Abstract
Antibody-mediated rejection (AMR) caused by donor-specific anti-human leukocyte antigen antibodies (DSA) is widely accepted to be a risk factor for decreased graft survival after kidney transplantation. This entity also plays a pathogenic role in other solid organ transplants as it appears to be an increasingly common cause of heart graft dysfunction and an emerging issue in lung transplantation. In contrast, the liver appears relatively resistant to DSA-mediated injury. This “immune-tolerance” liver property has been sustained by a low rate of liver graft loss in patients with preformed DSA and by the intrinsic liver characteristics that favor the absorption and elimination of DSA; however, alloantibody-mediated adverse consequences are increasingly being recognized, and several cases of acute AMR after ABO-compatible liver transplant (LT) have been reported. Furthermore, the availability of new solid-phase assays, allowing the detection of low titers of DSA and the refinement of objective diagnostic criteria for AMR in solid organ transplants and particularly in LT, have improved the recognition and management of this entity. A cost-effective strategy of DSA monitoring, avoidance of class II human leukocyte antigen mismatching, judicious immunosuppression attached to a higher level of clinical suspicion of AMR, particularly in cases unresponsive to conventional anti-rejection therapy, can allow a rational approach to this threat.
Keywords: Donor-specific anti-human leukocyte antigen antibodies, Liver transplantation, Rejection, Acute antibody-mediated rejection, C4d, Solid-phase immunoassays, Human leukocyte antigen single antigen bead
Core tip: The role of donor-specific anti-human leukocyte antigen antibodies (DSA) in liver transplant (LT) remains unclear. Alloantibody-mediated adverse consequences are increasingly being recognized, and several cases of acute antibody-mediated rejection after ABO-compatible LT have been reported. There is a need to investigate and quantify the potential adverse impact of DSA on LT outcomes. The present review addresses the current knowledge on this issue.
INTRODUCTION
Although human leukocyte antigen (HLA) antibodies (Abs) have been more extensively studied in kidney transplantation, they can be detected after any solid organ transplantation. As with renal transplantation, the presence of anti-HLA Abs in heart and lung transplants is associated with a worse graft survival[1]. The impact of donor-specific anti-HLA antibodies (DSA) on short- and long-term liver transplant (LT) outcome is not clearly defined. In LT, the presence of preformed DSA is well recognized, although in most cases, DSA disappear a few months after liver transplantation. In the setting of DSA persistence and evidence of complement activation after LT, no significant clinical impact in the first year post-transplantation has been described[2]; however, recent reports indicate that some LT recipients who develop de novo DSA result in lower graft survival and patient survival[3-7]. Thus, there is a need to investigate and quantify the potential adverse impact of DSA on LT outcomes. The present review addresses the current knowledge on this issue with a particular focus on LT.
IMPORTANCE OF ANTIBODY-MEDIATED REJECTION IN SOLID ORGAN TRANSPLANTATION
The detrimental effects of DSA on renal transplantation outcomes have been recognized since 1969[8], and since then, strong evidence has indicated longer kidney allograft survival among patients without DSA. In this setting, the incidence of hyperacute rejection caused by pre-existing DSA has been nearly eliminated by performing a complement-dependent cytotoxic cross-match prior to kidney transplantation; however, acute and chronic antibody-mediated rejection (AMR) plays an increasingly critical role in kidney allograft loss and is considered among the most important barrier that limits long-term outcomes[9-14]. In 2003, at the National Institutes of Health conference, acute AMR in renal transplantation was defined as an acute rejection with graft dysfunction, histological evidence of acute tissue injury and C4d deposition in the presence of DSA[15].
The negative impact of alloantibodies directed against donor HLA antigens was subsequently widely demonstrated and accepted not only in kidney but also in heart transplant, and recent evidence also endorses this notion in pancreatic and lung transplantation[16-24]. For instance, whereas the incidence and mortality of cardiac acute cellular rejection (ACR) have decreased in recent years as a result of advances in immunosuppression, the incidence of AMR appears to be increasing[25]. Furthermore, AMR also seems to be an increasingly common cause of graft dysfunction and cardiac allograft vasculopathy[26,27]. In fact, the presence of DSA in these types of solid organ transplant may contraindicate the transplant due to the increased risk of acute rejection and lower graft survival[28-30]. Moreover, in these patients the development of de novo DSA after transplantation has also been associated with an increased risk of rejection and lower survival[22,24,31,32]. As a consequence of the above-mentioned problems, different strategies-from prevention, DSA monitoring, and selection of adequate immunosuppressive regimens to therapeutic approaches-have been adopted to minimize the deleterious effects of AMR. In the next sections we will focus on these factors.
ANTIBODY-MEDIATED REJECTION IN LIVER TRANSPLANTATION
Human liver allografts are highly resistant to acute AMR from preformed human HLA alloantibodies in comparison with kidney allografts[33]. In LT, the presence of preformed DSA is well recognized, although in most cases, DSA disappear a few months after liver transplantation. Several separate mechanisms in isolation or in combination have been postulated to explain this state of “immune privilege” in the LT setting[34,35]: (1) the liver secretes soluble HLA class I molecules that form immune complexes with alloantibodies, which are then cleared by Kupffer cells; (2) Kupffer cell phagocytosis of platelet aggregates and immune-complexes limits complement activation; (3) the limited distribution of HLA class II expression in the microvasculature; (4) the great liver restorative and regenerative capacity before any insult, even mediated by the immune system; and (5) a large endothelial surface that is capable of absorbing circulating Abs. For example, in a rat model, DSA are cleared from the circulation in only 30 min when the serum is perfused through an extracorporeal liver of donor origin[36]. Other possible mechanisms proposed are related to the particular coagulation state in advanced liver diseases (the deficit of coagulation factors and thrombocytopenia-related portal hypertension can help reduce platelet aggregates and hence the formation of vascular thrombosis observed in humoral rejection mediated by DSA) that can facilitate the vascular flow, the hypocomplementemia of liver cirrhosis, and the dual hepatic vasculature that facilities improved flow during injury. This factor may decrease hepatic necrosis from arterial vasospasm and local intrahepatic coagulation that occur as result of DSA[34].
However, in the last years there have been different reports that highlight a potential deleterious role of preformed HLA Abs in liver graft survival[5,37-53]. Kozlowski et al[40] found that preformed DSA that persists after LT was associated with severe early rejection. Moreover, Krukemeyer et al[54] have revealed portal infiltration and proliferation of B lymphocytes (CD20) and plasma cells (CD138) as well as the expression of the B cell/plasma cell-activating chemokines MIP-3, CXCL9, CXCL10, CXCL11, and CXCL12 in acute liver allograft rejection. Recently, O’Leary et al[38] have found AMR to be a contributor to previously unexplained early liver allograft loss through the analysis of 60 patients with idiopathic early allograft loss when strict criteria for AMR diagnosis were fulfilled. The authors concluded that liver allograft recipients with preformed DSA with a high mean fluorescence intensity (MFI) seem to be at risk for clinically significant allograft injury and possibly for loss from AMR, often in combination with ACR. In addition, Musat et al[39] demonstrated that DSA is present in up to 75% of patients experiencing rejection, and both DSA and C4d staining was present in 54% of the patients diagnosed with ACR, demonstrating a previously unrecognized humoral component to these rejections. Furthermore, in this study 70% of the patients with ductopenia had DSA and 60% of the ductopenia cases had both circulating DSA in association with diffuse portal C4d deposition, supporting a role for AMR in the pathogenesis of interlobular bile duct injury and loss[39]. These results have been corroborated in other studies[31,37,40,51,55-59]. Morphometric studies have shown that portal tract microvasculature destruction precedes bile duct loss in the process of liver allograft rejection[38,57]. Thus, the following chain of events seems to occur: the formation of the DSA-HLA complex on endothelial cells of the portal tract microvasculature triggers complement activation (evidenced by C4d deposition) and destruction of the portal microvasculature/capillaries branching off the communicating artery from which the periductal vascular plexus arises[60], resulting in ischemic bile duct injury and loss. In fact, the resolution of cholestasis and ductopenia in association with a reduction of C4d deposition only after a decrease in circulating DSA with aggressive therapy specifically directed towards antibody removal further supports this role.
