Abstract
Nano-enabled products (NEPs) represent a growing economic global market that integrates nanotechnology into our everyday lives. Increased consumer use and disposal of NEPs at their end of life has led to increased environmental, health and safety (EHS) concerns, due to the potential environmental release of constituent engineered nanomaterials (ENMs) used in the production of NEPs. Although, there is an urgent need to assess particulate matter (PM) release scenarios and potential EHS implications, no current standardized methodologies exist across the exposure-toxicological characterization continuum. Here, an integrated methodology is presented, that can be used to sample, extract, disperse and estimate relevant dose of life cycle-released PM (LCPM), for in vitro and in vivo toxicological studies. The proposed methodology was utilized to evaluate two “real world” LCPM systems simulating consumer use and disposal of NEPs. This multi-step integrated methodology consists of: (1) real-time monitoring and sampling of size fractionated LCPM; (2) efficient extraction of LCPM collected on substrates using aqueous or ethanol extraction protocols to ensure minimal physicochemical alterations; (3) optimized LCPM dispersion preparation and characterization; (4) use of dosimetric techniques for in vitro and in vivo toxicological studies. This comprehensive framework provides a standardized protocol to assess the release and toxicological implications of ENMs released across the life cycle of NEPs and will help in addressing important knowledge gaps in the field of nanotoxicology.
Keywords: nano-enabled products, life cycle, nanosafety, nanotoxicology, engineered nanomaterials
The exponential growth of nanotechnology has revolutionized the landscape of several industries and research sectors due to the development of novel engineered nanomaterials (ENMs) and their envisioned innovative applications (Keller et al., 2013; Roco et al., 2011). In fact since 2009, the market potential of nano-enabled products (NEPs) has been predicted to have an annual growth rate of ∼50% (Limited, 2011). Consequentially, several NEPs are currently commercially available containing various classes of ENMs such as carbon nanotubes (CNTs), metal/metal oxides and metal alloy nanoparticles.
Although, ENMs improve the properties of several products, recent studies reveal the potential for ENMs to elicit adverse biological and environmental effects (Borm et al., 2006; Demokritou et al., 2013; Wiesner, 2006). One major concern is the potential of ENMs and nano-fibers to translocate into pulmonary connective tissues, lymphatics, or the circulating blood and organs (Cohen et al., 2014a). Nanoparticles may enter cells and possess greater bioactivity than their larger counterparts due to their smaller size and larger surface-to-volume ratio (Cohen et al., 2013; Hamilton et al., 2009). Indeed, nano-environmental health and safety (EHS) research has progressed over the last 10 years; however, primary mechanisms involving nanobiointeractions are not well defined resulting in major knowledge gaps (BBC, 2010; Demokritou, et al., 2013; RS, 2004).
Increased manufacturing and consumer use of NEPs have inevitably raised the urgent questions of nano-release during their synthesis, integration, processing, assembly, consumer usage, and eventually recycling or disposal at the end of their life (Keller, et al., 2013). Currently, only a handful of ENM release studies exist and focus primarily on CNTs embedded in polymer systems. For example, Bouillard and coworkers studied the potential release of CNTs from CNT–polymer nanocomposites upon their incineration (Bello et al., 2009; Bouillard et al., 2013; Wohlleben et al., 2011). A number of other studies have characterized the properties of LCPM from NEPs containing CNTs under a limited number of release scenarios such as sanding, drilling, and sawing (Wohlleben, et al., 2011). Within potential ‘cradle to grave’ scenarios, a mixture of possible pollutants that may include LCPM of different sizes, which may or may not be in the nanoscale regime, as well as volatile organic compounds (VOCs) and semi-volatile organic compounds (sVOCs) from matrices may be generated. Thus, the release of LCPM and other pollutants from NEPs across their life cycle (LC) may depend on composition and/or morphological properties of the utilized ENM and matrix, which further recapitulates the need for standardized methods to assess the release of LCPM from NEPs.
Both risk assessors and industry are struggling with the limited population exposure data across the LC of NEP and the fact that most of the current nano-EHS data focus on pristine (raw) nanomaterials rather than impacts associated with real world exposures across their LC. This important knowledge gap has been recently emphasized in both the National Research Council report, as well as the National Nanotechnology Initiative’s Strategy on Nano-EHS (NNI, 2011; NRC, 2012). The gap inhibits public health evaluators from assessing nano-related risk concerns and delays the exploration of emerging nanotechnology applications. These nano-EHS uncertainties involving NEPs, if unresolved, will also have major societal and economic consequences, which may influence the sustainable development of the nanotechnology industry.
In this study, the development and optimization, of a standardized integrated methodology across the release–capture–toxicological assessment continuum of LCPM is presented. Such a multi-step methodology can be used to sample LCPM released across LC of NEPs, extract and prepare biological media dispersions for in vitro or in vivo studies taking into consideration dosimetry. A number of ‘real world’ nanoparticles released from NEPs across LC scenarios were used as test particles to demonstrate the versatility of the proposed integrated methodology.
MATERIALS AND METHODS
Generation of ‘Real-World’ LCPM Test Particles Used in the Study
Two unique real-world LC scenarios that are associated with the release of LCPM were used as case studies for the development and validation of the proposed methodology: (1) consumer exposure to laser printer-emitted engineered particles (PEPs), associated with the use of nano-enabled toner; (2) LCPM released during the end of life incineration or thermo-decomposition scenario of a nanocomposite consisting of 0.1% CNTs embedded in polyurethane.
In case study 1, the recently developed printer exposure generation systems (PEGS) (Pirela et al., 2014a) was used for studying generation of real-world exposure to PEPs. Printer toner formulations are recently considered NEPs due to the presence of ENMs, which can be emitted during printing (Pirela, et al., 2014a, b; Sisler et al., 2014). The toner powder for the printer used in this study was found to have a complex chemical composition including organic/elemental carbon, nanoscale metals/metal oxides (e.g., iron oxide, alumina, titanium, nickel, copper, magnesium) and other elements, such as phosphorus, calcium and sulfur among others. A detailed description of the chemical composition of both the toner powder and the PEPs was recently published by Pirela et al. (2014b). The release of ENMs during consumer use from the toners and the preliminary toxicological data raise concerns for possible deleterious effects on the lung. Recent published studies by the authors ascertained the toxicological properties of PEPs, which include changes in cellular activity, as well as release of cytokines (inflammation) at low exposure levels of PEPs (Pirela, et al., 2014a; Sisler, et al., 2014).
In case study 2, an integrated exposure generation system (INEXS) (Sotiriou et al., 2015) recently developed by the authors was used for systematic investigation of thermodecomposition of polyurethane embedded with CNT (PU-CNT) nanocomposite. At their end of life, a large portion of NEPs will enter the waste stream and waste treatment plants and possibly incinerated. The thermodecomposition process of polymer nanocomposites would release nanoscale particulate matter (PM) and ENMs used in their synthesis. The byproducts of thermal decomposition (released aerosol and residual ash) could have broad environmental and societal impacts by creating new hazards in which the biological activity remains largely unknown. Here, PU-CNT (0.1wt% CNT) nanocomposite was thermally decomposed at 800°C using the INEXS exposure generation platform and the LCPM-released aerosol was size fractionated and sampled. More details on both the INEXS exposure generation platform as well on the physicochemical properties of the released PM can be found in Sotiriou et al. (2015).
Extensive details for the real-world exposure generation platforms used here can be found in Pirela et al. (2014a, b), Sisler et al. (2014), and Sotiriou et al. (2015). Here, we present a summary of data for completion purposes for the various steps of the presented sampling, extraction, dispersion, and dosimetry (SEDD) methodology.
Integrated SEDD Methodology for EHS Assessment of Released PM Across the LC
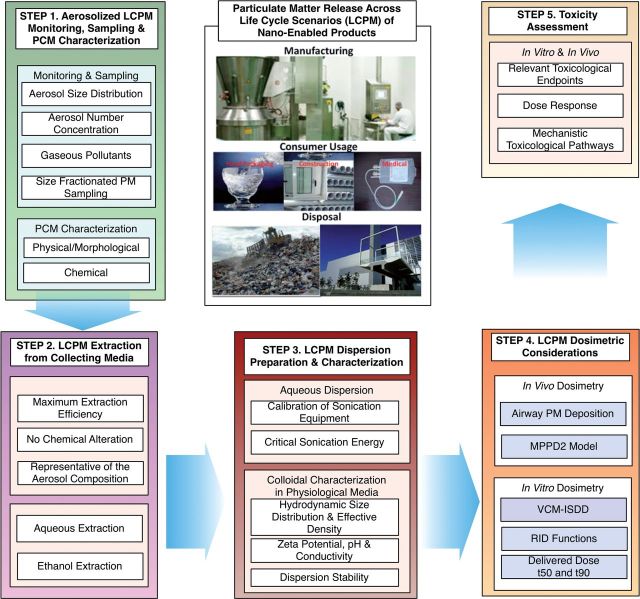
The SEDD framework is outlined in Figure 1 and consists of the following steps.
FIG. 1.
SEDD methodology for toxicity assessment of PM release across the life cycle of NEPs.
