Synopsis
In-hospital worsening heart failure represents a clinical scenario in which a patient hospitalized for treatment of acute heart failure experiences a worsening of their condition while in the hospital, requiring escalation of therapy. In-hospital worsening heart failure is associated with worse in-hospital and post-discharge outcomes. In-hospital worsening heart failure is increasingly being used as an endpoint, or as part of a combined endpoint, in many clinical trials in acute heart failure. This endpoint has advantages over other endpoints commonly used in acute and chronic heart failure trials, such as dyspnea relief and mortality or rehospitalization. Despite the extensive study of this condition, no treatment strategies have been approved for the prevention of this condition. However, several prediction models have been developed to identify worsening heart failure. Continued study in this area is warranted.
Keywords: worsening heart failure, clinical trials, outcomes, medications
Introduction and Definitions
Heart failure is a common condition in the United States. Over five million Americans have heart failure, with over 800,000 new cases diagnosed annually.1 This chronic condition is marked by episodes of acute decompensation, often requiring hospitalization. In the United States alone, there are more than >1 million hospitalizations annually for acute heart failure.1 Unfortunately, patient outcomes remain poor with a 5-year survival rate of approximately 50% and there is an urgent public health need to improve our understanding and treatment options for patients suffering with acute heart failure.1 Acute heart failure therapeutics remain largely homogenous and unchanged over the past 40 years in the United States.2,3
The term “worsening heart failure” has been used to indicate worsening of chronic heart failure, also termed “acute heart failure” or “acute decompensated heart failure.” This acute worsening of chronic heart failure often results in adjustment of chronic therapy or requires in-patient hospitalization and is associated with worse prognosis.4,5 Worsening heart failure has also been used to describe worsening of acute heart failure that occurs during a hospitalization for acute heart failure. For the purposes of this review, we will focus on the latter condition, in-hospital worsening heart failure.
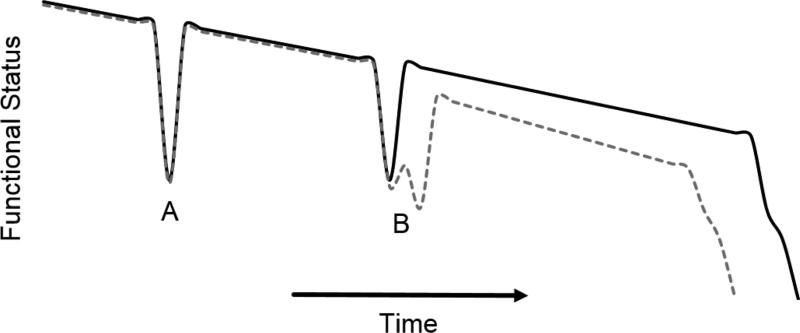
In-hospital worsening heart failure represents a clinical scenario in which a patient hospitalized for treatment of acute heart failure experiences a worsening of their condition while in the hospital, requiring escalation of therapy. This can occur in patients who don’t respond to initial therapy, or in patients who do respond to initial therapy but subsequently stop responding or worsen. Worsening heart failure can occur at any point throughout the hospitalization.6,7 There is a growing body of evidence that in-hospital worsening heart failure is also associated with a worse prognosis and signals an important change in the heart failure patient’s clinical course (Figure 1). These data suggest therapeutic and treatment strategies designed to reduce the incidence of in-hospital worsening heart failure could improve patient outcomes.
Figure 1.
Patient’s clinical trajectory after an episode of in-hospital worsening heart failure (dotted gray line) compared to clinical trajectory without worsening heart failure (solid black line). The heart failure syndrome is characterized by acute changes in status typically requiring a hospitalization (A). In-hospital worsening heart failure (B, dotted gray line) represents a more complicated and costly hospitalization associated with worse long-term outcomes.
Establishing a Definition for Worsening Heart Failure as a Clinical Trial Endpoint
Several clinical trials in acute heart failure have examined worsening heart failure as an endpoint. The first trial to define and examine worsening heart failure was published in 2004 and compared different doses of tezosentan, an endothelin receptor antagonist with vasodilating properties.8 This study included patients from centers in Europe, Israel, and the United States. Investigators examined both hemodynamic and clinical endpoints, including worsening heart failure, defined as “either failure to improve (persistent symptoms and signs of acute heart failure during the first 24 h of treatment) or recurrent symptoms and signs of acute heart failure, pulmonary edema, or cardiogenic shock after initial stabilization within 30 days after randomization, either of which required the initiation or increase of appropriate intravenous therapy or the implementation of mechanical circulatory or ventilatory support to treat the event.”8
The rationale for using worsening heart failure as an endpoint in acute heart failure trials was summarized in subsequent publications.9 Worsening heart failure during a hospitalization for acute heart failure was analogous to re-infarction after an episode of acute coronary syndrome—a failure of the initial treatment strategy. This endpoint was a departure from traditional acute heart failure studies that focused on acute symptoms—primarily dyspnea—or post-discharge outcomes. Neither acute symptoms nor post-discharge outcomes capture the inpatient clinical course, a critical time for heart failure patients. The purpose of worsening heart failure as an endpoint is to represent the inpatient course in a way that can be measured in a clinical trial. Furthermore, in-hospital worsening heart failure is unique to episodes of acute heart failure. Recognizing the different physiology of acute and chronic heart failure underscores the need for different outcomes in clinical trials of these disease states. 10
Despite the value identifying worsening heart failure, there are also some challenges with using worsening heart failure as a clinical trial endpoint. Most notably, it may be difficult to ascertain whether escalation of care is due to true worsening of a patient’s condition or due to initial under treatment. This is particularly challenging for patients who are deemed unresponsive to initial treatment and for patients classified as having worsening heart failure early in the hospital course.
