Abstract
Ambient ozone (O3) levels are associated with cardiovascular morbidity and mortality, but the underlying pathophysiological mechanisms driving extrapulmonary toxicity remain unclear. This study examined the coronary vascular bed of rats in terms of constrictive and dilatory responses to known agonists following a single O3 inhalation exposure. In addition, serum from exposed rats was used in ex vivo preparations to examine whether bioactivity and toxic effects of inhaled O3 could be conveyed to extrapulmonary systems via the circulation. We found that 24 h following inhalation of 1 ppm O3, isolated coronary vessels exhibited greater basal tone and constricted to a greater degree to serotonin stimulation. Vasodilation to acetylcholine (ACh) was markedly diminished in coronary arteries from O3-exposed rats, compared with filtered air-exposed controls. Dilation to ACh was restored by combined superoxide dismutase and catalase treatment, and also by NADPH oxidase inhibition. When dilute (10%) serum from exposed rats was perfused into the lumen of coronary arteries from unexposed, naïve rats, the O3-induced reduction in vasodilatory response to ACh was partially recapitulated. Furthermore, following O3 inhalation, serum exhibited a nitric oxide scavenging capacity, which may partially explain blunted ACh-mediated vasodilatory responses. Thus, bioactivity from inhalation exposures may be due to compositional changes of the circulation. These studies shed light on possible mechanisms of action that may explain O3-associated cardiac morbidity and mortality in humans.
Keywords: cardiovascular system, cardiopulmonary, inhalation toxicology, respiratory toxicology, endothelium
Ambient ozone (O3) exposure is associated with cardiovascular disease outcomes in several recent epidemiological studies (Nuvolone et al., 2013; Raza et al., 2014), although some controversy exists owing to covariate uncertainty and the shortage of documented biological plausibility. Recent toxicological studies with controlled exposures to O3 provide new evidence for systemic effects of O3, but the mechanism of vascular dysfunction is unclear (Chuang et al., 2009; Robertson et al., 2013). Controlled human exposures offer conflicting evidence as to a cardiovascular impact of O3. Devlin et al. (2012) exposed healthy human volunteers to 0.3 ppm O3 for 2 h and found slight, but significant, exposure-related alterations in circulating interleukin-8 and plasminogen activator inhibitor-1, along with reduced high frequency heart rate variability and modestly increased QT interval at up to 24-h postexposure (Devlin et al., 2012). More recently, however, Barath et al. (2013) found no alterations in endothelial function in humans up to 8-h postexposure to 0.3 ppm O3 for 75 min, using a well -established and sensitive clinical model (Barath et al., 2013). Thus, there remains considerable debate regarding the plausibility for O3 to promote extrapulmonary toxicity.
Numerous other pollutants, such as particulate matter (PM) and combustion–source mixtures (eg, diesel emissions, tobacco smoke), also induce endothelial dysfunction beyond the pulmonary bed (Campen et al., 2005; Conklin et al., 2009; Sun et al., 2005). While the cellular mechanisms of endothelial dysfunction include roles for endothelin receptors and nitric oxide (NO) synthase, the underlying pathways leading from the lungs to the systemic vasculature remain unknown (Cherng et al., 2009, 2011). While complex emissions contain compounds that can directly translocate into the blood and contact endothelial cells, O3 is rapidly scavenged in the epithelial lining fluid of the airways and only rarely comes in direct contact with airway cells, much less penetrates to the circulation (Postlethwait et al., 1994; Pryor 1992). Thus, in addition to being an environmental concern, O3 is also a model pollutant to better understand how pulmonary interactions with inhaled pollutants may result in systemic vascular outcomes.
Despite the lack of translocation and direct contact with vascular cells, O3 inhalation has been consistently found to induce vascular impairments (Chuang et al., 2009; Robertson et al., 2013) and functional hemodynamic and cardiac alterations. (Tankersley et al., 2013; Wagner et al., 2014; Watkinson et al., 2001) Thus, secondary reactants and by-products appear necessary to convey toxicity to the systemic vasculature. The scavenger receptor CD36 appears to have an important role in mediating the impaired endothelial function when activated by as yet unknown circulating factors that are generated following O3 exposure (Robertson et al., 2013). However, we have previously demonstrated a role for the lectin-like receptor for oxidized low-density lipoprotein (LOX-1) in mediating systemic effects of fresh engine emissions (Lund et al., 2011). Yet, it is unknown how selective the circulating ligand(s) are in terms of activating specific receptors. We hypothesize that the resultant loss of vasodilation is due to loss of NO resulting from either NO synthase impairment by NADPH oxidase-derived intracellular reactive oxygen species (ROS), as results from LOX-1 activation (Lund et al., 2011), or by scavenging of already-generated NO. To test this, we implemented a complementary in vivo/ex vivo study to ascertain mechanisms of endothelial cell dysfunction and the potential for circulating factors to alter endothelial cell physiology or affect NO bioavailability.
MATERIALS AND METHODS
Animals
A total of 65 male Sprague-Dawley rats, aged 8–12 weeks, were obtained from a commercial vendor (Charles River Laboratories) and used for all experiments. Upon arrival, rats were housed 2 per cage under controlled environmental conditions (21 ± 2°C; 12 h light/dark cycle) with access to tap water and standard chow ad libitum (Harlan). All protocols were reviewed and approved by the University of New Mexico Institutional Animal Care and Use Committee to ensure safety and humane treatment of animal subjects. Rats were euthanized by exsanguination via cardiac puncture while under anesthesia (isoflurane; concentration 1.5–2% in oxygen).
Exposures
O3 was generated using an OREC silent arc discharge O3 generator (Osmonics, Phoenix, Arizona) with room air supply. O3 concentrations, along with concentrations of carbon monoxide and oxides of nitrogen, were continuously monitored using a photometric O3 analyzer (TG-501, GrayWolf, Shelton, Connecticut) and temperature was maintained at 21 ± 2°C. Total oxides of nitrogen were monitored to confirm that no nitrogen-related contaminants were generated. Rats were randomly assigned a group and exposed to either filtered air (FA) or 1 ppm O3 for 4 h. During exposures, the rats were singly housed within a sealed chamber (Biospherics) with no bedding and an elevated, stainless steel flooring. Food, but not water, was withheld during the 4 h exposure period to preclude ingestion of ozonation products. Rats were euthanized for tissue collections 24 h after exposure.
