Abstract
The shortage of organ donors mandates the use of liver allograft from anti-HBc(+) donors, especially in areas highly endemic for hepatitis B virus (HBV) infection. The incidence of de novo hepatitis B infection (DNH) is over 30%-70% among recipients of hepatitis B core antibody (HBcAb) (+) grafts without any prophylaxis after liver transplantation (LT). Systematic reviews showed that prophylactic therapy [lamivudine and/or hepatitits B immunoglobulin (HBIG)] dramatically reduces the probability of DNH. However, there are limited studies regarding the effects of active immunization to prevent DNH, and the role of active vaccination is not well-defined. This review focuses on the feasibility and efficacy of pre- and post-LT HBV vaccination to prevent DNH in HBsAg(-) recipient using HBcAb(+) grafts. The presence of HBsAb in combination with lamivudine or HBIG results in lower incidence of DNH and may reduce the requirement of HBIG. There was a trend towards decreasing incidence of DNH with higher titers of HBsAb. High titers of HBsAb (> 1000 IU/L) achieved after repeated vaccination could eliminate the necessity for additional antiviral prophylaxis in pediatric recipients. In summary, active vaccination with adequate HBsAb titer is a feasible, cost-effective strategy to prevent DNH in recipients of HBcAb(+) grafts. HBV vaccination is advised for candidates on waiting list and for recipients after withdrawal of steroids and onset of low dose immunosuppression after transplantation.
Keywords: De novo hepatitis B, Vaccination, Liver transplantation, Core antibody positive donor
Core tip: De novo hepatitis B virus infection (DNH) can both result in significant morbidity and reduced graft survival after liver transplantation. Utilization of hepatitis B core antibody(+) grafts may increase the risk of DNH. Different approaches to mitigate this risk have been described. There is no widespread consensus regarding the prophylactic measures to reduce the incidence of DNH by active immunization. This review examines the important published studies on DNH, and presents the clinically relevant points in a lucid manner. It also presents an algorithm which is simple to follow, and which has been validated in pediatric patients at our center.
INTRODUCTION
There is a wide disparity between the number of patients awaiting liver transplantation (LT) and the pool of available donors. Strategies such as living donor LT and using extended criteria donors have been utilized to increase the number of liver grafts. Additionally, many LT programs use liver grafts from hepatitis B core antibody (HBcAb)-positive donors to increase donor organ availability. The acquisition of hepatitis B virus (HBV) infection after transplantation in recipients who are hepatitis B surface antigen (HBsAg)-negative before transplantation has been recognized[1]. The incidence of de novo HBV infection (DNH) among patients receiving HBcAb(-) grafts is low (0%-1.7%) but unacceptably high (38%-100%) among recipients receiving HBcAb(+) grafts without prophylaxis[2-5]. The mechanism has been well-established that HBV DNA may persist in the serum and liver in low replicating or non-replicative forms following serologic recovery from HBV infection, thereby presenting a risk of DNH[6]. Hence, some centers have suggested to exclude these grafts from HBcAb(+) donors or to limit its use in selected recipients[7] . This strategy is not practical in endemic areas for HBV infection[8,9].
Several strategies have been recommended to prevent DNH in non-HBV recipients who receive HBcAb(+) grafts. Hepatitis B immunoglobin (HBIG) and/or lamivudine have been most commonly used for prophylaxis. Recently, the results have been extensively reviewed[10,11]. However, the results of active immunization are still not well-documented due to heterogenous data resources and limited case numbers. In this review, we mostly focus on the role of active vaccination before and after LT to prevent DNH.
POST-TRANSPLANT PROPHYLAXIS AGAINST DE NOVO HBV INFECTION
Lamivudine monoprophylaxis
Studies show that lamivudine monoprophylaxis (100-150 mg/d for long periods) has been an effective strategy against DNH. During a median follow-up of 25 mo (range: 1-69 mo), the incidence of DNH was observed in 2.6% of recipients: 4.0% in recipients with past HBV infection, 3.4% in HBV naive recipients and 0% in recipients with successful pre-LT vaccination[10]. New generations of nucleos(t)ide analogs have been used to replace lamivudine in order to further reduce the probability of DNH. Tenofovir and entecavir seem to be more potent than lamivudine and adefovir in limited cases[12]. It is worth noting that the lowest probability was observed among recipients with the presence of HBsAb despite no statistical difference. HBV naive recipients were more likely to develop DNH after nucleos(t)ide discontinuation. These observations suggest that the presence of HBsAb alone may not completely prevent DNH but plays a role in combination with antiviral agents to prevent DNH.
