Abstract
Tissue-type plasminogen activator (tPA) a serine protease is constituted of five functional domains through which it interacts with different substrates, binding proteins, and receptors. In the last years, great interest has been given to the clinical relevance of targeting tPA in different diseases of the central nervous system, in particular stroke. Among its reported functions in the central nervous system, tPA displays both neurotrophic and neurotoxic effects. How can the protease mediate such opposite functions remain unclear but several hypotheses have been proposed. These include an influence of the degree of maturity and/or the type of neurons, of the level of tPA, of its origin (endogenous or exogenous) or of its form (single chain tPA versus two chain tPA). In this review, we will provide a synthetic snapshot of our current knowledge regarding the natural history of tPA and discuss how it sustains its pleiotropic functions with focus on excitotoxic/ischemic neuronal death and neuronal survival.
Keywords: tissue-type plasminogen activator, excitotoxicity, apoptosis, NMDA receptors, differential effects
The Natural History Of tPa
Morgagni (1761) noted that the blood of patients who died suddenly was not completely coagulated. Denis (1838) observed the spontaneous dissolution of blood clots. Fifty years later, Denys and de Marbaix (1889) postulated the existence of an endogenous fibrinolytic enzyme. Accordingly, Hedin (1903) revealed a proteolytic activity in serum globulin fraction, later identified as the fraction containing a precursor of plasmin. Christensen and Macleod (1945) proposed that this inactive circulating precursor, named plasminogen, could be activated by bacterial extracts like streptokinase. Macfarlane and Biggs (1948) completed the description of the plasminogen activation cascade. In parallel, Conradi (1902) identified tPA, at this time named fibrikinase, in different organs), later characterized to mediate fibrinolysis (Fleisher and Loeb, 1915; Astrup and Permin, 1947; Astrup and Stage, 1952). tPA was then purified from human vessels and uterus in Binder et al. (1979), Rijken et al. (1979) and in larger amounts from Bowes melanoma cell line allowing its biochemical characterization (Collen et al., 1982; Collen and Lijnen, 2009). Pennica et al. (1983) succeeded in cloning and expressing recombinant tPA, providing the primary structure of tPA. tPA is a protein of 527 amino-acids including three glycosylation sites and 17 disulfide bridges (Pennica et al., 1983). Collen and Lijnen (1991) then provided evidence that tPA could facilitate the dissolution of blood clots by inducing the degradation of fibrin in a plasminogen-dependent manner. tPA is now used in the clinic to promote fibrinolysis, especially at the acute phase of ischemic stroke either alone (NINDS, 1995) or combined with thrombectomy (Campbell et al., 2015; Goyal et al., 2015).
In addition to this fibrinolytic function at the origin of its discovery, an increasing number of studies have since the mid-90s, discovered functions of tPA within the brain parenchyma. In particular, tPA is believed to control neuronal fate during several CNS disorders, including multiple sclerosis, Alzheimer’s disease, and stroke. The aim of this review is to summarize and discuss structure-function studies related to the influence of tPA on neuronal death and survival.
tPA or tPAs?
The mature form of tPA is a mosaic protein of five distinct modules, which, from its N-terminal end to its C-terminal end, are: a finger domain (F), an epidermal growth factor-like domain (EGF), two kringle domains (K1 and K2), and a serine protease proteolytic domain (SP). The finger domain is involved in tPA binding to fibrin and is necessary to promote fibrinolytic activity at low plasminogen activator concentrations (Larsen et al., 1988). In the brain, other functions attributed to the finger domain include its ability to cross the blood–brain barrier (Benchenane et al., 2005), its astrocytic clearance (Cassé et al., 2012) and some of its signaling pathways (Siao and Tsirka, 2002; Pineda et al., 2012). The EGF-like domain shows homology with EGF. Both the trophic and mitogenic functions of tPA have been attributed to this domain (Liot et al., 2006; Ortiz-Zapater et al., 2007; Correa et al., 2011; Haile et al., 2012). The EGF-like domain has been also reported to contribute to the hepatic recapture of tPA (Hajjar and Reynolds, 1994). The kringle domains fold into large loops stabilized by three disulfide bridges. Because of the high-mannose-type glycosylation at Asn117, K1 is of major importance in the uptake of tPA by mannose receptors on liver endothelial cells in vivo and in vitro (Kuiper et al., 1996). The K2 domain and more specifically its lysine binding site (LBS) is involved in the capacity of tPA to bind and activate substrates and/or receptors such as plasminogen, PDGF-CC (platelet derived growth factor-CC; Fredriksson et al., 2004) and NMDAR (N-methyl-D-aspartate receptor; López-Atalaya et al., 2008). The K1 of tPA does not possess a LBS (Kim et al., 2003). The C-terminal domain supports the catalytic activity of tPA and forms the catalytic triad (His 322, Asp 371, and Ser 478) involving an aspartic acid residue (Asp371) hydrogen-bonded to a histidine (His322), which itself is hydrogen-bonded to a serine (Ser478).
As detailed here after, the literature suggests that there is not one but several forms of tPAs.
Long and Short Variants
The pro-form of tPA is a molecule of 562 amino acids. The signal peptide and a pro-peptide of, respectively, 22 and 10 amino acids should be removed before storage in vesicles and release. Three additional amino acids (Gly–Ala–Arg) at the N-terminal end of the molecule can be also removed leading to the release of either the long variant (L-tPA) or the short variant (S-tPA) of 530 and 527 amino acids, respectively (Jörnvall et al., 1983; Berg and Grinnell, 1991). These tPAs include 17 disulfide bridges.
sc-tPA vs. tc-tPA
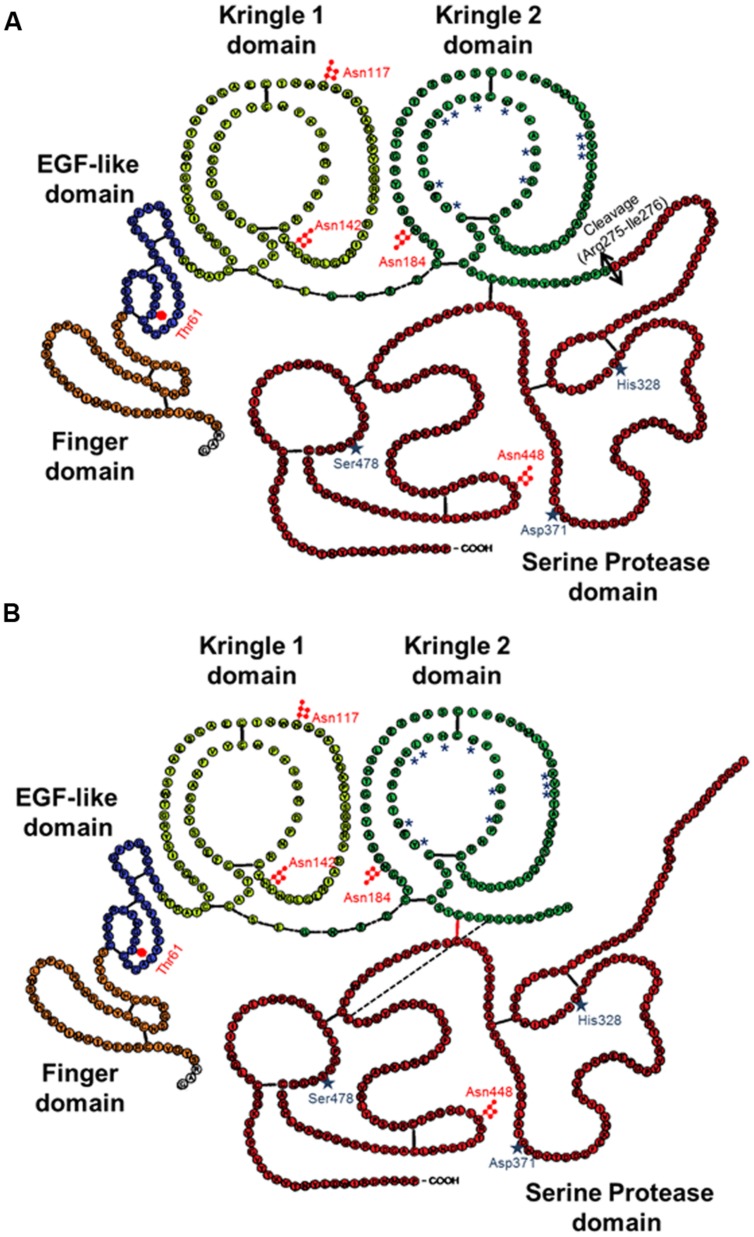
In contrast to the other members of the chymotrypsin family, tPA is not synthesized and secreted as a “true” zymogen (Madison et al., 1993). Like other members of the family, the secreted single-chain tPA (sc-tPA; Figure 1A) can be processed into a two-chain form tPA (tc-tPA; Figure 1B) by plasmin or kallikrein (Wallén et al., 1982; Ichinose et al., 1984). However, sc-tPA is an unusually active zymogen (high intrinsic proteolytic activity, low zymogenicity) that does not require proteolytic processing to be active but relies on the presence of an allosteric regulator, such as fibrin (Thelwell and Longstaff, 2007). The passage from the sc-tPA to the tc-tPA form results from the hydrolysis of the peptide bond linking the Arg275 and the Ile276, both parts of the protein remaining connected by a disulfide bridge between Cys299 (heavy chain A) and Cys430 (light chain B) and a novel salt bridge between Arg302 and Glu445 (Lamba et al., 1996). In the absence of an allosteric regulator such as fibrin, tc-tPA is fivefold catalytically more active than sc-tPA (Rånby et al., 1982; Wallén et al., 1982; Tate et al., 1987; Petersen et al., 1988; Boose et al., 1989). However, in the presence of fibrin, both sc-tPA and tc-tPA display the same catalytic activity (Thelwell and Longstaff, 2007).
FIGURE 1.
Schematic representations of the primary structure of sc-tPA (A) and tc-tPA (B). Each amino acid is represented by its single letter symbol. Sites of N- ( ) or O-glycosylation (
) or O-glycosylation ( ) are showed. The active site residues His322, Asp371, and Ser478 are marked by stars. The amino acids involved in the structure of the lysine binding site are noted with asterisks. The black bars indicate disulfide bonds. The black bar dotted indicate salt bond. The double-arrow indicates the cleavage site for conversion of sc-tPA to tc-tPA.