Collection and analysis of bronchoalveolar lavage fluid
Bronchoalveolar lavage (BAL) fluid was analyzed for pulmonary inflammatory responses of rats following inhalation. In brief, rats were euthanized and the lungs were lavaged 4 times with 8 ml of sterile saline. BAL fluid was centrifuged at 1800 × g for 5 min. The supernatant from the first lavage was stored at −80°C until required for biochemical determination. Total protein content was assessed with a bicinchoninic acid assay kit (Pierce, Rockford, Illinois). The cell pellets from all lavages were resuspended in 1.0 ml of physiological saline and combined. Total cell numbers were determined and 10 000 cells were centrifuged onto cytospin slides for differential staining as described (Robertson et al., 2013).
Blood analysis
Blood was taken from the right ventricle immediately before kill and stored in ice-cooled tubes containing EDTA. The mean values of different hematological parameters; white blood cells (WBC), red blood cells (RBC), and platelets; were measured with the CoulterAc.T diff2 analyzer (Beckman Coulter, Miami, Florida). Additional blood was collected into serum separator tubes and centrifuged at 10 000× g for 5 min to isolate serum, which was stored at −80°C until use. Serum nitrate/nitrite (Cayman Chemical, Ann Arbor, Michigan) and myeloperoxidase (Abcam, Cambridge, Massachusetts) levels were measured with commercially available kits, according to manufacturer’s instructions.
Ex vivo vascular function using myography
Septal coronary arteries were isolated 24 h after exposure, and cleaned of connective tissue in ice-cold PBS, as previously described (Campen et al., 2005; Cherng et al., 2009). Vessels were mounted between 2 opposing pipettes, secured with 12-0 monofilament silk suture, and luminally perfused with a physiological saline solution (PSS) and pressurized to physiologic levels (60 mmHg). Arteries were equilibrated at 37°C for 45 min prior to serotonin (5-HT) constriction or acetylcholine (ACh; 0.001–100 μmol/l) in U46619 (a thromboxane mimetic) preconstricted arteries (constricted to ∼50% of fully relaxed diameter). Measurements of vessel internal diameter were recorded and analyzed offline.
To assess myogenic tone responses to increasing intraluminal pressure, vessel diameter was assessed at progressive pressure steps (20, 40, 60, 80, 100, 120 mmHg) in normal PSS, then the steps were repeated in a Ca2+-free PSS (containing 3.7 mmol/l EGTA) to fully relax the vessel (Earley and Walker, 2002). The role of intracellular ROS in mediating endothelial dysfunction after O3 exposure was assessed by luminal perfusion with the cell-permeable superoxide dismutase (PEG-SOD, 150 U/ml), catalase (CAT), or NADPH oxidase inhibitor apocynin (30 and 100 μM). Active tone (myogenic or drug-induced) was calculated as the % change in inner diameter: myogenic tone = [diameter (−Ca2+) − diameter (+Ca2+)]/diameter (−Ca2+) × 100; percent constriction = [diameter (−drug) − diameter (+drug)]/diameter (−drug) × 100 (Cherng et al., 2009).
Measurement of NO by electron paramagnetic resonance
Serum samples from O3- and FA-exposed rats were incubated with the iron-chelate NO-spin trap, Fe2+-di(N-methyl-d-glutaminedithiocarbamate) (Fe2+(MGD)2; 1 mM, final concentration) and the NO donor, spermine NONOate (0.33 mM, final concentration), for 10 min. The iron-chelate, Fe2+(MGD)2, was freshly prepared by mixing a stock solution of ferrous sulfate (20 mM, dissolved in deionized water under N2) and an equal volume of Sodium N-methyl-d-glucamine dithiocarbamate (NaMGD; 100 mM, dissolved in deionized water under N2) to give a molar ratio of 1:5, respectively, prior to each experiment. Following the incubation period, the incubation medium (400 mL) containing spin trapped NO was immediately transferred into custom-made gas permeable Teflon tubing (Zeus Industries, Raritan, New Jersey), folded 4 times, and inserted into a quartz electron paramagnetic resonance (EPR) tube open at each end. The quartz EPR tube was inserted within the cavity of a Bruker EleXsys 540 X-band EPR spectrometer (Billerica, Massachusetts) operating at 9.8 GHz and 100 kHz field modulation and spectra was recorded after spectrometer tuning at room temperature. The EPR spectrum was acquired with a scan time of 40 s, and 10 scans were obtained and averaged to produce significant signal-to-noise ratio. Instrument settings were as follows: magnetic field, 3440 G; scan range, 100 G; microwave power, 21 mW; modulation frequency, 100 kHz; modulation amplitude, 1.0 G; time constant, 20 ms. The EPR spectra were collected, stored, and processed using the Bruker Software Xepr (Billerica). NO levels were quantified and peak-to-peak measurements were taken and expressed in relative units.
Biotin-switch assay detection of NO scavenging in serum
A modified biotin-switch assay based on Jaffrey and Snyder (2001) was performed using O3-exposed (n = 4) and FA-treated (n = 3) rat serum. In brief, free serum protein thiols were blocked and S–NOs were reduced and labeled with biotin using an S-nitrosylation Protein Detection Assay Kit (Cayman Chemical). Total protein in each serum sample was then quantified using a spectrophotometer (λ = 280 nm). Protein (100 µg) was loaded per well for SDS-PAGE prior to Western blotting. Membranes were blocked in 2% BSA for 1 h and subsequently incubated with S-Nitrosylation Detection Reagent (HRP) in 2% BSA for 1 h. Membranes were developed using HYGLO Chemiluminescent Quick Spray (Denville Scientific, Denville, New Jersey) and visualized using a ProteinSimple imager (ProteinSimple, San Jose, California). Bands were then quantified using ImageJ.
Statistical analysis
Data are expressed as the mean ± standard deviation of the mean (SD) for each group, except in figures where standard error of the mean (SEM) is used. Vascular responses to agonists are expressed as percentage of the preconstriction to EC50 U-46619, with 100% representing basal tension. Statistical comparisons for vascular responses were performed by Student’s 2-sample t test or 2-way repeated measures analysis of variance (ANOVA) with Bonferroni post hoc tests. Other data comparisons, such as serum-induced tone, were conducted with a Student’s ttest. Statistical analyses were performed using GraphPad Prism software (V5.0; GraphPad Software Inc., USA). P < .05 was considered to be statistically significant (with appropriate Bonferroni adjustments). Sample numbers for specific assays are described in the figure legends.