Step 1—Aerosolized LCPM monitoring, sampling, and characterization
To accurately derive a cause-effect association of LCPM release/exposure, it is critical to characterize the exposures using both real-time and integrated monitoring systems. Some of the key exposure LCPM parameters include, monitoring in real-time size distribution, particle number, and mass concentration. It is worth noting that particle size (nano to micron size range), total particle number, and mass concentration are important in predicting the potential fate and transport of LCPM along with the deposition of inhaled PM in the human respiratory track (Buzea et al., 2007). Therefore, for real-time monitoring of released LCPM, which is usually poly-dispersed in size, multiple particle detection instruments are needed. Table 1 summarizes real-time and integrated instrumentation and methods that can be used for both proper physicochemical and morphological (PCM) characterization of LCPM. Thus, for both PEGS and INEXS exposure generation platforms used in the case studies presented here, an extensive suite of instruments for monitoring and sampling of aerosolized PM and gaseous by-products were used. More specifically, a water-based condensation particle counter (WCPC Model 3785, TSI Inc., Shoreview, Minnesota) was used to monitor number concentration of released PM, from 5 to 1000 nm. A scanning mobility particle sizer (SMPS Model 3080, TSI Inc.) was also used to measure particle size distribution from 2.5 to 210 nm. To measure the particle size distribution and number concentration of the aerosolized PM as a function of time for particles from 0.5 to 20 μm, an aerodynamic particle sizer (APS Model 3321, TSI Inc.) was also employed. Real-time measurements of environmental conditions (temperature, relative humidity, and ozone concentration) during LCPM release were also performed using Q-track (Model 8551, TSI Inc.) Photo ionization-based system (Gray Wolf Sensing solutions, Shelton, Connecticut) was used for measuring total volatile organic compounds (tVOC) as well.
TABLE 1.
Indicative instrumentation details for SEDD methodology
| Instruments | Measures | Size Range (μm) | Key Features |
|---|---|---|---|
| Real-time monitoring of released PMa | |||
| Fast Mobility Particle Sizer (FMPS) (TSI 3091) | Size distribution; TNC; electrical mobility diameter | 0.0056–0.56 | Upper limit of 1.0 × 10 −9 particles/cm3 |
| Aerodynamic Particle Sizer (APS) (TSI 3321) | Size distribution; TNC; Aerodynamic diameter | 0.5–20 | Upper limit of 1.0 × 10−4 particles/cm3 |
| Condensation Particle Counter (CPC) (TSI 3785) | TNC | 0.01–1000 | Upper limit of 1.0 × 105 particles/cm3 |
| P-track (TSI 8525) | TNC | 0.02–1 | Upper limit of 5.0 × 105 particles/cm3 |
| Dust track (TSI 8520) | Mass Concentration | 0.10–1 | No size resolution; upper limit 100 mg/m3 |
| Scanning Mobility Particle Sizer (SMPS) (TSI 3080) | Size distribution; TNC | 0.0025–2 | Upper limit of 5.0 × 107 particles/cm3 |
| Total VOC (Gray Wolf Sensing solutions) | CO2, CO, NO2 | – | Parts per billion (ppb) |
| Q-track (TSI 8551) | Temp, RH, and Ozone conc. | – | |
| Integrated PM Samplers | |||
| Thermophoretic precipitator (TP) | For TEM grids analysis | 0.001 to >100 | Size dependent, highest for nanoparticles |
| Electrostatic precipitator (EP) | For TEM grids analysis | 0.001 to >100 | >80 (at 20 nm to 100% (at 400 nm) |
| WRASS (Nano-ID) | Mobility diameter based collection | 0.002–20 | <0.2% Penetration; Upper stage (0.25–20 μm); Lower stage (2–250 nm) |
| Naneum Ltd. | |||
| Harvard Compact Cascade Impactor (HCCI) (Demokritou et al., 2004) | Aerodynamic diameter based collection | PM2.5–10; PM0.1–2.5, and PM0.1 | Size dependent collection, collects up to mg quantities of PM. |
| Off-line PCM characterization | |||
| Scanning/Transmission Electron Microscopy (SEM/TEM-EDX) | Size, particle shape and elemental mapping | 0.001–10 | Particle morphology determination |
| X-ray diffraction (XRD) | Crystallinity and particle size | 0.001–10 | Inference about structure of material |
| Fourier transform infrared spectroscopy (FTIR) | Surface chemistry of particles | – | Chemical identification and mapping of materials. |
| Inductively coupled plasma mass spectrometry (ICPMS) | Elemental analysis | – | Elemental identification and quantitation |
| Gas Chromatography-Mass spectrometry (GC/MS) | SVOC | – | Chemical identification and quantitation |
| NMR | Functional groups analysis | – | Chemical identification and quantitation |
| Instrumentation for colloidal characterization | |||
| DLS (Malvern Zetasizer Nano-ZS) | Size; Size distribution and particle charge | <0.004–4 | Ensemble technique, 100 μl sample volume |
| Tunable resistive pulse sensing (qNANO, Izon Science) | Size; size distribution; particle charge and particle number concentration | 0.04–10 | Particle by particle measurement, 40 μl sample volume and particle concentration range (105–1012 particles/ml) |
| Harvard volumetric centrifugation method (VCM) (Deloid et al., 2014) | Effective density | – | Effective density of agglomerates in liquids |
| Dose model | |||
| MPPD2 (Anjilvel and Asgharian, 1995) | In vivo deposited dose | – | Deposited mass flux for human and murine airway |
| VCM-ISDD (Cohen et al., 2014) | In vitro deposited dose | – | Cellular deposited dose as a function of time |
Size fractionated sampling of aerosolized LCPM for off line PCM and toxicological characterization of particles requires the use of appropriate PM samplers. For extensive PCM characterization and toxicological assessment of LCPM, it is essential to collect large quantities of size fractionated LCPM mass (in the order of mgs), preferably using inert collection substrates in order to avoid interference with chemicals used as coatings on impaction surfaces to minimize particle bounce and re-entrainment (Demokritou et al., 2004). In the field of ambient PM, a number of impaction-based systems were developed and used for the PCM and toxicological characterization of PM. The authors have developed in the past such cascade impaction systems suitable for PCM and toxicological characterization of ambient PM. Size-fractionated released LCPM was sampled in our case studies using the Harvard Compact Cascade Impactor system (HCCI) (Demokritou et al., 2004). The HCCI sampler operates with four stages corresponding to the PM10, PM2.5, PM1.0, and PM0.1 (final filter) sizes. The major feature of this novel sampler is the ability to both fractionate by size and collect relatively large amounts of particles (mg quantities) onto inert polyurethane foam (PUF)/Teflon filter, impaction substrates without the use of any adhesives (Bello et al., 2009). The impaction media is weighed following a 48 h stabilization process in a temperature (22 ± 1°C) and humidity (43 ± 2%) controlled environmental chamber utilizing a Mettler Toledo XPE analytical microbalance. The difference between post-sampled filter media and clean filter media can then be used to determine the collected LCPM mass and its mass size distribution.
Offline PCM characterization of sampled LCPM
Post-sampling physicochemical characterization on collected LCPM size fractions (i.e., PM0.1, PM0.1–2.5, PM2.5) is also needed to understand the properties of the generated particles in greater detail. Offline PCM characterization can entail the use of various techniques for specific chemical species. Table 1 summarizes the various methods that can be used in offline PCM characterization of LCPM. Techniques commonly used for elemental analysis such as magnetic sector field inductively coupled plasma mass spectrometry (ICPMS, Thermo-Finnegan Element 2) can identify metals and several non-metals. Moreover, organic carbon/elemental carbon analysis (OC-EC) analysis can be used to estimate organic and elemental carbon content. The OC-EC method used in the presented case studies was adapted from the NIOSH 5040 method (Pirela et al., 2014b). Extensive PCM characterization was performed for both LCPM test particles used here, namely the PEPs and thermo-decomposed nano-enabled products (TNEPs), using the state-of-the-art methods described above. Furthermore, transmission and scanning electron microscopy (SEM/TEM) of sampled particles collected on TEM grids (100-mesh copper with carbon film, Electron Microscopy Sciences, Hatfield, Pennsylvania) can also be utilized to determine the morphological features and size of the sampled LCPM. Energy dispersive spectroscopy was also performed in our case studies to obtain LCPM elemental composition of surface chemistry of collected LCPM.
Step 2—LCPM extraction from collecting media
Extraction of sampled LCPM from collection impaction substrates is a critical step. An ideal extraction methodology will achieve the following: (a) maximum LCPM recovery of collected particulate mass; (b) minimum contamination by the components of collection substrate itself; and (c) LCPM-extracted sample that is representative of sampled LCPM, in terms of size and organic/inorganic composition (Biran et al., 1996).