HBIG monoprophylaxis
During a median follow-up of 31 mo (range: 3-86 mo), 18% of recipients with HBIG monoprophylaxis developed DNH, of which 27% had discontinued HBIG and 11% had low serum anti-HBs levels (< 50 IU/mL) despite HBIG administration[10]. As to HBV status, DNH developed in 27% of HBV naive recipients and 5.8% of recipients with past HBV infection (P = 0.10) during a median follow-up of 30 mo and 19 mo, respectively. Of importance, DNH developed in none of five recipients with successful pre-LT vaccination during a median follow-up of 35 mo. Although the impact of the recipients’ HBsAb status could not be determined, there was a trend toward decreasing incidence of DNH in combination with the presence of HBsAb. Moreover, a smaller number of recipients with cessation of HBIG had much higher incidence of DNH than recipients with long term HBIG administration (P = 0.0002)[11]. Most centers use HBIG indefinitely to prevent DNH; post-LT vaccination has therefore been utilized to replace the use of HBIG[13].
HBIG and lamivudine combination therapy
Since the combination of HBIG with lamivudine was the most widely used treatment approach to prevent post-LT HBV recurrence in patients transplanted for HBV-related liver disease, it was also used as prophylaxis against DNH. However, given the low probability of DNH with lamivudine or HBIG alone, the benefit of HBIG with lamivudine combined prophylaxis was similar to lamivudine alone[10]. There is no clear evidence showing the necessity of using combination therapy.
Pre-LT recipient HBV status
HBV naive recipients receiving HBcAb(+) grafts are highly susceptible to develop DNH without prophylaxis. Recipients of HBcAb(+) grafts had DNH rates of 18% without prophylaxis and 0% with prophylaxis. Recipients with HBsAb and received HBcAb(+) grafts had DNH rates of 4% without prophylaxis and 3% with prophylaxis; whereas, recipients with HBcAb positivity alone had DNH rates of 14% without prophylaxis and 3% with prophylaxis (P = 0.21)[11]. In addition, some centers allocated HBcAb(+) grafts to HBsAb(+) candidates if possible to minimize DNH[14]. These data suggest that the presence of HBsAb either from past infection or active vaccination provides some additional benefits against DNH.
Active immunization against DNH
The long-term use of lamivudine may raise the concern of mutant strain and HBIG is a costly and inconvenient regimen. Non-compliance is a significant factor for failure of prophylaxis. The difference between innate and exogenously administered antibody is unknown. The acquisition of immunity through active vaccination is preferred if both are equally effective. Active vaccination has been suggested against DNH. Although HBsAb alone is not able to eliminate DNH completely, its presence reduces the probability of DNH either in lamivudine or HBIG regimen. In addition, a study indicated that the level of pre-LT HBsAb in children was associated with the response of post-LT booster vaccine[15]. Therefore, pre-transplant active vaccination for candidates in waiting list is fundamental to avoid DNH.
Pre-transplant vaccination
HBV vaccines are extremely safe and have an efficacy of more than 90% in the general population; the response rate is lower in cirrhotic patients due to the impairment in T-cell dependent function. The results of vaccination in pre-LT candidates have been very disappointing, with response rates of 20%-30%[16]. The accelerated protocol, double-dose schedule and other more immunologic formulation have been proposed to increase the response. A study reported that vaccination with this double-dose schedule can achieve 65% response rate in candidates before transplantation[17]. Age plays an important role in the response to HBV vaccine; especially many pediatric patients for LT have non-parenchymal, cholestatic diseases. In our pediatric series, 72% of recipients were positive for HBsAb at the initial evaluation for transplantation due to the national vaccination program. After pre-LT booster vaccination, all recipients were positive for HBsAb with a median of 784 (8-18736) (IU/L)[18]. Likewise, 63% of our adult non-HBV patients were positive for HBsAb at the initial evaluation and 93% were positive after double-dose booster vaccination before transplant (unpublished data).