Certainly, no associations between donor-specific HLA alloantibodies with outcomes in liver or simultaneous liver-kidney transplant recipients (SLKT) have been demonstrated in large, randomized clinical trials[34]. Nonetheless, a panel of experts gathered in a recent meeting to discuss the different aspects regarding the consequences of DSA in liver transplantation agree that both acute AMR in liver transplantation recipients and an antibody-mediated renal allograft rejection observed in SLKT are two accepted associations on the basis of multiple case-control studies[34].
Regarding SLKT, “renal allograft protection” by the liver allograft occurs when the recipient harbors isolated preformed class I DSA in low-to-moderate amounts[34]; however, inferior outcomes have been demonstrated when preformed high MFI class II DSA is present[61,62]. In those cases, both the kidney and liver allografts are at a risk for rejection, especially when class II DSA persists post-transplantation[62,63]. Patients who undergo SLKT should ideally receive organs without class II antigens against which the recipient has DSA with an MFI > 5000.
Other potential associations described include the following: hyperacute rejection[64], de novo autoimmune hepatitis[65], anastomotic biliary strictures[66], and idiopathic fibrosis progression[60,67] (Figure 1).
Figure 1.
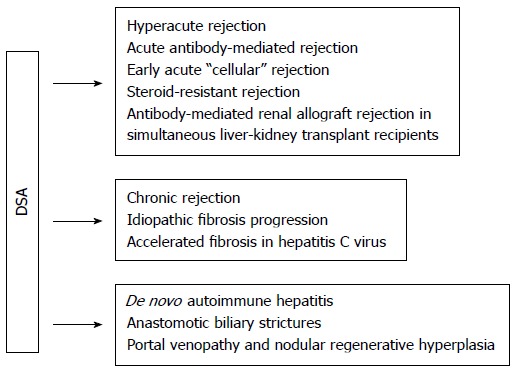
Potential associations of donor-specific human leukocyte antigen antibodies with outcomes in liver transplant or simultaneous liver-kidney transplant recipients. No associations have been confirmed in large randomized controlled trials. Adapted from O'Leary et al[34]. HLA: Human leukocyte antigen; DSA: Donor-specific anti-HLA antibodies.
DE NOVO DSA IN LIVER TRANSPLANTATION
The role of de novo DSA after LT remains unclear as the majority of studies have focused on preformed DSA. The risk of DSA development increases with a low immunosuppression load[60]. Infections and inflammatory events could alter the expression of class-I and class-II antigens and hence contribute to alloresponse induction and DSA development[68-70]. A recent report demonstrated that 8.1% of a cohort of 749 LT recipients developed de novo DSA one year after transplantation (most of them against HLA-II, especially HLA-DQ)[5]. De novo DSA resulted in lower graft and patient survival in a multivariate analysis. These findings were confirmed by Fontana et al[71] Moreover, 75% of the patients who developed de novo DSA had biliary complications. Furthermore, O’Leary et al[49] have shown the clinical relevance of de novo-specific antibodies on rejection and long-term survival. In addition, a higher rate of the novo DSA, especially of HLA-class-II, in pediatric patients with chronic rejection has recently been observed[72].
IDIOPATHIC FIBROSIS PROGRESSION
Evidence has shown that the humoral alloresponse may have a role in interstitial fibrosis and tubular athropy development after kidney transplantation[73]. In LT, graft fibrosis is frequently observed in late biopsies from pediatric patients with a normal or mild hepatic profile, and the severity of fibrosis correlates with the timing from LT to biopsy[74-77]. Miyagawa-Hayashino et al[78] are the first to suggest a role of DSA and the humoral response in long-term fibrosis in LT. The LT patients with de novo DSA and normal graft function had a higher grade of fibrosis and inflammation with a C4d-positive biopsy than patients free of DSA. Importantly, this study showed an association between DSA and fibrosis, but the cause-effect was not demonstrated. Although other potential issues could explain the fibrosis such as subclinical biliary obstruction or venous flow, recent publications have confirmed the observations of Miyagawa-Hayashino (Table 1 and Figure 2)[58,72,79].
Table 1.
Association of graft fibrosis and concomitant anti-human leukocyte antigen class II donor-specific anti-human leukocyte antigen antibodies
| Ref. | No. of patients | Positive for HLA Abs | Transplant type | Follow-up. median (yr) | Time detection DSA | Method detection DSA | MFI |
| Miyagawa-Hayashino et al[78] | 79 | 32 | LD | 11 | After LT | SAB | > 5000 |
| Salah et al[58] | 114 | 5 | LD | 2 | After LT | SAB | > 5000 |
| O´Leary et al[60] | 507 | 46 | DD | 6.4 | Pre and after LT | SAB | > 5000 |
| Grabhorn et al[72] | 19 | 16 | LD + DD | 4.5 | After LT | SAB | > 5000 |
| Iacob et al[79] | 174 | 34 | LD + DD | ND | After LT | SAB | > 5000 |
HLA: Human leukocyte antigen; DSA: Donor-specific anti-HLA antibodies; SAB: Single-antigen-bead; MFI: Mean fluorescence intensity; LT: Liver transplant.
Figure 2.
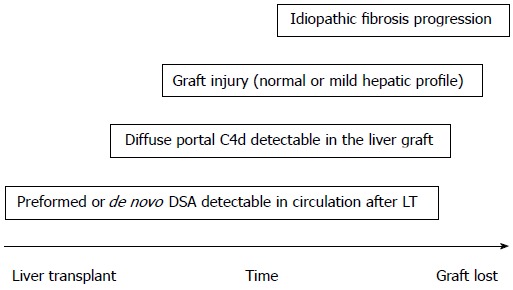
Idiopathic fibrosis progression. Hypothetical chain of events. DSA: Donor-specific anti-HLA antibodies; LT: Liver transplant.
MECHANISMS OF ANTIBODY-MEDIATED REJECTION
The mechanisms involved in the DSA-mediated graft damage (inflammation, necrosis and fibrosis) can be summarized as follows[17-19]: (1) the complement activation by the classical pathway that induces complex formation of the membrane attack (indirectly detected using immunohistochemistry for C4d -a degradation product of C4, present at the site of complement activation- attached to vascular endothelium); (2) direct damage to the vascular endothelial capillaries through the interaction of the Abs to HLA and non-HLA antigens expressed on their cell surface; (3) platelet activation and aggregation causing the release of their granules containing growth factors, cytokines, chemokines and adhesion molecules that promote the recruitment and activation of pro-inflammatory cells; and (4) the DSA facilitate the activation of pro-inflammatory cells such as natural killer (NK) cells, macrophages and neutrophils, which express at their surface the receptor for the crystallizable fragment (Fc) of immunoglobulin (Figure 3). This cascade of events is morphologically translated by the observation of platelet aggregates, neutrophil accumulation, and microangiopathic thrombosis, causing cell necrosis and early graft failure. Chronic antibody-mediated rejection is due to repetitive thrombotic events and inflammatory phenomena culminating in fibrotic changes. The following pathological damages have been described after liver transplantation: platelet aggregates in the portal and/or centrilobular areas, neutrophil infiltration, patchy necrosis and centrilobular hepatocyte ballooning, cholangiolar proliferation, acute cholangiolitis and cholestasis[6,50,51,80].
Figure 3.