Initial Descriptions of the Incidence of Worsening Heart Failure and Associated Outcomes
One of the first studies to examine the incidence of in-hospital worsening heart failure in patients admitted with acute heart failure was performed in a single-center in a community hospital in Israel.9 Patients were considered to have in-hospital early worsening heart failure if, from six hours after admission through the seventh day of admission, they had “unresolved or recurrent symptoms and signs of heart failure that required an increase in or institution of intravenous heart failure-specific therapy, or institution of mechanical ventilatory or circulatory support.”9 They found 29% of 337 patients experienced worsening heart failure, which was associated with increased mortality at 6 months (age adjusted Hazard Ratio 3.3 [95% CI 1.7-6.3]).
The incidence of worsening heart failure and associations with outcomes was retrospectively investigated in early studies of tezosentan using a slightly different definition of worsening heart failure, which captured clinical events occurring both during hospital admission and in the early post-discharge period.10 Specifically, worsening heart failure that occurred during the first seven days of in-patient hospital admission was termed early worsening heart failure. In their study of 120 patients, 35% experienced early worsening heart failure during the first seven days of hospitalization, and 7% required readmission within 30 days of discharge. Most in-hospital worsening heart failure events were treated with an increase dose of diuretics (82%). They found that patients with worsening heart failure were at higher risk of death at 6 months compared to patients without worsening heart failure (HR 4.1 [95% CI 1.3-13]).
Worsening Heart Failure as a Trial Endpoint
Given the importance of identifying a meaningful endpoint for acute heart failure trials, and the proven association between in-hospital worsening heart failure and long-term clinical outcomes, worsening heart failure was incorporated into subsequent trials in acute heart failure, including VERITAS,11,12 PROTECT,13 ASCEND-HF,14,15 DOSE,16 REVIVE,17 RELAX-AHF,18,19 ROSE-AHF,20 BLAST-AHF,21 and TRUE-AHF22 (Table 1). Each of these large clinical trials used different criteria to define worsening heart failure, but all with the same general framework of a worsening clinical condition. Most trials also specified that the worsening clinical condition required an escalation of therapy to be considered worsening heart failure. The REVIVE trials, however, used only the clinical condition and did not require escalation of therapy.
Table 1.
Worsening Heart Failure as a Clinical Trial Endpoint
| Trial | Year | Drug | WHF Endpoint | Definition/Time Course/Clinical Events | Treatment |
|---|---|---|---|---|---|
| Low dose Tezosentan study8 | 2004 | Tezosentan | Secondary endpoint: Incidence and time to WHF or death up to 30 days after the start of treatment |
Persistent signs or symptoms of HF in initial 24 hours of treatment or Recurrent signs of symptoms of HF, pulmonary edema, or cardiogenic shock after initial stabilization with 30 day of randomization | Initiation or increase of IV therapy Implementation of MCS or ventilator |
| VERITAS I and II12 | 2007 | Tezosentan | Primary endpoint: Death or WHF at 7 days (WHF during admission or after discharge) |
Persistent signs or symptoms of HF with treatment or Development of pulmonary edema, cardiogenic shock, or other evidence of WHF |
IV treatment for HF (diuretic, vasodilator, or inotrope) Implementation of MCS, ventilator, or CPAP Use of ultrafiltration, hemofiltration, or hemodialysis. |
| PROTECT Pilot25 | 2008 | Rolofylline | Primary endpoint: Treatment success, treatment failure, or no change in condition |
Worsening symptoms or signs of HF occurring > 24 hours after start of study drug to day 7 or discharge, whichever occurred first | |
| PROTECT13 | 2010 | Rolofylline | Primary endpoint: Treatment success, treatment failure, or no change in condition |
Death or readmission for HF through day 7 or Worsening symptoms and signs of HF occurring > 24 hours after start of study drug requiring intervention by day 7 or discharge |
|
| ASCEND15 | 2011 | Nesiritide | Secondary endpoint: Composite of persistent or WHF and all-cause death |
Typical clinical manifestations of worsening heart failure from randomization through hospital discharge | Addition or increase of IV pharmacologic agent Mechanical or surgical intervention Ultrafiltration, hemofiltration, or dialysis |
| REVIVE17 | 2013 | Levosimendan | Primary endpoint: Characterization of clinical course as improved, unchanged, or worse |
Death through day 5 Persistent symptoms of HF from >24 after start of study drug through day 5 |