) are showed. The active site residues His322, Asp371, and Ser478 are marked by stars. The amino acids involved in the structure of the lysine binding site are noted with asterisks. The black bars indicate disulfide bonds. The black bar dotted indicate salt bond. The double-arrow indicates the cleavage site for conversion of sc-tPA to tc-tPA.
Type I vs. Type II tPA
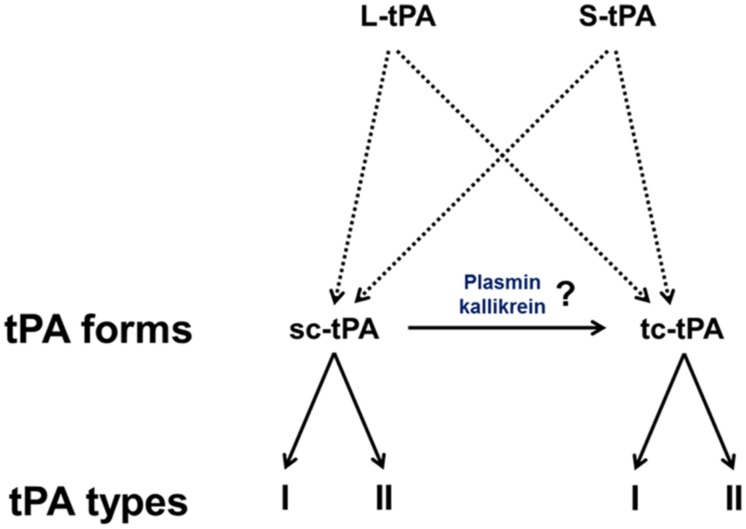
Type plasminogen activator is a glycoprotein containing three major N-glycosylation sites. Two glycosylations are constitutives at Asn117 within the kringle 1 domain and at Asn448 within the serine protease domain. A third one is alternative at Asn184 within the kringle 2 domain. Type I tPA is glycosylated at Asn117, Asn184, and Asn448, while type II tPA is glycosylated only at Asn117 and Asn448 (Pohl et al., 1984; Spellman et al., 1989; Mori et al., 1995; Jaques et al., 1996). Asn184 acts as a switch that enables long-distance communication between fibrin-binding residues (achieved by the finger domain) and the catalytic site in the protease domain (Rathore et al., 2012). Glycosylation of Asn184 (i.e., type I) reduces the ability of tPA to activate plasminogen as well as its binding to fibrin (Einarsson et al., 1985; Wittwer et al., 1989; Berg et al., 1993). Type I sc-tPA seems to be more stable than type II sc-tPA regarding its conversion to tc-tPA (Wittwer and Howard, 1990; Berg et al., 1993; Figure 2). tPA also contains a O-linked fucose at Thr61 (occupancy 100%) within the EGF domain (Harris et al., 1991) and potentially an additional N-glycosylation site at Asn142 within the K1 domain (occupancy 1%; Borisov et al., 2009).
FIGURE 2.
The diversity of tPAs. L-tPA and S-tPA are released under their single chain form (sc-tPA), possibly cleaved into their two-chain form (tc-tPA) by plasmin or kalikrein. Each form of tPA exists in two glycosylated states, types I or II.
Is tPA Good or Bad for Neuronal Survival?
The Facts
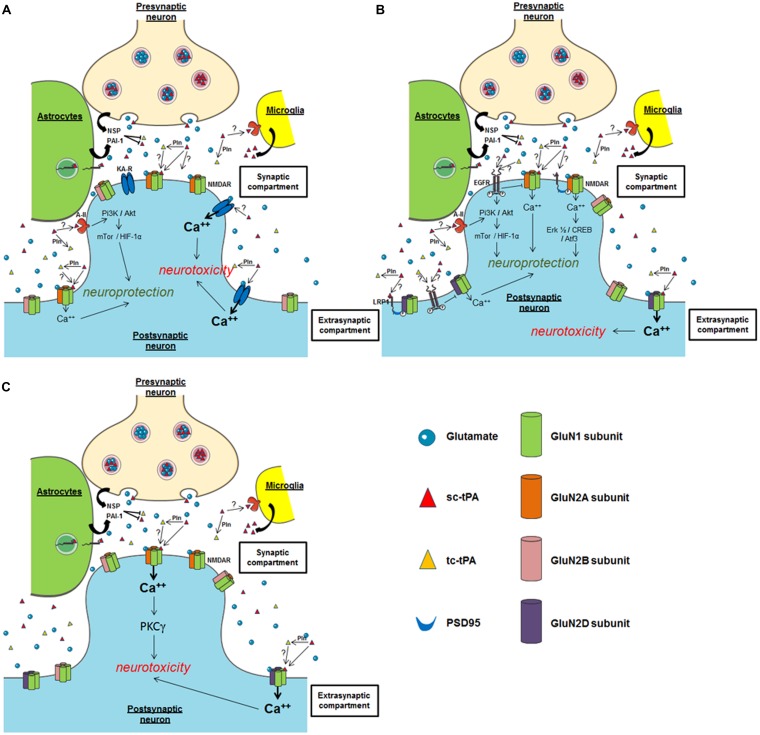
The group of Sidney Strickland was the first to demonstrate that tPA deficient mice were more sensitive to hippocampal neuronal death induced by both NMDAR- and non-NMDAR-agonists (Tsirka et al., 1995), an effect dependent of the ability of tPA to activate plasminogen into plasmin (Tsirka et al., 1997a,b; Figure 3A). Accordingly, several studies have reported that inhibitors of tPA, such as neuroserpin and type 1 plasminogen activator inhibitor (PAI-1) protect neurons against toxicity induced by the over-activation of NMDARs (Buisson et al., 1998; Zhang et al., 2002; Gabriel et al., 2003; Lebeurrier et al., 2005). Exogenous tPA was then reported pro-neurotoxic, on cortical neurons, in paradigms of in vitro or in vivo excitotoxicity mediated by over-activation of NMDAR (Nicole et al., 2001; Liberatore et al., 2003; Reddrop et al., 2005; Park et al., 2008; Figure 3B). The tPA was also reported to promote damages on Purkinje cells (Lu and Tsirka, 2002; Li et al., 2006, 2013; Cops et al., 2013; Figure 3C), especially by altering the neurotrophic mechanisms that control their postnatal development (Li et al., 2006, 2013).
FIGURE 3.
Possible mechanisms of tPA on neuronal survival. (A) Hippocampal neurons; (B) cortical neurons; (C) cerebellar neurons. NSP, neuroserpin; Pln, plasmin; PAI-1, plasminogen activator inhibitor-1; NMDAR, N-methyl-D-aspartate receptor; EGFR, epidermal growth factor receptor; KA-R, kainate receptor; A-II, annexin II receptor.
Both plasmin-dependent and plasmin-independent mechanisms have been proposed to explain the potentiation of NMDAR signaling by tPA (Nicole et al., 2001; Pawlak et al., 2002; Matys and Strickland, 2003), but several recent studies agree that it can occur independently of plasminogen activation (Samson et al., 2008; Echeverry et al., 2010; Parcq et al., 2012). For instance, tPA can interact with the GluN1 subunit of NMDAR involving the LBS of its K2 domain (Nicole et al., 2001; Fernández-Monreal et al., 2004; Kvajo et al., 2004; López-Atalaya et al., 2008; Parcq et al., 2012). Our group reported that the cleavage of the amino-terminal domain of GluN1 subunit is necessary for enhancement of NMDAR signaling by tPA (Nicole et al., 2001; Fernández-Monreal et al., 2004). In the brain of protease nexin-1 (PN-1, an inhibitor of tPA) deficient mice, Kvajo et al. (2004), demonstrated an increase in the proteolytic activity of tPA, correlated with a decrease in the amount of the GluN1 subunit of the NMDA receptor. However, no cleavage of GluN1 was observed despite the interaction of tPA with the GluN1 subunits of NMDAR (Kvajo et al., 2004). Other groups did not detect tPA-dependent cleavage of GluN1, despite enhancement of NMDAR function by exogenous tPA in cortical cultures (Samson et al., 2008). In a more recent study, it was reported that sc-tPA, but not tc-tPA can promote NMDAR signaling and neurotoxicity in cortical neurons (Parcq et al., 2012; Bertrand et al., 2015). These data were the first to describe a differential function of sc-tPA and tc-tPA. tPA would also act on neuronal death by engaging Low density lipoprotein related protein (LRP) receptors, which in turn would enhance Ca2+ downstream of NMDAR (Samson et al., 2008). More recent data obtained from Schwann cells showed that tPA can promote NMDAR signaling independently of LRP1 (Mantuano et al., 2015). Similarly, in PC12 and N2a neuron-like cells, tPA may signal through a complex containing NMDAR, LPR1, and Trk receptors (Mantuano et al., 2013). Plasmin, which is generated by the tPA-dependent conversion of plasminogen, has also been reported to cleave NMDARs, specifically the GluN2 subunit. This cleavage can occur at two sites: Lys317 on GluN2A, which relieves Zn2+ inhibition and thereby increases NMDAR function (Yuan et al., 2009), and Arg67 on GluN2B, which increases sensitivity of the NMDAR to glycine (Ng et al., 2012). Whether tPA-dependent plasmin formation counteracts or interferes with tPA-dependent NMDAR activation is still under debate. Whatever the mechanism, all these studies showed that tPA can increase NMDAR signaling.
By contrast, other studies, in particular using transgenic mice over-expressing tPA in neurons (T4 transgenic mice) or tPA KO mice, suggested that tPA can also have neuroprotective effects (Haile et al., 2012; Wu et al., 2012). These two studies also proposed a mechanism dependent on the activation of NMDAR and independent on plasmin. In vitro and ex vivo studies also reported pro-survival effects of tPA on neurons (Liot et al., 2006; Lee et al., 2007; Polavarapu et al., 2007; Bertrand et al., 2015; Lemarchand et al., 2015), mainly anti-apoptotic effects. Also interesting, tPA was reported to attenuate zinc-induced neuronal cell death independently of its proteolytic action (Kim et al., 1999; Siddiq and Tsirka, 2004). Despite the heterogeneity of the paradigms used in these different studies, they all showed that this effect of tPA occurs independently of its proteolytic activity, with the activation of either PI3K/Akt, AMPK- or mTor-HIF-1alpha-dependent signaling pathways needed (Correa et al., 2011; Wu et al., 2012; Figure 3B). Two candidates have been proposed as the receptors mediating the pro-survival effects of tPA: Annexin II and EGF receptor (Siao and Tsirka, 2002; Wu et al., 2012; Bertrand et al., 2015; Lemarchand et al., 2015). The ability of tPA to convert the pro-neurotrophins (BDNF, NGF) to their active forms (Pang et al., 2004) is also a possible explanation to the pro-survival effects of tPA.