RESULTS
Ozone Inhalation Induces Pulmonary Inflammation
Inhalation of 1 ppm O3 for 4 h caused predictable increases in BAL cellularity 24 h post exposure. Significant elevation in total cells, driven predominantly by neutrophil influx, was observed, along with elevations in total lavage protein (Fig. 1A–C). Furthermore, a modest but significant neutrophilia was observed in the whole blood, indicating a systemic inflammation (Fig. 1D). The neutrophilia was accompanied by a nonsignificant trend (P = .053 by Student’s t test) toward higher myeloperoxidase levels in O3-exposed rats (mean ± SD = 1.03 ± 0.48, n = 12) compared with control rats (0.73 ± 0.15, n = 12).
FIG. 1.
Induction of pulmonary inflammation and circulating neutrophilia by O3 exposure. O3 significantly induced bronchoalveolar lavage cellularity (A), neutrophil count (B), and total protein (C). In addition, O3 induced a significant increase in the percentage of circulating neutrophils (NE) and macrophages (MO), but not lymphocytes (LY), eosinophils (EO), or basophils (BA) in whole blood analysis (D). Asterisks (*) indicate significant difference from FA control rats by unpaired Student’s t test (P < .05; N = 5 per group for A and C; N = 3 per group for B and D).
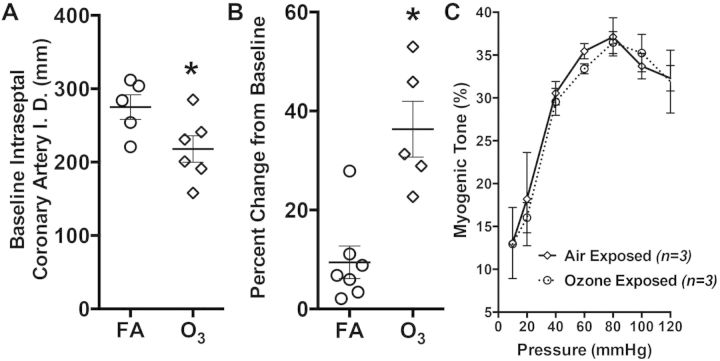
Ozone Inhalation Enhances Tone at Baseline
We first compared basal tone in coronary arteries obtained from FA or O3-exposed rats. Following a 30-min recovery from the isolation, cleaning, and mounting process, we noted that the overall diameters of vessels obtained from O3-exposed rats (217 ± 44 μm) were significantly smaller than the FA-exposed control arteries (275 ± 38 μm; Fig. 2A). Because the rats were randomized upon delivery and the exposures were acute, unlikely to induce remodeling, we examined more closely the change in diameter from initial (pretone) mounting to the complete development of resting tone. Here, coronary arteries from O3-exposed rats displayed a greater change in tone (34.3 ± 12.6 μm) compared with FA-exposed controls (9.4 ± 8.7 μm; Fig. 2B). Thus, initial resting diameters of the vessels were not substantially different, but O3 exposure led to a phenotype of enhanced basal tone during equilibration. Myogenic tone in response to pressurization did not appear different between the FA- and O3-exposed rats across a range of luminal pressures (20–120 mmHg; Fig. 2C).
FIG. 2.
A, Baseline coronary artery internal diameter (I.D.) and B, percent increase in tone following isolation and mounting in vessels obtained from rats exposed to FA or O3. Asterisks (*) indicate significant difference from FA control rats by unpaired Student’s t test (P < .05; N = 5–6 per group). C, Myogenic tone in coronary arteries did not appear affected by O3 across luminal pressures ranging from 20 to 120 mmHg. No significant differences were noted by a 2-factor (pressure, exposure) repeated measures ANOVA (N = 3 per group).
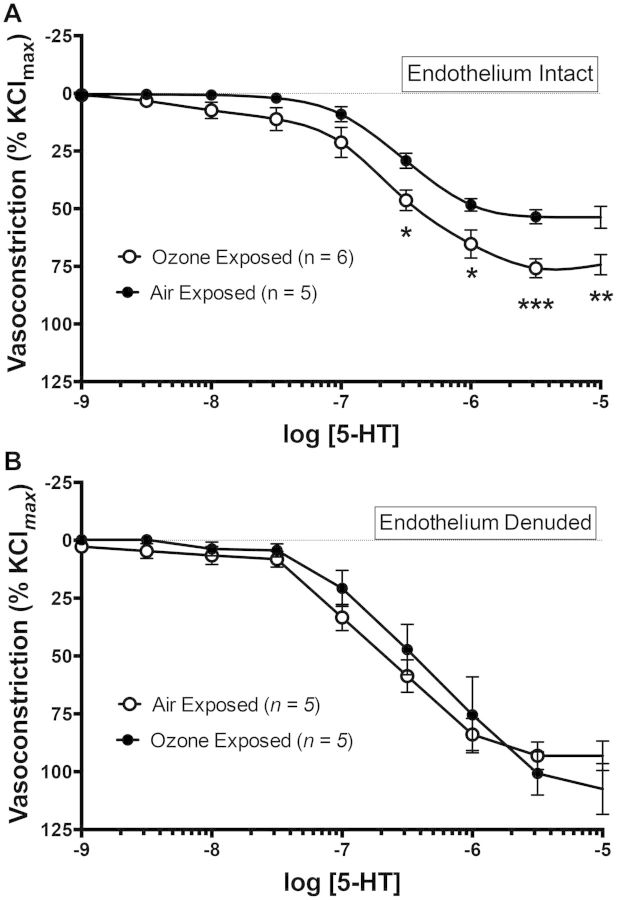
Ozone Inhalation Enhances Constrictive Response to Serotonin
Coronary arteries from both FA- and O3-exposed rats were treated with increasing concentrations of 5-HT (10−9–10−5 M), leading to a concentration-dependent reduction in internal diameter (Fig. 3A). This constriction was significantly enhanced in coronaries from O3-exposed rats compared with coronaries from FA-exposed rats. A 2-way repeated measures ANOVA revealed a significant exposure effect of P < .001 and a significant interaction between exposure and agonist of P = .0023, with specific concentrations of 5-HT ranging from 10−6.5 to 10−5 M causing significantly greater constriction in the O3-exposed coronaries as assessed by a Bonferroni multiple comparison test. EC50 values for 5-HT appeared relatively similar between FA-exposed (−6.51 ± 0.08 logM) and O3-exposed rats (−6.61 ± 0.09 logM).
