Abstract
Edoxaban, an oral direct inhibitor of factor Xa, was recently approved in the United States and Japan for prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation and for treatment of venous thromboembolism (VTE). It is also licensed in Japan for prevention of VTE after major orthopedic surgery. Although routine laboratory monitoring of edoxaban is not required, laboratory measurement may be desirable in special circumstances. Our objective was to provide a systematic review of current evidence on laboratory measurement of the anticoagulant activity of edoxaban. PubMed and the Cochrane Library were searched for studies that reported a relationship between coagulation tests and plasma edoxaban levels. Study quality was assessed using Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2). We identified 9 eligible studies. Anti-Xa activity is linear across a broad range of drug levels (R2>0.95) and may be used for edoxaban quantification. The assay shows greater variability at above on-therapy drug concentrations. The PT is less sensitive to edoxaban. A normal prothrombin time may not exclude clinically relevant on-therapy drug levels. The activated partial thromboplastin time has insufficient sensitivity to edoxaban for measurement of its anticoagulant activity. Edoxaban exhibits variable effects on coagulation assays. Understanding these effects facilitates interpretation of test results in edoxaban-treated patients. More data on the relationship between drug levels, coagulation test results, and clinical outcomes in patients are needed.
Keywords: Activated partial thromboplastin time (APTT), Anti-Xa, Edoxaban, Monitoring, Prothrombin time (PT)
Introduction
Edoxaban (DU-176b, Lixiana™, Savaysa™), an oral direct inhibitor of factor Xa, was recently approved in the United States and Japan for prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation (NVAF) and for treatment of venous thromboembolism (VTE) on the basis of two large phase III clinical trials [1, 2]. Edoxaban is also licensed for prevention of VTE following total knee and hip arthroplasty in Japan.
Edoxaban has an oral bioavailability of approximately 60 %; 35 % of ingested drug is excreted by the kidneys. It exhibits linear pharmacokinetics with a terminal half-life of 8–10 h. Peak plasma concentration is achieved 1–2 h after ingestion [3, 4]. Edoxaban has been studied as a once daily drug at a dose of 30 or 60 mg in phase III clinical trials [1, 2]. In a pharmacokinetic analysis involving predominantly Caucasian patients with NVAF, the interquartile range for steady-state peak and trough levels was approximately 60–120 and 5–20 ng/mL for subjects receiving edoxaban 30 mg daily and 120–250 and 10–40 ng/mL for subjects receiving 60 mg daily [5]. Exposure was somewhat greater in Asian patients with NVAF [6].
Unlike warfarin and other vitamin K antagonists, edoxaban was administered in fixed doses and did not require routine laboratory monitoring or dose adjustment in phase III clinical trials [1, 2]. However, measurement of the anticoagulant activity of edoxaban may be advantageous in special situations including bleeding, the preoperative state, treatment failure, suspected non-compliance or overdose, renal insufficiency, advanced age, extremes of body weight and drug interactions. A systematic review on the laboratory measurement of dabigatran, rivaroxaban, and apixaban was recently published [7]. The purpose of this review is to summarize published evidence on laboratory measurement of the anticoagulant activity of edoxaban, to provide guidance to clinicians on the interpretation of coagulation tests in edoxaban-treated patients, and to highlight areas where further research is needed.
Methods
Literature search
A search of PubMed and the Cochrane Library from inception through October 31, 2014 was undertaken using the following keyword search: [(monitoring OR measurement OR laboratory OR prothrombin time OR partial thromboplastin time OR activated partial thromboplastin time OR PT OR aptt OR anti-xa OR anti factor xa) AND (edoxaban OR DU-176b OR Lixiana OR Savaysa)].
Study selection
Articles were examined by one reviewer, first by title and abstract and then by review of the complete manuscript as indicated. Additional articles were sought by reviewing references of eligible studies and by contacting the manufacturer of edoxaban (Daiichi Sankyo, Parsippany, NJ). Liquid chromatography/tandem mass spectrometry (LC–MS/MS) is the reference method for measurement of plasma edoxaban concentration. Studies that reported a relationship between drug levels in human plasma, as measured directly by LC–MS/MS or by preparation of stock solutions of edoxaban of known concentration, and one or more commercially available coagulation assays, were eligible for inclusion. Animal studies, abstract-only and non-English language publications were excluded.
Data extraction
Key characteristics of eligible studies were extracted by one reviewer and recorded in an evidence table. These included author, year of publication, reference method for measurement of edoxaban levels, range of edoxaban concentrations studied, test material (i.e. spiked normal plasma, spiked patient plasma, ex vivo normal plasma, ex vivo patient plasma), dose (for studies using ex vivo plasma), indication (for studies using ex vivo patient plasma), coagulation assays and reagents, and descriptors of the relationship between drug level and coagulation assay.