What are the Possible Explanations of the Differential Effects of tPA on Neuronal Survival? (Table 1)
Table 1.
Reported effects of tPA on challenged neurons.
| Reference | Model(s) | tPA | Mechanism(s) | |
|---|---|---|---|---|
| Beneficial | ||||
| Kim et al., 1999 | In vitro: cortical cultures exposure to 300 mM zinc (mice) | Exogenous 10 μg/ml | Independently of its proteolytic action, tPA attenuated zinc-induced cell death | |
| In vivo: kainate injection (10 mg/kg) in rats | Intracerebroventricular tPA 1 mg/ml | tPA attenuated kainate seizure-induced neuronal death in the hippocampus | ||
| Flavin and Zhao, 2001 |
In vitro: OGD, 2.5 h Cultured hippocampal neurons from rats (DIV 7–10) |
Exogenous 1,000 IU | tPA protects neurons from oxygen glucose deprivation (OGD) by a non-proteolytic action | |
| Centonze et al., 2002 | Ex vivo: striatal neurons WT and tPA –/– mice subjected to OGD | Endogenous | tPA enhanced ischemia-induced neuronal damage by facilitating apoptosis rather than necrosis | |
| Yi et al., 2004 |
In vitro: mixed cortical cell cultures (mice) Treatment: zinc (35 μmol/l) |
Exogenous 10 μg/ml | tPA attenuated zinc-induced neuronal death, independently of its proteolytic activity | |
| Head et al., 2009 |
In vitro: primary cultures of neurons (DIV 5–21) exposed to 1.4% isoflurane for 4 h In vivo: 1.4% isoflurane (anesthetic mediated neurotoxicity in mice) |
Exogenous 0.03–3 μg/ml | Isoflurane induced apoptosis at DIV 5 (but not DIV 14 or DIV 21) in cultured neurons tPA decreases isoflurane-induced cell death in primary cultures of neurons (DIV 5) Isoflurane-induced neurotoxicity in the developing rodent brain is mediated by reduced tPA synaptic release and enhanced proBDNF/p75NTR-mediated apoptosis | |
| Echeverry et al., 2010 | In vitro: cultures of hippocampal neurons (OGD conditions for 30 min (preconditioning) or not, followed 24 h later by incubation under OGD conditions for 55 min) | Endogenous (tPA KO mice) and exogenous (0–1 μM) | Treatment after OGD (early preconditionning). Beneficial effect of tPA involving a LRP1 dependent signaling pathway and independent of its proteolytic activity. Treatment 24 h after OGD (delayed preconditioning): beneficial effect of tPA via a NMDA-dependent signaling pathway (activation of pAkt), and activation of plasmin |
|
| Wu et al., 2012 | In vitro: cultures of cortical neurons (55 min OGD and then exposed 10 min later to a second episode of hypoxia (10 min OGD, post-conditioning)) | Endogenous (transgenic mice T4) | Decrease of the activation of mTor- HIFα, involving NMDAR | |
| Wu et al., 2013a |
In vivo: excitotoxin-induced neuronal death T4 mice and WT Intrastriatal injection of NMDA (50 mM) |
T4 mice or IV 1 mg/Kg on WT mice | tPA protected the brain from excitotoxin-induced cell death Dose-dependent effect of tPA on NMDA-induced neuronal death – 5 and 10 nM beneficial – 100 at 500 nM deleterious |
|
| (1) The neuroprotective effect of tPA was mediated by activation of synaptic GluN2A containing NMDAR via a plasminogen-independent mechanism (2) ERK activation mediated the protective effect of tPA against excitotoxin-induced neuronal death |
||||
| In vitro: cerebral cortical neurons (mice) NMDA induced neuronal death (50 M) | Exogenous 5–500 nM | (3) tPA activated the ERK -CREB-Atf3 pathway (4) Atf3-mediated the protective effect of tPA against excitotoxin-induced neuronal death |
||
| Wu et al., 2013b | In vitro: cultures of cortical neurons (OGD 55 min) | Endogenous (transgenic mice T4) | Adaptation to metabolic stress – AMPK activation involving NMDAR | |
| Henry et al., 2013 | Ex vivo: cortical brain slices from postnatal P10 mice | Exogenous (20 μg/mL) | tPA significantly reduced caspase-3 activity In superficial layers (less mature), tPA alone inhibited apoptosis via EGFR |
|
| No effects | ||||
| Vandenberghe et al., 1998 |
In vitro: spinal cords cultures of mice tPA –/– and WT (DIV 10–12) Kainate-induced death of motoneurons (20 and 100 μM for 24 h) |
Endogenous | tPA did not affect the vulnerability of cultured neurons to kainite | |
| Tucker et al., 2000 |
In vivo: primary cultures of rat cortical neurons Treatment: Aβ (16 or 25 μM) and plasminogen (30 nM) |
Exogenous 10 μg/ml | tPA required plasminogen to inhibit Aβ toxicity and to block Aβ deposition Degradation of Aβ fibrils is dependent on tPA and Plg proteolytic activity |
|
| Flavin and Zhao, 2001 |
In vitro: cultured hippocampal neurons from rats (DIV 7–10) ± NMDA 10 μM |
Exogenous 1,000 IU | tPA resulted in a modest exaggeration of this injury | |
| Yi et al., 2004 |
In vitro: mixed cortical cell cultures (mice) Treatment: NMDA (30 μmol/l) |
Exogenous 10 μg/ml | Calcium-mediated neuronal death was not attenuated by tPA | |
| Deleterious | ||||
| Tsirka et al., 1995 | In vivo: kainate induced neuronal death | Mouse tPA –/– | Endogenous | tPA is required to promote neuronal degeneration |
| Mouse WT | 120 μg tPA for 3 days (intra-parenchymal) | |||
| Wang et al., 1999 | In vitro: PC12 cells and primary cultures of cortical neurons (rats; DIV 12–14) | Exogenous 50 μg/ml | tPA significantly increased hemoglobin-induced cell death | |
| Flavin and Zhao, 2001 | In vitro: cultured hippocampal neurons on rats (DIV 7–10) ± plasminogen | Exogenous 100 IU | Proteolytic action | |
| Nicole et al., 2001 | In vitro: mixed cortical cultures or near-pure neuronal cultures (mice) | Exogenous 0.2–20 μg/ml | tPA failed to modify the neurotoxicity induced by the exposure to a non-NMDA agonist (kainate) | |
| Excitotoxicity: NMDA (10 or 12.5 μM) or 50 μM kainate Calcium imaging |
The catalytic activity of tPA enhanced neuronal death induced by exposure to NMDA tPA cleaves the GluN1 subunit of the NMDAR |
|||
| In vivo: NMDA induced excitotoxic lesions (rats) (50 nmol) | Exogenous 3.0 μg (intra-parenchymal) | |||
| Gabriel et al., 2003 |
In vitro: cultured cortical neurons (mice) Mixed cortical cultures of neurons and astrocytes (mice) |
Apoptosis: serum deprivation (DIV 7)Nifedipine (50 μM, DIV 14) Excitotoxicity (DIV 13–14) 12.5 μM of NMDA |
Endogenous | TGF-α rescued neurons from NMDA-induced excitotoxicity in mixed cultures through inhibition of tPA activity, involving PAI-1 overexpression by an ERK-dependent pathway in astrocytes |
| Liberatore et al., 2003 | In vivo: kainate-induced excitotoxicity on tPA –/– and WT mice (1.5 nmol of kainate) | Exogenous 1.85 μmol/L | Infusion of tPA into tPA –/– mice restored sensitivity to kainate-mediated neurotoxicity and activation of microglia | |
| In vivo: NMDA-induced excitotoxicity in mice (50 mmol/L NMDA) | Exogenous 46 μmol/L | tPA increased the lesion volumes induced by NMDA injection into the striatum | ||
| Liot et al., 2004 | In vitro: pure cultures of mouse cortical neurons exposed to NMDA (12.5 μmol/L) | Exogenous 20 μg/ml | Proteolytic activity | |
| Liu et al., 2004 |
In vitro: primary neuronal cultures (mice; DIV 14) NMDA treatment–induced apoptosis in neurons |
Exogenous 20 μg/ml | tPA potentiated apoptosis in mouse cortical neurons treated with N-methyl-D-aspartate (NMDA) by shifting the apoptotic pathway | |
| Benchenane et al., 2005 | In vivo: striatal excitotoxic lesions (rats; NMDA 50 nmol) | Exogenous IV 1 mg/kg | tPA potentiated excitotoxic lesions | |
| Lebeurrier et al., 2005 | In vivo: excitotoxic lesions in mice induced by NMDA (10 nmol in striatum or 20 nmol in cortex) | Endogenous | Overexpression of neuroserpin in the brain parenchyma might limit the deleterious effect of tPA on NMDAR-mediated neuronal death | |
| In vitro: neuronal cortical cultures from mice | Serum deprivation (DIV 7) | |||
| Treatment: neuroserpin (0.5–1 μM) | Excitotoxic paradigms (DIV 13–14) NMDA (12.5 μmol/l) AMPA (10 μmol/l) Calcium videomicroscopy |
|||
| Medina et al., 2005 | In vitro: mouse neuroblastoma N2a cells; primary cultures of hippocampal neurons tPA –/– or WT (mouse) | Exogenous 20 μg/ml | tPA induced Erk1/2 activation in neurons (independently of plasmin), tau phosphorylation and promoted A-beta mediated apoptosis tPA treatments induced GSK3 activation, tau hyperphosphorylation, microtubule destabilization and apoptosis in hippocampal neurons |
|
| Benchenane et al., 2007 |
In vivo studies: Excitotoxic lesions in mice performed by injection of NMDA (10 nmol) into the striatum In vivo studies: permanent MCAO in mice |
Exogenous 1 mg/kg |
Immunization against the NTD of the GluN1 subunit of NMDAR prevented the neurotoxic effect of endogenous and exogenous tPA | |