Rescue intervention specifically to relieve HF symptoms or Moderately or markedly worsened global assessment at 6 h, 24 h, or 5 days. |
| Pre-RELAX36 | 2009 | Serelaxin | Exploratory endpoint: In-hospital WHF from baseline to day 5 |
Physician-determined assessment on the basis of worsening symptoms or signs of HF | Addition or institution of IV medications or mechanical support to treat acute HF |
| RELAX-AHF19 | 2013 | Serelaxin | Additional endpoint: Time to WHF through day 5 and through day 14 |
Worsening signs or symptoms of HF | Institution or uptitration of IV therapy (furosemide, nitrates, other HF medications) Institution of MCS or ventilatory support |
| DOSE16 | 2011 | Furosemide | Secondary endpoint: Worsening or persistent heart failure |
Need for rescue therapy within 72 hours from randomization | Additional loop diuretic, addition of thiazide, IV vasoactive agent for HF Ultrafiltration MCS or respiratory support |
| ROSE-AHF20 | 2013 | Dopamine or Nesiritide | Secondary endpoint: Worsening or persistent heart failure |
Need for rescue therapy within 72 hours from randomization | Additional IV vasoactive agent for HF Ultrafiltration MCS or respiratory support |
| BLAST-AHF21 ClinicalTrials.gov NCT01966601 |
Ongoing | TRV027 | Composite primary endpoint: Time from randomization to WHF through day 5 |
Worsening signs or symptoms of HF during hospitalization, or rehospitalization for HF after discharge | Intensification of IV therapy including loop diuretics, nitrates, or other medications for HF MCS or ventilator support (including CPAP/BiPAP if used for HF). |
| TRUE-AHF22 ClinicalTrials.gov NCT01661634 |
Ongoing | Ularitide | Co-primary endpoint: Improvement in a clinical composite including WHF |
Persistent or worsening HF requiring an intervention | Initiation or intensification of IV therapy MCS or ventilatory support, surgical intervention, ultrafiltration, hemofiltration, or dialysis |
Abbreviations: CPAP continuous positive airway pressure, HF heart failure, IV intravenous, MCS mechanical circulatory support, WHF worsening heart failure
The other differences between the trial definitions are mostly related to the setting and timing of worsening heart failure. VERITAS, RELAX-AHF, and BLAST defined worsening heart failure as occurring either during admission or early after discharge, while other trials defined worsening heart failure as occurring during index admission only. However, different trials focused on different timing of the occurrence of worsening heart failure.—ASCEND-HF collected worsening heart failure events throughout the entire hospitalization, while DOSE and ROSE-AHF collected events from randomization through 72 hours, the REVIVE studies evaluated events that occurred after the first 24 hours and through day 5 of the hospitalization, and RELAX -AHF evaluated events that occurred through day 5 and also through day 14. Despite these differences, all of the trials included similar requirements for what constituted escalation of care. These treatments included initiation or increase of intravenous therapies for heart failure (including diuretics, vasodilators, and inotropes), implementation of mechanical circulatory support or ventilatory support, or the initiation of ultrafiltration, hemofiltration, or hemodialysis.
Recent guidelines from the European Medicines Agency (EMA) regarding studies of medications for the treatment of acute heart failure state that for worsening heart failure to be used as a clinical trial endpoint, clear and objective criteria must be pre-specified to reduce variability and inconsistency.23 While the Food and Drug Administration (FDA) has no such guidelines, they have raised similar issues for the worsening heart failure endpoint.24
Association of Worsening Heart Failure with Clinical Outcomes
From the clinical trial datasets detailed above, secondary analyses were performed to examine the association of worsening heart failure with clinical outcomes (Table 2). Worsening heart failure was found to be associated with worse in-hospital outcomes, including a longer length of stay, based on observations from the PROTECT pilot study and the VERITAS studies.25,26 Data from the PROTECT trial showed the association between worsening heart failure and an increased risk of all-cause mortality at 14 days and 30 days.27 The increased risk of 30-day all-cause mortality was also observed in data from the Pre-RELAX trial.28 In addition, worsening heart failure was associated with increased all-cause mortality at 60 days, 90 days, and 180 days, and cardiovascular mortality at 60 days and 180 days. 26,28-30 A recent analysis of ASCEND-HF confirmed increased risk of 30-day and 180-day mortality in patients with worsening heart failure compared to those without worsening heart failure.7
Table 2.
