.55, 1.32) | 1.12 (0.82, 1.54) |
aWeighted for episode number, socioeconomic status,29,30 maternal education, child sex, caesarean birth, low birthweight, preterm birth, hospitalization at birth, antibiotics given at birth and characteristics of the last diarrhoea episode: age, Vesikari score,25 duration, hospitalization, fever, dehydration, bloody diarrhoea, underweight, stunted, wasted, exclusive and any breastfeeding, zinc given, number of previous antibiotic courses for any illnesses, number of sick days between episodes and other antibiotics given between episodes. The mean weight was 1.01 with range 0.29–16.18; after censoring at the 0.05th and 99.5th percentiles, the mean was 0.99 with range 0.31–5.77.
bAssumes proportional hazards.
The effect of antibiotic treatment of the second diarrhoea episode on time to third diarrhoea was similar, whereas effects in later episode pairs were smaller (Figure 2B, Table 3). The overall adjusted time difference and ratio when collapsing all episode pairs were −4 weeks (95% CI: −9, 0) and 0.71 (95% CI: 0.44, 0.96), respectively (Figure 2C, Table 3).
Effect measure modification
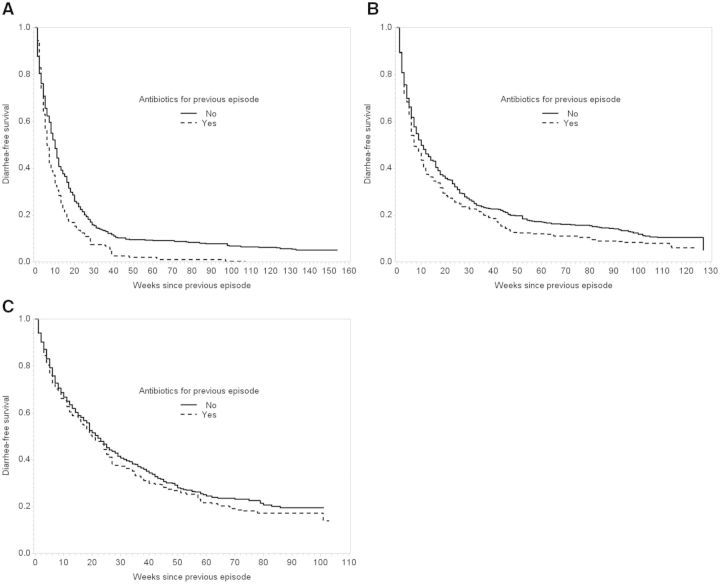
The effect of antibiotics on time to next diarrhoea was greatest among children who were treated with antibiotics for diarrhoea under 6 months of age compared with antibiotic treatment between 6 months and 1 year and after 1 year of age (Figure 3, Table 3). A shorter time to next diarrhoea was observed for both cotrimoxazole (MTD: −1, 95% CI: −7, 2) and cephalosporins (MTD: −3, 95% CI: −9, 0) compared with no antibiotics, though the effect was smaller for cotrimoxazole (Figure S2, Table S1, available as Supplementary data at IJE online).
Figure 3.
Stratified by age at first diarrhoea episode, inverse probability of treatment-weighted Kaplan-Meier curves for time to next diarrhoea episode among 430 children from Vellore, Tamil Nadu, 2009–13. A. First diarrhoea and antibiotic treatment below 6 months of age. B. First diarrhoea and antibiotic treatment between 6 months and 1 year of age. C. First diarrhoea and antibiotic treatment after 1 year of age.
Sensitivity analyses
Results under alternative exposure definitions were consistent with the main analyses, though the effect size diminished as the definitions became less sensitive and more specific (Figure S1, Table S1, available as Supplementary data at IJE online). When excluding all previous episodes with greater than 7 days’ duration, diarrhoea-free survival curves were similar to main analyses, and time differences and ratios were slightly larger in magnitude (Table S2, available as Supplementary data at IJE online).
When subsequent diarrhoea occurred, the average Vesikari score and duration of the second episode were slightly lower among children who were treated with antibiotics during their first episode compared with those who were not (Table S3, available as Supplementary data at IJE online). Correspondingly, the risks for a severe or prolonged/persistent second diarrhoea episode were lower among these children. However, the absolute differences in severity and duration were small (less than one point on the Vesikari scale and less than 1 day, respectively) and imprecise since few episodes were severe (10.4%) or of long duration (11.5%). The results were consistent when restricting to episodes which occurred under 6 months of age and when including all episode pairs (not shown).