Post-LT vaccination
Although the presence of HBsAb in recipients is protective to a certain degree, a small number of recipients with pre-LT serologic immunity do develop DNH, particularly those whose immunity was based on vaccination (HBsAb alone) rather than prior exposure to HBV[11]. Active vaccination was not absolutely effective to prevent DNH, because it was impossible to maintain an optimal HBsAb titer to prevent DNH during the early post-LT period.
The HBV vaccination response rates are lower during the early post-LT period with intense immunosuppression. Although a double-dose schedule was used, it was difficult to maintain an adequate HBsAb titer[19]. It is generally accepted that the immune system is less capable of mounting effective immune responses in recipients receiving high dose immunosuppression. T- and B-cell responses to antigenic stimulation are impaired through blockade of cellular proliferation by calcineurin inhibitors and steroids as well as by inhibition of cytokine production[20]. To optimize the response, the timing of vaccination appears to be critical. The first 6 mo post-LT appears to be a period where mounting immune responses are lowest since the recipients are usually on high dose immunosuppression.
The key factors for successful vaccination are the vaccine doses, the rapid schedule and administration during the low immunosuppression period[21]. As a general rule, vaccination should be administered when the dosages of immunosuppressants have been reduced post-LT, especially after steroid withdrawal. Repeated vaccination after LT in pediatric patients at our center resulted in HBsAb titers higher than 100 IU/L in about 80% cases and over 1000 IU/L in 40% cases[18]. Similarly, all adult recipients were positive for HBsAb, and approximately 80% patients achieved titers higher than 100 IU/L and 38% had titers over 1000 IU/L (unpublished data). It is not uncommon to observe decline of protective antibodies after LT, particularly if HBsAb is acquired by vaccination. Therefore, routine HBV booster vaccination is advised after steroid withdrawal and when recipients are on low-dose immunosuppression after transplant.
How high of HBsAb is enough
HBsAb level > 10 IU/L after vaccination is considered protective in immunocompetent patients. A plausible explanation for recipients developing DNH despite presence of HBsAb may be that their titers were insufficient to confer protection. HBIG prophylaxis to maintain HBsAb level > 500 IU/L against DNH has been advised[22,23]. However, the adequate titer of HBsAb to be achieved by vaccination, in order to protect immunosuppressed recipients against DNH is still not well defined (Table 1). HBsAb < 20 IU/L was obviously not protective against DNH[19,24]. Su et al[25] found that HBsAb > 200 IU/L by vaccination significantly protects pediatric recipients receiving HBcAb(+) positive grafts from DNH, reducing its incidence to 11%. Another group demonstrated the similar results by HBIG administration[26]. In our study, in combination with lamivudine, vaccination with HBsAb > 1000 IU/L significantly reduced the incidence of DNH from 15.4% to 0% in pediatric patients[18]. Notably, without antiviral agent prophylaxis, HBsAb > 1000 IU/L completely prevented the development of DNH in pediatric patients with a follow-up of more than 5 years[27]. Likewise, there was no case of DNH in 10 (24.3%) adult recipients with HBsAb > 1000 IU/L[28]. It is generally accepted that the level of HBsAb naturally declines and may even drop rapidly during heavy immunosuppression. These observations theoretically suggest that the higher the levels of HBsAb that can be achieved, the lower the incidence of DNH. In over 107 recipients with HBsAb > 1000 IU/L (Table 1), only one (approximately 0.9%) developed DNH due to escape mutant when post-LT HBsAb dropped to 362 IU/L[26].
Table 1.