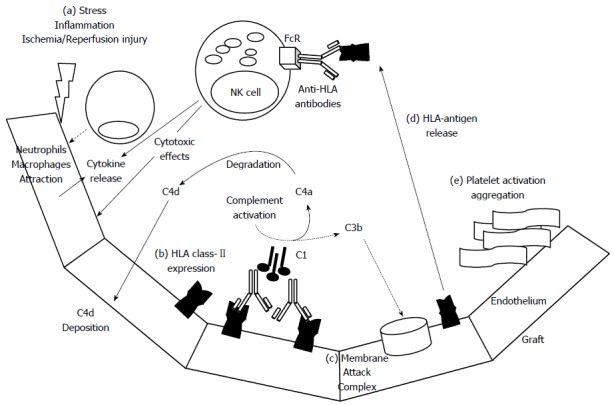
Mechanisms involved in humoral graft damage. Early post transplantation after ischemia/reperfusion injury (a) the endothelium can release several chemokines and cytokines to gather innate immune cells as neutrophils, macrophages. In this inflammatory setting, the graft endothelium could be activated and expressed human leukocyte antigen (HLA) class-II antigens (b), subsequently, these antigens could be recognized by anti-HLA class-II antibodies. If the antibodies are able to fix complement factors could trigger classical complement pathway that finally induce the membrane attack complex (c) on targeted endothelial cells. During complement activation, C4a component is degraded in C4d and finally deposited on capillaries. After destruction of endothelial cells, the HLA class-II molecules could be released and directly detected by circulating anti-HLA antibodies that once recognized by FC receptors on NK cells could direct cytotoxic actions and cytokine production. Another potential mechanism of humoral graft damage could be driven by platelet activation and thrombi formation (e).
DIAGNOSIS OF DSA-RELATED AMR
Because of the overwhelming evidence for antibody-mediated injury to kidney allografts, a consensus conference was held in 2003 to define the diagnostic criteria for antibody-mediated rejection in solid organ transplantation[15]. This group developed diagnostic criteria for AMR after kidney, heart or lung transplantation. Accordingly, the diagnosis of AMR requires clinical evidence of graft dysfunction, histologic evidence of tissue injury, immunopathologic evidence of an antibody response [complement component 4d (C4d) or immunoglobulin deposition] and serologic evidence of anti-HLA or anti-donor antibody at the time of biopsy.
In the setting of liver transplantation there are stringent criteria for the diagnosis of acute AMR that include the following (Table 2)[34,38]: (1) the presence of DSA in the serum; (2) histopathologic evidence of diffuse microvascular endothelial cell injury and microvasculitis; (3) strong and diffuse C4d positivity in the tissue; and (4) reasonable exclusion of other causes of injury that might result in similar findings.
Table 2.
Diagnostic criteria of acute antibody-mediated rejection in liver transplantation
| The presence of DSA in serum |
| Histopathologic evidence of diffuse microvascular endothelial cell injury and microvasculitis |
| Strong and diffuse C4d positivity in tissue1 |
| Reasonable exclusion of other causes of injury that might result in similar findings |
Diffuse portal microvascular positivity in formalin-fixed, paraffin-embedded samples (although detection of C4d is more sensitive in fresh tissue) is emerging as most strongly correlated with donor-specific anti-HLA antibodies-induced injury. DSA: Donor-specific anti-HLA antibodies.
Pre-transplantation cross-matching of the recipient’s serum and the donor’s lymphocytes has become a requirement of kidney transplant programs throughout the world on the basis of the known deleterious effects on kidney allografts of antibody-mediated graft injury[81]. In the setting of LT, there is a need to develop a cost-effective DSA monitoring algorithm, but a panel of experts has recently recommended a DSA monitoring schedule that includes testing all liver allograft recipients in the pre-transplant setting and, afterwards retesting all positive patients 1-2 wk post-transplantation to determine persistence[34]. There have been notable technological advances in the available assays to determine DSA. Earlier cell-based assays for DSA detection (i.e., cytotoxic crossmatch) had several limitations in terms of sensitivity and specificity and the ability to differentiate between IgG from IgM Abs and between HLA from non-HLA Abs. Flow cytometry cross-matching is another cell-based assay that relies on the detection of Abs binding to the surface of donor lymphocytes and is more sensitive than cytotoxic crossmatch. The first solid-phase immunoassay (SPI) used to test anti-HLA Abs was based on an enzyme-linked immune assay (ELISA), but recently SPI is being replaced by single-antigen-bead (SAB) assays. Acquired by LuminexTM, this technology offers a new approach in the detection and quantification of post-transplantation anti-HLA Abs, which can be present in any solid transplant. This immunoassay allows the detection of low titters of HLA Abs that were undetectable by former assays, specifically and semiquantitatively[23,34,81]. The fluorescence signals detected are expressed as MFI or molecules of equivalent soluble fluorochrome (MESF). The isolated finding of HLA DSA is not specific for AMR because it has been found in 60% of LT recipients without rejection[37]. Certainly, most patients with preformed low-to-moderate levels of isolated class I DSA in the absence of recurrent liver disease appear to have few, if any, short- or long-term consequences. Moreover, the significance of DSA late after liver transplantation without allograft dysfunction is uncertain[34]. As an isolated finding it does not represent an indication for intervention, although the long-term outcomes of such patients are thus far unknown.
C4d is a component of the complement cascade that is considered a marker of complement regulation. The complement system is a part of the innate immunological response and becomes activated in a variety of immunological events, such as ACR and viral and autoimmune hepatitis[82,83]. Different C4d staining patterns have been described in liver allografts. Even diffuse endothelial and sinusoidal C4d staining alone cannot be considered specific for the diagnosis of AMR as it has been found in AMR and other common allograft disorders such as ACR, chronic rejection, biliary obstruction and recurrent viral or autoimmune hepatitis[50,84]. Although there is no consensus, the diffuse portal microvascular positivity in formalin-fixed, paraffin-embedded samples (although detection of C4d is more sensitive in fresh tissue) is emerging to be most strongly correlated with DSA-induced injury[38-40,50,52,85]. Otherwise, C4d-negative AMR has been identified in renal allografts and likely occurs in the liver, although experts favor the above described conservative approach until more is learned about liver AMR[20,38].
Finally, the clinical presentation of liver allograft AMR is nonspecific, and many etiologies, such as ACR, ischemic injury, pharmacological toxicity, infections, initial graft dysfunction, hepatic artery thrombosis, biliary complications, and disease recurrence, can explain increases in aspartate aminotransferase (AST), alanine aminotransferase (ALT), and cholestasis[59,86,87]. AMR should be considered as part of the differential diagnosis if DSA are present. These observations have prompted the design of a multicenter study of specific features that could be used to screen patients for acute AMR via routine HE staining[88].
WHEN MUST AMR BE SUSPECTED?
Acute AMR occurs most commonly during the first several weeks after liver transplantation and consists of an otherwise unexplained liver allograft dysfunction associated with falling platelets and complement levels and increased levels of circulating immune complexes in patients with preformed, persistent DSA[67]. The liver biopsy shows microvascular injury in addition to other characteristics associated with allograft rejection, which is observed in approximately 1% of all early (< 90 d) liver allograft failures. Notwithstanding, acute AMR could explain up to 10% of idiopathic early liver allograft failures in DSA-positive patients[38].
Therefore, a high suspicion of DSA-induced AMR would theoretically be raised for a liver recipient with high titers of preformed anti-donor HLA class II Abs who presents graft dysfunction in the early post-transplant period (first 90 d) that is otherwise not explained and is associated with falling platelets and complement levels and increased levels of circulating immune complexes. Furthermore, a negative response to conventional antirejection therapy is also associated[89]. SLKT recipients who receive crossmatch-positive organs are also the patients in which a high level of alert must be maintained, especially when the recipient has DSA with an MFI > 5000[62,63]; however, as stated above, there are other possible clinical presentations where DSA can play a pathogenic effect and thus could indicate the use of a diagnostic approach (i.e., DSA assay, liver biopsy, etc.).
RISK FACTORS FOR DSA-RELATED AMR IN LIVER TRANSPLANT RECIPIENTS
Together with class II HLA mismatching and prior cellular rejection, inadequate immunosuppression (particularly minimization and non-adherence to immunosuppressive medication) is a risk factor for the development of DSA[23].