| López-Atalaya et al., 2007 | In vivo: striatal excitotoxic lesions (rats; 50 nmol) | Exogenous IV 1 mg/kg | tPA increased lesion volumes induced by NMDA (+40%) | |
| López-Atalaya et al., 2008 | In vitro: pure neuronal cultures (mice) | Excitotoxicity (NMDA 10 μmol/L) Calcium videomicroscopy (NMDA 12.5–100 μmol/L) |
Exogenous 0.3 μmol/L | Interaction of tPA with GluN1 led to a subsequent potentiation of NMDA-induced calcium influx and neurotoxicity |
| Wiegler et al., 2008 |
In vitro: hippocampal slices from P12 rats (OGD 30 min) Treatment: c-Jun N-terminal kinase inhibitor (XG-102; 12 nM 6 h after OGD) |
Exogenous 0.9 μg/ml | Addition of tPA after OGD enhanced neuronal death in CA1 and XG-102 administration reduced neuronal death, alone or in the presence of tPA | |
| Sun et al., 2009 |
In vitro: cultured dopaminergic neuroblasts (rat; N27 line) Treatments: aprotinine (200 KIU/ml), 𝜀-aminocaproic acid (2 mM), EGRck (Glu–Gly–Arg–CH2Cl, 100 mg/ml), FPRck (Phe–Pro–Arg–CH2Cl, 100 mg/ml), bivalirudin (20 mg/ml) |
Exogenous 10–20 μg/ml | tPA induced N27 neuroblast cell death. Aprotinin and other protease inhibitors led to an inhibition of tPA-mediated neurotoxicity Aprotinin, FPRck, and EGRck directly antagonized the proteolytic activity of tPA, whereas 𝜀-aminocaproic acid inhibited the binding of tPA to lysine residues on the cell surface |
|
| Baron et al., 2010 | In vitro study: cortical and hippocampal neurons from mice (DIV 7 or DIV 12–14). Excitotoxic neuronal death (NMDA 50 μM) | Exogenous 20 μg/ml | Catalytic tPA promoted NMDAR-induced Erk(1/2) MAPK activation tPA failed to potentiate excitotoxicity of hippocampal neurons lacking GluN2D tPA exacerbated neurotoxicity through GluN2D-containing NMDAR via Erk 1/2 |
|
| In vivo: excitotoxic lesions. Male Swiss mice Hippocampal or cortical bilateral injections of NMDA | Exogenous IV 10 mg/kg | |||
| Guo et al., 2011 |
In vitro: mouse cortical neurons (DIV14) Neuronal apoptosis model |
Exogenous 20 μg/ml | The anticoagulant factor protein S (PS) protects mouse cortical neurons from tPA/NMDA induced injury. PS blocks the extrinsic apoptotic cascade | |
| Jullienne et al., 2011 |
In vitro: cortical and hippocampal neurons (mice; DIV 12–13) Excitotoxic neuronal death: NMDA (10 μM) Treatment: UBP145 (0.2 μM) |
Exogenous 20 μg/mL | tPA increased NMDA-mediated neurotoxicity in cortical neuronal cultures but not in hippocampal neuronal cultures UBP145 had no effect on NMDA-mediated neurotoxicity in hippocampal neurons but prevented tPA-induced potentiation of NMDA-mediated neurotoxicity in cortical neurons |
|
|
In vivo: cortical excitotoxic lesions NMDA (mice; 2.5 nmol) Treatment: UBP145 (0.05 nmol) |
Exogenous IV 10 mg/kg | Inhibition of GluN2D-containing NMDAR with UBP145 can fully prevent the pro-excitotoxic effect of intravenously administered tPA | ||
| Rodríguez-González et al., 2011 | In vitro: primary mixed cortical cell cultures from rats (OGD 150 min) | Exogenous 5 mg/mL | Treatment with tPA after OGD increased LDH release, active MMP-9, MCP-1, and MIP-2 Treatment with neuroserpin after OGD decreased LDH release and active MMP-9 |
|
| Roussel et al., 2011 |
In vitro: primary cultures of cortical neurons (mice; DIV 10) Excitotoxicity induced by 10 μM NMDA Treatment: HMGB-1 0.3 μM |
Exogenous 0.3 μM | HMGB-1 reversed the pro-neurotoxic effect of tPA HMGB-1 prevented tPA from potentiating NMDA-evoked Ca2+ influx |
|
| Ma et al., 2012 |
In vitro: cultures of cortical neurons (rats; OGD/R) Treatment: neuroserpin |
Endogenous | Neuroserpin protected neurons against OGD/R. mainly by inhibiting tPA-mediated acute neuronal excitotoxicity | |
| Montagne et al., 2012 |
In vitro: cortical cultures of neurons from mice (DIV 12–13) Treatment: memantine (1–10 μmol/L) |
Excitotoxicity NMDA (10 μmol/L) OGD (30 min) Calcium videomicroscopy NMDA (50 μmol/L) |
Exogenous 0.3 μmol/L | Memantine prevented the potentiation of excitotoxic neuronal death induced by rtPA Memantine prevented rtPA-exacerbated calcium influx through activated NMDAR |
|
In vitro: cultures of cortical neurons from mice (DIV 15–16) Excitotoxic neuronal death: NMDA 50 μM |
Exogenous 0.3 μM | In contrast to WT tPA, tPA mutants including deletion of the kringle 2 domain and point mutation of the LBS-containing kringle 2 domain did not promote NMDAR-mediated neurotoxicity | ||
| Parcq et al., 2012 | In vitro | Excitotoxicity induced by exposure of cortical neurons to NMDA (mice; 50 μM) at DIV 14 | Exogenous 0.3 μM | sc-tPA promoted NMDAR-mediated neurotoxicity through its proteolytic activity, tc-tPA did not sc-tPA promoted both NMDA-induced calcium influx and Erk (½) activation, tc-tPA did not |
| NMDA-induced calcium influx recorded from cultured cortical neurons (mice; DIV 12–14) exposed to NMDA (50 μM) | ||||
| In vivo | NMDA-induced excitotoxic brain lesions (NMDA 10 mM) | Exogenous 45 μM | ||
| Henry et al., 2013 | Ex vivo: cortical brain slices from postnatal P10 mice | Exogenous 20 μg/mL | In deeper layers (more mature), tPA was associated with glutamate-promoted neuronal necrosis | |
| Omouendze et al., 2013 |
In vivo: excitotoxic insult by intra-cortical injection of Ibotenate in rats PAI-1 or tPA –/– or WT Ex vivo: brain sections |
Endogenous or exogenous 20 μg/ml | Neonatal brain lesions | |
Are Target Receptors the Explanations?
In the brain parenchyma, pro-survival and pro-neurotoxic effects of tPA have been shown to involve key receptors/pathways, including NMDAR (Nicole et al., 2001), LRP-mediated PSD95 activation (Martin et al., 2008), annexin-II (Siao and Tsirka, 2002), and EGF receptor (Liot et al., 2006; Lemarchand et al., 2015). Focusing on NMDARs, the fact that tPA induces toxic or protective effects could also depend on the different subtypes of GluN subunits involved, and/or their location (synaptic versus extrasynaptic; Paoletti et al., 2013). For instance, based on the current literature, it could be postulated that exogenous tPA could promote neurotoxicity on cortical neurons by activating extrasynaptic GluN2D-containing NMDARs (Baron et al., 2010; Jullienne et al., 2011; Montagne et al., 2012), but could lead to a neuroprotective effect by activating synaptic GluN2A-containing NMDARs (Wu et al., 2013a; Figure 3B). Several studies also propose that the neuroprotective activity of tPA, even in a paradigm involving NMDARs, is NMDAR-independent (Correa et al., 2011), independent of its proteolytic activity (Liot et al., 2006). In a model of apoptosis induced by serum deprivation (Liot et al., 2006) or when subjected to OGD, the neuroprotective effect of tPA is mediated by an activation of either EGFR (Correa et al., 2011; Bertrand et al., 2015; Lemarchand et al., 2015) or annexin II (Lee et al., 2007). Whether LRP is also involved is still under debate, again dependent on the paradigm used (Martin et al., 2008). Up to now, it is not clear how these different receptors contribute to the differential effects of tPA in neuronal survival. Additional studies are needed including investigations about possible crosstalks between these different receptors.
Are Protocols of Neuronal Injury the Explanation?
Type plasminogen activator-dependent over-activation of NMDARs has been proposed as a mechanism that could mediate both neuroprotective (Wu et al., 2013b) and neurotoxic (Baron et al., 2010) effects of tPA (Figure 3B). This discrepancy may be explained by the use of different models to induce neuronal death, either pure NMDAR-mediated excitotoxicity (Baron et al., 2010) or oxygen glucose deprivation (OGD; Wu et al., 2013b). Whether OGD induces excitotoxicity and/or apoptosis is not well documented and might depend on the severity/duration of the stress. Pathways such as autophagy or endoplasmic reticulum stress may also occur (Badiola et al., 2011; Shi et al., 2012). Another explanation could be the use of differential strategies to block tPA-induced potentiation of NMDAR signaling, MK-801 as a broad irreversible antagonist of NMDARs on one hand (Terro et al., 2000) and an antibody previously characterized to specifically prevent the tPA-dependent potentiation of NMDARs signaling without affecting their basal activity (Benchenane et al., 2007; Macrez et al., 2010) on the other hand. It is interesting to note that either over-activation and blockage of NMDARs are neurotoxic, the first one leading to excitotoxic neuronal death (Nicole et al., 2001), the second one inducing apoptosis (Mattson and Duan, 1999; Henry et al., 2013).
Is Neuronal Maturity an Explanation?