Published studies with HBsAb titers in patients receiving HBcAb(+) grafts
| Study | Patients | Lamivudine/HBIG | Vaccination | HBsAb | DNH | Follow-up |
| Pre/post-LT | cutoff (IU/L) | (mo) | ||||
| Chang et al[24] | 9/pediatric | HBIG | Yes/Yes | 20 | 50% vs 0%1 | 26 |
| Lin et al[18] | 30/pediatric | lamivudine | Yes/Yes | 1000 | 15.7% vs 0% | 57 |
| Soejima et al[13] | 11/adult | HBIG | No/Yes | 1000 | 0%2 | 15 |
| Park et al[26] | 14/pediatric | HBIG | No/Yes | 100 | 7.1%3 | 26.5 |
| Su et al[25] | 36/pediatric | N | Yes/Yes | 200 | 30.7% vs 4.3% | 52 |
| Liu et al[28] | 41/adult | Lamivudine | Yes/Yes | 1000 | 6.4% vs 0% | 52 |
| Lin et al[27] | 34/pediatric | N | Yes/Yes | 1000 | 0%4 | 65 |
Six out of 7 patients had > 200 IU/L;
All > 1000 IU/L, 4 patients with cessation of HBIG;
All > 100 IU/L, one patient with 362 IU/L of HBsAb and escape mutant;
All > 1000 IU/L without additional prophylaxis. HBIG: Hepatitis B immunoglobulin; DNH: de novo hepatitis B infection; LT: Liver transplantation.
Escape mutant
Another major concern for vaccination is the escape mutant of “a” determinant within HBsAg which can develop after HBV vaccination and use of HBIG post-LT[29,30]. A change of amino acids in the “a” determinant could lead to a structural variation in the epitope of the surface antigen recognized by HBsAb, which affects virus binding with a loss of immunoreactivity despite the presence of HBsAb. The incidence of escape mutant was below 4% in HBV-infected infants who received HBV vaccine or HBIG to an HBV-infected mother but up to 11%-66% in LT recipients who experienced HBV reinfection after HBIG prophylaxis[31]. Although the incidence of escape mutant in transplant recipients is uncertain, it would be lower compared to the administration of HBIG. Thus, we believe that the benefits of HBV vaccination for prophylaxis against DNH outweigh its potential side effects.
Donor HBsAb status
Whether it is possible to obtain adaptive transfer from donor HBsAb to recipient is not well-established. Both animal and human studies have demonstrated the adoptive transfer of immunity against HBV through LT that may be attributed to the presence of HBV-specific immunocompetent cells of donor origin in liver grafts[32]. However, most studies showed that donor HBsAb did not provide any additional benefits against DNH[18,25].
CONCLUSION
Despite the scarcity of evidence-based data due to heterogeneous prophylactic strategy and limited case numbers, some conclusions can be drawn based on these reviews. The presence of HBsAb in combination with antiviral agents or HBIG reduces the incidence of DNH. High titers of HBsAb (> 1000 IU/L) is an effective strategy to prevent DNH without additional prophylaxis, which can be achieved by repeated pre- and post-LT booster vaccination. Therefore, we recommend the following prophylactic strategy to prevent DNH in LT recipients receiving HBcAb(+) grafts (Figure 1). For recipients with pre-LT HBsAb titers > 1000 IU/L, no antiviral agent prophylaxis is required but only a booster vaccination after steroid withdrawal. However, recipients with HBsAb titers below 1000 IU/L require prophylaxis with nucleos(t)ide analogs in addition to booster HBV vaccinations after steroid withdrawal. Nucleos(t)ide analogs may be discontinued when the HBsAb is > 1000 IU/L after post-LT booster vaccination.
Figure 1.
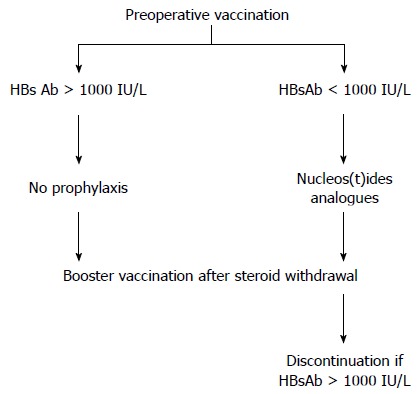
Algorithm for prophylaxis to prevent de novo hepatitis B virus for recipients of HBcAb(+) grafts. For recipients with pretransplant HBsAb > 1000 IU/L, no antiviral prophylaxis is required. Recipients require nucleos(t)ide analogs if HBsAb is < 1000 IU/L. Post-transplant vaccination is given after steroid withdrawal. Nucleos(t)ide analogs may be discontinued if HBsAb is > 1000 IU/L.