Recognized risk factors favoring DSA-mediated liver damage were identified before the use of SAB technology allowed more accurate DSA determinations and included high-titer preformed Abs, the persistence of anti-donor Abs after transplantation, and otherwise unexplained thrombocytopenia and hypocomplementemia[38,51,65,90-92]. Thereafter, adverse outcomes have been associated with strongly positive flow cytometry cross-matches versus weakly positive cross-matches and strong preformed DSA evaluated for their complement fixing ability with a complement component 1q (C1q) assay[86]. C1q-binding DSA are expected to have the potential to assess cytotoxicity and have been associated with a greater risk of acute rejection and allograft lost in patients undergoing renal and heart transplantation[93-95]. Thus, in a recently proposed algorithm, a patient with strong DSA and C1q-positive DSA is considered at a higher risk and should be monitored for post-transplant DSA[59]. If persistent DSA are detected, the patient is monitored as being at a higher risk for AMR.
Furthermore, the effects of DSA can vary depending on cofactors, some of which may promote immune stimulatory/profibrogenic effects and some of which could promote tolerogenic effects[34]. Thus, on the one hand, the up-regulation of DSA targets in allografts of patients with infections or inflammatory-mediated tissue damage[68-70] as occurs in patients with recurrent hepatitis C chronic infection, as a consequence, appears to be associated with fibrosis progression[60]. On the other hand, HLA class II-restricted regulatory T cell (Treg) epitopes in IgG (also called “Tregitopes”) that suppress immune responses to co-administered antigens may be formed as a result of DSA, thereby promoting tolerance[96].
PREVENTION AND MANAGEMENT OF LIVER DSA-RELATED AMR
As previously mentioned, the advent of new diagnostic technologies, particularly SAB assays, has allowed the assessment of the immunological risk in potential recipients of a particular donor by means of the identification and characterization of HLA Abs. In the kidney transplant setting, a detailed serological follow-up is of critical importance in the decision-making process because it can help determine whether to proceed with the transplantation, desensitize or follow a standard immunosuppressive (IS) therapy[23]. Efficient desensitization protocols have enabled successful transplantations, overcoming immunological barriers in patients including the barrier of a positive complement-dependent cytotoxic cross-match[97-99]. Anti-humoral therapy is based on two complementary approaches: (1) the removal of harmful Abs from the blood stream through plasmapheresis or immunoadsorption; and (2) the modulation of various components of specific and/or innate immunity using strategies including intravenous immunoglobulin, anti-CD20 antibody (rituximab), antithymocyte globulin (ATG), proteasome inhibitor (bortezomib), anti-C5 antibody (eculizumab), or even splenectomy[97-99].
In the setting of liver transplantation, the routine assessment of DSA pre-transplantation, with a retest of positive patients 1-2 wk post-transplantation, has been recommended by a panel of experts[34]. This fact is of particular interest when a SLKT is being considered and in the case of anti-donor HLA class II Abs; however, there are several shortcomings with this strategy that need to be solved[34]: (1) only a small percentage of sensitized patients before transplantation will have severe, adverse consequences after transplantation; and (2) the significance of DSA late after liver transplantation without allograft dysfunction is uncertain and, in general, this finding does not merit any intervention. Taking into account these shortcomings, a panel of experts have recently proposed to investigate the design of cost-effective DSA monitoring strategies that allow one to detect the first group of patients and that identifies DSA characteristics late after transplantation that indicate inadequate immunosuppression or an unacceptable risk of chronic allograft injury[34].
Patients who undergo SLKT should ideally receive organs without class II antigens against which the recipient has DSA with an MFI > 5000[34]; however, if a patient must receive cross-match positive organs after balancing the risks of a DSA-mediated rejection against those related to a protracted waiting list period in terms of progression of the liver disease, postoperative testing to determine antibody persistence and close follow-up are desirable[34].
Otherwise, the IS regimen and drug exposure can be relevant in terms of prevention of DSA-mediated allograft damage. In the kidney transplantation setting, the selection of an adequate IS can prevent subclinical inflammation and hence fibrosis progression[23]. For instance, in a case-control study, Moreso et al[100] confirmed the lower prevalence of subclinical inflammation associated with a regimen based on tacrolimus, mycophenolate mofetil, and prednisone than with a regimen based on cyclosporine, mycophenolate mofetil, and prednisone. In addition, lower exposure to tacrolimus between 3 and 12 mo after transplantation was independently associated with higher increases in chronic pathology in patients also treated with mycophenolate mofetil, and prednisone[101]. In the liver transplantation setting, de novo DSA prevention strategies also include a strict adherence to immunosuppression and the use of tacrolimus (rather than cyclosporine)[5,102,103].
The treatment of acute AMR in ABO-compatible liver transplants is not clearly determined because of the limited number of cases[34,104]. Most of the evidence in this field derives from studies in kidney transplantation where different anti-humoral therapies similar those mentioned above have been used. Bortezomib, a proteasome inhibitor effective in depleting plasma cells that in turn are responsible of producing the offending Abs, has been successfully used in three cases of severe AMR in ABO-compatible LT recipients[104]; however, concerns have been raised about the anti-humoral therapies in LT recipients because of their potent immunosuppressive effects that may exacerbate chronic viral hepatitis or increase infectious risks. Thus, experts currently advise that a strategy based on the combination of avoidance/prevention when possible may be the best strategy[34].
CONCLUSION
There has been a recent resurgence of interest in AMR in liver transplantation based on an increasingly number of reports indicating DSA-mediated allograft dysfunction and a better characterization of this entity in terms of diagnostic tools and diagnostic criteria. Although AMR is a less frequent cause of liver allograft dysfunction, it must be taken into account not only from a diagnostic/therapeutic point of view but also from a preventive standpoint.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest to report.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 19, 2015
First decision: June 23, 2015
Article in press: September 13, 2015
P- Reviewer: Boin IFSF, Inal A S- Editor: Yu J L- Editor: A E- Editor: Wang CH
References
- 1.Morales-Buenrostro LE, Castro R, Terasaki PI. Impact of immunosuppression on HLA-antibody formation. Clin Transpl. 2006:227–240. [PubMed] [Google Scholar]