To discuss the differential impact of tPA on neuronal survival, how neurons are mature is also an important issue including whether experiments were performed in vitro (neuronal cultures performed from E16 embryo and maintained different times in vitro, 5–14 days (Buisson et al., 1998; Samson et al., 2008), ex vivo (hippocampal slices harvested at P3 and maintained different times in vitro; Lemarchand et al., 2015) or in vivo (young versus aged animals; Roussel et al., 2009). For example, it was well-demonstrated that mouse primary cultures of cortical neurons become sensitive to NMDA-induced neuronal death only after 10 days in vitro, an effect potentiated by exogenous tPA (Launay et al., 2008). At early times (days in vitro), they require trophic factors contained in the culture media (serum) to survive (Hetman et al., 2000; Terro et al., 2000). When removed, serum deprivation led to neuronal apoptosis with a protective effect of exogenous tPA (Liot et al., 2006). Type of neurons may also be critical, with neurotoxic effects of tPA mainly described in cortical neurons (Nicole et al., 2001) or Purkinje neurons (Cops et al., 2013; Li et al., 2013; Figures 3B,C). The protective effect of tPA was described on hippocampal neurons (Flavin and Zhao, 2001; Echeverry et al., 2010; Lemarchand et al., 2015; Figure 3A) and on cortical neurons (Liot et al., 2006; Wu et al., 2013a; Figure 3B).
Does the Origin of tPA (Endogenous vs. Exogenous) make the Difference?
Another important point of discussion is to know whether exogenous and endogenous tPA have differential effects on neuronal survival. The most recent literature in this field demonstrates that endogenous tPA displays neuroprotective activities (Wu et al., 2013a; Lemarchand et al., 2015) and exogenous tPA is neurotoxic (Parcq et al., 2012). Nevertheless, using tPA deficient mice, exogenous tPA may also protect hippocampal neurons subjected to OGD (Lemarchand et al., 2015). These data suggest that tPA (exogenous or endogenous) may have either pro-neurotoxic or pro-survival effects depending of the type of stress paradigms used and/or the type of neurons. Thus, whether experiments are performed on wild type neurons, tPA deficient neurons, tPA over-expressing neurons, in vitro and in vivo, is important to understand the impacts of tPA on neuronal survival (Tsirka et al., 1995; Wang et al., 1998; Nicole et al., 2001; Liot et al., 2006; Echeverry et al., 2010; Wu et al., 2013a).
What about the Level of tPA?
Some authors suggest that low levels of tPA are neuroprotective (Wu et al., 2013a), either exogenous (Baron et al., 2010) or produced by stressed cells (Lemarchand et al., 2015). In contrast, high levels of tPA (mainly exogenous) are neurotoxic (Nicole et al., 2001; Parcq et al., 2012).
Finally, Why not the Form of tPA?
There is so far only one study which discriminated tPA isoforms in the context of neuronal survival, with a clear evidence that sc-tPA is the only one capable to activate NMDAR and to promote excitotoxicity (in mouse cortical neurons subjected to NMDA exposure) both in vitro and in vivo (Parcq et al., 2012; Bertrand et al., 2015). It is thus interesting to note, that complexes formed between sc-tPA and neuroserpin (NSP) were reported more stable than those formed between tc-tPA and NSP, with no differences when complexes are formed with PAI-1 (Barker-Carlson et al., 2002). Whether conversion of sc-tPA into tc-tPA (by plasmin like activity) may influence the functions of tPA on neuronal survival, especially in the context of brain injuries, need to be investigated.
Conclusion
Depending on the study, endogenous tPA was reported as deleterious or beneficial for neurons. Although it is difficult to reconcile these findings, some propose that tPA is neuroprotective at low levels, but neurotoxic at higher levels. Assays of extracellular levels of tPA under specific conditions should be provided to support this hypothesis. Undoubtedly, the target involved is also a key trigger in the effect of tPA. In general, the pro-survival effects of tPA are independent on its proteolytic activity involving, interconnected or independently, EGF receptors, annexin II, PI-3 kinase-, AMPK-, mTor-HIF-1alpha-dependent signaling pathways. In the adult, the neurotoxic effects of tPA seem to be dependent on its proteolytic activity, targeting either plasminogen, NMDARs, components of the extracellular matrix, inflammatory mediators, and/or other proteases. However, indirect neurotoxicity might also occur via a non-proteolytic activation of microglia (Siao and Tsirka, 2002). For now, there is no clear clinical data to determine, in human, whether tPA is neurotrophic or neurotoxic and in what conditions. Additional studies are needed to understand further the possible differential functions of tPA on neuronal survival. To address this question, we should consider the different isoforms of tPA (type I sc-tPA, type I tc-tPA, type II sc-tPA, and type II tc-tPA), the possibility that tPA may activate its substrates and/or receptors with differential affinities and that these substrates and/or receptors could be differentially expressed in cortical versus hippocampal neurons depending on their maturity.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Astrup T., Permin P. M. (1947). Fibrinolysis in the animal organism. Nature 159:681 10.1038/159681b0 [DOI] [PubMed] [Google Scholar]
- Astrup T., Stage A. (1952). Isolation of a soluble fibrinolytic activator from animal tissue. Nature 170:929 10.1038/170929a0 [DOI] [PubMed] [Google Scholar]
- Badiola N., Penas C., Miñano-Molina A., Barneda-Zahonero B., Fadó R. Sánchez-Opazo et al. (2011). Induction of ER stress in response to oxygen-glucose deprivation of cortical cultures involves the activation of the PERK and IRE-1 pathways and of caspase-12. Cell Death Dis. 2:e149 10.1038/cddis.2011.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker-Carlson K., Lawrence D. A., Schwartz B. S. (2002). Acyl-enzyme complexes between tissue-type plasminogen activator and neuroserpin are short-lived in Vitro. J. Biol. Chem. 277 46852–46857. 10.1074/jbc.M207740200 [DOI] [PubMed] [Google Scholar]
- Baron A., Montagne A., Cassé F., Launay S., Maubert E., Ali C., et al. (2010). NR2D-containing NMDA receptors mediate tissue plasminogen activator-promoted neuronal excitotoxicity. Cell Death Differ. 17 860–871. 10.1038/cdd.2009.172 [DOI] [PubMed] [Google Scholar]
- Benchenane K., Berezowski V., Ali C., Fernández-Monreal M., López-Atalaya J. P., Brillault J., et al. (2005). Tissue-type plasminogen activator crosses the intact blood-brain barrier by low-density lipoprotein receptor-related protein-mediated transcytosis. Circulation 111 2241–2249. 10.1161/01.CIR.0000163542.48611.A2 [DOI] [PubMed] [Google Scholar]
- Benchenane K., Castel H., Boulouard M., Bluthé R., Fernández-Monreal M., Roussel B. D., et al. (2007). Anti-NR1 N-terminal-domain vaccination unmasks the crucial action of tPA on NMDA-receptor-mediated toxicity and spatial memory. J. Cell Sci. 120 578–585. 10.1242/jcs.03354 [DOI] [PubMed] [Google Scholar]
- Berg D. T., Burck P. J., Berg D. H., Grinnell B. W. (1993). Kringle glycosylation in a modified human tissue plasminogen activator improves functional properties. Blood 81 1312–1322. [PubMed] [Google Scholar]
- Berg D. T., Grinnell B. W. (1991). Signal and propeptide processing of human tissue plasminogen activator: activity of a pro-tPA derivative. Biochem. Biophys. Res. Commun. 179 1289–1296. 10.1016/0006-291X(91)91713-M [DOI] [PubMed] [Google Scholar]
- Bertrand T., Lesept L., Chevilley A., Lenoir S., Aimable M., Briens A., et al. (2015). Conformations of tissue plasminogen activator (tPA) orchestrate neuronal survival by a crosstalk between EGFR and NMDAR. Cell Death Dis. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder B. R., Spragg J., Austen K. F. (1979). Purification and characterization of human vascular plasminogen activator derived from blood vessel perfusates. J. Biol. Chem. 254 1998–2003. [PubMed] [Google Scholar]
- Boose J. A., Kuismanen E., Gerard R., Sambrook J., Gething M. J. (1989). The single-chain form of tissue-type plasminogen activator has catalytic activity: studies with a mutant enzyme that lacks the cleavage site. Biochemistry 28 635–643. 10.1021/bi00428a033 [DOI] [PubMed] [Google Scholar]
- Borisov O. V., Field M., Ling V. T., Harris R. J. (2009). Characterization of oligosaccharides in recombinant tissue plasminogen activator produced in Chinese hamster ovary cells: two decades of analytical technology development. Anal. Chem. 81 9744–9754. 10.1021/ac901498k [DOI] [PubMed] [Google Scholar]
- Buisson A., Nicole O., Docagne F., Sartelet H., Mackenzie E. T., Vivien D. (1998). Up-regulation of a serine protease inhibitor in astrocytes mediates the neuroprotective activity of transforming growth factor beta1. FASEB J. 12 1683–1691. [PubMed] [Google Scholar]
- Campbell B. C. V., Mitchell P. J., Kleinig T. J., Dewey H. M., Churilov L., Yassi N., et al. (2015). Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 372 1009–1018. 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- Cassé F., Bardou I., Danglot L., Briens A., Montagne A., Parcq J., et al. (2012). Glutamate controls tPA recycling by astrocytes, which in turn influences glutamatergic signals. J. Neurosci. 32 5186–5199. 10.1523/JNEUROSCI.5296-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D., Saulle E., Pisani A., Bonsi P., Tropepi D., Bernardi G., et al. (2002). Tissue plasminogen activator is required for striatal post-ischemic synaptic potentiation. Neuroreport 13 115–118. 10.1097/00001756-200201210-00027 [DOI] [PubMed] [Google Scholar]