FIG. 3.
A, Coronary artery constriction to serotonin (5-HT) is enhanced in rats exposed to 1 ppm O3 for 4 h in endothelium-intact vessels (ANOVA interaction P = .0023). B, This difference was abolished in endothelium-denuded coronary arteries, suggesting that endothelial cells are the primary source of O3-induced vascular dysfunction. Asterisks (*) indicate significant difference from FA control rats by a repeated measures 2-way ANOVA with Bonferroni’s multiple comparison post hoc test (*P < .05, **P < .01, ***P < .001; N = 5–6 per group).
This finding was tested in a second cohort wherein the endothelium of mounted coronary arteries was disrupted by gently rubbing the lumen with a coarse fiber of moose mane. In endothelium-disrupted coronary arteries from both FA- and O3-exposed rats, 5-HT responses were significantly enhanced compared with endothelium-intact vessels (Fig. 3B). However, the difference between exposure groups was no longer observed, suggesting that the sensitizing effect of O3 on 5-HT-induced constriction was endothelium dependent.
Ozone Impairs Dilatory Response to ACh via a Superoxide-Related Mechanism
O3 exposure led to a substantial impairment in the vasodilatory response to ACh. While FA control vessels responded to ACh with an almost 100% reversal of the pre-constriction tone, coronary arteries from O3-exposed rats exhibited little if any response to this agonist (Fig. 4A). Cotreatment with polyethylene glycol-superoxide dismutase (PEG-SOD) led to a partial restoration of the dilatory response to ACh (Fig. 4B), and combined PEG-SOD with CAT fully restored the dilation (Fig. 4C), suggesting that superoxide is formed intracellularly and acts to impair normal activation of the downstream dilatory pathways. Lastly, we assessed the ability of apocynin, an NAPDH oxidase inhibitor, to improve vasodilatory response to ACh. At a sufficient concentration (100 μM, but not 30 μM), full vasodilation was restored, suggesting that NADPH oxidase-derived radicals may play a role in O3-induced endothelial dysfunction (Fig. 4D).
FIG. 4.
A, Coronary artery dilation to ACh is diminished in rats exposed to 1 ppm O3 for 4 h in endothelium-intact vessels (ANOVA interaction P < .0001). B, O3-induced impairments in coronary artery dilation to ACh is partially restored by ex vivo treatment with superoxide dismutase (PEG-SOD; A, N = 4 per group; ANOVA interaction P = .0382 comparing FA PEG-SOD vs O3 PEG-SOD). C, Addition of CAT fully restores normal vasodilation (N = 4 per group), suggesting that endothelial dysfunction is related to endothelial ROS. D, O3-induced impairment of vasodilation was restored by treatment with 100 μM apocynin, suggesting that endothelial dysfunction may be in part due to NADPH oxidase-derived ROS. Asterisks (*) indicate significant difference from FA control rats by a repeated measures 2-way ANOVA with Bonferroni’s multiple comparison post hoc test (*P < .05, **P < .01, ***P < .001).
Ozone Inhalation Creates Serum Bioactivity That Impairs Vascular Function
We have previously observed that serum, to which endothelial cells are constantly in contact, from pollutant-exposed humans and mice can cause bioactivity that negatively impacts endothelial cells (Channell et al., 2012; Robertson et al., 2013). Here, we luminally infused serum derived from FA or O3-exposed (1 ppm × 4 h) rats into naïve isolated coronary arteries (ie, coronaries were obtained from nonexposed rats) to test whether the serum contents can diminish ACh-induced dilation. Following luminal incubation for 30 min, we found that serum from FA-exposed rats, diluted to 10% in PSS, had minimal effects on the ability of the coronary artery to dilate in response to ACh. However, a significant diminution in this response was observed in the presence of serum from O3-exposed rats (Fig. 5A). ACh EC50 values were significantly reduced in coronaries treated with serum from O3-exposed rats (−6.10 ± 0.21), compared with vessels perfused with serum from FA-exposed rats (−6.42 ± 0.09). Interestingly, we also noticed a general increase in vascular tone upon infusion with the 10% serum (ie, before ACh treatment), and ultimately this constriction was greater in the presence of serum from O3-exposed rats than serum from FA-exposed rats (Fig. 5B).
FIG. 5.
A, Infusion of 10% serum (in PSS) from rats exposed to O3 into the lumen of isolated naïve (unexposed) coronary arteries induced a significant impairment in vasodilation to ACh compared with vessels infused with 10% serum from FA-exposed rats (ANOVA interaction P = .005). B, Similarly, the serum from O3-exposed rats, in a 10% mixture with PSS, induced spontaneous constriction in naïve coronary arteries that was significantly greater than that induced by serum from FA-exposed rats (P < .05 by Student’s t test).
Ozone-Induced Serum Modifications May Impact NO Species Bioavailability
Serum nitrite+nitrate levels were found to be significantly reduced in O3-exposed rats compared with controls (Fig. 6A). Previous studies with titanium dioxide nanoparticles suggested that NO may be directly scavenged or consumed (Nurkiewicz et al., 2009), thus we tested whether the serum itself following O3 exposure could impact the bioavailability of NO using an acellular EPR spin trap assay. Concentrations of FA- or O3-exposed serum ranging from 0 to 6.67% in an iron-free media were prepared and incubated with the NO donor, spermine NONOate, for 10 min. NO concentrations were found to be significantly reduced in the presence of serum from O3-exposed rats compared with serum from FA-exposed rats (Fig. 6B). A decreasing trend in NO concentrations with increasing percentage of serum was noted, suggesting that this assay may be optimal with dilute serum (<10%), but the magnitude of effect appeared consistent at several concentrations attempted. The reduced NO concentrations may explain the loss of vasodilatory function in isolated vessels, owing to an increased scavenging of NO.
FIG. 6.