In conclusion, active vaccination in combination with nucelos(t)ide analogues is a simple and cost-effective strategy to prevent DNH. This strategy may reduce the incidence of DNH even if high titers of HBsAb are not achieved. It is advised that active vaccination be administered for non-HBV patients before and after transplantation.
Footnotes
Conflict-of-interest statement: No conflicts of interest were declared for all authors.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 16, 2015
First decision: May 18, 2015
Article in press: September 13, 2015
P- Reviewer: Bock CT, Panduro A S- Editor: Yu J L- Editor: A E- Editor: Liu XM
References
- 1.Theilmann L, Arnold J, Töx U, Datsis K, Otto G, Hofmann W, Köck J, Schlicht HJ. Liver transplantation and de novo hepatitis B infection. Lancet. 1994;343:677–678. [PubMed] [Google Scholar]
- 2.Dickson RC, Everhart JE, Lake JR, Wei Y, Seaberg EC, Wiesner RH, Zetterman RK, Pruett TL, Ishitani MB, Hoofnagle JH. Transmission of hepatitis B by transplantation of livers from donors positive for antibody to hepatitis B core antigen. The National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Gastroenterology. 1997;113:1668–1674. doi: 10.1053/gast.1997.v113.pm9352871. [DOI] [PubMed] [Google Scholar]
- 3.Prieto M, Gómez MD, Berenguer M, Córdoba J, Rayón JM, Pastor M, García-Herola A, Nicolás D, Carrasco D, Orbis JF, et al. De novo hepatitis B after liver transplantation from hepatitis B core antibody-positive donors in an area with high prevalence of anti-HBc positivity in the donor population. Liver Transpl. 2001;7:51–58. doi: 10.1053/jlts.2001.20786. [DOI] [PubMed] [Google Scholar]
- 4.Roque-Afonso AM, Feray C, Samuel D, Simoneau D, Roche B, Emile JF, Gigou M, Shouval D, Dussaix E. Antibodies to hepatitis B surface antigen prevent viral reactivation in recipients of liver grafts from anti-HBC positive donors. Gut. 2002;50:95–99. doi: 10.1136/gut.50.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas DD, Rakela J, Wright TL, Krom RA, Wiesner RH. The clinical course of transplantation-associated de novo hepatitis B infection in the liver transplant recipient. Liver Transpl Surg. 1997;3:105–111. doi: 10.1002/lt.500030202. [DOI] [PubMed] [Google Scholar]
- 6.Michalak TI, Pasquinelli C, Guilhot S, Chisari FV. Hepatitis B virus persistence after recovery from acute viral hepatitis. J Clin Invest. 1994;94:907. doi: 10.1172/JCI116950C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton JR, Shaw-Stiffel TA. Use of hepatitis B core antibody-positive donors in recipients without evidence of hepatitis B infection: a survey of current practice in the United States. Liver Transpl. 2003;9:837–842. doi: 10.1053/jlts.2003.50157. [DOI] [PubMed] [Google Scholar]
- 8.Sung JL. Hepatitis B virus infection and its sequelae in Taiwan. Gastroenterol Jpn. 1984;19:363–366. doi: 10.1007/BF02779126. [DOI] [PubMed] [Google Scholar]
- 9.Lo CM, Fan ST, Liu CL, Yong BH, Wong Y, Ng IO, Wong J. Safety and outcome of hepatitis B core antibody-positive donors in right-lobe living donor liver transplantation. Liver Transpl. 2003;9:827–832. doi: 10.1053/jlts.2003.50115. [DOI] [PubMed] [Google Scholar]