- Christensen L. R., Macleod C. M. (1945). A proteolytic enzyme of serum: characterization, activation, and reaction with inhibitors. J. Gen. Physiol. 28 559–583. 10.1085/jgp.28.6.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collen D., Lijnen H. R. (1991). Basic and clinical aspects of fibrinolysis and thrombolysis. Blood 78 3114–3124. [PubMed] [Google Scholar]
- Collen D., Lijnen H. R. (2009). The tissue-type plasminogen activator story. Arterioscler. Thromb. Vasc. Biol. 29 1151–1155. 10.1161/ATVBAHA.108.179655 [DOI] [PubMed] [Google Scholar]
- Collen D., Rijken D. C., Van Damme J., Billiau A. (1982). Purification of human tissue-type plasminogen activator in centigram quantities from human melanoma cell culture fluid and its conditioning for use in vivo. Thromb. Haemost. 48 294–296. [PubMed] [Google Scholar]
- Conradi H. (1902). Über die Beziehung der Autolyse zur Blutgerinnung. Beitr. chem. Physiol. Path. 1:136. [Google Scholar]
- Cops E. J., Sashindranath M., Daglas M., Short K. M., da Fonseca Pereira C., Pang T. Y., et al. (2013). Tissue-type plasminogen activator is an extracellular mediator of Purkinje cell damage and altered gait. Exp. Neurol. 249 8–19. 10.1016/j.expneurol.2013.08.001 [DOI] [PubMed] [Google Scholar]
- Correa F., Gauberti M., Parcq J., Macrez R., Hommet Y., Obiang P., et al. (2011). Tissue plasminogen activator prevents white matter damage following stroke. J. Exp. Med. 208 1229–1242. 10.1084/jem.20101880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis P. S. (1838). Essai sur l’Application de la Chimie a l’Etude Physiologique Du sang de l’Homme. Paris: JB Ballièere. [Google Scholar]
- Denys J., de Marbaix H. (1889). Les peptonisations provoquees par le chloroforme. Cellule 5 197–251. [Google Scholar]
- Echeverry R., Wu J., Haile W. B., Guzman J., Yepes M. (2010). Tissue-type plasminogen activator is a neuroprotectant in the mouse hippocampus. J. Clin. Invest. 120 2194–2205. 10.1172/JCI41722.2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarsson M., Brandt J., Kaplan L. (1985). Large-scale purification of human tissue-type plasminogen activator using monoclonal antibodies. Biochim. Biophys. Acta 830 1–10. 10.1016/0167-4838(85)90123-2 [DOI] [PubMed] [Google Scholar]
- Fernández-Monreal M., López-Atalaya J. P., Benchenane K., Cacquaevel M., Dulin F., Le Caer J. P., et al. (2004). Arginine 260 of the amino-terminal domain of NR1 subunit is critical for tissue-type plasminogen activator-mediated enhancement of N-methyl-D-aspartate receptor signaling. J. Biol. Chem. 279 50850–50856. 10.1074/jbc.M407069200 [DOI] [PubMed] [Google Scholar]
- Flavin M. P., Zhao G. (2001). Hippocampal neurons from oxygen- glucose deprivation injury. J. Neurosci. Res. 63 388–394. 10.1002/1097-4547(20010301)63:5 [DOI] [PubMed] [Google Scholar]
- Fleisher M. S., Loeb L. (1915). Further investigations on the mode of action of substances inhibiting tumor grozcth and on immunisation against these substances. J. Exp. Med. 21 155–163. 10.1084/jem.21.2.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson L., Li H., Fieber C., Li X., Eriksson U. (2004). Tissue plasminogen activator is a potent activator of PDGF-CC. EMBO J. 23 3793–3802. 10.1038/sj.emboj.7600397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel C., Ali C., Lesné S., Fernández-Monreal M., Docagne F., Plawinski L., et al. (2003). Transforming growth factor alpha-induced expression of type 1 plasminogen activator inhibitor in astrocytes rescues neurons from excitotoxicity. FASEB J. 17 277–279. 10.1096/fj.02-0403fje [DOI] [PubMed] [Google Scholar]
- Goyal M., Demchuk A. M., Menon B. K., Eesa M., Rempel J. L., Thornton J., et al. (2015). Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 372 1019–1030. 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- Guo H., Barrett T. M., Zhong Z., Fernández J. A., Griffin J. H., Freeman R. S., et al. (2011). Protein S blocks the extrinsic apoptotic cascade in tissue plasminogen activator/N-methyl D-aspartate-treated neurons via Tyro3-Akt-FKHRL1 signaling pathway. Mol. Neurodegener. 6:13 10.1186/1750-1326-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile W. B., Wu J., Echeverry R., Wu F., An J., Yepes M. (2012). Tissue-type plasminogen activator has a neuroprotective effect in the ischemic brain mediated by neuronal TNF-α. J. Cereb. Blood Flow Metab. 32 57–69. 10.1038/jcbfm.2011.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar K. A., Reynolds C. M. (1994). alpha-Fucose-mediated binding and degradation of tissue-type plasminogen activator by HepG2 cells. J. Clin. Invest. 93 703–710. 10.1172/JCI117023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. J., Leonard C. K., Guzzetta A. W., Spellman M. W. (1991). Tissue plasminogen activator has an O-linked fucose attached to threonine-61 in the epidermal growth factor domain. Biochemistry 30 2311–2314. 10.1021/bi00223a004 [DOI] [PubMed] [Google Scholar]
- Head B. P., Patel H. H., Niesman I. R., Drummond J. C., Roth D. M., Patel P. M. (2009). Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology 110 813–825. 10.1097/ALN.0b013e31819b602b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedin S. G. (1903). On the presence of a proteolytic enzyme in the normal serum of the ox. J. Physiol. 30 195–201. 10.1113/jphysiol.1903.sp000989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry V. J., Lecointre M., Laudenbach V., Ali C., Macrez R., Jullienne A., et al. (2013). High t-PA release by neonate brain microvascular endothelial cells under glutamate exposure affects neuronal fate. Neurobiol. Dis. 50 201–208. 10.1016/j.nbd.2012.10.020 [DOI] [PubMed] [Google Scholar]
- Hetman M., Cavanaugh J. E., Kimelman D., Xia Z. (2000). Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J. Neurosci. 20 2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose A., Kisiel W., Fujikawa K. (1984). Proteolytic activation of tissue plasminogen activator by plasma and tissue enzymes. FEBS Lett. 175 412–418. 10.1016/0014-5793(84)80779-6 [DOI] [PubMed] [Google Scholar]
- Jaques A. J., Opdenakker G., Rademacher T. W., Dwek R. A., Zamze S. E. (1996). The glycosylation of Bowes melanoma tissue plasminogen activator: lectin mapping, reaction with anti-L2/HNK-1 antibodies and the presence of sulphated/glucuronic acid containing glycans. Biochem. J. 316 427–437. 10.1042/bj3160427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H., Pohl G., Bergsdorf N., Wallén P. (1983). Differential proteolysis and evidence for a residue exchange in tissue plasminogen activator suggest possible association between two types of protein microheterogeneity. FEBS Lett. 156 47–50. 10.1016/0014-5793(83)80245-2 [DOI] [PubMed] [Google Scholar]
- Jullienne A., Montagne A., Orset C., Lesept F., Jane D. E., Monaghan D. T., et al. (2011). Selective inhibition of GluN2D-containing N-methyl-D-aspartate receptors prevents tissue plasminogen activator-promoted neurotoxicity both in vitro and in vivo. Mol. Neurodegener. 6:68 10.1186/1750-1326-6-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. K., Lee S. Y., Oh H. K., Kang B. H., Ku H. J., Lee Y., et al. (2003). Inhibition of endothelial cell proliferation by the recombinant kringle domain of tissue-type plasminogen activator. Biochem. Biophys. Res. Commun. 304 740–746. 10.1016/S0006-291X(03)00656-9 [DOI] [PubMed] [Google Scholar]
- Kim Y., Park J. H., Hong S. H., Koh J. Y. (1999). Nonproteolytic neuroprotection by human recombinant tissue plasminogen activator. Science 284 647–650. 10.1126/science.284.5414.647 [DOI] [PubMed] [Google Scholar]
- Kuiper J., Van’t Hof A., Otter M., Biessen E. A., Rijken D. C., van Berkel T. J. (1996). Interaction of mutants of tissue-type plasminogen activator with liver cells: effect of domain deletions. Biochem. J. 313 775–780. 10.1042/bj3130775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvajo M., Albrecht H., Meins M., Hengst U., Troncoso E., Lefort S., et al. (2004). Regulation of brain proteolytic activity is necessary for the in vivo function of NMDA receptors. J. Neurosci. 24 9734–9743. 10.1523/JNEUROSCI.3306-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba D., Bauer M., Huber R., Fischer S., Rudolph R., Kohnert U., et al. (1996). The 2.3 A crystal structure of the catalytic domain of recombinant two-chain human tissue-type plasminogen activator. J. Mol. Biol. 258 117–135. 10.1006/jmbi.1996.0238 [DOI] [PubMed] [Google Scholar]
- Larsen G. R., Henson K., Blue Y. (1988). Variants of human tissue-type plasminogen activator. J. Biol. Chem. 263 1023–1029. [PubMed] [Google Scholar]
- Launay S., Maubert E., Lebeurrier N., Tennstaedt A., Campioni M., Docagne F., et al. (2008). HtrA1-dependent proteolysis of TGF-beta controls both neuronal maturation and developmental survival. Cell Death Differ. 15 1408–1416. 10.1038/cdd.2008.82 [DOI] [PubMed] [Google Scholar]
- Lebeurrier N., Liot G., Lopez-Atalaya J. P., Orset C., Fernandez-Monreal M., Sonderegger P., et al. (2005). The brain-specific tissue-type plasminogen activator inhibitor, neuroserpin, protects neurons against excitotoxicity both in vitro and in vivo. Mol. Cell. Neurosci. 30 552–558. 10.1016/j.mcn.2005.09.005 [DOI] [PubMed] [Google Scholar]
- Lee H. Y., Hwang I. Y., Im H., Koh J. Y., Kim Y. H. (2007). Non-proteolytic neurotrophic effects of tissue plasminogen activator on cultured mouse cerebrocortical neurons. J. Neurochem. 101 1236–1247. 10.1111/j.1471-4159.2007.04417.x [DOI] [PubMed] [Google Scholar]