A, Serum levels of nitrate and nitrite from FA and ozone-exposed mice. B, Serum from O3-exposed rats exhibits a nitric oxide scavenging property as measured by EPR. At varying concentrations in an iron-free culture media, significantly less nitric oxide (donated by spermine NONOate) could be measured in solutions with serum from O3-exposed rats, as compared with serum from FA-exposed rats (N = 3 per exposure per concentration). However, the concentration of serum also appeared to affect NO availability, indicating that optimal concentrations exist to delineate this effect. C, In addition, a biotin-switch assay was performed on the serum, revealing numerous potential proteins that may contribute to the NO scavenging in O3-exposed rats (N = 4) compared with control rats (N = 3). D, Quantification of biotin-switch assay. Bands between 75 and 100 kDa (band 2), 25 and 37 kDa (band 6), and at 25 kDa (band 7) indicate significant biotin-labeled nitrosylated sites in the O3-exposed group compared to FA controls. Asterisks indicate significant difference by Student’s t test (*P < .05; **P < .001).
A biotin-switch assay was employed to explore whether specific proteins in the serum may be altered in concentration or modified by exposure to become more potent at scavenging NO. Figure 6C shows electrophoretically separated serum visualized with biotin-labeling of nitrosylation sites, along with quantification of specific bands (Fig. 6D). Overall, this assay confirms that there is some potential for increased NO scavenging by thiol groups, but it remains unconfirmed whether this change specifically accounts for the coronary vasodilatory impairments arising from the serum.
DISCUSSION
Coronary vascular dysfunction occurs 24 h following exposure to O3, an effect that appears to be driven at least in part by a circulating factor or factors. Consistent with inhaled O3 impacts on other vascular beds (Chuang et al., 2009; Robertson et al., 2013), the principal dysfunction involves a loss of endothelial function, assessed by vasodilatory response to ACh. Endothelial oxidative stress appears central to this outcome, and is mechanistically linked to the pathogenesis of vascular dysfunction following inhalation of numerous pollutants (Campen 2009; Cherng et al., 2011; Nurkiewicz et al., 2010). Within the context of the present experiment, we also observed that serum from O3-exposed rats was able to partially recapitulate vasodilatory impairments, even in a dilute application, suggesting that circulating factors may directly interact with the endothelium to manifest the reduced responsiveness to ACh. While the longer range temporal dynamics of this response were not characterized, the present findings suggest that O3 can induce at least a temporary window during which major coronary events may be either more likely to occur or more severe, as a result of the impaired endothelial function.
Whether or not O3 has an important cardiovascular effect in the general population remains a matter of contention (Campen, 2013). Recent animal studies clearly demonstrate vascular dysfunction and pathology (Chuang et al., 2009; Robertson et al., 2013), and other studies have shown cardiac effects (Farraj et al., 2012; Tankersley et al., 2013; Wagner et al., 2014; Watkinson et al., 2001). Nonvascular systemic effects of O3 inhalation have also been shown, including exacerbated liver injury and metabolic dysfunction (Aibo et al., 2010; Sun et al., 2013). However, a recent human exposure study failed to observe endothelial dysfunction in a well-established experimental model (Barath et al., 2013) and epidemiological reports are often conflicting. Perhaps the most thorough account of the population-level association revealed a 0.2–0.3% increase in daily cardiovascular and respiratory mortality for every 10 ppb increase in ambient O3 levels, an effect that persisted over a 2-day lag (Bell et al., 2004). Similar outcomes have been noted in various other epidemiological studies (Yan et al., 2013). However, when multiple pollutants are included in models of associative comparisons, the impact of O3 on cardiac events (heart failure or myocardial infarction) is often diminished relative to PM, oxides of nitrogen (NOx), or other pollutants (Mustafic et al., 2012; Shah et al., 2013). Unfortunately, while epidemiological studies are unmatched for revealing the scope of the global health challenges, they provide little insight into mechanisms of action or the temporal dynamics of complex exposures. O3 exhibits relatively unique circadian trends compared with most other pollutants, as PM, NOx, and carbon monoxide tend to peak during high traffic volume hours, O3 is formed in the peak sunlight hours and is derived from the NOx and volatile organic compounds emitted from vehicular fossil fuel combustion.
Timing of exposure and endpoint assessment, along with the concentration of O3, are essential factors in defining the relevance of this study. In our studies, we found that the coronary arteries from O3-exposed rats (1.0 ppm × 4 h) displayed a significant increase in tone 24-h postexposure, and prior to the application of any specific agonist (Fig. 1). This observation is similar to seminal vascular tone research by Brook et al. (2002) in humans exposed to combined PM and O3. Subsequent studies have elucidated a predominant role for PM in driving such effects, not O3, but dose may be a likely factor. Moreover, combined dose of PM and O3 may be additive or synergistic. Another recent human exposure study by Barath et al. (2013) failed to observe acute vascular effects of O3 at 0.3 ppm for 2 h (Barath et al., 2013). However, in both of these studies, a second-day assessment (ie, 24–48-h postexposure) was not conducted, whereas most rodent research has shown vascular effects at ∼24-h post-O3 exposure. Recent studies with episodic O3 exposure noted a delayed hyperthermic, potentially inflammatory, response in rats, which persisted ∼48 h after exposure (Gordon et al., 2014). In addition, Chuang et al. (2009) observed impaired endothelial-dependent vasorelaxation in aortic rings of mice following a repeated exposure (5 days) at a lower level of O3 (0.5 ppm), suggesting that acclimation/adaptation may not occur with regard to this vascular effect. We suspect that, despite the rapid scavenging of O3 in the lung, persistent effects may be observed both in the airways and systemically due to secondary and tertiary metabolites and inflammation.
Our findings of impaired endothelial response to agonists are consistent with findings of diesel emissions exposure from our laboratory and others (Cherng et al., 2009, 2011; Hansen et al., 2007; Mills et al., 2011). In follow-up studies, diesel-induced vascular impairments appeared related to dysfunctional eNOS (Knuckles et al., 2008). Antioxidant treatment of vessels improved eNOS and dilatory function, and in situ examination of oxygen radicals by dihydroethidium suggested that eNOS itself becomes a superoxide generator, likely owing to uncoupling of the protein dimer (either physically or electrochemically; Cherng et al., 2011). Thus, inhibition of eNOS actually improved vasodilation (via alternate cyclooxygenase pathways). In this study with O3, the improvement in dilation due to SOD, SOD/CAT, and apocynin are consistent with an uncoupled eNOS and/or scavenging of free NO; thus, more specific studies need to be conducted to better understand the upstream circulating factors and downstream signaling that drive vascular dysfunction following O3 inhalation.