Quality assessment
Quality of eligible studies was evaluated by two reviewers using Quality Assessment of Diagnostic Accuracy Studies, version 2 (QUADAS-2) [8]. QUADAS-2 is a standardized tool for quality assessment of studies of diagnostic accuracy. The tool includes four domains: patient selection, index test, reference standard, and flow and timing. Clinical applicability is assessed across the first 3 domains. Risk of bias is assessed across all domains. Disagreements between reviewers were resolved by discussion and consensus.
Results
Study selection
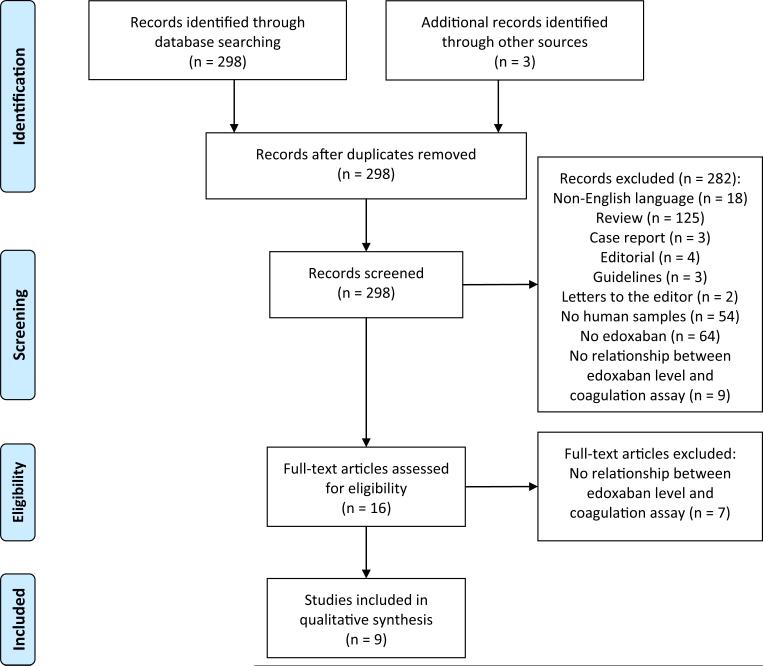
Results of the literature search are summarized in Fig. 1 [9]. A total of 298 articles were identified in the database query. Three duplicate articles were identified by the manufacturer. Bibliography review did not yield additional articles. A total of 289 articles were excluded: 18 were non-English language, 137 were not research studies, 54 did not involve human samples, 64 did not involve edoxaban, and 16 did not report a relationship between drug levels and one or more coagulation assays. The remaining nine articles met eligibility criteria [4, 10–17]. Collectively, eligible studies were conducted across a range of edoxaban concentrations from 0 to >1200 ng/mL. All eligible studies used either ex vivo plasma from edoxaban-treated healthy volunteers or normal plasma spiked in vitro with edoxaban. No studies used patient plasma as the test material (Table 1).
Fig. 1.
PRISMA diagram. This PRISMA (Preferred Reporting Items of Systematic Reviews and Meta-Analyses) flow diagram illustrates the results of the literature search [9]
Table 1.
Study characteristics
| Reference | Range of drug concentration (ng/mL) | Test material | Dose (mg) | Coagulation test (reagent) | R 2 |
|---|---|---|---|---|---|
| Zafar et al. 2007 [10] | 34 to 256 | Ex vivo healthy volunteer plasma | 60 SD | APTT (NR) | 0.40 |
| PT (NR) | 0.79 | ||||
| Anti-Xa (STA-Rotachrom) | 0.85 | ||||
| Furugohri et al. 2008 [11] | NR | Spiked normal plasma | NA | APTT (Platelin LS) | NR |
| PT (Thromboplastin C Plus) | NR | ||||
| TT (Calbiochem human thrombin) | NR | ||||
| Anti-Xa (Chromogenix) | NR | ||||
| Ogata et al. 2010 [4] | 0 to > 1200 | Ex vivo healthy volunteer plasma | Variousa | APTT (Hemochron Jr.) | 0.89 |
| PT (NR) | 0.94–0.96 | ||||
| Anti-Xa (NR) | 0.96 | ||||
| Mendell et al. 2011 [12] | 9 to 567 | Ex vivo healthy volunteer plasma | 60 SD | APTT (NR) | NR |
| PT (NR) | NR | ||||
| Wolzt et al. 2011 [13] | 0 to > 600 | Ex vivo healthy volunteer plasma | 30, 60, or 120 SD | APTT (STA aPTT) | 0.74 |
| PT (STA Neoplastin Plus) | 0.96 | ||||
| Anti-Xa (STA-Rotachrom) | 0.96 | ||||
| Fukuda et al. 2012 [14] | 150 to 300 | Spiked normal plasma | NA | PT (Thromboplastin C Plus) | NR |
| Samama et al. 2012 [15] | 0 to 500 | Spiked normal plasma | NA | APTT (PTT-A) | NR |
| APTT (CK-Prest) | NR | ||||
| APTT (PTT-LA) | NR | ||||
| APTT (Actin FS) | NR | ||||
| PT (Neoplastin) | NR | ||||
| PT (Neoplastin Plus) | NR | ||||
| PT (TriniCLOT) | NR | ||||
| PT (RecombiPlasTin) | NR | ||||
| PT (Innovin) | NR | ||||
| PT (Thromborel S) | NR | ||||
| Anti-Xa (Rotachrom HBPM) | 0.99 | ||||
| Anti-Xa (Factor Xa inhibitors) | 0.99 | ||||
| PiCT (PiCT activator reagent) | NR | ||||
| DRVVT (American Diagnostica) | 0.97 | ||||
| Mendell et al. 2013 [16] | NR | Ex vivo healthy volunteer plasma | 60 QD × 5 days | Anti-Xa (STA-Rotachrom) | >0.95 |
| Noguchi et al. 2014 [17] | 0 to 720 | Spiked normal plasma | NA | APTT (Actin-activated cephaloplastin) | NR |
| PT (Thromborel S) | NR |