- Lemarchand E., Maubert E., Haelewyn B., Ali C., Rubio M., Vivien D. (2015). Stressed neurons protect themselves by a tissue-type plasminogen activator-mediated EGFR-dependent mechanism. Cell Death Differ. 10.1038/cdd.2015.76 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ma Y., Teng Y. D., Zheng K., Vartanian T. K., Snyder E. Y., et al. (2006). Purkinje neuron degeneration in nervous (nr) mutant mice is mediated by a metabolic pathway involving excess tissue plasminogen activator. Proc. Natl. Acad. Sci. U.S.A. 103 7847–7852. 10.1073/pnas.0602440103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Yu L., Gu X., Ma Y., Pasqualini R., Arap W., et al. (2013). Tissue plasminogen activator regulates Purkinje neuron development and survival. Proc. Natl. Acad. Sci. U.S.A. 110 E2410–E2419. 10.1073/pnas.1305010110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore G. T., Samson A., Bladin C., Schleuning W. D., Medcalf R. L. (2003). Vampire bat salivary plasminogen activator (desmoteplase): a unique fibrinolytic enzyme that does not promote neurodegeneration. Stroke 34 537–543. 10.1161/01.STR.0000049764.49162.76 [DOI] [PubMed] [Google Scholar]
- Liot G., Benchenane K., Léveillé F., López-Atalaya J. P., Fernández-Monreal M., Ruocco A., et al. (2004).2,7-Bis-(4-amidinobenzylidene)-cycloheptan-1-one dihydrochloride, tPA stop, prevents tPA-enhanced excitotoxicity both in vitro and in vivo. J. Cereb. Blood Flow Metab. 24 1153–1159. 10.1097/01.WCB.0000134476.93809.75 [DOI] [PubMed] [Google Scholar]
- Liot G., Roussel B. D., Lebeurrier N., Benchenane K., López-Atalaya J. P., Vivien D., et al. (2006). Tissue-type plasminogen activator rescues neurones from serum deprivation-induced apoptosis through a mechanism independent of its proteolytic activity. J. Neurochem. 98 1458–1464. 10.1111/j.1471-4159.2006.03982.x [DOI] [PubMed] [Google Scholar]
- Liu D., Cheng T., Guo H., Fernández J. A., Griffin J. H., Song X., et al. (2004). Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat. Med. 10 1379–1383. 10.1038/nm1122 [DOI] [PubMed] [Google Scholar]
- López-Atalaya J. P., Roussel B. D., Ali C., Maubert E., Petersen K. U., Berezowski V., et al. (2007). Recombinant Desmodus rotundus salivary plasminogen activator crosses the blood-brain barrier through a low-density lipoprotein receptor-related protein-dependent mechanism without exerting neurotoxic effects. Stroke 38 1036–1043. 10.1161/01.STR.0000258100.04923.84 [DOI] [PubMed] [Google Scholar]
- López-Atalaya J. P., Roussel B. D., Levrat D., Parcq J., Nicole O., Hommet Y., et al. (2008). Toward safer thrombolytic agents in stroke: molecular requirements for NMDA receptor-mediated neurotoxicity. J. Cereb. Blood Flow Metab. 28 1212–1221. 10.1038/jcbfm.2008.14 [DOI] [PubMed] [Google Scholar]
- Lu W., Tsirka S. E. (2002). Partial rescue of neural apoptosis in the Lurcher mutant mouse through elimination of tissue plasminogen activator. Development 129 2043–2050. [DOI] [PubMed] [Google Scholar]
- Ma J., Yu D., Tong Y., Mao M. (2012). Effect of neuroserpin in a neonatal hypoxic-ischemic injury model ex vivo. Biol. Res. 45 357–362. 10.4067/S0716-97602012000400005 [DOI] [PubMed] [Google Scholar]
- Macfarlane R. G., Biggs R. (1948). Fibrinolysis; its mechanism and significance. Blood 3 1167–1187. [PubMed] [Google Scholar]
- Macrez R., Bezin L., Le Mauff B., Ali C., Vivien D. (2010). Functional occurrence of the interaction of tissue plasminogen activator with the NR1 Subunit of N-methyl-D-aspartate receptors during stroke. Stroke. 41 2950–2955. 10.1161/STROKEAHA.110.592360 [DOI] [PubMed] [Google Scholar]
- Madison E. L., Kobe A., Gething M. J., Sambrook J. F., Goldsmith E. J. (1993). Converting tissue plasminogen activator to a zymogen: a regulatory triad of Asp-His-Ser. Science 262 419–421. 10.1126/science.8211162 [DOI] [PubMed] [Google Scholar]
- Mantuano E., Lam M. S., Gonias S. L. (2013). LRP1 assembles unique co-receptor systems to initiate cell-signaling in response to tissue-type plasminogen activator and myelin-associated glycoprotein. J. Biol. Chem. 288 34009–34018. 10.1074/jbc.M113.509133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantuano E., Lam M. S., Shibayama M., Campana W. M., Gonias S. L. (2015). The NMDA receptor functions independently and as an LRP1 co-receptor to promote Schwann cell survival and migration. J. Cell Sci. 128 3478–3488. 10.1242/jcs.173765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. M., Kuhlmann C., Trossbach S., Jaeger S., Waldron E., Roebroek A., et al. (2008). The functional role of the second NPXY motif of the LRP1 β-chain in tissue-type plasminogen activator-mediated activation of N-methyl-D-aspartate receptors. J. Biol. Chem. 283 12004–12013. 10.1074/jbc.M707607200 [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Duan W. (1999). “Apoptotic” biochemical cascades in synaptic compartments: roles in adaptive plasticity and neurodegenerative disorders. J. Neurosci. Res. 58 152–166. 10.1002/(SICI)1097-4547(19991001)58 [DOI] [PubMed] [Google Scholar]
- Matys T., Strickland S. (2003). Tissue plasminogen activator and NMDA receptor cleavage. Nat. Med. 9 371–372. 10.1038/nm0403-371 [DOI] [PubMed] [Google Scholar]
- Medina M. G., Ledesma M. D., Domínguez J. E., Medina M., Zafra D., Alameda F., et al. (2005). Tissue plasminogen activator mediates amyloid-induced neurotoxicity via Erk1/2 activation. EMBO J. 24 1706–1716. 10.1038/sj.emboj.7600650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A., Hébert M., Jullienne A., Lesept F., Le Béhot A., Louessard M., et al. (2012). Memantine improves safety of thrombolysis for stroke. Stroke 43 2774–2781. 10.1161/STROKEAHA.112.669374 [DOI] [PubMed] [Google Scholar]
- Morgagni J. B. (1761). De Sedibus, et Causis Morborum per Anatomen Indagatis. Venezia: Remondini. [Google Scholar]
- Mori K., Dwek R. A., Downing K. A., Opdenakker G., Pauline M., Rudd P. M. (1995). The activation of Type 1 and Type 2 plasminogen by Type I and Type II tissue plasminogen activator. Biochem. J. 270 3261–3267. 10.1074/jbc.270.7.3261 [DOI] [PubMed] [Google Scholar]
- Ng K. S., Leung H. W., Wong P. T. H., Low C. M. (2012). Cleavage of the NR2B subunit amino terminus of N-methyl-D-aspartate (n.d.) Receptor by tissue plasminogen activator: identification of the cleavage site and characterization of ifenprodil and glycine affinities on truncated NMDA receptor. J. Biol. Chem. 287 25520–25529. 10.1074/jbc.M112.374397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicole O., Docagne F., Ali C., Margaill I., Carmeliet P., MacKenzie E. T., et al. (2001). The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat. Med. 7 59–64. 10.1038/83358 [DOI] [PubMed] [Google Scholar]
- NINDS (1995). Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 333 1581–1587. 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- Omouendze P. L., Henry V. J., Porte B., Dupré N., Carmeliet P., Gonzalez B. J., et al. (2013). Hypoxia-ischemia or excitotoxin-induced tissue plasminogen activator- dependent gelatinase activation in mice neonate brain microvessels. PLoS ONE 8:e71263 10.1371/journal.pone.0071263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Zapater E., Peiró S., Roda O., Corominas J. M., Aguilar S., Ampurdanés C., et al. (2007). Tissue plasminogen activator induces pancreatic cancer cell proliferation by a non-catalytic mechanism that requires extracellular signal-regulated kinase 1/2 activation through epidermal growth factor receptor and annexin A2. Am. J. Pathol. 170 1573–1584. 10.2353/ajpath.2007.060850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang P. T., Teng H. K., Zaitsev E., Woo N. T., Sakata K., Zhen S., et al. (2004). Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 306 487–491. 10.1126/science.1100135 [DOI] [PubMed] [Google Scholar]
- Paoletti P., Bellone C., Zhou Q. (2013). NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 14 383–400. 10.1038/nrn3504 [DOI] [PubMed] [Google Scholar]
- Parcq J., Bertrand T., Montagne A., Baron A. F., Macrez R., Billard J. M., et al. (2012). Unveiling an exceptional zymogen: the single-chain form of tPA is a selective activator of NMDA receptor-dependent signaling and neurotoxicity. Cell Death. Differ. 19 1983–1991. 10.1038/cdd.2012.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L., Gallo E. F., Anrather J., Wang G., Norris E. H., Paul J., et al. (2008). Key role of tissue plasminogen activator in neurovascular coupling. Proc. Natl. Acad. Sci. U.S.A. 105 1073–1078. 10.1073/pnas.0708823105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak R., Nagai N., Urano T., Napiorkowska-Pawlak D., Ihara H., Takada Y., et al. (2002). Rapid, specific and active site-catalyzed effect of tissue-plasminogen activator on hippocampus-dependent learning in mice. Neuroscience 113 995–1001. 10.1016/S0306-4522(02)00166-5 [DOI] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., et al. (1983). Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature 301 214–221. 10.1038/301214a0 [DOI] [PubMed] [Google Scholar]
- Petersen L. C., Johannessen M., Foster D., Kumar A., Mulvihill E. (1988). The effect of polymerised fibrin on the catalytic activities of one-chain tissue-type plasminogen activator as revealed by an analogue resistant to plasmin cleavage. Biochim. Biophys. Acta 952 245–254. 10.1016/0167-4838(88)90123-9 [DOI] [PubMed] [Google Scholar]