Observational Studies and Secondary Analyses of Clinical Trials Assessing Worsening Heart Failure
| Trial | Year | Drug | WHF Definition | Outcome | Hazard Ratio (95% Confidence Interval) |
|---|---|---|---|---|---|
| PROTECT pilot37 | 2010 | Tezosentan | Physician Determined WHF: Worsening signs and symptoms of HF AND initiation or uptitration of IV treatment or MCS for HF |
LOS 60 day CV/RF readmission and death |
Mean (SD): WHF vs No WHF 13.8 (6.8) vs 9.3 (5.9) 49.7% vs 19.5% in patients without WHF |
| PROTECT27 | 2011 | Rolofylline | Worsening signs and symptoms of HF with resulting intensification of IV therapy for HF or MCS or ventilator support | 14 day all-cause mortality 30 day all-cause mortality |
6.84 (4.12, 11.35) 4.78 (3.10, 7.37) |
| PROTECT30 | 2015 | Rolofylline | Worsening signs and symptoms of HF with resulting intensification of therapy: high intensity therapy: initiation of inotropes, vasopressors and inodilators; MCS, ventilator support, and ultrafiltration low intensity therapy: restarting/increasing diuretics or initiating vasodilators without high intensity interventions |
60 day CV/RF rehospitalization and death 60 day all-cause rehospitalization or death 180 day all-cause mortality 60 day CV/RF rehospitalization and death 60 day all-cause rehospitalization or death 180 day all-cause mortality |
1.54(1.22, 1.95), p<0.001 1.55(1.25, 1.93) , p<0.001 2.46 (1.87, 3.25) , p<0.001 High vs Low intensity 1.41 (0.88, 2.26), p=0.15 1.32 (0.85, 2.05), p=0.22 1.55 (0.93, 2.60), p=0.096 |
| Pre RELAX-AHF28 | 2010 | Serelaxin | Worsening signs or symptoms of HF requiring the increase or re-institution of IV therapy or MCS for HF | 60 day HF/RF readmission or death 30 day all-cause mortality 60 day CV mortality 60 day all-cause mortality 180 day CV death |
3.93 (1.72-8.98), p=0.001 7.70 (1.72-34.41), p=0.008 4.56 (1.02-20.20), p=0.05 3.76 ( 1.23-11.50), p=0.02 6.04 (1.75-20.87), p=0.004 |
| RELAX-AHF38 | 2013 | Serelaxin | Worsening signs or symptoms of HF requiring reinstitution or intensification of IV therapy or MCS for HF | 180 day all-cause mortality | 1.90 (1.11-3.22), p=0.016 |
| PROTECT and RELAX-AHF29 | 2015 | Physician assessment of worsening signs or symptoms of HF requiring intensification of IV therapy or MCS The treatment required was categorized as IV loop diuretic alone, IV inotrope (e.g., dobutamine, norepinephrine, levosimendan, phenylephrine) or mechanical therapy (e.g., mechanical ventilation, MCS, ultrafiltration), or other treatment (e.g., IV nitrates, nesiritide, nonloop diuretic) |
60 day HF/RF rehospitalization or CV death 180 day all-cause mortality |
2.19 (1.80-2.67), p=0.58 2.61 (2.20-3.10, p = 0.45 |
|
| VERITAS26 | 2014 | Tezosentan | WHF could occur during the index admission or after discharge. In-hospital: either: (i) the development of pulmonary edema, cardiogenic shock, or other evidence of WHF; or (ii) failure of the patient's HF condition to improve with treatment (treatment failure) Required at least one of the following: (i) initiation of new IV therapy; (ii) re-institution of prior IV therapy; (iii) increase in current IV therapy for HF; (iv) implementation of MCS or ventilatory support; or (v) use of ultrafiltration, hemofiltration, or hemodialysis. |
LOS 30 day HF rehospitalization or death 90 day mortality |
4.33 (3.54-5.13), p<0.001 2.45 (1.75-3.40), p<0.001 2.57 (1.81-3.65), p<0.001 |
| ADHERE31 | 2014 | Registry | Any of the following criteria: initiated inotropic medications or an IV vasodilator more than 12 hours after hospital presentation, were transferred to the ICU, or received advanced medical therapy after the first inpatient day. | 30 day mortality 1 year mortality 30 day all-cause readmission 1 year all-cause readmission 30 day HF readmission 1 year HF readmission 30 day Medicare payments 1 year Medicare payments |
Hazard Ratio (99% CI) 2.78 (2.55-3.04), p<0.001 1.84 (1.75-1.93), p<0.001 1.47 (1.35-1.59), p<0.001 1.27 (1.21-1.34), p<0.001 1.62 (1.43-1.84), p<0.001 1.36 (1.26-1.47), p<0.001 Cost Ratio (99% CI) 1.70 (1.57-1.84), p<0.001 1.43 (1.37-1.49), p<0.001 |
| ASCEND7 | 2015 | Nesiritide | At least 1 sign, symptom, or radiologic evidence of new, persistent, or worsening acute HF requiring addition of a new IV therapy (inotrope or vasodilator) or mechanical support during index hospitalization targeted specifically at HF symptoms. | 30 day all-cause mortality or HF hospitalization 30 day all-cause mortality 180 day all-cause mortality |
8.43 (6.70-10.60), p<0.001 16.56 (12.58-21.79), p<0.001 5.05 (4.23-6.03), p<0.001 |
| ADHERE6 | 2015 | Registry | Any of the following criteria: use of IV inotropes or vasodilators; mechanical support including ventilator, dialysis, IABP or LVAD; or an ICU stay during the index hospitalization Early WHF: occurred during day 1 of hospitalization Late WHF: occurred after day 1 of hospitalization |