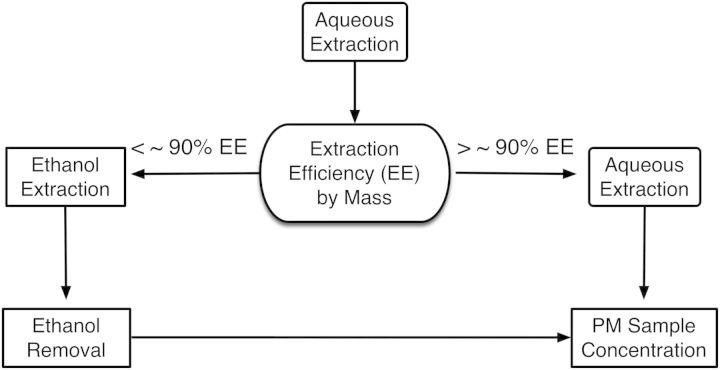
Figure 2 summarizes in detail the various sub-steps necessary for extraction of size fractionated LCPM from both Teflon filters (PTFE, Pall Corp., Port Washington, New York) and poly-urethane foam (PUF) (Pall Corp.) substrates used as collection substrates for HCCI. In summary, LCPM from Teflon filters and PUFs were extracted utilizing a cup-horn sonicator (Branson Ultrasonics, Danbury, Connecticut). Two different extraction protocols, aqueous and 75% ethanol in DI water (v/v) were evaluated in order to ensure that the particle extraction efficiency (EE%) from substrates is greater than ∼90% by mass. First, the use of an aqueous extraction as a biologically inert solvent is assessed and if the EE% is below ∼90% limit then the 75% ethanol (v/v) extraction approach is followed. Sonication of collection substrate in aqueous/organic mediums, such as ethanol, may lead to disintegration of substrate and possible contamination of recovered PM suspensions. Thus, it is necessary to first determine the rupture sonication energy of the particular filter media using blank filters prior to extraction. Supplementary Table S1 presents data on this process, for the two filter media used in this study. However, the same methodology can be applied for other substrate/filter materials. In more detail the two proposed extraction protocols are as follows:
FIG. 2.
Schematic illustrating LCPM extraction protocol in the SEDD methodology (Step 2).
Aqueous Extraction: Teflon filter containing particles were placed facing up in a beaker with the same diameter as the filter. Next, 7–10 ml of deionized (DI) water was added to cover the filter and the beaker was placed in a bath sonicator, and sonicated for 15 min to remove collected particles that are not highly retained by the Teflon filter fibers. Subsequently, a cup-horn sonicator was employed to deliver 418 J/ml of energy, so as to promote the release of the remaining collected particles. Resulting particle suspensions were transferred to a glass vial. With regards to PM collected on PUF substrate, the edges of the PUF were removed leaving only the area with the visible particles. The leftover foam piece was then cut with a razor blade into two pieces and placed into a glass vial with 4 ml of DI water and cup sonicated to deliver 466 J/ml of energy. After sonication, foam pieces were removed and dried using forceps to squeeze out water completely. Aqueous extraction was performed for PEPs extraction, for both PM0.1 and PM2.5 size fractions.
Ethanol Extraction: For collected particle with hydrophobic chemistries (i.e., TNEPs, see ‘Results’ section), where EE% of >90% by mass may not be reached, the use of organic solvents such as ethanol may be necessary. For performing ethanol extraction, the PM0.1 Teflon filters were placed particle side up in beakers containing (15 ml), 75% ultrapure ethanol followed by sonication in a cup-horn sonicator (Branson sonicator) for delivering 418 J/ml of energy. Likewise, polyurethane foam of PUF substrates were cut revealing particle containing areas and immersed in 75% ethanol and sonicated for delivering 466 J/ml of energy. The collected particle suspensions were concentrated to the desired volume/concentration by evaporating water under reduced pressure by utilizing a simple rotary evaporator system (Rotavapor R215, Buchi). The particle suspension was diluted to minimize residual ethanol by re-adding DI water and repeating the concentration step (at 19°C at 40 Torr pressure) several times, with final suspension left in DI water. Moreover, as described in later section, a blank filter solution (without particles), extracted, and processed following exact 75% ethanol extraction protocol was used as control in cellular studies for the presence of miniscule by mass ethanol content which will remain in the suspension (see ‘Results’ section below). NMR (described in later section) was performed to assess the levels of ethanol remained in the suspension. It is worth noting that only for the case study of TNEPs ethanol extraction was required, for both PM0.1 and PM0.1–2.5 size fractions.
Gravimetric Analysis: To determine the amount of mass extracted (i.e., % Extraction Efficiency), each substrate is weighed both pre- and post-extraction in a temperature and humidity-controlled weighing chamber (described in the previous section). The subtraction of the post-extraction weight from the pre-extraction weight equals the total amount of mass collected.
Calculation of Particle Extraction Efficiency: Equation (1) was used to estimate the mass of the extracted particles, and Equation (2) was used to determine the extraction efficiency (EE%):
| (1) |
| (2) |
where Wα = pre-extraction weight, Wω = post-extraction weight, and Mass = total mass extracted (mg). The mass extracted divided by the total volume of deionized water provides the concentration of PM suspension.
Experimental Validation of LCPM Ethanol Extraction Process: In order to compare, the chemical composition of extracted LCPM using both the aqueous and ethanol extraction protocols, nuclear magnetic resonance spectroscopy (NMR) functional composition analysis was utilized using the TNEPs as test particles. In brief, the 1H NMR spectra in DMSO-d6 were obtained on a Bruker Avance 500 MHz instrument equipped with a 5-mm double resonance broad band (BBFO Plus Smart) probe at 298 K with 8192 scans, using spin-lock, acquisition time of 2.0 s, relaxation delay (d1) of 2.0 s, and 1 Hz exponential line broadening and water suppression using excitation sculpting with gradients using 180 water selective pulses. Spectra were apodized by multiplication with an exponential decay corresponding to 1 Hz line broadening in the spectrum and a zero filling factor of 2. The baseline was manually corrected and integrated using the Advanced Chemistry Development NMR processor (Version 12.01 Academic Edition). The determination of chemical shifts (δ1Η) was done relative to that of trimethylsilane (TMS) (set at 0.0).
Step 3—LCPM dispersion preparation and characterization for cellular studies
Extracted LCPM dispersion preparation and colloidal characterization in liquid suspension are essential for understanding in vitro cellular toxicity of particles. Early contributions in the nanotoxicology field documented the impact of adequate nanoparticle dispersion, characterization and dosing on toxicological studies (Hinderliter et al., 2010; Powers et al., 2006). Moreover, suitable characterization techniques are necessary to be employed for accurately and reliably measuring particle size (d(h, z-ave)), size distribution (PdI), particle charge (ζ), pH, conductivity in dispersion medium, agglomeration potential, and effective density of formed agglomerates in order to assess cellular responses to LCPM (Table 1) (Cohen et al., 2013, 2014b; Pal et al., 2014; Powers et al., 2006). Table 1 summarizes methods and instruments commonly used for the proper colloidal characterization of LCPM suspensions in culture media. A variety of instruments are needed including both dynamic light scattering (DLS) and tunable resistance particle sizer (TRPS) (Cohen et al., 2013; Pal et al., 2015).
PEPs and TNEPs suspensions from the two case studies were characterized here extensively. Properties measured include hydrodynamic diameter, polydispersivity, zeta potential, conductivity, and pH. In addition, the effective density of formed agglomerates was determined using the Harvard Volumetric Centrifugation Method (VCM) recently developed by the authors. (Cohen et al., 2013, 2014b; DeLoid et al., 2014; Demokritou, 2012; Pal et al., 2015). Effective density of particle suspensions in culture medium is an important dosimetry determinant (see below) (Cohen et al., 2014b; Pal et al., 2015). It is also worth noting that in our case studies, the recently developed by the authors optimized dispersion preparation and characterization protocol was adopted and used (Cohen et al., 2013; Pal et al., 2015). In summary, the calibration of sonication equipment and standardized reporting of delivered sonication energy (DSE) is utilized. The LCPM-specific critical sonication energy, DSEcr, was then calculated as described by Cohen et al. (2013) to determine the energy required to ensure “disentanglement” of extracted LCPM particles with minimal agglomeration. Each size fraction PM was dispersed via sonication at levels greater than the critical sonication energy, initially in DI water to minimize reactive oxygen species production via sonolysis, minimize ionic strength, and to avoid protein denaturing in the final cell delivery media. Stock solutions in DI water were then diluted to desired concentrations in cell culture media.
Step 4—LCPM dosimetric considerations
Adjusting in vitro and in vivo doses to the same scale is a major challenge in nanotoxicology and various dosimetric models were developed and utilized recently. The Multiple-Path Particle Dosimetry 2 (MPPD2) (Anjilvel and Asgharian, 1995; Demokritou et al., 2013) model can be implemented to determine the dose deposited in the head region, conducting zone, the transitional and respiratory zones of human respiratory system for the case of inhaled LCPM. The airborne LCPM distribution values (count mean diameter, geometric standard deviation, and mass concentration), as well as the human breathing parameters (tidal volume, breathing frequency, inspiratory fraction, pause fraction, functional residual capacity, head volume, and breathing route) as described in detail Pirela et al. (2014a, b) were used in the simulations for the PEPs as well as TNEPs case studies and presented here in Table 2. The breathing frequency used in the MPPD2 simulation was that of a resting individual (12 breaths/min). Please note that the MPPD2 model provides the deposition mass flux for all the generations of the human respiratory tree. Thus, the total deposition mass flux of the entire human airways comprised of the conducting zone and the transitional and respiratory zones (excluding the head airway region) was calculated here and used in the computation of the in vitro equivalent volumetric dose, in vitroeq (μg/ml), which represents dose delivered to cells.
TABLE 2.