To validate our findings, we analysed data from two previous cohorts conducted at this site.35–37 One study36 was conducted from 2008 to 2011 and included 160 children with at least one diarrhoea episode. Prevalence of antibiotic treatment of diarrhoea was lower, at 6.4% (50 of 785 total episodes with antibiotic treatment information). The second study,35,37 conducted from 2002 to 2006, included 390 children who had at least one diarrhoea episode. Of 1812 diarrhoea episodes with known antibiotic treatment, 27.7% (n = 502) were treated with antibiotics. Information on antibiotic treatment for other illnesses was missing. The weighted Kaplan-Meier curves including all episode pairs from these earlier studies were consistent with the results from the main study. Combining all three cohorts, children who were treated with antibiotics for their first diarrhoea episode had their second episode 3 weeks (MTD: −3, 95% CI: −7, 1) or 20% (MTR: 0.80, 95% CI: 0.53, 1.07) earlier than children who were not treated with antibiotics (Figure S3, available as Supplementary data at IJE online).
Discussion
This study provides the first evidence that antibiotic treatment of diarrhoea may shorten the time between episodes, especially among younger infants. These results are directly applicable to diarrhoea treatment decisions, since antibiotic treatment is not essential for most cases of diarrhoea. Specifically, according to Integrated Management of Childhood Illness (IMCI) protocols,38 antibiotic treatment was likely not indicated for a majority of cases in this study since only few episodes (0.9%) were associated with bloody stools. Antibiotics are a well-known cause of antibiotic-associated diarrhoea,21 and we provide further support for a sustained impact of antibiotics on diarrhoeal risk. These results, which focus on antibiotic treatment of diarrhoea specifically, are consistent with our recent work demonstrating that any antibiotic exposure early in life is associated with increased diarrhoeal rates.39
Antibiotic treatment of diarrhoea had the greatest impact on time to next episode during the first two diarrhoea episodes. This difference in effect may be due to young age at earlier episodes and high overall antibiotic exposure by the time of later episodes. The substantial increases in magnitude of the adjusted effects compared with the crude effect are largely due to removing confounding by age. Because the microbiota is underdeveloped and more susceptible to disturbances during infancy, antibiotic exposures at the youngest ages may have the largest impact on the microbiota, and correspondingly on diarrhoeal risk.12,40 In addition, because of the high rates of antibiotic use in this population, four-fifths (83%) of the population had prior exposure to antibiotics by the third diarrhoea episode. We hypothesize that antibiotics for diarrhoea are likely to have the largest impact when they represent a majority of total antibiotic exposures, which occurs at earlier episodes and younger ages.
The difference in effect on diarrhoeal risk between cotrimoxazole and cefixime may result from their different spectrums of activity. Cotrimoxazole is broad-spectrum, but notably does not affect anaerobes41 which dominate the gut microbiota.42 Conversely, anaerobes are sensitive to cefixime, and this drug is also more effective against Gram-negative bacteria (especially Enterobacteriaceae) common in the gut.41 Correspondingly, diarrhoea as a drug-related adverse event is more commonly reported for cefixime (15–20%) compared with cotrimoxazole (< 1–10%).41,43 Similarly, cephalosporins are one of the predominant drug classes noted to cause antibiotic-associated diarrhoea.44,45 The activity of cefixime against intestinal anaerobes may result in greater disruption of the gut microbiota and increased susceptibility to diarrhoeal pathogens.
In the minority of diarrhoea episodes of bacterial aetiology and for which antibiotics may have been indicated, the reduction in time to subsequent diarrhoea may alternatively have been due to a temporary benefit of antibiotics followed by the recrudescence of the causative and antibiotic-susceptible agents, resulting in a second diarrhoea episode.
As in any observational study, there is the potential for bias due to uncontrolled confounding, including by local environmental factors associated with force of transmission and pathogen-specific effects on the microbiome. However, this cohort has the advantage of a detailed record of illness characteristics that were likely the main indications for treatment. This study was limited by potential misclassification of exposure due to caregiver-reported treatment information. However, we also incorporated antibiotic prescriptions from clinic records, which likely captured the majority of antibiotic exposures since the clinic was located in the study area and provided free care and medicines. There was concordance between caregiver-reported and antibiotic prescriptions for diarrhoea: 78% of antibiotic prescriptions during diarrhoea episodes were associated with caregiver-reported antibiotic treatment. Further, our results were consistent when we used alternative definitions of antibiotic exposure in sensitivity analyses.