In addition to intracellular redox alterations that can impair eNOS function, loss of NO bioavailability and possibly NO scavenging (Fig. 6) by the serum following inhaled O3 may augment vasomotor dysfunction. Reductions in overall NOx levels suggest either a global reduction in eNOS activity or an increased scavenging or consumption of NO species. This observation is consistent with a study on the impact of O3 exposure on exercise in rats, wherein O3 diminish plasma NOx levels in resting and exercising groups (Martinez-Campos et al., 2012). Mice lacking eNOS have reduced circulating NOx and are more prone to vasomotor impairment. In a chimeric model of eNOS deletion specific to erythrocytes (and all bone marrow-derived cells), plasma NOx was reduced and greater severity of infarct pathologies were noted (Merx et al., 2014; Wood et al., 2013). Investigating the overall protein composition of the serum, several bands containing nitrosylation sites appeared to be enhanced after O3 inhalation, which may explain the scavenging of free NO. Whether such alterations would also be observed in interstitial fluid, where interception of NO transiting through the myoendothelial cleft would be postulated to impair smooth muscle relaxation remains unclear. In addition, loss of NO bioavailability in the serum may also be mediated by enzymes such as peroxidases, rather than thiol-binding (Rees et al., 2014), and myeloperoxidases have been previously documented in the vasculature of PM-exposed rodents (Nurkiewicz et al., 2006). NO scavenging in the serum may have a greater impact on platelet activation and coagulative events than on vasodilation, but this was beyond the focus of this study. These assays related to NO bioavailability are observational in nature, and further biomolecular studies will be needed to verify a role for this mechanism in vivo.
O3 concentrations used in rodent studies are frequently higher than that used in human studies, owing to a well-documented species insensitivity (Tsujino et al., 2005; Wiester et al., 1996a, b). For one, the nares of the rat are far more complex in the obligate nasal-breathing rodents, allowing for greater initial scavenging of the highly reactive O3 (Tsujino et al., 2005). In addition, the lung surfactant chemistry of the rodent is different, with greater levels of antioxidants (Wiester et al., 1996b). Using radiolabelled (18O) O3, pulmonary uptake has been shown to be greater in humans than rats, as were markers of toxic effects (Hatch et al., 1994). Thus, the use of the 1 ppm concentration for this study is considered a moderate level for rodent studies, as it causes a mild pulmonary inflammation that would likely be observed in humans breathing 0.3–0.4 ppm O3 (Hatch et al., 1994). Indeed, Devlin et al. (2012) show comparable airway neutrophilia, along with circulatory changes, in humans exposed to 0.3 ppm O3.
The nature of the bioactive serum factor(s) remains unknown. Several recent studies have addressed the composition of plasma following O3 exposure, with largely negative results. Kadiiska et al. (2013) conducted an extensive panel of assays for oxidation products, including malondialdehyde, F2-isoprostanes, 15-HETE, and protein carbonyls, but found no significant changes at 2, 7, or 16-h postexposure. In human exposures, Barath et al. (2013) observed no O3 exposure-related trends for TNF α, IL-6, CD40, soluble P-selectin, or soluble ICAM-1. However, 18O-labeled O3 studies recently were conducted by Hatch et al. (2013) demonstrating that plasma-borne 18O concentrations peaked at 7-h postinhalation, with very little 18O observed at 2 h post, suggesting some complexity to the reactions and uptake beyond that of simple passive diffusion. With 18O levels restored to baseline at a 16 h time point, these findings do not seem to explain this study outcomes of serum bioactivity, but overall it is clear that a temporally complex chemistry occurs in the circulation following inhalation of a gas too reactive to penetrate beyond 0.1 µm of the airway epithelial lining fluid (Postlethwait et al., 1994; Pryor 1992). In parallel research, we have observed that the serum factor(s) arising from O3 inhalation appear to act through vascular CD36 receptors, but the generation of these unknown factors is not dependent on the presence of CD36 or pulmonary inflammation (Robertson et al., 2013).
In conclusion, O3 inhalation led to significant perturbations of rat coronary vascular function, characterized by increased constrictive responses to serotonin and diminished vasodilation to ACh, which appeared related to intracellular redox disturbances. Our ex vivo experiments with serum from exposed rats reveal a bioactivity conferred to the circulatory milieu, which may explain the present extrapulmonary findings, as well as shed light on the mechanisms underlying cardiovascular morbidity from inhaled pollutants. These findings further provide biological plausibility for epidemiologically observed cardiovascular morbidity and mortality resulting from O3 exposure in large populations. Further research into the compositional alterations of the circulation resulting from O3 inhalation, and the altered capacity for scavenging bioavailable NO, will further inform the systemic pathobiology.
ACKNOWLEDGMENTS
This work was supported in part by grants from National Institute of Environmental Health Sciences R01ES014639 (M.J.C.), National Institutes of Health Center of Biomedical Research Excellence P30GM103400 (J.W.), and from the Environmental Protection Agency RD-83479601-0 (M.J.C. and L.S.). All authors have read and approved the manuscript and declare no financial interests with the contents of the manuscript.