Summary of parameters used in the in vivo lung multiple path particle deposition model for both PEPs and TNEPs (MPPD2, Anjilvel and Asgharian, 1995)
| Human model | Breathing parameters |
Airborne nanoparticle distribution* |
|
|---|---|---|---|
| PEPs | TNEPs | ||
| Functional residual capacity: 3300 ml | Tidal volume: 625 ml | Count mean diameter: | |
| 36.3 nm | 107 nm | ||
| Head volume: 50 ml | Breathing frequency: 12 breaths/min | Geometric standard deviation: | |
| 1.74 | 1.52 | ||
| Breathing route: Nasal |
|
Mass concentration: | |
| 32.5 µg/m3 | 23.9 µg/m3 | ||
The estimation of the delivered to cell in vitro dose as a function of in vitro exposure time was obtained using the recently developed by the authors integrated in vitro dosimetric methodology (Cohen et al., 2014b; DeLoid et al., 2014). It is worth noting that for most ENMs, the administered dose in vitro is not necessarily the dose that will be deposited on the cells as a function of time with some particle systems settling faster than others (Demokritou et al., 2013; Pirela et al., 2014b; Sisler et al., 2014). In summary, the relative in vitro dose (RID) functions, which calculate delivered dose in terms of mass (µg), surface area (cm2), and particle number concentration (particles/cm2) as a function of exposure time, were derived as detailed in Cohen et al. (2014b). The deposition fraction constant, a (h−1), needed for the RID functions, was derived from curve fitting of the VCM-ISDD numerical model output (Cohen et al., 2014a, b; Pirela et al., 2014b; Sisler et al., 2014). Furthermore, the time required for the delivery of 50 and 90% of the administered dose, t50 and t90, respectively, can be calculated.
Step 5—LCPM cellular toxicity assessment
In vitro and in vivo mechanistic toxicological pathway studies are routinely conducted for assessment of PM. These mechanistic pathways can be based on generation of oxidative stress, eliciting cytotoxicity, and genotoxicity among others in different cellular and animal models (Borm et al., 2006). One important inquiry in any toxicological evaluation is elucidating the strength of association in the dose–response relationship (Pal et al., 2015). In in vitro systems, this relationship should be adjusted to take into account the effective dose delivered to cells rather than the administered cell dose (Cohen et al., 2014b; Pal et al., 2015). To evaluate these in vitro dose–response relationships and assess mechanistic pathways, well-characterized human cell lines for toxicity screening applications can be employed. In this study, only one endpoint (metabolic activity) and cell line were reported for demonstration purposes only of the SEDD methodology. For the PEPs, a detailed in vitro characterization study was completed and manuscript is currently under review (Pirela, 2015). In summary here, normal small airway epithelial cells (SAEC) were utilized, which are physiologically relevant to likely routes such as inhalation exposure. The cells were cultured in small airway basal media (SABM) (Lonza, supplemented with bovine pituitary extract—BPE) 2 ml, hydrocortisone 0.50 ml, human epidermal growth factor (hEGF) 0.50 ml, epinephrine 0.50 ml, transferrin 0.50 ml, insulin 0.50 ml, retinoic acid 0.50 ml, triiodothyronine 0.50 ml, gentimicin, amphoteracin-B (GA-1000). The metabolic activity was measured using the CellTiter 96 Aqueous One Solution (MTS) (Promega, Madison, Wisconsin) assay. In brief, cells were seeded at a density of 600 cells/well in 96-well plates (Corning Inc., New York, New York) and were maintained until 70–80% confluency. Culture media was replaced with PEPs and TNEP LCPM suspensions, respectively, in supplement free SABM and incubated for 24 h. A blank filter solution (without particles), extracted and processed following exact 75% ethanol extraction protocol was also used as a control to assess the effect of ethanol on the toxicological experiments. Following exposure (0.5–50 µg/ml), the remaining exposure media was removed from the treated cells, followed by two washes with 1× phosphate buffered saline (PBS) and fresh media containing MTS reagent was added for 1 h. The absorbance was promptly read using a fluorescent plate reader (Molecular Devices) at 490 nm. The quantity of formazan product formed, as measured by the amount of absorbance at 490 nm, is directly proportional to the number of living cells in culture. Media only and PM only controls were performed to ensure reagent integrity. Statistical differences between the means were determined by performing one-way analysis of variance (ANOVA) using Prism version 5 (GraphPad Software, La Jolla, California) and a treatment effect with P-value of ≤0.05 was considered significant. More detailed in vitro toxicological assessment studies for the two case studies have been presented in detail in our recently published and submitted manuscripts (Pirela et al., 2014a, 2015; Sisler et al., 2014). The endpoints utilized in this study are not meant to fully assess biological responses of the two tested particle systems described but to simply validate the methodology.
RESULTS
Results from the application of the SEDD methodology for the case studies are presented as follows.
Case Study 1: Release of ENMs During Use of a Nano-Enabled Toner Used in Laser Printers: EHS Implications of PEPs
Step 1—Aerosolized Monitoring, Sampling, and PCM Characterization of PEPs
Supplementary Figure S1A shows a unimodal particle size distribution throughout the printing process. Mean particle diameters ranged from 39 to 138 nm for printer B1. A noticeable variation was observed in the mobility diameter of the PEPs at the three different time points of the printing (modal diameter varies from 50 to 110 nm). In addition, the particle mass size concentration as a function of particle size reveals that most particles by mass are <2.5 μm in size. There were also no observable differences in ozone levels, which ranged from 9.54 to 23.84 parts per billion by volume (ppbv). The levels of tVOCs were found to be 1900 ppb (Supplementary Fig. S1B).
Off line PCM characterization of PEPs
The size of PEPs as determined by electron microscopy (Supplementary Fig. S1C) was in agreement with that of real-time particle size distribution confirming nano-sized PM was released during printing process. Moreover, EDX analysis indicated the presence of several inorganic elemental components in released PM (zinc, copper, titanium, cerium, silicon, calcium, and sulfur). The pie chart in Supplementary Figure S1D shows the ICP-MS analysis on PEPs and it confirms the presence of copper, cerium, chromium, nickel, iron, and titanium. These observations confirm the fact that the ENMs incorporated in printer toner formulations were aerosolized during the printing process. Interested readers can find the extensive PCM characterization details on toner powder and PEPs in recent publications from Pirela et al. (2014b).
Step 2—PEPs Extraction From Collecting Media
The collected PEPs (both PM0.1 and PM2.5 fractions) were extracted from HCCI impactor substrates. The aqueous extraction protocol described previously in the ‘Methods’ section was used. As shown in Supplementary Table S1, DSE below 466 J/ml was used for PEPs aqueous extraction for both PM0.1 and PM2.5 fractions. Control experiments (without LCPM) indicated that delivered extraction energy below 466 J/ml had no impact on mass differences of blank Teflon and PUF substrates, an indication that both types of substrates were intact during the extraction process and no fibers/debris were released. Using the protocol described in Figure 2, the aqueous extraction was efficient to extract ∼90% collected LCPM mass from collection media.
Step 3 — PEPs Dispersion Preparation and Characterization
Critical DSE, DSEcr, was found to be 275 J/ml (Table 3). Observed values of ζ were strongly negative for the PM0.1 PEPs size fraction in DI water (−20.6 mV) and became positive when dispersed in SABM (9.97 mV). The opposite was observed for the larger PEPs counterpart (PM2.5), whose ζ was −16 mV in DI water and remained negative when suspended in SABM (−17.7 mV). The colloidal stability of the particle suspensions was subsequently evaluated 24 h post-sonication to DSEcr. The size of PEPs (PM0.1) suspended in SABM remained stable with an average diameter ranging from 381 to 402 nm over 24 h. Contrasting stability was observed for the bigger size fraction of PEPs (PM2.5), whose size increased at 24 h post-sonication going from ∼200 to 300 nm.
TABLE 3.
Characterization (d(h,z-ave), PdI and zeta) of extracted particles in DI water at DSEcr and after particle dispersion in SABM
| Material label |
In DI water |
In SABM |
Dispersion stability (24h post dispersion) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| d(h, z-ave) (nm) | PdI | Zeta ζ (mV) | d(h, z-ave) (nm) | PdI | Zeta ζ (mV) | d(h, z-ave) (nm) | PdI | Zeta ζ (mV) | |
| PEPs PM0.1 | 178.3 ± 3.5 | 0.40 ± 0.05 | −20.6 ± 1.9 | 381.7 ± 40.2 | 0.58 ± 0.05 | 9.97 ± 2.8 | 402.9 | 0.48 ± 0.25 | 1.22 ± 2.24 |
| PEPs PM2.5 | 197.8 ± 17.4 | 0.44 ± 0.06 | −16.0 ± 1.0 | 231.1 ± 2.9 | 0.35 ± 0.05 | −17.7 ± 3.6 | 294.6 | 0.89 ± 0.10 | – |
| TNEPs PM0.1 | 269 ± 5 | 0.34 ± 0.09 | −28.7 ± 2.25 | 283 ± 46 | 0.45 ± 0.06 | −7.85 ± 0.6 | 254 ± 44 | 0.44 ± 0.03 | −8.73 ± 0.63 |
| TNEPs PM0.1–2.5 | 298 ± 47 | 0.26 ± 0.06 | −19.6 ± 1.54 | 375 ± 82 | 0.48 ± 0.3 | −11.50 ± 0.8 | 390 ± 24 | 0.75 ± 0.24 | −12.1 ± 0.87 |
The VCM-ISDD measured effective density of the formed agglomerates in SABM culture media was 2.39 and 3.10 g/cm3 for PEPs (PM0.1) and PEPs (PM2.5), respectively (Table 4). Interested readers can find the extensive colloidal characterization details for multiple culture media used in detailed in vitro toxicological assessment studies from Pirela et al. (2015) and Sisler et al. (2014).