Because there were few severe illnesses in our cohort, we considered diarrhoea incidence the main outcome of interest. Antibiotic treatment was associated with slightly lower severity and duration of subsequent diarrhoea episodes, but the differences were small and imprecise. Antibiotic treatment of diarrhoea may also have unintended consequences for other illnesses such as respiratory infections, and other potential effects should be taken into account when making treatment decisions.
By providing evidence that antibiotics may cause direct harm to children through an association with decreased time to future diarrhoea episodes, these findings counter a commonly held assumption among doctors and caregivers that even if antibiotics are not strictly indicated, ‘at least they can’t hurt’.11 Rational use of antibiotics has been advocated to reduce antimicrobial resistance at the population level, and rational use might also decrease future diarrhoeal risk among treated patients.
Supplementary Data
Supplementary data are available at IJE online.
Funding
The parent study was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health [5-R01-AI072222 to H.D.W.]. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health [5-T32-AI070114-08 to E.T.R and D43-TW007392 to D.K.].
Supplementary Material
Acknowledgements
We thank the participants, study staff members and medical teams for their participation and support. We also thank the study clinic doctors and nurses, Jenipha Elizabeth for clinic record data entry, and Stephen R Cole for guidance with the analysis.
Conflicts of interest: S.B.D. has held investigator-initiated research grants with Pfizer, and from Merck for research studies completely unrelated to the submitted work. D.J.W. engages in occasional, ad hoc consulting on epidemiological methods for NIH/NICHD—there is no overlap with the present work. All other authors have no conflicts of interest.
References
- 1.Walker CLF, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013;381:1405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers . Geneva: WHO, 2005. [Google Scholar]
- 3.Bhatnagar S, Lodha R, Choudhury P, et al. IAP Guidelines 2006 on management of acute diarrhoea. Indian Pediatr 2007;44:380–89. [PubMed] [Google Scholar]
- 4.World Health Organization. The Rational Use of Drugs in the Management of Acute Diarrhoea in Children . Geneva: WHO, 1990. [Google Scholar]
- 5.Diniz-Santos DR, Silva LR, Silva N. Antibiotics for the empirical treatment of acute infectious diarrhoea in children. Braz J Infect Dis 2006;10:217–27. [DOI] [PubMed] [Google Scholar]
- 6.Indira KSK, Chandy SJ, Jeyaseelan L, Kumar R, Suresh S. Antimicrobial prescription patterns for common acute infections in some rural and urban health facilities of India. Indian J Med Res 2008;128:165–71. [PubMed] [Google Scholar]
- 7.Mishra D, Sethi M, Mantan M. Factors affecting antibiotic prescribing pattern in pediatric practice. Indian J Pediatr 2007;74:513. [DOI] [PubMed] [Google Scholar]
- 8.Chakraborti S, Barik KL, Singh AK, Nag SS. Prescribing practices of doctors in management of acute diarrhoea. Indian Pediatr 2011;48:811–12. [DOI] [PubMed] [Google Scholar]
- 9.Kotwani A, Chaudhury RR, Holloway K. Antibiotic-prescribing practices of primary care prescribers for acute diarrhoea in New Delhi, India. Value Health 2012;15(Suppl 1):S116–19. [DOI] [PubMed] [Google Scholar]
- 10.International Institute for Population Sciences (IIPS) and Macro International. National Family Health Survey (NFHS-3), 2005-06. Vol I Mumbai, India: IIPS, 2007. [Google Scholar]
- 11.Stewardson AJ, Huttner B, Harbarth S. At least it won’t hurt: the personal risks of antibiotic exposure. Curr Opin Pharmacol 2011;11:446–52. [DOI] [PubMed] [Google Scholar]
- 12.Johnson CL, Versalovic J. The human microbiome and its potential importance to pediatrics. Pediatrics 2012;129:950–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol 2011;9:233–43. [DOI] [PubMed] [Google Scholar]
- 14.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 2008;6:e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010;156(Pt 11):3216–23. [DOI] [PubMed] [Google Scholar]
- 16.Martin R, Nauta AJ, Ben Amor K, Knippels LMJ, Knol J, Garssen J. Early life: gut microbiota and immune development in infancy. Benef Microbes 2010;1:367–82. [DOI] [PubMed] [Google Scholar]