- 10.Cholongitas E, Papatheodoridis GV, Burroughs AK. Liver grafts from anti-hepatitis B core positive donors: a systematic review. J Hepatol. 2010;52:272–279. doi: 10.1016/j.jhep.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Skagen CL, Jou JH, Said A. Risk of de novo hepatitis in liver recipients from hepatitis-B core antibody-positive grafts - a systematic analysis. Clin Transplant. 2011;25:E243–E249. doi: 10.1111/j.1399-0012.2011.01409.x. [DOI] [PubMed] [Google Scholar]
- 12.Chang MS, Olsen SK, Pichardo EM, Stiles JB, Rosenthal-Cogan L, Brubaker WD, Guarrera JV, Emond JC, Brown RS. Prevention of de novo hepatitis B in recipients of core antibody-positive livers with lamivudine and other nucleos(t)ides: a 12-year experience. Transplantation. 2013;95:960–965. doi: 10.1097/TP.0b013e3182845f97. [DOI] [PubMed] [Google Scholar]
- 13.Soejima Y, Ikegami T, Taketomi A, Yoshizumi T, Uchiyama H, Harada N, Yamashita Y, Maehara Y. Hepatitis B vaccination after living donor liver transplantation. Liver Int. 2007;27:977–982. doi: 10.1111/j.1478-3231.2007.01521.x. [DOI] [PubMed] [Google Scholar]
- 14.Manzarbeitia C, Reich DJ, Ortiz JA, Rothstein KD, Araya VR, Munoz SJ. Safe use of livers from donors with positive hepatitis B core antibody. Liver Transpl. 2002;8:556–561. doi: 10.1053/jlts.2002.33451. [DOI] [PubMed] [Google Scholar]
- 15.Kwon CH, Suh KS, Yi NJ, Chang SH, Cho YB, Cho JY, Lee HJ, Seo JK, Lee KU. Long-term protection against hepatitis B in pediatric liver recipients can be achieved effectively with vaccination after transplantation. Pediatr Transplant. 2006;10:479–486. doi: 10.1111/j.1399-3046.2006.00540.x. [DOI] [PubMed] [Google Scholar]
- 16.Castells L, Esteban R. Hepatitis B vaccination in liver transplant candidates. Eur J Gastroenterol Hepatol. 2001;13:359–361. doi: 10.1097/00042737-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Domínguez M, Bárcena R, García M, López-Sanroman A, Nuño J. Vaccination against hepatitis B virus in cirrhotic patients on liver transplant waiting list. Liver Transpl. 2000;6:440–442. doi: 10.1053/jlts.2000.8313. [DOI] [PubMed] [Google Scholar]
- 18.Lin CC, Chen CL, Concejero A, Wang CC, Wang SH, Liu YW, Yang CH, Yong CC, Lin TS, Jawan B, et al. Active immunization to prevent de novo hepatitis B virus infection in pediatric live donor liver recipients. Am J Transplant. 2007;7:195–200. doi: 10.1111/j.1600-6143.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee KW, Lee DS, Lee HH, Kim SJ, Joh JW, Seo JM, Choe YH, Lee SK. Prevention of de novo hepatitis B infection from HbcAb-positive donors in living donor liver transplantation. Transplant Proc. 2004;36:2311–2312. doi: 10.1016/j.transproceed.2004.08.139. [DOI] [PubMed] [Google Scholar]
- 20.Stark K, Günther M, Schönfeld C, Tullius SG, Bienzle U. Immunisations in solid-organ transplant recipients. Lancet. 2002;359:957–965. doi: 10.1016/S0140-6736(02)08028-5. [DOI] [PubMed] [Google Scholar]
- 21.Bárcena R, Fernandez-Braso M, Urman J, López-San Román A, del Campo S, Moreno N, Lopez P, Garcia M, Plaza MP, Garcia Plaza A. Response to hepatitis B virus vaccine in patients transplanted for HBV-related liver disease under specific gammaglobulin prophylaxis. Transplant Proc. 1999;31:2459–2460. doi: 10.1016/s0041-1345(99)00417-0. [DOI] [PubMed] [Google Scholar]