Early WHF vs Late WHF 30 day mortality 1 year mortality 30 day all-cause readmission 1 year all-cause readmission 30 day HF readmission 1 year HF readmission 30 day Medicare payments 1 year Medicare payments |
Hazard Ratio (99% CI) 0.69 (0.57-0.83), p<0.001 0.84 (0.75-0.94), p<0.001 1.04 (0.91-1.20), p=0.44 1.08(1.01-1.16), p=0.003 0.95 (0.75-1.19), p=0.54 0.99 (0.86-1.13), p=0.81 Cost Ratio (99% CI) 1.09(0.94-1.28), p=0.14 1.26 (1.16-1.37), p<0.001 |
Abbreviations: CPAP continuous positive airway pressure, HF heart failure, IABP intra-aortic balloon pump, ICU intensive care unit, IV intravenous, LVAD left ventricular assist device, MCS mechanical circulatory support, RF renal failure, WHF worsening heart failure
Extending the study of worsening heart failure out of the clinical trial space in order to provide real-world data from patients in the United States, worsening heart failure was recently examined in the Acute Decompensated Heart Failure National Registry (ADHERE).31 This analysis confirmed the findings of increased all-cause mortality at 30 days, and also found an increased mortality at 1 year. In addition to the mortality findings, there was an increased risk of all-cause and heart failure readmissions at 30 days and 1 year. Using Medicare claims data the investigators also examined the financial implications of worsening heart failure. Because of increased readmissions for patients who experience worsening heart failure, it is not surprising that after discharge from the index hospitalization, post-discharge Medicare payments were shown to be higher at 30 days and 1 year for patients with worsening heart failure compared to those without worsening heart failure.31
In an effort to further examine the condition of worsening heart failure, several analyses examined timing of worsening heart failure. Two studies, one using data from ASCEND-HF and one using data from PROTECT, stratified worsening heart failure by whether it occurred before day four of hospitalization or after that time.7,30 There was no difference between timing of worsening heart failure and outcomes in these studies. A study using data from ADHERE categorized the timing of worsening heart failure differently. In this study, early in-hospital worsening heart failure was defined as occurring during the first hospital day, and late worsening heart failure occurring after the first inpatient day. 6 When defined by these time point, early worsening heart failure was associated with lower all-cause mortality but similar all-cause and heart failure rehospitalizations at 30 days and 1 year, compared to late in-hospital worsening heart failure.
As worsening heart failure is defined by the need for escalation of therapy, one study delineated therapy as high intensity (initiation of inotropes, vasopressors, or inodilators; or initiation of mechanical support including circulatory support, ventilator support, or ultrafiltration) or low intensity (increasing diuretics or initiating vasodilators).30 There was no difference between the groups in the risk of death or hospitalization at 60 days or death at 180 days.
Treatment of Worsening Heart Failure
While many trials have included worsening heart failure as an endpoint, most treatments have not been shown to be associated with decreased worsening heart failure. Compared to placebo, tezosentan was not associated with decreased incidence of worsening heart failure (Odds Ratio: 0.99, CI: 0.92-1.21), nor was rollofylline (OR: 1.13, CI: 0.90-1.42). Similarly, in ASCEND-HF, the composite endpoint of worsening heart failure or death during index hospitalization was similar for the nesiritide group (4.2%) and placebo group (4.8%).
Two drugs have shown an improvement in worsening heart failure in adequately powered clinical trials—levosimendan and serelaxin. In the REVIVE trials, the placebo groups had more patients classified as worsening clinical course compared to the levosimendan groups. However, this study noted serious adverse events associated with the drug. Levosimendan has not been approved by the FDA for an indication in the acute decompensated heart failure patient population; however, this drug is currently being studied for use in patients with reduced left ventricular ejection fraction undergoing cardiac surgery (ClinicalTrials.gov NCT 02025621). In the RELAX-AHF trial, treatment with serelaxin decreased worsening heart failure through day 5 (Hazard Ratio: 0.3, 95% CI: 0.1-0.4) and day 14 (HR: 0.7, 95% CI: 0.51-0.96).19 The worsening heart failure endpoint was exploratory and not prepecified. When serelaxin was evaluated by the FDA in 2014, the FDA report noted that the endpoint of worsening heart failure was not well characterized in the RELAX-AHF trial.24 A larger trial of serelaxin is ongoing, with worsening heart failure as a prespecified secondary endpoint. (ClinicalTrials.gov NCT NCT01870778).