TABLE 4.
Mass, surface area and particle concentration as cellular dose metric, based on administered and deposited doses for all particles dispersed in SABM
| Material | Effective density, ρe (g/cm3) | Deposition fraction constant, α (h−1) | t90 (h) | Administered dose at t = 24 h |
Delivered dose at t = 24 h |
|||
|---|---|---|---|---|---|---|---|---|
| Mass (μg/ml) | SA (cm2) | Mass (μg) | SA (cm2) | Particles /cm2 | ||||
| PEPs PM0.1 | 2.39 | 0.027 | 85.2 | 0.5 | 3.29E + 04 | 0.5 | 1.57E + 04 | 7.22E + 12 |
| PEPs PM2.5 | 3.10 | 0.016 | 143.9 | 0.5 | 4.19E + 04 | 0.5 | 1.34E + 04 | 7.97E + 12 |
| TNEPs PM0.1 | 1.148 | 0.02 | 99.0 | 0.5 | 9.23E + 04 | 0.19 | 3.94E + 04 | 1.57E + 13 |
| TNEPS PM0.1–2.5 | 1.141 | 0.03 | 83.3 | 0.5 | 7.01E + 04 | 0.25 | 3.4E + 04 | 7.69E + 12 |
The dose estimates derived from the VCM-ISDD model calculations.
SA, Surface area.
Note Surface area and particle number concentration dose metrics are based on VCM-ISDD model calculations. RID functions can be calculated by inserting appropriate alpha parameter, effective density and hydrodynamic diameter reported in Table 3 into Equations (3)–(5) as below for delivered mass, surface area, and particle number after a given exposure duration. The table reports the RID functions for 24 h exposure duration of PEPs and TNEPs.
| (3) |
| (4) |
| (5) |
Step 4—PEPs Dosimetric Considerations
In order to ensure that the in vitro doses used in this study are equivalent to the in vivo doses from real-world consumer inhalation exposures, the dosimetric approach described in ‘Methods’ section was followed. The MPPD2 model was used to estimate the total lung deposition mass flux (1.732 μg/m2 min). The in vitro equivalent delivered to cell dose volumetric dose, in vitroeq (μg/ml), was found to be 0.033, 0.27, and 0.79 μg/ml for exposure durations of 1, 8, and 24 h of corresponding inhalation to PEPs, respectively. Using the effective density of PM0.1 (2.39 g/cm3), 100% of the administered dose would reach the cell monolayer when suspended in complete SABM after 24 h. The simple mathematical equations that allow for estimation of the delivered to cell dose metrics as a function of time are also presented in Table 4. Based on RID functions for PEPs PM0.1 size fraction, the resulting delivered dose at 24 h in vitro exposure was 0.5 μg of mass, 7.22E+12 particles/cm2, and surface area of 1.57E+04 cm2 (Table 4). For PM2.5 the delivered dose was 7.97E+12 particles/cm2 and 1.34E+04 cm2.
Step 5—PEPs Cellular Toxicity Assessment
To understand the toxicological response of PEPs exposures during a possible printing job lasting ∼13 h, we utilized a concentration of 0.5 µg/ml as derived by the MPPD2 simulation model (see Step 4). We also included 2- and 5-fold higher (1.0 and 2.5 µg/ml) concentrations, to establish a dose response. The administered mass of PEPs were 0.05, 0.1, 0.25 µg for both PM0.1 and PM2.5. There was 100% deposition of the administered mass at 24 h for both size fractions, thus the delivered mass was equivalent to the administered mass. As illustrated in Figure 3A, the results from the MTS assay show that PM0.1 size fraction of PEPs was cytotoxic to SAEC at the delivered dose of 0.25 μg. Conversely, PEPs (PM2.5) was non-toxic at 0.25 μg, but caused an increase in metabolic activity. At the low dose of 0.1 μg, PEPs (PM0.1) or PEPs (PM2.5) showed no significant changes in metabolic activity. There were also no significant changes observed when SAEC were treated with 0.05 μg of either size fraction. Interested readers can find the extensive cellular characterization details for multiple cell lines and endpoints in recent publications from authors (Pirela et al., 2015; Sisler et al., 2014).
FIG. 3.
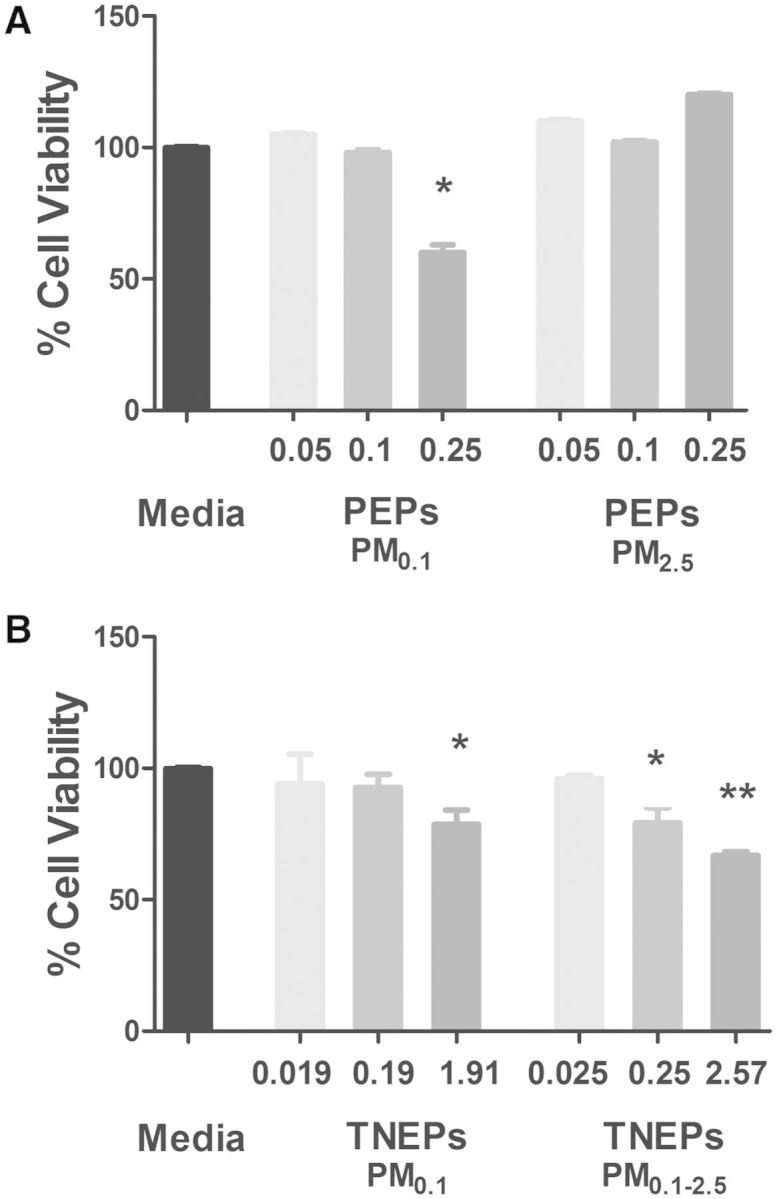
Cellular viability of SAEC exposed to A, PEPs and B, TNEPs for 24 h. Doses presented are the delivered masses for each LCPM, which were calculated from the fraction deposited of the administered mass. The administered mass of PEPs were 0.05, 0.1, 0.25 µg for both PM0.1 and PM2.5 size fractions. The administered doses of the TNEPs concentrations were 0.05, 0.5, 5 µg for both PM0.1 and PM0.1–25 size fractions. Results represent the mean ± SD of three individual experiments performed in triplicate. The P values were determined by one-way ANOVA followed by Bonferroni’s post-test, where *P ≤ 0.05, **P ≤ 0.01 versus media control.
Case Study #2: TNEPs: End of Life Release Evaluations of Incinerated CNT Embedded Polyurethane
Step 1—Aerosolized Monitoring, Sampling and PCM Characterization of TNEPs
Polyurethane containing 0.1% multi-wall CNTs was selected as the representative test NEP. The effect of temperature on PM release using the INEXS has been reported in detail by Sotiriou et al. In summary, the particle evolution starts occurring around 300°C, independent of the Td,final. The maximum release occurs ∼400°C and then for increasing time and temperature, the particle concentration decreases. The particle size distribution as well as the mode diameter shifts from low to high values for increasing time (and particle concentration) reaching a maximum at ∼400°C and then subsequently decreases over time. The average size of the released aerosol ranges from ∼30 to ∼100 nm (Supplementary Fig. S2A), is in agreement with the sizes observed in the literature (Bouillard et al., 2013). The particle mass concentration in the micro size regime (0.5–20 μm) is rather low (<3 wt%), but not negligible. This indicates that the released aerosol is rather polydisperse with some particles having aerodynamic diameters >2.5 μm. The levels of tVOCs were found to be up to 1200 ppb (Supplementary Fig. S2B).