- 2.Taner T, Gandhi MJ, Sanderson SO, Poterucha CR, De Goey SR, Stegall MD, Heimbach JK. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am J Transplant. 2012;12:1504–1510. doi: 10.1111/j.1600-6143.2012.03995.x. [DOI] [PubMed] [Google Scholar]
- 3.Chan KM, Lee CS, Wu TJ, Lee CF, Chen TC, Lee WC. Clinical perspective of acute humoral rejection after blood type-compatible liver transplantation. Transplantation. 2011;91:e29–e30. doi: 10.1097/TP.0b013e318208138c. [DOI] [PubMed] [Google Scholar]
- 4.Kamar N, Lavayssière L, Muscari F, Selves J, Guilbeau-Frugier C, Cardeau I, Esposito L, Cointault O, Nogier MB, Peron JM, et al. Early plasmapheresis and rituximab for acute humoral rejection after ABO-compatible liver transplantation. World J Gastroenterol. 2009;15:3426–3430. doi: 10.3748/wjg.15.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaneku H, O’Leary JG, Banuelos N, Jennings LW, Susskind BM, Klintmalm GB, Terasaki PI. De novo donor-specific HLA antibodies decrease patient and graft survival in liver transplant recipients. Am J Transplant. 2013;13:1541–1548. doi: 10.1002/ajt.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson R, Kozlowski T, Nickeleit V, Woosley JT, Schmitz JL, Zacks SL, Fair JH, Gerber DA, Andreoni KA. Isolated donor specific alloantibody-mediated rejection after ABO compatible liver transplantation. Am J Transplant. 2006;6:3022–3029. doi: 10.1111/j.1600-6143.2006.01554.x. [DOI] [PubMed] [Google Scholar]
- 7.Wilson CH, Agarwal K, Carter V, Burt AD, Hübscher S, Talbot D, Jaques BC, Manas DM. Late humoral rejection in a compliant ABO-compatible liver transplant recipient. Transplantation. 2006;82:988–989. doi: 10.1097/01.tp.0000229939.85412.27. [DOI] [PubMed] [Google Scholar]
- 8.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735–739. doi: 10.1056/NEJM196904032801401. [DOI] [PubMed] [Google Scholar]
- 9.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, Goldberg A, Birk PE, Rush DN, Nickerson PW. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12:1157–1167. doi: 10.1111/j.1600-6143.2012.04013.x. [DOI] [PubMed] [Google Scholar]
- 10.Stegall MD, Gloor J, Winters JL, Moore SB, Degoey S. A comparison of plasmapheresis versus high-dose IVIG desensitization in renal allograft recipients with high levels of donor specific alloantibody. Am J Transplant. 2006;6:346–351. doi: 10.1111/j.1600-6143.2005.01178.x. [DOI] [PubMed] [Google Scholar]
- 11.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 12.Jordan SC, Pescovitz MD. Presensitization: the problem and its management. Clin J Am Soc Nephrol. 2006;1:421–432. doi: 10.2215/CJN.01651105. [DOI] [PubMed] [Google Scholar]
- 13.El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, Cosio FG. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9:527–535. doi: 10.1111/j.1600-6143.2008.02519.x. [DOI] [PubMed] [Google Scholar]
- 14.Djamali A, Kaufman DB, Ellis TM, Zhong W, Matas A, Samaniego M. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant. 2014;14:255–271. doi: 10.1111/ajt.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takemoto SK, Zeevi A, Feng S, Colvin RB, Jordan S, Kobashigawa J, Kupiec-Weglinski J, Matas A, Montgomery RA, Nickerson P, et al. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004;4:1033–1041. doi: 10.1111/j.1600-6143.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 16.Torrealba JR, Samaniego M, Pascual J, Becker Y, Pirsch J, Sollinger H, Odorico J. C4d-positive interacinar capillaries correlates with donor-specific antibody-mediated rejection in pancreas allografts. Transplantation. 2008;86:1849–1856. doi: 10.1097/TP.0b013e3181902319. [DOI] [PubMed] [Google Scholar]
- 17.Terasaki PI, Cai J. Human leukocyte antigen antibodies and chronic rejection: from association to causation. Transplantation. 2008;86:377–383. doi: 10.1097/TP.0b013e31817c4cb8. [DOI] [PubMed] [Google Scholar]
- 18.Morrell MR, Patterson GA, Trulock EP, Hachem RR. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant. 2009;28:96–100. doi: 10.1016/j.healun.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Morales-Buenrostro LE, Castro R, Terasaki PI. A single human leukocyte antigen-antibody test after heart or lung transplantation is predictive of survival. Transplantation. 2008;85:478–481. doi: 10.1097/TP.0b013e3181605cd9. [DOI] [PubMed] [Google Scholar]
- 20.Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Cendales L, Demetris AJ, Drachenberg CB, et al. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563–570. doi: 10.1111/j.1600-6143.2011.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kfoury AG, Renlund DG, Snow GL, Stehlik J, Folsom JW, Fisher PW, Reid BB, Clayson SE, Gilbert EM, Everitt MD, et al. A clinical correlation study of severity of antibody-mediated rejection and cardiovascular mortality in heart transplantation. J Heart Lung Transplant. 2009;28:51–57. doi: 10.1016/j.healun.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Cantarovich D, De Amicis S, Akl A, Devys A, Vistoli F, Karam G, Soulillou JP. Posttransplant donor-specific anti-HLA antibodies negatively impact pancreas transplantation outcome. Am J Transplant. 2011;11:2737–2746. doi: 10.1111/j.1600-6143.2011.03729.x. [DOI] [PubMed] [Google Scholar]
- 23.Arias M, Rush DN, Wiebe C, Gibson IW, Blydt-Hansen TD, Nickerson PW, Sellarés J, López-Hoyos M, San Segundo D, Crespo-Leiro MG, et al. Antibody-mediated rejection: analyzing the risk, proposing solutions. Transplantation. 2014;98 Suppl 3:S3–S21. doi: 10.1097/TP.0000000000000218. [DOI] [PubMed] [Google Scholar]
- 24.Abu-Elmagd KM, Wu G, Costa G, Lunz J, Martin L, Koritsky DA, Murase N, Irish W, Zeevi A. Preformed and de novo donor specific antibodies in visceral transplantation: long-term outcome with special reference to the liver. Am J Transplant. 2012;12:3047–3060. doi: 10.1111/j.1600-6143.2012.04237.x. [DOI] [PubMed] [Google Scholar]
- 25.Subherwal S, Kobashigawa JA, Cogert G, Patel J, Espejo M, Oeser B. Incidence of acute cellular rejection and non-cellular rejection in cardiac transplantation. Transplant Proc. 2004;36:3171–3172. doi: 10.1016/j.transproceed.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 26.Berry GJ, Angelini A, Burke MM, Bruneval P, Fishbein MC, Hammond E, Miller D, Neil D, Revelo MP, Rodriguez ER, et al. The ISHLT working formulation for pathologic diagnosis of antibody-mediated rejection in heart transplantation: evolution and current status (2005-2011) J Heart Lung Transplant. 2011;30:601–611. doi: 10.1016/j.healun.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Topilsky Y, Gandhi MJ, Hasin T, Voit LL, Raichlin E, Boilson BA, Schirger JA, Edwards BS, Clavell AL, Rodeheffer RJ, et al. Donor-specific antibodies to class II antigens are associated with accelerated cardiac allograft vasculopathy: a three-dimensional volumetric intravascular ultrasound study. Transplantation. 2013;95:389–396. doi: 10.1097/TP.0b013e318273878c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith JD, Danskine AJ, Laylor RM, Rose ML, Yacoub MH. The effect of panel reactive antibodies and the donor specific crossmatch on graft survival after heart and heart-lung transplantation. Transpl Immunol. 1993;1:60–65. doi: 10.1016/0966-3274(93)90060-l. [DOI] [PubMed] [Google Scholar]
- 29.Gloor JM, Winters JL, Cornell LD, Fix LA, DeGoey SR, Knauer RM, Cosio FG, Gandhi MJ, Kremers W, Stegall MD. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010;10:582–589. doi: 10.1111/j.1600-6143.2009.02985.x. [DOI] [PubMed] [Google Scholar]