- Pineda D., Ampurdanés C., Medina M. G., Serratosa J., Tusell J. M., Saura J., et al. (2012). Tissue plasminogen activator induces microglial inflammation via a noncatalytic molecular mechanism involving activation of mitogen-activated protein kinases and Akt signaling pathways and AnnexinA2 and Galectin-1 receptors. Glia 60 526–540. 10.1002/glia.22284 [DOI] [PubMed] [Google Scholar]
- Pohl G., Källström M., Bergsdorf N., Wallén P., Jörnvall H. (1984). Tissue plasminogen activator: peptide analyses confirm an indirectly derived amino acid sequence, identify the active site serine residue, establish glycosylation sites, and localize variant differences. Biochemistry 23 3701–3707. 10.1021/bi00311a020 [DOI] [PubMed] [Google Scholar]
- Polavarapu R., Gongora M. C., Yi H., Ranganthan S., Lawrence D. A., Strickland D., et al. (2007). Tissue-type plasminogen activator – mediated shedding of astrocytic low-density lipoprotein receptor – related protein increases the permeability of the neurovascular unit. Blood 109 3270–3278. 10.1182/blood-2006-08-043125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rånby M., Bergsdorf N., Nilsson T. (1982). Enzymatic properties of the one-and two-chain form of tissue plasminogen activator. Thromb. Res. 27 175–183. 10.1016/0049-3848(82)90197-9 [DOI] [PubMed] [Google Scholar]
- Rathore Y. S., Rehan M., Pandey K., Ashish S. G. (2012). First structural model of full-length human tissue-plasminogen activator: a SAXS data-based modeling study. J. Phys. Chem. B. 116 496–502. 10.1021/jp207243n [DOI] [PubMed] [Google Scholar]
- Reddrop C., Moldrich R. X., Beart P. M., Farso M., Liberatore G. T., Howells D. W., et al. (2005). Vampire bat salivary plasminogen activator (desmoteplase) inhibits tissue-type plasminogen activator-induced potentiation of excitotoxic injury. Stroke 36 1241–1246. 10.1161/01.STR.0000166050.84056.48 [DOI] [PubMed] [Google Scholar]
- Rijken D. C., Wijngaards G., Jong M. Z., Welbergen J. (1979). Purification and partial characterization of plasminogen activator from human uterine tissue. Biochim. Biophys. Acta 580 140–153. 10.1016/0005-2795(79)90205-8 [DOI] [PubMed] [Google Scholar]
- Rodríguez-González R., Agulla J., Pérez-Mato M., Sobrino T., Castillo J. (2011). Neuroprotective effect of neuroserpin in rat primary cortical cultures after oxygen and glucose deprivation and tPA. Neurochem. Int. 58 337–343. 10.1016/j.neuint.2010.12.006 [DOI] [PubMed] [Google Scholar]
- Roussel B. D., Macrez R., Jullienne A., Agin V., Maubert E., Dauphinot L., et al. (2009). Age and albumin D site-binding protein control tissue plasminogen activator levels: neurotoxic impact. Brain 132 2219–2230. 10.1093/brain/awp162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel B. D., Mysiorek C., Rouhiainen A., Jullienne A., Parcq J., Hommet Y., et al. (2011). HMGB-1 promotes fibrinolysis and reduces neurotoxicity mediated by tissue plasminogen activator. J. Cell Sci. 124 2070–2076. 10.1242/jcs.084392 [DOI] [PubMed] [Google Scholar]
- Samson A. L., Nevin S. T., Croucher D., Niego B., Daniel P. B., Weiss T. W., et al. (2008). Tissue-type plasminogen activator requires a co-receptor to enhance NMDA receptor function. J. Neurochem. 107 1091–1101. 10.1111/j.1471-4159.2008.05687.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R., Weng J., Zhao L., Li X. M., Gao T. M., Kong J. (2012). Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci. Ther. 18 250–260. 10.1111/j.1755-5949.2012.00295.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siao C. J., Tsirka S. E. (2002). Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. J. Neurosci. 22 3352–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiq M. M., Tsirka S. E. (2004). Modulation of zinc toxicity by tissue plasminogen activator. Mol. Cell. Neurosci. 25 162–171. 10.1016/j.mcn.2003.10.007 [DOI] [PubMed] [Google Scholar]
- Spellman M. W., Basa L. J., Leonard C. K., Chakel J. A., O’Connor J. V., Wilson S., et al. (1989). Carbohydrate structures of human tissue plasminogen activator expressed in Chinese hamster ovary cells. J. Biol. Chem. 264 14100–14111. [PubMed] [Google Scholar]
- Sun H. Y., Szlam F., Levy J. H., Csete M. E., Tanaka K. A. (2009). Antifibrinolytic agents reduce tissue plasminogen activator-mediated neuronal toxicity in vitro. Acta Anaesthesiol. Scand. 53 325–331. 10.1111/j.1399-6576.2008.01858.x [DOI] [PubMed] [Google Scholar]
- Tate K. M., Higgins D. L., Holmes W. E., Winkler M. E., Heyneke H. L., Vehar G. A. (1987). Functional role of proteolytic cleavage at arginine-275 of human tissue plasminogen activator as assessed by site-directed mutagenesis. Biochemistry 26 338–343. 10.4049/jimmunol.1100412 [DOI] [PubMed] [Google Scholar]
- Terro F., Esclaire F., Yardin C., Hugon J. (2000). N-methyl-D-aspartate receptor blockade enhances neuronal apoptosis induced by serum deprivation. Neurosci. Lett. 278 149–152. 10.1016/S0304-3940(99)00911-8 [DOI] [PubMed] [Google Scholar]
- Thelwell C., Longstaff C. (2007). The regulation by fibrinogen and fibrin of tissue plasminogen activator kinetics and inhibition by plasminogen activator inhibitor 1. J. Thromb. Haemost. 5 804–811. 10.1111/j.1538-7836.2007.02422.x [DOI] [PubMed] [Google Scholar]
- Tsirka S. E., Bugge T. H., Degen J. L., Strickland S. (1997a). Neuronal death in the central nervous system demonstrates a non-fibrin substrate for plasmin. Proc. Natl. Acad. Sci. U.S.A. 94 9779–9781. 10.1073/pnas.94.18.9779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirka S. E., Rogove A. D., Bugge T. H., Degen J. L., Strickland S. (1997b). An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J. Neurosci. 17 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirka S. E., Gualandris A., Amaral D. G., Strickland S. (1995). Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature 377 340–344. 10.1038/377340a0 [DOI] [PubMed] [Google Scholar]
- Tucker H. M., Kihiko M., Caldwell J. N., Wright S., Kawarabayashi T., Price D., et al. (2000). The plasmin system is induced by and degrades amyloid-beta aggregates. J. Neurosci. 20 3937–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe W., Van Den Bosch L., Robberecht W. (1998). Tissue-type plasminogen activator is not required for kainate-induced motoneuron death in vitro. Neuroreport 9 2791–2796. 10.1097/00001756-199808240-00020 [DOI] [PubMed] [Google Scholar]
- Wallén P., Bergsdorf N., Rånby M. (1982). Purification and identification of two structural variants of porcine tissue plasminogen activator by affinity adsorption on fibrin. Biochim. Biophys. Acta 719 318–328. 10.1016/0304-4165(82)90105-2 [DOI] [PubMed] [Google Scholar]
- Wang X., Asahi M., Lo E. H. (1999). Tissue type plasminogen activator amplifies hemoglobin-induced neurotoxicity in rat neuronal cultures. Neurosci. Lett. 274 79–82. 10.1016/S0304-3940(99)00682-5 [DOI] [PubMed] [Google Scholar]
- Wang Y. F., Tsirka S. E., Strickland S., Stieg P. E., Soriano S. G., Lipton S. A. (1998). Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat. Med. 4 228–231. 10.1038/ng0598-56 [DOI] [PubMed] [Google Scholar]
- Wiegler K., Bonny C., Coquoz D., Hirt L. (2008). The JNK inhibitor XG-102 protects from ischemic damage with delayed intravenous administration also in the presence of recombinant tissue plasminogen activator. Cerebrovasc. Dis. 26 360–366. 10.1159/000151639 [DOI] [PubMed] [Google Scholar]
- Wittwer A. J., Howard S. C. (1990). Glycosylation at Asn-184 inhibits the conversion of single-chain to two-chain tissue-type plasminogen activator by plasmin. Biochemistry 29 4175–4180. 10.1021/bi00469a021 [DOI] [PubMed] [Google Scholar]
- Wittwer A. J., Howard S. C., Carr L. S., Harakas N. K., Feder J., Parekh R. B., et al. (1989). Effects of N-glycosylation on in vitro activity of Bowes melanoma and human colon fibroblast derived tissue plasminogen activator. Biochemistry 28 7662–7669. 10.1021/bi00445a022 [DOI] [PubMed] [Google Scholar]
- Wu F., Echeverry R., Wu J., An J., Haile W. B., Cooper D. S., et al. (2013a). Tissue-type plasminogen activator protects neurons from excitotoxin-induced cell death via activation of the ERK1/2-CREB-ATF3 signaling pathway. Mol. Cell. Neurosci. 52 9–19. 10.1016/j.mcn.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Nicholson A. D., Haile W. B., Torre E., An J., Chen C., et al. (2013b). Tissue-type plasminogen activator mediates neuronal detection and adaptation to metabolic stress. J. Cereb. Blood Flow Metab. 33 1761–1769. 10.1038/jcbfm.2013.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Wu J., Nicholson A. D., Echeverry R., Haile W. B., Tong F. C., et al. (2012). Tissue-type plasminogen activator regulates the neuronal uptake of glucose in the ischemic brain. J. Neurosci. 32 9848–9858. 10.1523/JNEUROSCI.1241-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J. S., Kim Y. H., Koh J. Y. (2004). Infarct reduction in rats following intraventricular administration of either tissue plasminogen activator (tPA) or its non-protease mutant S478A-tPA. Exp. Neurol. 189 354–360. 10.1016/j.expneurol.2004.05.032 [DOI] [PubMed] [Google Scholar]
- Yuan H., Vance K. M., Junge C. E., Geballe M. T., Snyder J. P., Hepler J. R., et al. (2009). The serine protease plasmin cleaves the amino-terminal domain of the NR2A subunit to relieve zinc inhibition of the N-methyl-D-aspartate receptors. J. Biol. Chem. 284 12862–12873. 10.1074/jbc.M805123200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zhang L., Yepes M., Jiang Q., Li Q., Arniego P., et al. (2002). Adjuvant treatment with neuroserpin increases the therapeutic window for tissue-type plasminogen activator administration in a rat model of embolic stroke. Circulation 106 740–745. 10.1161/01.CIR.0000023942.10849.41 [DOI] [PubMed] [Google Scholar]