Off line PCM characterization of released TNEPs
The size of TNEPs as determined by electron microscopy (Supplementary Fig. S2C) was in nano-size range and in agreement with the real time instrumentation measurements. This PM mainly consists of organic carbon (99.2%) that is expected as a byproduct from the thermo-decomposition of polymers. Moreover, as shown in pie-chart (Supplementary Fig. S2D), ICP-MS analysis on TNEPs revealed low amounts of zinc, arsenic, and boron. However, electron microscopy (SEM and TEM) analysis on TNEPs did not reveal any CNTs. Thus, there was no detectable ENM (0.1% CNT) release during thermodecomposition at this condition. More PCM characterization details of the released PM can be found in Sotiriou et al.
Step 2—TNEPs Extraction From Collecting Media
TNEPs proved to be highly resilient to aqueous extraction protocol resulting in low extraction efficiency (EE% = 26.16 ± 12.07%), thus ethanol extraction protocol was used in this case. Ethanol extraction resulted in higher EE% (90.65%). As shown in Supplementary Table S1, DSE below 466 J/ml for 75% ethanol extraction conditions, did not compromise the integrity of Teflon or PUF collection substrates and no substrate material was released during the ethanol extraction process.
Step 3—TNEPs Dispersion, Preparation, and Characterization
Table 3 summarizes the TNEPs particle behavior in both DI water and SABM cell culture media, as described by hydrodynamic diameter and zeta potential. Both the PM0.1 and PM0.1–2.5 TNEPs suspensions in DI water showed a decrease in size as the calculated DSE increased, toward a horizontal asymptote, representing a marginal state of agglomeration (data not shown). The estimated DSEcr value was 418 J/ml. The values for ζ were strongly negative for the PM0.1 TNEPs size fraction in DI water (−28.7 mV) and were slightly reduced when dispersed in SABM (−7.8 mV). Similar decrease in ζ was observed for the larger TNEPs (PM0.1–2.5), whose ζ was −19.6 mV in DI water and remained negative when suspended in SABM (−11.5 mV). The colloidal stability of the particle suspensions was subsequently evaluated 24 h post-sonication to DSEcr. The size of TNEPs (PM0.1) suspended in SABM remained stable with an average diameter ranging from 250 to 280 nm. Stable SABM suspensions in terms of size distribution of formed agglomerates were observed for the larger size fraction of TNEPs (PM0.1–2.5) also (size distribution data not shown) for 24 h period. The VCM-ISDD measured effective densities of the SABM suspensions were found to be 1.148 and 1.141 g/cm3 for PM0.1 and PM0.1–2.5, respectively.
Step 4—TNEPs Dosimetric Considerations
As described previously, the MPPD2 model was used (model parameters similar to that for PEPs—Table 2) to estimate the total lung deposition mass flux of (0.0605 μg/m2 min) for TNEPs. The in vitro cell delivered, equivalent volumetric dose, in vitroeq (μg/ml), for simulating inhalation exposure durations of 1, 8, and 24 h to TNEPs was found to be 0.017, 0.135, and 0.407 μg/ml, respectively. Using the effective density of PM0.1 (1.148 g/cm3), within 24 h of in vitro exposure, 38.2% of the administered dose would be delivered to the monolayer surface. In regards to TNEPs of PM0.1–2.5, 51.4% of the administered dose would reach the cell monolayer after 24 h exposure. Based on RID functions the resulting delivered dose was 1.57E+13 particles/cm2 and 3.94E+04, respectively for PM0.1 fraction (Table 4). For PM0.1–2.5 the delivered dose was 7.69E+12 particles/cm2 and 3.4E+04.
Step 5—TNEPs Cellular Toxicity Assessment
In our toxicological assessments, the lowest concentration utilized was 0.5 µg/ml to simulate a 24 h exposure to TNEPs as derived by the MPPD2 simulation model (see Step 4). We included 10- and 100-fold doses (5 and 50 µg/ml) to obtain a dose–response relationship. The administered doses of the TNEP concentrations was 0.05, 0.5, 5 µg. The deposited doses of PM0.1 was 0.019, 0.19, 1.91 µg and for PM0.1–2.5 0.025, 0.25, 2.57 µg due to 38.2 and 51.4% deposition of administered doses at 24 h, respectively. SAEC exposed to TNEPs (PM0.1) (1.91 µg (delivered dose)) displayed a significant reduction in metabolic activity (21.3%) after 24 h exposure in comparison to media only controls (Fig. 3B). Exposure to TNEPs (PM0.1–2.5) (2.57 μg (delivered dose)) decreased metabolic activity of SAEC by 33.2%, suggesting the larger size TNEPs are more toxic than their smaller counterparts. When dosimetry is considered, the SAEC were exposed to 34.5% more of TNEPs (PM0.1–2.5) (delivered mass) in comparison to TNEPs (PM0.1), which contributed to the additional toxicity observed.
To account for the presence of minimal ethanol concentrations from solvent extraction in toxicological evaluations, an ethanol control of the same (%v/v) of the highest TNEP concentration was used. The MTS assay indicated no reduction in viability due to ethanol extraction solution diluted in media (1:10) at the same %v/v of TNEP exposures, which suggests the ethanol extraction method imposes no additional toxicity to LCPM suspensions. Thus, the control cellular toxicological experiments for evaluating the effect of ethanol on the outcome were negative and the results were similar to media only controls (Supplementary Fig. S3).
Validation experiments for the V/V extraction process
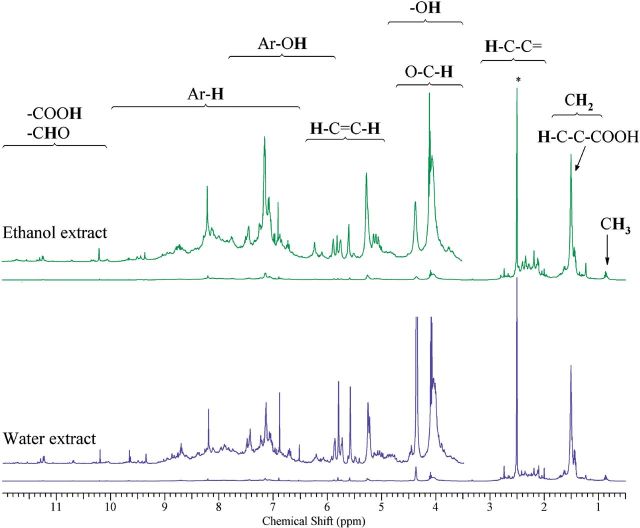
NMR analysis on extracted particles and collection substrates after extraction was conducted to compare the chemical content between the two extraction protocols. Resonances of aliphatic (H-C; 0.5–6.5 ppm), aromatic (Ar-H; 6.5–9 ppm), and carboxylic/aldehyde protons (H-C=O; 9–12 ppm) were observed (Fig. 4). The resonances in the 3.5–5.0 ppm region indicated the presence of hydroxyl groups (–OH), and protons in carbons adjacent to OH– (H–C–O) and ether– (H–C–O–C) functional groups. Vinylic–Hs (=C–H) (5.0–6.5 ppm) overlap with the region containing phenol (Ar–OH) signals (3.5–7.5 ppm). H–C protons accounted for 38.7 and 40.1% and H–C–C = protons accounted for 42.7 and 44.5% of H-atoms for the ethanol and water extracts, respectively. The percentages of =C–H and –OH protons were 8.2 and 8.4% for the ethanol and water extract, respectively. Aromatic–Hs content was slightly different between the ethanol and water extract (10.2 and 6.8%, respectively).
FIG. 4.
500 MHz 1H NMR spectrums in DMSO-d6 for TNEPs extract, following extraction protocol in ethanol (upper) and water (lower). Asterisk indicates the residual DMSO signal.
These NMR results indicated that the water soluble and the ethanol soluble fraction was a mixture of carbonyl, carboxyl, and aliphatic polyols and the relatively high contribution from saturated compounds (allylic, vinylic compounds) and aromatic compounds, compared with atmospheric aerosols (Supplementary Fig. S4). The similarities in the relative distribution of functional groups suggested that the chemical content of extracted organic aerosol did not change for the two extraction solvents. A peak-by-peak analysis also showed that <0.5% by mass, ethanol was identified in the ethanol extract that was not present in the water extract. The estimated non-exchangeable organic hydrogen concentration for the water and ethanol extracts were 26.94 and 78.60 μmol, respectively, resulting to an (Ethanol/Water)H ration of 2.91. This was comparable (within 11%) to the ratio of extracted mass for the two solvents (Ethanol/Water)mass (3.29) suggesting that the quantitative differences between the two extracts were due to more efficient extraction by ethanol rather than the extraction of other organic species.
DISCUSSION
Assessing the environmental, health, and safety (EHS) of released LCPM across the LC of NEPs is an area of research still in the initial developmental phase (Gavankar et al., 2012; Klöpffer et al., 2007). Ongoing efforts have focused on addressing this issue by borrowing existing traditional concepts of aerosol science and ambient particle toxicology (Bein and Wexler, 2014; Froggett et al., 2014). However, there is a critical need to develop a standardized integrated methodology that can be used for sampling, extraction, dispersion, and dosing associated with toxicological assessment of LCPM (Gavankar et al., 2012; Klöpffer et al., 2007). Using two different LCPM release case studies, one simulating consumer use of NEPs (Pirela et al., 2014a, b) and the other related to disposal and subsequent thermodecomposition of NEPs (end of life) (Sotiriou et al., 2015), the proposed SEDD methodology was evaluated and validated.