- 17.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012;489:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev 2010;90:859–904. [DOI] [PubMed] [Google Scholar]
- 19.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature 2012;489:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 2013;13:790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McFarland LV. Antibiotic-associated diarrhoea: epidemiology, trends and treatment. Future Microbiol 2008;3:563–78. [DOI] [PubMed] [Google Scholar]
- 22.Coté GA, Buchman AL. Antibiotic-associated diarrhoea. Expert Opin Drug Saf 2006;5:361–72. [DOI] [PubMed] [Google Scholar]
- 23.Alam S, Mushtaq M. Antibiotic associated diarrhoea in children. Indian Pediatr 2009;46:491–96. [PubMed] [Google Scholar]
- 24.Kattula D, Sarkar R, Sivarathinaswamy P, et al. The first 1000 days of life: Pre- and post-natal risk factors for morbidity and growth in a birth cohort in southern India. BMJ Open 2014;4:e005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis 1990;22:259–67. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. WHO Ghild Growth Standards: Length/height-for-age, Weight-for-age, Weight-for-length, Weight-for-height and Body Mass Index-for-age, Methods and Development. Geneva:WHO, 2006. [Google Scholar]
- 27.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004;75:45–49. [DOI] [PubMed] [Google Scholar]
- 28.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10(1):37–48. [PubMed] [Google Scholar]
- 29.Kuppuswami B. Manual of Socioeconomic scale (Urban). New Delhi: Manasayan, 1981. [Google Scholar]
- 30.Mahajan B, Gupta M. Textbook of Preventive and Social Medicine . 2nd edn Delhi: Jaypee, 1995. [Google Scholar]
- 31.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ. Splines for trend analysis and continuous confounder control. Epidemiology 2011;22:874–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008Sep;168:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westreich D, Cole SR, Tien PC, et al. Time scale and adjusted survival curves for marginal structural Cox models. Am J Epidemiol 2010;171:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cole SR. Simple bootstrap statistical inference using the SAS system. Comput Methods Programs Biomed 1999;60:79–82. [DOI] [PubMed] [Google Scholar]
- 35.Gladstone BP, Das AR, Rehman AM, et al. Burden of illness in the first 3 years of life in an Indian slum. J Trop Pediatr 2010;56:221–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarkar R, Sivarathinaswamy P, Thangaraj B, et al. Burden of childhood diseases and malnutrition in a semi-urban slum in southern India. BMC Public Health 2013;13:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gladstone BP, Muliyil JP, Jaffar S, et al. Infant morbidity in an Indian slum birth cohort. Arch Dis Child 2008;93:479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization (WHO). Integrated Management of Childhood Illness. Geneva: World Health Organization, 2014. [Google Scholar]
- 39.Rogawski ET, Westreich DJ, Becker-Dreps S, et al. The effect of early life antibiotic exposures on diarrhoeal rates among children in Vellore, India. Pediatr Infect Dis 2015. PMID: 25742244. (Epub ahead of print.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saavedra JM, Dattilo AM. Early development of intestinal microbiota: implications for future health. Gastroenterol Clin 2012;41:717–31. [DOI] [PubMed] [Google Scholar]
- 41.Gilbert D, Moellering Jr R, Eliopoulos G, Chambers H, Saag M. (eds). The Sanford Guide to Antimicrobial Therapy. 40th edn Sperryville, VA: Antimicrobial Therapy Inc., 2010. [Google Scholar]
- 42.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl 2003;91:48–55. [DOI] [PubMed] [Google Scholar]
- 43.Mitropoulos IF, Rotschafer JC, Rodvold KA. Adverse events associated with the use of oral cephalosporins/cephems. Diagn Microbiol Infect Dis 2007;57(Suppl 3):67S–76S. [DOI] [PubMed] [Google Scholar]
- 44.Bergogne-Bérézin E. Treatment and prevention of antibiotic associated diarrhoea. Int J Antimicrob Agents 2000;16:521–26. [DOI] [PubMed] [Google Scholar]
- 45.Chassany O, Michaux A, Bergmann JF. Drug-induced diarrhoea. Drug Saf 2000;22:53–72. [DOI] [PubMed] [Google Scholar]
- 46.Brick T, Primrose B, Chandrasekhar R, Roy S, Muliyil J, Kang G. Water contamination in urban south India: household storage practices and their implications for water safety and enteric infections. Int J Hyg Environ Health 2004;207:473–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.