- 30.Farmer DG, Venick RS, Colangelo J, Esmailian Y, Yersiz H, Duffy JP, Cortina GR, Artavia K, Ngo K, McDiarmid SV, et al. Pretransplant predictors of survival after intestinal transplantation: analysis of a single-center experience of more than 100 transplants. Transplantation. 2010;90:1574–1580. doi: 10.1097/TP.0b013e31820000a1. [DOI] [PubMed] [Google Scholar]
- 31.Ho EK, Vlad G, Vasilescu ER, de la Torre L, Colovai AI, Burke E, Deng M, Schwartz J, Marboe C, Mancini D, et al. Pre- and posttransplantation allosensitization in heart allograft recipients: major impact of de novo alloantibody production on allograft survival. Hum Immunol. 2011;72:5–10. doi: 10.1016/j.humimm.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Terasaki PI, Ozawa M, Castro R. Four-year follow-up of a prospective trial of HLA and MICA antibodies on kidney graft survival. Am J Transplant. 2007;7:408–415. doi: 10.1111/j.1600-6143.2006.01644.x. [DOI] [PubMed] [Google Scholar]
- 33.Andres GA, Ansell ID, Halgrimson CG, Hsu KC, Porter KA, Starzl TE, Accinni L, Calne RY, Herbertson BM, Penn I, et al. Immunopathological studies of orthotopic human liver allografts. Lancet. 1972;1:275–280. doi: 10.1016/s0140-6736(72)90288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Leary JG, Demetris AJ, Friedman LS, Gebel HM, Halloran PF, Kirk AD, Knechtle SJ, McDiarmid SV, Shaked A, Terasaki PI, et al. The role of donor-specific HLA alloantibodies in liver transplantation. Am J Transplant. 2014;14:779–787. doi: 10.1111/ajt.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piazza A, Adorno D, Torlone N, Valeri M, Poggi E, Monaco PI, Pisani F, Tisone G, Casciani CU. Flow cytometric analysis of antidonor-specific antibodies in liver transplant. Transplant Proc. 1997;29:2975–2976. doi: 10.1016/s0041-1345(97)00751-3. [DOI] [PubMed] [Google Scholar]
- 36.Gugenheim J, Amorosa L, Gigou M, Fabiani B, Rouger P, Gane P, Reynes M, Bismuth H. Specific absorption of lymphocytotoxic alloantibodies by the liver in inbred rats. Transplantation. 1990;50:309–313. doi: 10.1097/00007890-199008000-00027. [DOI] [PubMed] [Google Scholar]
- 37.O’Leary JG, Kaneku H, Susskind BM, Jennings LW, Neri MA, Davis GL, Klintmalm GB, Terasaki PI. High mean fluorescence intensity donor-specific anti-HLA antibodies associated with chronic rejection Postliver transplant. Am J Transplant. 2011;11:1868–1876. doi: 10.1111/j.1600-6143.2011.03593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Leary JG, Kaneku H, Demetris AJ, Marr JD, Shiller SM, Susskind BM, Tillery GW, Terasaki PI, Klintmalm GB. Antibody-mediated rejection as a contributor to previously unexplained early liver allograft loss. Liver Transpl. 2014;20:218–227. doi: 10.1002/lt.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musat AI, Agni RM, Wai PY, Pirsch JD, Lorentzen DF, Powell A, Leverson GE, Bellingham JM, Fernandez LA, Foley DP, et al. The significance of donor-specific HLA antibodies in rejection and ductopenia development in ABO compatible liver transplantation. Am J Transplant. 2011;11:500–510. doi: 10.1111/j.1600-6143.2010.03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozlowski T, Rubinas T, Nickeleit V, Woosley J, Schmitz J, Collins D, Hayashi P, Passannante A, Andreoni K. Liver allograft antibody-mediated rejection with demonstration of sinusoidal C4d staining and circulating donor-specific antibodies. Liver Transpl. 2011;17:357–368. doi: 10.1002/lt.22233. [DOI] [PubMed] [Google Scholar]
- 41.Kasahara M, Kiuchi T, Takakura K, Uryuhara K, Egawa H, Asonuma K, Uemoto S, Inomata Y, Ohwada S, Morishita Y, et al. Postoperative flow cytometry crossmatch in living donor liver transplantation: clinical significance of humoral immunity in acute rejection. Transplantation. 1999;67:568–575. doi: 10.1097/00007890-199902270-00014. [DOI] [PubMed] [Google Scholar]
- 42.Demetris AJ, Markus BH, Burnham J, Nalesnik M, Gordon RD, Makowka L, Starzl TE. Antibody deposition in liver allografts with chronic rejection. Transplant Proc. 1987;19:121–125. [PubMed] [Google Scholar]
- 43.O’Leary JG, Kaneku H, Jennings LW, Bañuelos N, Susskind BM, Terasaki PI, Klintmalm GB. Preformed class II donor-specific antibodies are associated with an increased risk of early rejection after liver transplantation. Liver Transpl. 2013;19:973–980. doi: 10.1002/lt.23687. [DOI] [PubMed] [Google Scholar]
- 44.Kaneku H, O’Leary JG, Taniguchi M, Susskind BM, Terasaki PI, Klintmalm GB. Donor-specific human leukocyte antigen antibodies of the immunoglobulin G3 subclass are associated with chronic rejection and graft loss after liver transplantation. Liver Transpl. 2012;18:984–992. doi: 10.1002/lt.23451. [DOI] [PubMed] [Google Scholar]
- 45.Ashihara E, Tsuji H, Sakashita H, Haga H, Yurugi K, Kimura S, Egawa H, Manabe T, Uemoto S, Maekawa T. Antidonor antibody in patients receiving ABO-identical and HLA-mismatched living donor liver transplants: effect on survival. Transplantation. 2007;83:506–509. doi: 10.1097/01.tp.0000251361.12249.a1. [DOI] [PubMed] [Google Scholar]
- 46.Castillo-Rama M, Castro MJ, Bernardo I, Meneu-Diaz JC, Elola-Olaso AM, Calleja-Antolin SM, Romo E, Morales P, Moreno E, Paz-Artal E. Preformed antibodies detected by cytotoxic assay or multibead array decrease liver allograft survival: role of human leukocyte antigen compatibility. Liver Transpl. 2008;14:554–562. doi: 10.1002/lt.21408. [DOI] [PubMed] [Google Scholar]
- 47.Goh A, Scalamogna M, De Feo T, Poli F, Terasaki PI. Human leukocyte antigen crossmatch testing is important for liver retransplantation. Liver Transpl. 2010;16:308–313. doi: 10.1002/lt.21981. [DOI] [PubMed] [Google Scholar]
- 48.Muro M, Marin L, Miras M, Moya-Quiles R, Minguela A, Sánchez-Bueno F, Bermejo J, Robles R, Ramírez P, García-Alonso A, et al. Liver recipients harbouring anti-donor preformed lymphocytotoxic antibodies exhibit a poor allograft survival at the first year after transplantation: experience of one centre. Transpl Immunol. 2005;14:91–97. doi: 10.1016/j.trim.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 49.O’Leary JG, Kaneku H, Banuelos N, Jennings LW, Klintmalm GB, Terasaki PI. Impact of IgG3 subclass and C1q-fixing donor-specific HLA alloantibodies on rejection and survival in liver transplantation. Am J Transplant. 2015;15:1003–1013. doi: 10.1111/ajt.13153. [DOI] [PubMed] [Google Scholar]
- 50.Lunz J, Ruppert KM, Cajaiba MM, Isse K, Bentlejewski CA, Minervini M, Nalesnik MA, Randhawa P, Rubin E, Sasatomi E, et al. Re-examination of the lymphocytotoxic crossmatch in liver transplantation: can C4d stains help in monitoring? Am J Transplant. 2012;12:171–182. doi: 10.1111/j.1600-6143.2011.03786.x. [DOI] [PubMed] [Google Scholar]
- 51.Demetris AJ, Nakamura K, Yagihashi A, Iwaki Y, Takaya S, Hartman GG, Murase N, Bronsther O, Manez R, Fung JJ. A clinicopathological study of human liver allograft recipients harboring preformed IgG lymphocytotoxic antibodies. Hepatology. 1992;16:671–681. doi: 10.1002/hep.1840160310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakashita H, Haga H, Ashihara E, Wen MC, Tsuji H, Miyagawa-Hayashino A, Egawa H, Takada Y, Maekawa T, Uemoto S, et al. Significance of C4d staining in ABO-identical/compatible liver transplantation. Mod Pathol. 2007;20:676–684. doi: 10.1038/modpathol.3800784. [DOI] [PubMed] [Google Scholar]