Prediction of Worsening Heart Failure
The frequent occurrence of worsening heart failure in patients hospitalized with acute heart failure, and the association of worsening heart failure with poor outcomes including rehospitalization and death, has motivated the development of worsening heart failure as an important outcome in the care of patients with acute heart failure and highlighted the importance of prevention of worsening heart failure in these patients. Using clinical trials and registries, several groups have developed prediction models for the development of worsening heart failure (Table 3). Predictors of death, heart failure rehospitalization, or worsening heart failure at 7 days in PROTECT and validated in VERITAS include blood urea nitrogen, albumin, cholesterol, as well as heart rate, systolic blood pressure, and respiratory rate, with blood urea nitrogen being the strongest predictor. Using patients from both PROTECT and RELAX-AHF, predictors of death or worsening heart failure through day 5 included blood urea nitrogen, hematocrit, respiratory rate and systolic blood pressure. A risk model developed in ADHERE and validated in ASCEND, found several variables that were independently associated with the development of in-hospital worsening heart failure, including age, heart rate, systolic blood pressure, left ventricular ejection fraction, and the laboratory values of brain natriuretic peptide, troponin, sodium, blood urea nitrogen and creatinine. The strongest predictors were troponin and creatinine. Taken together, it appears that renal dysfunction, cardiac injury, and markers of decompensation, all predict worse outcomes.
Table 3.
Prediction Models for Worsening Heart Failure
| Trial | Year | Outcome | Predictors | C-statistic | External Validation (C-statistic) |
|---|---|---|---|---|---|
| PROTECT and RELAX-AHF29 | 2015 | Death or WHF through day 5 | higher blood urea nitrogen respiratory rate hematocrit systolic blood pressure |
0.67 | |
| PROTECT39 | 2012 | Death, heart failure rehospitalization, or WHF through day 7 |
higher blood urea nitrogen lower serum albumin lower serum cholesterol lower systolic blood pressure higher heart rate higher respiratory rate |
0.67 | VERITAS (0.67) |
| ADHERE40 | 2015 | In-hospital WHF | age heart rate systolic blood pressure left ventricular ejection fraction brain natriuretic peptide troponin (positive or negative) sodium blood urea nitrogen creatinine |
0.74 | ASCEND (0.63) |
Abbreviations: WHF worsening heart failure
Developing Therapeutics and Treatment Strategies that Decrease Worsening Heart Failure
From the first use of worsening heart failure as an endpoint in 2004, there is now a robust body of evidence linking worsening heart failure with poor future outcomes. This endpoint may offer significant advantage over other symptom-based and clinical endpoints.32,33
Acute heart failure is currently recognized as a heterogeneous disorder characterized by symptoms, typically dyspnea, and findings of congestion and/or poor perfusion that clearly represent a deviation from the typical journey of a patient with chronic heart failure (Figure 1). The reasons for this remain incompletely understood but therapeutics and treatment strategies are focused on restoring the clinical status of the patient, i.e., relieving symptoms and improving congestion and perfusion. Inhospital worsening heart failure represents a failure of initial treatment strategies to achieve all of these clinical goals and is characterized by increased level of care, either medical therapy or mechanical circulatory support. While clinical judgment is necessary for changing a level of care this clearly-documented and measurable event is representative of the many facets of the heart failure syndrome and incorporates resource utilization, an often overlooked aspect of clinical care.
We believe worsening heart failure may have advantages over other acute heart failure endpoints, such as dyspnea. There are challenges with standardizing dyspnea measurements, and furthermore, while dyspnea is a patient-centered outcome it only represents one aspect of the acute heart failure syndrome.34 As worsening heart failure continues to be incorporated into clinical trials, we must recognize that a standardized definition is required to compare results across trials.18,22 Given the continued emphasis on pragmatic clinical trials, this standardized definition of worsening heart failure should be simple and easily ascertained from electronic health records.35
Summary
In-hospital worsening heart failure occurs when a patient hospitalized for treatment of acute heart failure experiences a worsening of their condition while in the hospital, requiring escalation of therapy. In-hospital worsening heart failure is associated with a worse prognosis, including worse inhospital outcomes, increased short- and long-term post-discharge mortality, increased readmissions, and higher healthcare spending. Renal dysfunction, cardiac injury, and markers of decompensation are the strongest predictors of worsening heart failure. No drugs have been approved by the FDA for the prevention of worsening heart failure, but trials are ongoing. In acute heart failure trials, worsening heart failure has advantages over other endpoints commonly used in acute and chronic heart failure trials, such as dyspnea relief and mortality or rehospitalization, and should continue to be used as an endpoint in these trials. We must continue to test and evaluate treatment strategies and therapeutics for acute heart failure and we believe there is substantial evidence to support worsening heart failure as a trial endpoint in these clinical trials.
Key Points.
In-hospital worsening heart failure is an increasingly important endpoint in trials of acute heart failure.
In-hospital worsening heart failure is associated with increased short and long-term mortality, increased rehospitalization, and increased health care costs.
Renal dysfunction and cardiac dysfunction predict worsening heart failure in patients hospitalized with acute heart failure.
A standardized definition of worsening heart failure should be established for use in future clinical trials.
Acknowledgments
Funding Source: Dr Cooper was supported by grant T32HL069749-11A1 from the National Institutes of Health.