Glossary
ABBREVIATIONS
- 5-HT
serotonin
- Ach
acetylcholine
- ANOVA
analysis of variance
- BAL
bronchoalveolar lavage
- BSA
bovine serum albumin
- CAT
catalase
- CD36
cluster of differentiation 36
- EDTA; eNOS
endothelial nitric oxide synthase
- EPR
electron paramagnetic resonance
- FA
filtered air
- ICAM-1
intracellular adhesion molecule-1
- IL
interleukin
- LOX-1
lectin-like receptor for oxidized low density lipoprotein
- MGD
di(N-methyl-d-glutaminedithiocarbamate)
- NADPH; NO
nitric oxide
- NOx
oxides of nitrogen
- PEG-SOD
polyethylene glycol-superoxide dismutase
- PM
particulate matter
- PSS
physiological saline solution
- O3
Ozone
- ppm
parts per million
- RBC
red blood cells
- TNF-alpha
tumor necrosis factor-alpha
- WBC
white blood cells
REFERENCES
- Aibo D. I., Birmingham N. P., Lewandowski R., Maddox J. F., Roth R. A., Ganey P. E., Wagner J. G., Harkema J. R. (2010). Acute exposure to ozone exacerbates acetaminophen-induced liver injury in mice. Toxicol. Sci. 115, 267–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barath S., Langrish J. P., Lundback M., Bosson J. A., Goudie C., Newby D. E., Sandström T., Mills N. L., Blomberg A. (2013). Short-term exposure to ozone does not impair vascular function or affect heart rate variability in healthy young men. Toxicol. Sci. 135, 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M. L., McDermott A., Zeger S. L., Samet J. M., Dominici F. (2004). Ozone and short-term mortality in 95 US urban communities, 1987–2000. JAMA 292, 2372–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook R. D., Brook J. R., Urch B., Vincent R., Rajagopalan S., Silverman F. (2002). Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation 105, 1534–1536. [DOI] [PubMed] [Google Scholar]
- Campen M. J. (2009). Nitric oxide synthase: “Enzyme zero” in air pollution-induced vascular toxicity. Toxicol. Sci. 110, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen M. J. (2013). To breathe or not to breathe: negative data on ozone and vascular function in an established research model. Toxicol. Sci. 135, 263–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen M. J., Babu N. S., Helms G. A., Pett S., Wernly J., Mehran R., McDonald J. D. (2005). Nonparticulate components of diesel exhaust promote constriction in coronary arteries from ApoE-/- mice. Toxicol. Sci. 88, 95–102. [DOI] [PubMed] [Google Scholar]
- Channell M. M., Paffett M. L., Devlin R. B., Madden M. C., Campen M. J. (2012). Circulating factors induce coronary endothelial cell activation following exposure to inhaled diesel exhaust and nitrogen dioxide in humans: evidence from a novel translational in vitro model. Toxicol. Sci. 127,179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherng T. W., Campen M. J., Knuckles T. L., Gonzalez Bosc L., Kanagy N. L. (2009). Impairment of coronary endothelial cell et(b) receptor function after short-term inhalation exposure to whole diesel emissions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R640–R647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherng T. W., Paffett M. L., Jackson-Weaver O., Campen M. J., Walker B. R., Kanagy N. L. (2011). Mechanisms of diesel-induced endothelial nitric oxide synthase dysfunction in coronary arterioles. Environ. Health Perspect. 119, 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang G. C., Yang Z., Westbrook D. G., Pompilius M., Ballinger C. A., White C. R., Krzywanski D. M., Postlethwait E. M., Ballinger S. W. (2009). Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. Am. J. Physiol. Lung Cell Mol. Physiol. 297, L209–L216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin D. J., Haberzettl P., Prough R. A., Bhatnagar A. (2009). Glutathione-s-transferase p protects against endothelial dysfunction induced by exposure to tobacco smoke. Am. J. Physiol. Heart Circ. Physiol. 296, H1586–H1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin R. B., Duncan K. E., Jardim M., Schmitt M. T., Rappold A. G., Diaz-Sanchez D. (2012). Controlled exposure of healthy young volunteers to ozone causes cardiovascular effects. Circulation 126, 104–111. [DOI] [PubMed] [Google Scholar]
- Earley S., Walker B. R. (2002). Endothelium-dependent blunting of myogenic responsiveness after chronic hypoxia. Am. J. Physiol. Heart Circ. Physiol. 283, H2202–H2209. [DOI] [PubMed] [Google Scholar]
- Farraj A. K., Hazari M. S., Winsett D. W., Kulukulualani A., Carll A. P., Haykal-Coates N., Lamb C. M., Lappi E., Terrell D., Cascio W. E., et al. (2012). Overt and latent cardiac effects of ozone inhalation in rats: evidence for autonomic modulation and increased myocardial vulnerability. Environ. Health Perspect. 120, 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C. J., Johnstone A. F., Aydin C., Phillips P. M., MacPhail R. C., Kodavanti U. P., Ledbetter A. D., Jarema K. A. (2014). Episodic ozone exposure in adult and senescent brown norway rats: acute and delayed effect on heart rate, core temperature and motor activity. Inhal. Toxicol. 26, 380–390. [DOI] [PubMed] [Google Scholar]
- Hansen C. S., Sheykhzade M., Moller P., Folkmann J. K., Amtorp O., Jonassen T., Loft S. (2007). Diesel exhaust particles induce endothelial dysfunction in ApoE-/- mice. Toxicol. Appl. Pharmacol. 219, 24–32. [DOI] [PubMed] [Google Scholar]
- Hatch G. E., Slade R., Harris L. P., McDonnell W. F., Devlin R. B., Koren H. S., Costa D. L., McKee J. (1994). Ozone dose and effect in humans and rats. A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am. J. Respir. Crit. Care Med. 150, 676–683. [DOI] [PubMed] [Google Scholar]
- Hatch G. E., Slade R., McKee J. (2013). Fate of pathologically bound oxygen resulting from inhalation of labeled ozone in rats. Environ. Health Insights 7, 43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffrey S. R., Snyder S. H. (2001). The biotin switch method for the detection of S-nitrosylated proteins. Sci. STKE 2001, l1. [DOI] [PubMed] [Google Scholar]
- Kadiiska M. B., Basu S., Brot N., Cooper C., Saari Csallany A., Davies M. J., George M. M., Murray D. M., Jackson Roberts L., Shigenaga M. K., et al. (2013). Biomarkers of oxidative stress study v: ozone exposure of rats and its effect on lipids, proteins, and DNA in plasma and urine. Free Radic. Biol. Med. 61C, 408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuckles T. L., Lund A. K., Lucas S. N., Campen M. J. (2008). Diesel exhaust exposure enhances venoconstriction via uncoupling of enos. Toxicol. Appl. Pharmacol. 230, 346–351. [DOI] [PubMed] [Google Scholar]