Real-time monitoring and size fractionated sampling of the LCPM release from NEPs is an important element of the SEDD methodology. As clearly shown in the two real-world case studies outlined here, a poly-dispersed aerosol, which may or may not contain the pure form of ENMs used in the synthesis of NEPs, is expected to be released across their LC. A suite of instruments (Table 1) are needed to measure important LCPM parameters such as size distribution, total particle mass and number concentration as a function of size, volatile/semi-volatile organic components, temperature, and humidity. In the presented case studies, SMPS and APS real-time instrumentation was used in tandem enabled the detection of broad size ranges and VOC monitor for quantifying released gaseous pollutants.
Another important element of the SEDD methodology is to perform size selective sampling and to collect large amounts of each size fraction to evaluate biological properties of PM (Bello et al., 2009). For example, the Nano-ID sampler can be used to sample PM from 0.002–20 μm, and provide size fractionation of sampled particles across the nano to micron range (Pfefferkorn et al., 2010), but cannot be used to collect large quantities of particles needed for toxicological assessment studies. Similarly, samplers such as the thermophoretic and electrostatic precipitators used to collect PM samples for morphological analysis cannot provide proper size fractionation and collect large amounts for offline PCM characterization and toxicological assessment (Pfefferkorn et al., 2010). In both case studies presented here, the authors used the HCCI sampler, which provided size fractionation of LCPM and the collection of relatively large amounts of particles (mg quantities) onto inert polyurethane foam PUF/Teflon filter, impaction substrates (Demokritou et al., 2004).
Offline physicochemical analysis of sampled LCPM is warranted to link potential toxicological outcomes to specific chemical species present in the LCPM (i.e., number of total and water soluble metals, sVOCs, and organic and elemental carbon) (Solomon et al., 2011). Table 1 lists the description of such routinely used techniques. ICP-MS analysis on LCPM provides trace metal elemental mapping and quantification, whereas FTIR aids in functional groups analysis. FTIR measures the absorption frequencies of sample and the absorption intensity peaks for determining concentration. NMR used here can provide sensitive chemical functional group analysis, quantification, and structural studies on LCPM. GC-MS is also a highly useful and preferred technique for analysis and estimation of gaseous pollutants, VOCs, and sVOCs.
Proper extraction and characterization of collected LCPM post-sampling is important to further explore and link the effect of PCM properties on toxicological outcomes (Bein and Wexler, 2014; Solomon et al., 2011). Bein and Wexler (2014) highlighted the importance of using an extraction protocol for ambient PM that (a) maximizes extraction efficiency, (b) minimizes compositional biases in extracted PM, relative to sampled PM, and (c) minimizes extraction artifacts. Moreover, their work extensively summarizes the critical steps and various methods that can be used in extracting PM for subsequent PCM and toxicological evaluations. In brief, in their developed protocol the PM substrate is ultra-sonicated in water (that has 70% extraction by mass) followed by sequential ultra-sonication of filters in solvents of varying polarity, including water, dichloromethane (DCM), and hexane (for remaining mass). Water extract is lyophilized to recover dry PM whereas for liquid–liquid extraction in organic compounds, the solvents were evaporated under a nitrogen atmosphere to recover the solvent soluble fraction. Subsequently, the water solution is lyophilized and solvent soluble fractions are added back to the dry PM from lyophilization. The method is extensive and relevant for atmospheric particles; however, modifications are needed when applied to LCPM. While such an extraction protocol could be used on LCPM, use of strong organic solvent potentially lead to particle solubilization and changes in PCM properties. As in the case of TNEPs, use of DCM will be problematic and might lead to solubilization of polyurethane, thus changing the particle characteristics (morphology, potential particle fusion, and aggregation). Another limitation of this approach is related to possible generation of fragmented filter particles (FFP) on sonication thus sample contamination and impact of FFP on toxicological evaluation. The extraction protocol used in the SEDD methodology follows a simpler approach that minimizes possible extraction artifacts while maximizing EE% (over 90%). It is worth mentioning that the extraction procedure in SEDD methodology has been optimized for delivering sonication energy that maintains the physical integrity of filter (reducing FFP, Supplementary Table S1).
As mentioned in the study of Bein and Wexler (2014) no PM extraction protocol is perfect and may not lead to 100% recovery of all sampled PM compounds. As shown here, the SEDD methodology resulted in high by mass extraction efficiency for both impaction types (>90%), which is indicative of minimum extraction artifacts. Also, it is also worth noting that in the case of using the ethanol extraction protocol, since ethanol is water miscible, the final LCPM suspension in water will still contain minimal ethanol quantities. As shown here in the presented case study, the final suspension in DI water contains <0.5% by mass ethanol. While this amount is minimal, control toxicological experiments would need to be included in the toxicological evaluations to assess the effect of ethanol on the outcome. As indicated in the presented TNEP case study the toxicological controls were negative and at same levels as the media only control (Supplementary Fig. S3). It is also worth noting that the final LCPM suspension can be further diluted a few times followed by repetition of ethanol removal step in order to reduce the ethanol component to 0.001% by mass.
In nanotoxicology, studies with ‘raw’ ENMs have shown the importance of proper particle dispersion and characterization in physiological medium. The authors have previously shown that particle sonication using the critical sonication energy or DSEcr can minimize agglomeration and if combined with the serum proteins, fairly stable, and monodispersed colloidal conditions (Cohen et al., 2013; Pal et al., 2015). In both case studies presented here, as part of SEDD methodology, we utilized the DSEcr for dispersing LCPM and achieved reduced agglomerate size and stable suspensions in culture media.
Recent studies that highlight the impact of dispersion and in vitro dosimetry on toxicity outcomes have been recently published from our group, demonstrating the importance of in vitro dosimetry in toxicological ranking of ENMs (Cohen et al., 2013, b; Pal et al., 2014, 2015). It was shown that strength of dose–response association and rank order of toxicity of nanomaterials changes when dose–response data are corrected for the delivered dose (Pal et al., 2015). In this study, fate and transport numerical models enabled accurate calculations of the rate at which LCPM particles interact with cell monolayers and the portion of administered LCPM mass deposited on the cell monolayer surface over time. Furthermore, as illustrated in the two case studies presented here, bringing in vivo and in vitro doses on same scale is of paramount importance in nanotoxicology studies. As indicated in the TNEPs case study, it was shown that larger PM was more toxic compared with the smaller size fraction PM for same administered dose. However, when dosimetry is considered, the delivered mass or dose of the TNEPs PM0.1–2.5 was greater than that of PM0.1, which contributed to the toxicity profile observed. Conversely, we saw a different trend in the PEPs biological responses. The marked decrease in cell viability observed due to increasing dose of PEPs (PM0.1) was not found in the PEPs (PM2.5) exposures even with identical delivered masses. This larger size fraction may not elicit the same cellular responses as PM0.1 because of lower surface-area-to-volume ratios or differences in chemical composition. It is worth noting that the effect of gaseous pollutants such as VOCs and ozone released from NEPs across their LC may have some synergistic effects on toxicity. More importantly, such VOCs and sVOCs may condense on particle surfaces and render those particles more bioactive.
In summary, the increased use of NEPs in our society will inevitably lead to environmental and human exposures. Evidence of environmental health and safety concerns from LCPM released across the LC of NEPs will continue to grow. The current risk assessment paradigm used in nanotoxicology, which focuses on the toxicological characterization of ‘raw’ ENMs, should be revised to include assessments of particles released across the LC of NEPs. Toxicity assessment will play an important role in regulating as well as designing safer NEPs. The comprehensive SEDD framework discussed here can be implemented for designing relevant studies for studying LCPM release during NEPs manufacturing, usage and disposal.
FUNDING
This work was supported by the National Institute for Occupational Safety and Health (NIOSH) (Grant # 200-2013-M-57393) and the National Science Foundation (NSF) (Grant # 1436450). C.Y. Watson was funded by the National Institute of Health (NIH) National Heart Lung Blood Institute (NHLBI) Ruth L. Kirschstein T32 training grant (NIH HL007118). NIOSH (Grant # 200-2013-M-57393) and NSF (Grant # 1436450).
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
ACKNOWLEDGMENTS
The authors like to acknowledge Dr. Georgios Sotiriou (HSPH) for his helpful discussion with the TNEPs study. The authors have no conflicts of interest to disclose.