Disclosures: Dr Cooper reports receiving research support from Novartis. Dr DeVore reports receiving research support from Amgen, the American Heart Association, Novartis, Maquet, and Thoratec, and serving as a consultant for Maquet. Dr Felker reports receiving grant support from the National Heart, Lung, and Blood Institute, Novartis, Roche Diagnostics, Otsuka, and Amgen, and serving as a consulting for Trevena, Amgen, Novartis, Celladon, Sorbent, Bristol-Myers Squibb, Singlulex, St. Jude Medical, and Medtronic.
Abbreviations
- ADHERE
Acute Decompensated Heart Failure National Registry
- ASCEND-HF
Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure
- BLAST-AHF
Biased Ligand of the Angiotensin Receptor Study in Acute Heart Failure
- DOSE
Diuretic Strategies in Patients with Acute Decompensated Heart Failure
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- PROTECT
Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function
- RELAX-AHF
Relaxin for the Treatment of Acute Heart Failure
- REVIVE
Randomized Evaluation of Intravenous Levosimendan Efficacy
- ROSE-AHF
Renal Optimization Strategies Evaluation in Acute Heart Failure
- TRUE-AHF
Trial to Evaluate the Efficacy and Safety of Ularitide Intravenous Infusion in Patients Suffering from Acute Decompensated Heart Failure
- VERITAS
Value of Endothelin Receptor Inhibition With Tezosentan in Acute Heart Failure Studies
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, or the National Institutes of Health.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2015 Update: A Report From the American Heart Association. Circulation. 2014 doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Ramirez A, Abelmann WH. Cardiac decompensation. The New England journal of medicine. 1974;290(9):499–501. doi: 10.1056/NEJM197402282900906. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 4.Solomon SD, Dobson J, Pocock S, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116(13):1482–1487. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 5.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. American heart journal. 2007;154(2):260–266. doi: 10.1016/j.ahj.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 6.Cooper LB, Hammill BG, Sharma PP, et al. Differences in Health Care Utilization and Outcomes Based on Timing of In-Hospital Worsening Heart Failure. Circulation: Cardiovascular Quality and Outcomes. 2015;8(Suppl 2):A324. [Google Scholar]
- 7.Kelly JP, Mentz RJ, Hasselblad V, et al. Worsening heart failure during hospitalization for acute heart failure: Insights from the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF). American heart journal. 2015 doi: 10.1016/j.ahj.2015.04.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotter G, Kaluski E, Stangl K, et al. The hemodynamic and neurohormonal effects of low doses of tezosentan (an endothelin A/B receptor antagonist) in patients with acute heart failure. European journal of heart failure. 2004;6(5):601–609. doi: 10.1016/j.ejheart.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Weatherley BD, Milo-Cotter O, Felker GM, et al. Early worsening heart failure in patients admitted with acute heart failure--a new outcome measure associated with long-term prognosis? Fundamental & clinical pharmacology. 2009;23(5):633–639. doi: 10.1111/j.1472-8206.2009.00697.x. [DOI] [PubMed] [Google Scholar]
- 10.Torre-Amione G, Milo-Cotter O, Kaluski E, et al. Early worsening heart failure in patients admitted for acute heart failure: time course, hemodynamic predictors, and outcome. Journal of cardiac failure. 2009;15(8):639–644. doi: 10.1016/j.cardfail.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Teerlink JR, McMurray JJ, Bourge RC, et al. Tezosentan in patients with acute heart failure: design of the Value of Endothelin Receptor Inhibition with Tezosentan in Acute heart failure Study (VERITAS). American heart journal. 2005;150(1):46–53. doi: 10.1016/j.ahj.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 12.McMurray JJ, Teerlink JR, Cotter G, et al. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. JAMA : the journal of the American Medical Association. 2007;298(17):2009–2019. doi: 10.1001/jama.298.17.2009. [DOI] [PubMed] [Google Scholar]
- 13.Massie BM, O'Connor CM, Metra M, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. The New England journal of medicine. 2010;363(15):1419–1428. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez AF, O'Connor CM, Starling RC, et al. Rationale and design of the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND-HF). American heart journal. 2009;157(2):271–277. doi: 10.1016/j.ahj.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 15.O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. The New England journal of medicine. 2011;365(1):32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 16.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. The New England journal of medicine. 2011;364(9):797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Packer M, Colucci W, Fisher L, et al. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC. Heart failure. 2013;1(2):103–111. doi: 10.1016/j.jchf.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Ponikowski P, Metra M, Teerlink JR, et al. Design of the RELAXin in acute heart failure study. American heart journal. 2012;163(2):149–155. e141. doi: 10.1016/j.ahj.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Teerlink JR, Cotter G, Davison BA, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381(9860):29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 20.Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA : the journal of the American Medical Association. 2013;310(23):2533–2543. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felker GM, Butler J, Collins SP, et al. Heart failure therapeutics on the basis of a biased ligand of the angiotensin-2 type 1 receptor. Rationale and design of the BLAST-AHF study (Biased Ligand of the Angiotensin Receptor Study in Acute Heart Failure). JACC. Heart failure. 2015;3(3):193–201. doi: 10.1016/j.jchf.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Anker SD, Ponikowski P, Mitrovic V, Peacock WF, Filippatos G. Ularitide for the treatment of acute decompensated heart failure: from preclinical to clinical studies. European heart journal. 2015;36(12):715–723. doi: 10.1093/eurheartj/ehu484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agency EM, editor. Guideline on clinical investigation of medicinal products for the treatment of acute heart failure. London: United Kingdom: 2015. [Google Scholar]
- 24.FDA Briefing Document. Cardiovascular and Renal Drugs Advisory Committee Meeting; FDA. March 27, 2014.2014. [Google Scholar]
- 25.Cotter G, Dittrich HC, Davison Weatherley B, et al. The PROTECT Pilot Study: A Randomized, Placebo-Controlled, Dose-Finding Study of the Adenosine A1 Receptor Antagonist Rolofylline in Patients With Acute Heart Failure and Renal Impairment. Journal of cardiac failure. 2008;14(8):631–640. doi: 10.1016/j.cardfail.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Cotter G, Metra M, Davison BA, et al. Worsening heart failure, a critical event during hospital admission for acute heart failure: results from the VERITAS study. European journal of heart failure. 2014;16(12):1362–1371. doi: 10.1002/ejhf.186. [DOI] [PubMed] [Google Scholar]
- 27.Metra M, O'Connor CM, Davison BA, et al. Early dyspnoea relief in acute heart failure: prevalence, association with mortality, and effect of rolofylline in the PROTECT Study. 322011 doi: 10.1093/eurheartj/ehr042. [DOI] [PubMed] [Google Scholar]
- 28.Metra M, Teerlink JR, Felker GM, et al. Dyspnoea and worsening heart failure in patients with acute heart failure: results from the Pre-RELAX-AHF study. European journal of heart failure. 2010;12(10):1130–1139. doi: 10.1093/eurjhf/hfq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davison BA, Metra M, Cotter G, et al. Worsening Heart Failure Following Admission for Acute Heart Failure: A Pooled Analysis of the PROTECT and RELAX-AHF Studies. JACC. Heart failure. 2015;3(5):395–403. doi: 10.1016/j.jchf.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Mentz RJ, Metra M, Cotter G, et al. Early vs. late worsening heart failure during acute heart failure hospitalization: insights from the PROTECT trial. European journal of heart failure. 2015 doi: 10.1002/ejhf.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeVore AD, Hammill BG, Sharma PP, et al. In-hospital worsening heart failure and associations with mortality, readmission, and healthcare utilization. Journal of the American Heart Association. 2014;3(4) doi: 10.1161/JAHA.114.001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen LA, Hernandez AF, O'Connor CM, Felker GM. End points for clinical trials in acute heart failure syndromes. Journal of the American College of Cardiology. 2009;53(24):2248–2258. doi: 10.1016/j.jacc.2008.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felker GM, Pang PS, Adams KF, et al. Clinical trials of pharmacological therapies in acute heart failure syndromes: lessons learned and directions forward. Circulation. Heart failure. 2010;3(2):314–325. doi: 10.1161/CIRCHEARTFAILURE.109.893222. [DOI] [PubMed] [Google Scholar]
- 34.Gheorghiade M, Adams KF, Cleland JG, et al. Phase III clinical trial end points in acute heart failure syndromes: a virtual roundtable with the Acute Heart Failure Syndromes International Working Group. American heart journal. 2009;157(6):957–970. doi: 10.1016/j.ahj.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Butler J, Fonarow GC, O'Connor C, et al. Improving cardiovascular clinical trials conduct in the United States: recommendation from clinicians, researchers, sponsors, and regulators. American heart journal. 2015;169(3):305–314. doi: 10.1016/j.ahj.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Teerlink JR, Metra M, Felker GM, et al. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet. 2009;373(9673):1429–1439. doi: 10.1016/S0140-6736(09)60622-X. [DOI] [PubMed] [Google Scholar]
- 37.Cotter G, Metra M, Weatherley BD, et al. Physician-determined worsening heart failure: a novel definition for early worsening heart failure in patients hospitalized for acute heart failure--association with signs and symptoms, hospitalization duration, and 60-day outcomes. Cardiology. 2010;115(1):29–36. doi: 10.1159/000249280. [DOI] [PubMed] [Google Scholar]
- 38.Metra M, Cotter G, Davison BA, et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. Journal of the American College of Cardiology. 2013;61(2):196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 39.O'Connor CM, Mentz RJ, Cotter G, et al. The PROTECT in-hospital risk model: 7-day outcome in patients hospitalized with acute heart failure and renal dysfunction. European journal of heart failure. 2012;14(6):605–612. doi: 10.1093/eurjhf/hfs029. [DOI] [PubMed] [Google Scholar]
- 40.DeVore AD, Greiner MA, Sharma PP, et al. The ADHERE Risk Model for In-hospital Worsening Heart Failure. Circulation: Cardiovascular Quality and Outcomes. 2015;8(Suppl 2) [Google Scholar]