- Lund A. K., Lucero J., Harman M., Madden M. C., McDonald J. D., Seagrave J. C., Campen M. J. (2011). The oxidized low-density lipoprotein receptor mediates vascular effects of inhaled vehicle emissions. Am. J. Respir. Crit. Care Med. 184, 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Campos C., Lara-Padilla E., Bobadilla-Lugo R. A., Kross R. D., Villanueva C. (2012). Effects of exercise on oxidative stress in rats induced by ozone. ScientificWorldJournal 2012, 135921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merx M. W., Gorressen S., van de Sandt A. M., Cortese-Krott M. M., Ohlig J., Stern M., Rassaf T., Gödecke A., Gladwin M. T., Kelm M. (2014). Depletion of circulating blood nos3 increases severity of myocardial infarction and left ventricular dysfunction. Basic Res. Cardiol. 109, 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills N. L., Miller M. R., Lucking A. J., Beveridge J., Flint L., Boere A. J., Fokkens P. H., Boon N. A., Sandstrom T., Blomberg A., et al. (2011). Combustion-derived nanoparticulate induces the adverse vascular effects of diesel exhaust inhalation. Eur. Heart J. 32, 2660–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafic H., Jabre P., Caussin C., Murad M. H., Escolano S., Tafflet M., Périer M. C., Marijon E., Vernerey D., Empana J. P., et al. (2012). Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA 307, 713–721. [DOI] [PubMed] [Google Scholar]
- Nurkiewicz T. R., Porter D. W., Barger M., Millecchia L., Rao K. M., Marvar P. J., Hubbs A. F., Castranova V., Boegehold M. A. (2006). Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environ. Health Perspect. 114, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurkiewicz T. R., Porter D. W., Hubbs A. F., Stone S., Chen B. T., Frazer D. G., Boegehold M. A., Castranova V. (2009). Pulmonary nanoparticle exposure disrupts systemic microvascular nitric oxide signaling. Toxicol. Sci. 110, 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurkiewicz T. R., Wu G., Li P., Boegehold M. A. (2010). Decreased arteriolar tetrahydrobiopterin is linked to superoxide generation from nitric oxide synthase in mice fed high salt. Microcirculation 17, 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuvolone D., Balzi D., Pepe P., Chini M., Scala D., Giovannini F., Cipriani F., Barchielli A. (2013). Ozone short-term exposure and acute coronary events: a multicities study in Tuscany (Italy). Environ. Res. 126, 17–23. [DOI] [PubMed] [Google Scholar]
- Postlethwait E. M., Langford S. D., Bidani A. (1994). Determinants of inhaled ozone absorption in isolated rat lungs. Toxicol. Appl. Pharmacol. 125, 77–89. [DOI] [PubMed] [Google Scholar]
- Pryor W. A. (1992). How far does ozone penetrate into the pulmonary air/tissue boundary before it reacts? Free Radic. Biol. Med. 12, 83–88. [DOI] [PubMed] [Google Scholar]
- Raza A., Bellander T., Bero-Bedada G., Dahlquist M., Hollenberg J., Jonsson M., Lind T., Rosenqvist M., Svensson L., Ljungman P. L. (2014). Short-term effects of air pollution on out-of-hospital cardiac arrest in Stockholm. Eur. Heart J. 35, 861–868. [DOI] [PubMed] [Google Scholar]
- Rees M. D., Maiocchi S. L., Kettle A. J., Thomas S. R. (2014). Mechanism and regulation of peroxidase-catalyzed nitric oxide consumption in physiological fluids: critical protective actions of ascorbate and thiocyanate. Free Radic. Biol. Med. 72, 91–103. [DOI] [PubMed] [Google Scholar]
- Robertson S., Colombo E. S., Lucas S. N., Hall P. R., Febbraio M., Paffett M. L., Campen M. J. (2013). CD36 mediates endothelial dysfunction downstream of circulating factors induced by o3 exposure. Toxicol. Sci. 134, 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A. S., Langrish J. P., Nair H., McAllister D. A., Hunter A. L., Donaldson K., Newby D. E., Mills N. L. (2013). Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet 382, 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Liu C., Xu X., Ying Z., Maiseyeu A., Wang A., Allen K., Lewandowski R. P., Bramble L. A., Morishita M., et al. (2013). Ambient fine particulate matter and ozone exposures induce inflammation in epicardial and perirenal adipose tissues in rats fed a high fructose diet. Part. Fibre Toxicol. 10, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Wang A., Jin X., Natanzon A., Duquaine D., Brook R. D., Aguinaldo J. G., Fayad Z. A., Fuster V., Lippmann M., et al. (2005). Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 294, 3003–3010. [DOI] [PubMed] [Google Scholar]
- Tankersley C. G., Georgakopoulos D., Tang W. Y., Abston E., Bierman A., Sborz N. (2013). Effects of ozone and particulate matter on cardiac mechanics: role of the atrial natriuretic peptide gene. Toxicol. Sci ., 131, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujino I., Kawakami Y., Kaneko A. (2005). Comparative simulation of gas transport in airway models of rat, dog, and human. Inhal. Toxicol. 17, 475–485. [DOI] [PubMed] [Google Scholar]
- Wagner J. G., Allen K., Yang H. Y., Nan B., Morishita M., Mukherjee B., Dvonch J. T., Spino C., Fink G. D., Rajagopalan S., et al. (2014). Cardiovascular depression in rats exposed to inhaled particulate matter and ozone: effects of diet-induced metabolic syndrome. Environ. Health Perspect. 122, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkinson W. P., Campen M. J., Nolan J. P., Costa D. L. (2001). Cardiovascular and systemic responses to inhaled pollutants in rodents: effects of ozone and particulate matter. Environ. Health Perspect. 109(Suppl 4), 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiester M. J., Stevens M. A., Menache M. G., McKee J. L., Jr., Gerrity T. R. (1996a). Ozone uptake in healthy adult males during quiet breathing. Fundam. Appl. Toxicol. 29,102–109. [DOI] [PubMed] [Google Scholar]
- Wiester M. J., Tepper J. S., Winsett D. W., Crissman K. M., Richards J. H., Costa D. L. (1996b). Adaptation to ozone in rats and its association with ascorbic acid in the lung. Fundam. Appl. Toxicol. 31, 56–64. [DOI] [PubMed] [Google Scholar]
- Wood K. C., Cortese-Krott M. M., Kovacic J. C., Noguchi A., Liu V. B., Wang X., Raghavachari N., Boehm M., Kato G. J., Kelm M., et al. (2013). Circulating blood endothelial nitric oxide synthase contributes to the regulation of systemic blood pressure and nitrite homeostasis. Arterioscler. Thromb. Vasc. Biol. 33, 1861–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M., Liu Z., Liu X., Duan H., Li T. (2013). Meta-analysis of the Chinese studies of the association between ambient ozone and mortality. Chemosphere 93, 899–905. [DOI] [PubMed] [Google Scholar]