REFERENCES
- Anjilvel S., Asgharian B. (1995). A Multiple-path model of particle deposition in the rat lung. Fund. Appl. Toxicol. 28, 41–50. [DOI] [PubMed] [Google Scholar]
- BBC (2010). Nanotechnology: A Realistic Market Assessment Mc Williams, A. Nanotechnology: Realistic Market Assessment. BBC Research, http://www.bccresearch.com/report/NAN031D.html, Published July 1, 2010. [Google Scholar]
- Bein K. J., Wexler A. S. (2014). A high-efficiency, low-bias method for extracting particulate matter from filter and impactor substrates. Atmos. Environ. 90, 87–95. [Google Scholar]
- Bello D., Wardle B., Yamamoto N., Guzman deVilloria R., Garcia E., Hart A., Ahn K., Ellenbecker M., Hallock M. (2009). Exposure to nanoscale particles and fibers during machining of hybrid advanced composites containing carbon nanotubes. J. Nanopart. Res. 11, 231–249. [Google Scholar]
- Biran R., Tang Y.-Z., Brook J. R., Vincent R., Keeler G. J. (1996). Aqueous extraction of airborne particulate matter collected on Hi-Vol Teflon filters. Int. J. Environ. Anal. Chem. 63, 315–322. [Google Scholar]
- Borm P. J., Robbins D., Haubold S., Kuhlbusch T., Fissan H., Donaldson K., Schins R., Stone V., Kreyling W., Lademann J., et al. (2006). The potential risks of nanomaterials: a review carried out for ECETOC. Part. Fibre Toxicol. 3, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillard J., R’Mili B., Moranviller D., Vignes A., Le Bihan O., Ustache A., Bomfim J. S., Frejafon E., Fleury D. (2013). Nanosafety by design: risks from nanocomposite/nanowaste combustion. J. Nanopart. Res. 15, 1–11. [Google Scholar]
- Buzea C., Pacheco I. I., Robbie K. (2007). Nanomaterials and nanoparticles: sources and toxicity. Biointerphases 2, MR17–MR71. [DOI] [PubMed] [Google Scholar]
- Cohen J., Deloid G., Pyrgiotakis G., Demokritou P. (2013). Interactions of engineered nanomaterials in physiological media and implications for in vitro dosimetry. Nanotoxicology 7, 417–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. M., Derk R., Wang L., Godleski J., Kobzik L., Brain J., Demokritou P. (2014a). Tracking translocation of industrially relevant engineered nanomaterials (ENMs) across alveolar epithelial monolayers in vitro. Nanotoxicology 8(Suppl 1), 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. M., Teeguarden J. G., Demokritou P. (2014b). An integrated approach for the in vitro dosimetry of engineered nanomaterials. Part. Fibre Toxicol. 11, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLoid G., Cohen J. M., Darrah T., Derk R., Rojanasakul L., Pyrgiotakis G., Wohlleben W., Demokritou P. (2014). Estimating the effective density of engineered nanomaterials for in vitro dosimetry. Nat. Commun. 5, 3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demokritou P., DeLoid G., Cohen J. (2012). Novel methods of measuring effective density of nanoparticles in fluids. U.S. Patent Application 61/661,895 [Google Scholar]
- Demokritou P., Gass S., Pyrgiotakis G., Cohen J. M., Goldsmith W., McKinney W., Frazer D., Ma J., Schwegler-Berry D., Brain J., et al. (2013). An in vivo and in vitro toxicological characterisation of realistic nanoscale CeO(2) inhalation exposures. Nanotoxicology 7, 1338–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demokritou P., Lee S. J., Ferguson S. T., Koutrakis P. (2004). A compact multistage (cascade) impactor for the characterization of atmospheric aerosols. J. Aerosol Sci. 35, 281–299. [Google Scholar]
- Froggett S., Clancy S., Boverhof D., Canady R. (2014). A review and perspective of existing research on the release of nanomaterials from solid nanocomposites. Part. Fibre Toxicol. 11(1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavankar S., Suh S., Keller A. (2012). Life cycle assessment at nanoscale: review and recommendations. Int. J. Life Cycle Assess. 17, 295–303. [Google Scholar]
- Hamilton R. F., Jr, Wu N., Porter D., Buford M., Wolfarth M., Holian A. (2009). Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Part. Fibre Toxicol. 6, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinderliter P. M., Minard K. R., Orr G., Chrisler W. B., Thrall B. D., Pounds J. G., Teeguarden J. G. (2010). ISDD: a computational model of particle sedimentation, diffusion and target cell dosimetry for in vitro toxicity studies. Part. Fibre Toxicol. 7, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A., McFerran S., Lazareva A., Suh S. (2013). Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 15, 1–17. [Google Scholar]
- Klöpffer W., Curran M. A., Frankl P., Heijungs R., Köhler A., Olsen S. I. (2007). In: Nanotechnology and Life Cycle Assessment . A Systems Approach to Nanotechnology and the Environment: Synthesis of Results Obtained at a workshop, Washington, DC, 2–3 October 2006. [Google Scholar]
- Limited R. E.-S. P. (2011). Nanotechnology Market Forecast to 2013. RNCOS E-Services Private Limited, 2011. [Google Scholar]
- NNI (2011). Environmental, Health, and Safety (EHS) Research Strategy. http://www.nano.gov/node/681 Published October 20, 2011. [Google Scholar]
- NRC (2012). A Research Strategy for Environmental, Health, and Safety Aspects of Engineered Nanomaterials. Committee to Develop a Research Strategy for Environmental, Health, and Safety Aspects of Engineered Nanomaterials; National Research Council. A Research Strategy for Environmental, Health, and Safety Aspects of Engineered Nanomaterials. Washington (DC): National Academies Press (US); 2012 Jan 25. Available from: http://www.ncbi.nlm.nih.gov/books/NBK189509/ [PubMed] [Google Scholar]
- Pal A. K., Aalaei I., Gadde S., Gaines P., Schmidt D., Demokritou P., Bello D. (2014). High resolution characterization of engineered nanomaterial dispersions in complex media using tunable resistive pulse sensing technology. ACS Nano 8, 9003–9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A. K., Cohen J., Bello D., Demokritou P. (2015). Implications of in-vitro dosimetry on toxicological ranking of low aspect ratio engineered nanomaterials. Nanotoxicology 1–15. (doi:10.3109/17435390.2014.986670). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn F. E., Bello D., Haddad G., Park J.-Y., Powell M., Mccarthy J., Bunker K. L., Fehrenbacher A., Jeon Y., Virji M. A., et al. (2010). Characterization of exposures to airborne nanoscale particles during friction stir welding of aluminum. Ann. Occup. Hyg. 54, 486–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirela S. V., Miousse I. R., Lu X., Castranova V., Thomas T., Qian Y., Bello D., Kobzik L., Koturbash I., Demokritou P. (2015). Laser printer-emitted engineered nanoparticles lead to cytotoxicity, inflammation and changes in dna methylation in human cells. Environ. Health Perspect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirela S. V., Pyrgiotakis G., Bello D., Thomas T., Castranova V., Demokritou P. (2014a). Development and characterization of an exposure platform suitable for physico-chemical, morphological and toxicological characterization of printer-emitted particles (PEPs). Inhal. Toxicol. 26, 400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirela S. V., Sotiriou G. A., Bello D., Shafer M., Bunker K. L., Castranova V., Thomas T., Demokritou P. (2014b). Consumer exposures to laser printer-emitted engineered nanoparticles: a case study of life-cycle implications from nano-enabled products. Nanotoxicology 11, 1–9. DOI: 10.3109/17435390.2014.976602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers K. W., Brown S. C., Krishna V. B., Wasdo S. C., Moudgil B. M., Roberts S. M. (2006). Research strategies for safety evaluation of nanomaterials. Part VI. Characterization of nanoscale particles for toxicological evaluation. Toxicol. Sci. 90, 296–303. [DOI] [PubMed] [Google Scholar]
- Roco M., Mirkin C., Hersam M. (2011). Nanotechnology research directions for societal needs in 2020. J. Nanopart. Res. 13, 897–919. [Google Scholar]
- RS (2004). Nanoscience and Nanotechnologies: Opportunities and Uncertainties. Royal Society, Royal Academy of Engineering. Nanoscience and nanotechnologies: Opportunities and Uncertainties, http://www.nanotech.org.uk/finalReport, 2004. [Google Scholar]
- Sisler J. D., Pirela S. V., Friend S., Farcas M., Schwegler-Berry D., Shvedova A., Castranova V., Demokritou P., Qian Y. (2014). Small airway epithelial cells exposure to printer-emitted engineered nanoparticles induces cellular effects on human microvascular endothelial cells in an alveolar-capillary co-culture model. Nanotoxicology 11, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon P. A., Fraser M. P., Herckes P. (2011). Methods for Chemical Analysis of Atmospheric Aerosols. In P. Kulkarni, P. A. Baron and K. Willeke (eds), Aerosol Measurement: Principles, Techniques, and Applications. Hoboken, NJ: John Wiley & Sons, Inc. doi: 10.1002/9781118001684.ch9 [Google Scholar]
- Sotiriou G. A., Singh D., Zhang F., Wohllenben W., Demokritou P. (2015). An integrated exposure generation platform for the thermal decomposition of nano-enabled products. ES:Nano. Advance Access published March 2, 2015. DOI: 10.1039/C4EN00210E. [Google Scholar]
- Wiesner M. R. (2006). Responsible development of nanotechnologies for water and wastewater treatment. Water Sci. Technol. 53, 45–51. [DOI] [PubMed] [Google Scholar]
- Wohlleben W., Brill S., Meier M. W., Mertler M., Cox G., Hirth S., von Vacano B., Strauss V., Treumann S., Wiench K., et al. (2011). On the lifecycle of nanocomposites: comparing released fragments and their in-vivo hazards from three release mechanisms and four nanocomposites. Small 7, 2384–2395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.