- 53.Mañez R, Kelly RH, Kobayashi M, Takaya S, Bronsther O, Kramer D, Duquesnoy RJ, Iwaki Y, Fung JJ, Starzl TE. Immunoglobulin G lymphocytotoxic antibodies in clinical liver transplantation: studies toward further defining their significance. Hepatology. 1995;21:1345–1352. doi: 10.1002/hep.1840210519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krukemeyer MG, Moeller J, Morawietz L, Rudolph B, Neumann U, Theruvath T, Neuhaus P, Krenn V. Description of B lymphocytes and plasma cells, complement, and chemokines/receptors in acute liver allograft rejection. Transplantation. 2004;78:65–70. doi: 10.1097/01.tp.0000132324.14207.8b. [DOI] [PubMed] [Google Scholar]
- 55.Troxell ML, Higgins JP, Kambham N. Evaluation of C4d staining in liver and small intestine allografts. Arch Pathol Lab Med. 2006;130:1489–1496. doi: 10.5858/2006-130-1489-EOCSIL. [DOI] [PubMed] [Google Scholar]
- 56.Martelius T, Halme L, Arola J, Höckerstedt K, Lautenschlager I. Vascular deposition of complement C4d is increased in liver allografts with chronic rejection. Transpl Immunol. 2009;21:244–246. doi: 10.1016/j.trim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 57.Matsumoto Y, McCaughan GW, Painter DM, Bishop GA. Evidence that portal tract microvascular destruction precedes bile duct loss in human liver allograft rejection. Transplantation. 1993;56:69–75. doi: 10.1097/00007890-199307000-00012. [DOI] [PubMed] [Google Scholar]
- 58.Salah A, Fujimoto M, Yoshizawa A, Yurugi K, Miyagawa-Hayashino A, Sumiyoshi S, Minamiguchi S, Uemoto S, Maekawa T, Haga H. Application of complement component 4d immunohistochemistry to ABO-compatible and ABO-incompatible liver transplantation. Liver Transpl. 2014;20:200–209. doi: 10.1002/lt.23789. [DOI] [PubMed] [Google Scholar]
- 59.Leonard GR, Shike H, Uemura T, Gaspari JL, Ruggiero FM, Shah RA, Riley TR, Kadry Z. Liver transplantation with a strongly positive crossmatch: case study and literature review. Liver Transpl. 2013;19:1001–1010. doi: 10.1002/lt.23694. [DOI] [PubMed] [Google Scholar]
- 60.O’Leary JG, Kaneku H, Jennings L, Susskind BM, Terasaki PI, Klintmalm GB. Donor-specific alloantibodies are associated with fibrosis progression after liver transplantation in hepatitis C virus-infected patients. Liver Transpl. 2014;20:655–663. doi: 10.1002/lt.23854. [DOI] [PubMed] [Google Scholar]
- 61.Saidman SL, Duquesnoy RJ, Demetris AJ, McCauley J, Ramos H, Mazariegos G, Shapiro R, Starzl TE, Fung JJ. Combined liver-kidney transplantation and the effect of preformed lymphocytotoxic antibodies. Transpl Immunol. 1994;2:61–67. doi: 10.1016/0966-3274(94)90080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Leary JG, Gebel HM, Ruiz R, Bray RA, Marr JD, Zhou XJ, Shiller SM, Susskind BM, Kirk AD, Klintmalm GB. Class II alloantibody and mortality in simultaneous liver-kidney transplantation. Am J Transplant. 2013;13:954–960. doi: 10.1111/ajt.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dar W, Agarwal A, Watkins C, Gebel HM, Bray RA, Kokko KE, Pearson TC, Knechtle SJ. Donor-directed MHC class I antibody is preferentially cleared from sensitized recipients of combined liver/kidney transplants. Am J Transplant. 2011;11:841–847. doi: 10.1111/j.1600-6143.2011.03467.x. [DOI] [PubMed] [Google Scholar]
- 64.Starzl TE, Demetris AJ, Todo S, Kang Y, Tzakis A, Duquesnoy R, Makowka L, Banner B, Concepcion W, Porter KA. Evidence for hyperacute rejection of human liver grafts: The case of the canary kidneys. Clin Transplant. 1989;3:37–45. [PMC free article] [PubMed] [Google Scholar]
- 65.Castillo-Rama M, Sebagh M, Sasatomi E, Randhawa P, Isse K, Salgarkar AD, Ruppert K, Humar A, Demetris AJ. “Plasma cell hepatitis” in liver allografts: identification and characterization of an IgG4-rich cohort. Am J Transplant. 2013;13:2966–2977. doi: 10.1111/ajt.12413. [DOI] [PubMed] [Google Scholar]
- 66.Iacob S, Cicinnati VR, Dechêne A, Lindemann M, Heinemann FM, Rebmann V, Ferencik S, Sotiropoulos GC, Popescu I, Horn PA, et al. Genetic, immunological and clinical risk factors for biliary strictures following liver transplantation. Liver Int. 2012;32:1253–1261. doi: 10.1111/j.1478-3231.2012.02810.x. [DOI] [PubMed] [Google Scholar]
- 67.Mañez R, Bronsther O, Kusne S, Llull R, Aguado JM, Starzl TE. Vanishing bile duct syndrome after liver transplantation: alloreactivity or viral reactivity? Transplant Proc. 1995;27:2280. [PubMed] [Google Scholar]
- 68.Hubscher SG, Adams DH, Elias E. Changes in the expression of major histocompatibility complex class II antigens in liver allograft rejection. J Pathol. 1990;162:165–171. doi: 10.1002/path.1711620210. [DOI] [PubMed] [Google Scholar]
- 69.Steinhoff G, Wonigeit K, Pichlmayr R. Analysis of sequential changes in major histocompatibility complex expression in human liver grafts after transplantation. Transplantation. 1988;45:394–401. doi: 10.1097/00007890-198802000-00030. [DOI] [PubMed] [Google Scholar]
- 70.Gouw AS, Huitema S, Grond J, Slooff MJ, Klompmaker IJ, Gips CH, Poppema S. Early induction of MHC antigens in human liver grafts. An immunohistologic study. Am J Pathol. 1988;133:82–94. [PMC free article] [PubMed] [Google Scholar]
- 71.Fontana M, Moradpour D, Aubert V, Pantaleo G, Pascual M. Prevalence of anti-HLA antibodies after liver transplantation. Transpl Int. 2010;23:858–859. doi: 10.1111/j.1432-2277.2009.01022.x. [DOI] [PubMed] [Google Scholar]
- 72.Grabhorn E, Binder TM, Obrecht D, Brinkert F, Lehnhardt A, Herden U, Peine S, Nashan B, Ganschow R, Briem-Richter A. Long-term Clinical Relevance of De Novo Donor-Specific Antibodies After Pediatric Liver Transplantation. Transplantation. 2015;99:1876–1881. doi: 10.1097/TP.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 73.Lachmann N, Terasaki PI, Budde K, Liefeldt L, Kahl A, Reinke P, Pratschke J, Rudolph B, Schmidt D, Salama A, et al. Anti-human leukocyte antigen and donor-specific antibodies detected by luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation. 2009;87:1505–1513. doi: 10.1097/TP.0b013e3181a44206. [DOI] [PubMed] [Google Scholar]
- 74.Scheenstra R, Peeters PM, Verkade HJ, Gouw AS. Graft fibrosis after pediatric liver transplantation: ten years of follow-up. Hepatology. 2009;49:880–886. doi: 10.1002/hep.22686. [DOI] [PubMed] [Google Scholar]
- 75.Ekong UD, Melin-Aldana H, Seshadri R, Lokar J, Harris D, Whitington PF, Alonso EM. Graft histology characteristics in long-term survivors of pediatric liver transplantation. Liver Transpl. 2008;14:1582–1587. doi: 10.1002/lt.21549. [DOI] [PubMed] [Google Scholar]
- 76.Martin SR, Russo P, Dubois J, Alvarez F. Centrilobular fibrosis in long-term follow-up of pediatric liver transplant recipients. Transplantation. 2002;74:828–836. doi: 10.1097/00007890-200209270-00017. [DOI] [PubMed] [Google Scholar]
- 77.Fouquet V, Alves A, Branchereau S, Grabar S, Debray D, Jacquemin E, Devictor D, Durand P, Baujard C, Fabre M, et al. Long-term outcome of pediatric liver transplantation for biliary atresia: a 10-year follow-up in a single center. Liver Transpl. 2005;11:152–160. doi: 10.1002/lt.20358. [DOI] [PubMed] [Google Scholar]
- 78.Miyagawa-Hayashino A, Yoshizawa A, Uchida Y, Egawa H, Yurugi K, Masuda S, Minamiguchi S, Maekawa T, Uemoto S, Haga H. Progressive graft fibrosis and donor-specific human leukocyte antigen antibodies in pediatric late liver allografts. Liver Transpl. 2012;18:1333–1342. doi: 10.1002/lt.23534. [DOI] [PubMed] [Google Scholar]