- 22.Uemoto S, Sugiyama K, Marusawa H, Inomata Y, Asonuma K, Egawa H, Kiuchi T, Miyake Y, Tanaka K, Chiba T. Transmission of hepatitis B virus from hepatitis B core antibody-positive donors in living related liver transplants. Transplantation. 1998;65:494–499. doi: 10.1097/00007890-199802270-00007. [DOI] [PubMed] [Google Scholar]
- 23.Rokuhara A, Tanaka E, Yagi S, Mizokami M, Hashikura Y, Kawasaki S, Kiyosawa K. De novo infection of hepatitis B virus in patients with orthotopic liver transplantation: analysis by determining complete sequence of the genome. J Med Virol. 2000;62:471–478. [PubMed] [Google Scholar]
- 24.Chang SH, Suh KS, Yi NJ, Choi SH, Lee HJ, Seo JK, Lee KU. Active immunization against de novo hepatitis B virus infection in pediatric patients after liver transplantation. Hepatology. 2003;37:1329–1334. doi: 10.1053/jhep.2003.50227. [DOI] [PubMed] [Google Scholar]
- 25.Su WJ, Ho MC, Ni YH, Chen HL, Hu RH, Wu YM, Chang MH, Lee PH. High-titer antibody to hepatitis B surface antigen before liver transplantation can prevent de novo hepatitis B infection. J Pediatr Gastroenterol Nutr. 2009;48:203–208. doi: 10.1097/MPG.0b013e3181819ad4. [DOI] [PubMed] [Google Scholar]
- 26.Park JB, Kwon CH, Lee KW, Choi GS, Kim DJ, Seo JM, Kim SJ, Joh JW, Lee SK. Hepatitis B virus vaccine switch program for prevention of de novo hepatitis B virus infection in pediatric patients. Transpl Int. 2008;21:346–352. doi: 10.1111/j.1432-2277.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- 27.Lin CC, Liu YW, Chen CL, Wang CC, Wang SH, Yong CC, Yang CH, Concejero AM, Lin TS, Lin TL, et al. Immunogenicity and long-term effects of active immunization against de novo hepatitis B virus infection in pediatric patients after living donor liver transplantation. Proceedings of the 16th Annual Metting of International Liver Transplantation Society; 2010 June 16-19; Hongkong. Liver Transpl. 2010;16 Suppl 1:S197. [Google Scholar]
- 28.Liu YW, Chen CL, Lin CC, Wang CC, Wang SH, Yong CC, Lin TL, Concejero AM, Jawade K, Yap AQ. High level active immunization is a simple practical strategy to prevent de novo hepatitis B virus infection in non-HBV adults after living donor liver transplantation. Proceedings of the 16th Annual Metting of International Liver Transplantation Society; 2010 June 16-19; Hongkong. Liver Transpl. 2010;16 Suppl 1:S94. [Google Scholar]
- 29.Carman WF, Zanetti AR, Karayiannis P, Waters J, Manzillo G, Tanzi E, Zuckerman AJ, Thomas HC. Vaccine-induced escape mutant of hepatitis B virus. Lancet. 1990;336:325–329. doi: 10.1016/0140-6736(90)91874-a. [DOI] [PubMed] [Google Scholar]
- 30.Ghany MG, Ayola B, Villamil FG, Gish RG, Rojter S, Vierling JM, Lok AS. Hepatitis B virus S mutants in liver transplant recipients who were reinfected despite hepatitis B immune globulin prophylaxis. Hepatology. 1998;27:213–222. doi: 10.1002/hep.510270133. [DOI] [PubMed] [Google Scholar]
- 31.Tabor E. Infections by hepatitis B surface antigen gene mutants in Europe and North America. J Med Virol. 2006;78 Suppl 1:S43–S47. doi: 10.1002/jmv.20606. [DOI] [PubMed] [Google Scholar]
- 32.Luo Y, Lo CM, Cheung CK, Lau GK, Fan ST, Wong J. Identification of hepatitis B virus-specific lymphocytes in human liver grafts from HBV-immune donors. Liver Transpl. 2007;13:71–79. doi: 10.1002/lt.20887. [DOI] [PubMed] [Google Scholar]