- 79.Iacob S, Cicinnati VR, Lindemann M, Heinemann FM, Radtke A, Kaiser GM, Kabar I, Schmidt HH, Baba HA, Beckebaum S. Donor-Specific Anti-HLA Antibodies and Endothelial C4d Deposition-Association With Chronic Liver Allograft Failure. Transplantation. 2015;99:1869–1875. doi: 10.1097/TP.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 80.Demetris AJ, Murase N, Nakamura K, Iwaki Y, Yagihashi A, Valdivia L, Todo S, Iwatsuki S, Takaya S, Fung JJ. Immunopathology of antibodies as effectors of orthotopic liver allograft rejection. Semin Liver Dis. 1992;12:51–59. doi: 10.1055/s-2007-1007376. [DOI] [PubMed] [Google Scholar]
- 81.Taner T, Stegall MD, Heimbach JK. Antibody-mediated rejection in liver transplantation: current controversies and future directions. Liver Transpl. 2014;20:514–527. doi: 10.1002/lt.23826. [DOI] [PubMed] [Google Scholar]
- 82.Schmeding M, Dankof A, Krenn V, Krukemeyer MG, Koch M, Spinelli A, Langrehr JM, Neumann UP, Neuhaus P. C4d in acute rejection after liver transplantation--a valuable tool in differential diagnosis to hepatitis C recurrence. Am J Transplant. 2006;6:523–530. doi: 10.1111/j.1600-6143.2005.01180.x. [DOI] [PubMed] [Google Scholar]
- 83.Bouron-Dal Soglio D, Rougemont AL, Herzog D, Soucy G, Alvarez F, Fournet JC. An immunohistochemical evaluation of C4d deposition in pediatric inflammatory liver diseases. Hum Pathol. 2008;39:1103–1110. doi: 10.1016/j.humpath.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 84.Ali S, Ormsby A, Shah V, Segovia MC, Kantz KL, Skorupski S, Eisenbrey AB, Mahan M, Huang MA. Significance of complement split product C4d in ABO-compatible liver allograft: diagnosing utility in acute antibody mediated rejection. Transpl Immunol. 2012;26:62–69. doi: 10.1016/j.trim.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 85.Kozlowski T, Andreoni K, Schmitz J, Hayashi PH, Nickeleit V. Sinusoidal C4d deposits in liver allografts indicate an antibody-mediated response: diagnostic considerations in the evaluation of liver allografts. Liver Transpl. 2012;18:641–658. doi: 10.1002/lt.23403. [DOI] [PubMed] [Google Scholar]
- 86.Corbani A, Burroughs AK. Intrahepatic cholestasis after liver transplantation. Clin Liver Dis. 2008;12:111–129, ix. doi: 10.1016/j.cld.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 87.Ben-Ari Z, Pappo O, Mor E. Intrahepatic cholestasis after liver transplantation. Liver Transpl. 2003;9:1005–1018. doi: 10.1053/jlts.2003.50212. [DOI] [PubMed] [Google Scholar]
- 88.O’Leary JG, Michelle Shiller S, Bellamy C, Nalesnik MA, Kaneku H, Jennings LW, Isse K, Terasaki PI, Klintmalm GB, Demetris AJ. Acute liver allograft antibody-mediated rejection: an inter-institutional study of significant histopathological features. Liver Transpl. 2014;20:1244–1255. doi: 10.1002/lt.23948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mauiyyedi S, Crespo M, Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Tolkoff-Rubin NE, Williams WW, Delmonico FL, Cosimi AB, et al. Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol. 2002;13:779–787. doi: 10.1681/ASN.V133779. [DOI] [PubMed] [Google Scholar]
- 90.Nakamura K, Yagihashi A, Iwaki Y, Takaya S, Hartman GG, Murase N, Bronsther O, Manez R, Fung JJ, Iwatsuki S. The lymphocytotoxic crossmatch in liver transplantation: a clinicopathologic analysis. Transplant Proc. 1991;23:3021–3022. [PMC free article] [PubMed] [Google Scholar]
- 91.Mañez R, Kobayashi M, Takaya S, Bronsther O, Kramer D, Bonet H, Iwaki Y, Fung JJ, Demetris AJ, Starzl TE. Humoral rejection associated with antidonor lymphocytotoxic antibodies following liver transplantation. Transplant Proc. 1993;25:888–890. [PMC free article] [PubMed] [Google Scholar]
- 92.Kobayashi M, Yagihashi A, Manez R, Takaya S, Noguchi K, Konno A, Kita Y, Yoshida Y, Terasawa K, Starzl TE. Posttransplant donor-specific T-lymphocytotoxic antibody in liver transplant patients with a positive crossmatch. Transplant Proc. 1992;24:2510–2511. [PMC free article] [PubMed] [Google Scholar]
- 93.Zeevi A, Lunz J, Feingold B, Shullo M, Bermudez C, Teuteberg J, Webber S. Persistent strong anti-HLA antibody at high titer is complement binding and associated with increased risk of antibody-mediated rejection in heart transplant recipients. J Heart Lung Transplant. 2013;32:98–105. doi: 10.1016/j.healun.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sutherland SM, Chen G, Sequeira FA, Lou CD, Alexander SR, Tyan DB. Complement-fixing donor-specific antibodies identified by a novel C1q assay are associated with allograft loss. Pediatr Transplant. 2012;16:12–17. doi: 10.1111/j.1399-3046.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 95.Chin C, Chen G, Sequeria F, Berry G, Siehr S, Bernstein D, Rosenthal D, Reinhartz O, Tyan D. Clinical usefulness of a novel C1q assay to detect immunoglobulin G antibodies capable of fixing complement in sensitized pediatric heart transplant patients. J Heart Lung Transplant. 2011;30:158–163. doi: 10.1016/j.healun.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 96.Cousens LP, Su Y, McClaine E, Li X, Terry F, Smith R, Lee J, Martin W, Scott DW, De Groot AS. Application of IgG-derived natural Treg epitopes (IgG Tregitopes) to antigen-specific tolerance induction in a murine model of type 1 diabetes. J Diabetes Res. 2013;2013:621693. doi: 10.1155/2013/621693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rogers NM, Eng HS, Yu R, Kireta S, Tsiopelas E, Bennett GD, Brook NR, Gillis D, Russ GR, Coates PT. Desensitization for renal transplantation: depletion of donor-specific anti-HLA antibodies, preservation of memory antibodies, and clinical risks. Transpl Int. 2011;24:21–29. doi: 10.1111/j.1432-2277.2010.01138.x. [DOI] [PubMed] [Google Scholar]
- 98.Levine MH, Abt PL. Treatment options and strategies for antibody mediated rejection after renal transplantation. Semin Immunol. 2012;24:136–142. doi: 10.1016/j.smim.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bartel G, Schwaiger E, Böhmig GA. Prevention and treatment of alloantibody-mediated kidney transplant rejection. Transpl Int. 2011;24:1142–1155. doi: 10.1111/j.1432-2277.2011.01309.x. [DOI] [PubMed] [Google Scholar]
- 100.Moreso F, Serón D, Carrera M, Gil-Vernet S, Cruzado JM, Hueso M, Fulladosa X, Ramos R, Ibernon M, Castelao AM, et al. Baseline immunosuppression is associated with histological findings in early protocol biopsies. Transplantation. 2004;78:1064–1068. doi: 10.1097/01.tp.0000137268.85155.11. [DOI] [PubMed] [Google Scholar]
- 101.Naesens M, Lerut E, Damme BV, Vanrenterghem Y, Kuypers DR. Tacrolimus exposure and evolution of renal allograft histology in the first year after transplantation. Am J Transplant. 2007;7:2114–2123. doi: 10.1111/j.1600-6143.2007.01892.x. [DOI] [PubMed] [Google Scholar]
- 102.Turgeon NA, Kirk AD, Iwakoshi NN. Differential effects of donor-specific alloantibody. Transplant Rev (Orlando) 2009;23:25–33. doi: 10.1016/j.trre.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 103.Heidt S, Roelen DL, Eijsink C, Eikmans M, van Kooten C, Claas FH, Mulder A. Calcineurin inhibitors affect B cell antibody responses indirectly by interfering with T cell help. Clin Exp Immunol. 2010;159:199–207. doi: 10.1111/j.1365-2249.2009.04051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Paterno F, Shiller M, Tillery G, O’Leary JG, Susskind B, Trotter J, Klintmalm GB. Bortezomib for acute antibody-mediated rejection in liver transplantation. Am J Transplant. 2012;12:2526–2531. doi: 10.1111/j.1600-6143.2012.04126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