APTT activated partial thromboplastin time, DRVVT dilute Russell viper venom time, INR international normalized ratio, NA not applicable, NR not reported, PiCT prothrombinase-induced clotting time, PT prothrombin time, SD single dose, TT thrombin time
10, 30, 60, 90, 120, 150 SD; 90, 120 QD × 10 days; 60 BID × 10 days
Activated partial thromboplastin time
Seven studies reported a relationship between the activated partial thromboplastin time (APTT) and edoxaban levels (Table 1) [4, 10–13, 15, 17]. One study compared four different APTT reagents [15]. Edoxaban prolonged the APTT in a dose-dependent manner in both in vitro and ex vivo studies. Correlation between APTT results and drug levels was modest with R2 values ranging between 0.40 and 0.89. The APTT was relatively insensitive to on-therapy levels of edoxaban. The mean APTT increased by only 21 and 4 % at 1.5 and 12 h, respectively, after ingestion of a single 60 mg dose [10]. In an in vitro study of normal spiked plasma, an edoxaban concentration of 500 ng/mL (well above the peak levels of 120–250 ng/mL noted in patients taking edoxaban 60 mg daily [5] ) was required to double the APTT from baseline [15].
Prothrombin time/international normalized ratio
Eight eligible studies reported a relationship between prothrombin time (PT) and plasma edoxaban concentration (Table 1) [4, 10–15, 17]. Edoxaban prolonged the PT in a dose-dependent, linear fashion with R2 values of 0.79–0.96. The PT was more sensitive than the APTT to edoxaban, but still exhibited insufficient sensitivity at low on-therapy drug levels. At peak levels of edoxaban 1.5 h after ingestion of a single 30 or 60 mg dose, the PT increased by 25 and 40–50 %, respectively [10, 12, 13]. However, the PT 12 h after a 60 mg dose was only 10 % above baseline [10]. Normal plasma needed to be spiked to an edoxaban concentration greater than 200 ng/mL to achieve a doubling of the baseline PT [14].
Different thromboplastin reagents varied in their sensitivity to edoxaban. One study compared the sensitivity of 6 different PT reagents [15]. At a plasma concentration of 500 ng/mL, the most sensitive reagent was associated with a 150 % increase in the PT from baseline whereas the least sensitive reagent was associated with only a 70 % increase. Correction of the PT ratio using the reagent-specific warfarin international sensitivity index and expression as an international normalized ratio did not reduce variability between reagents [15].
Anti-Xa activity
A relationship between edoxaban levels and anti-Xa activity was reported in 6 studies (Table 1) [4, 10, 11, 13, 15, 16]. Anti-Xa activity increased in a concentration-dependent linear fashion. The R2 value exceeded 0.95 in 4 of 5 studies that reported a coefficient of determination. Measurable anti-Xa activity was observed with low doses of edoxaban. Peak activity after ingestion of a 10 mg single dose was 0.4 IU/mL [4]. Greater variability in anti-Xa activity was observed at plasma edoxaban levels above 200–300 ng/mL [4, 13]. In most eligible studies, low molecular weight heparin standards were used for calibration of the anti-Xa assay. Use of edoxaban calibrators may improve linearity and enhance accuracy of quantification at high edoxaban concentrations.
Other assays
One study each reported a relationship between edoxaban levels and the thrombin time [11], dilute Russell viper venom time (DRVVT) [15], and prothrombinase-induced clotting time (PiCT) (Table 1) [15]. Edoxaban is a weak inhibitor of thrombin (it is a >10,000-fold more potent inhibitor of factor Xa than thrombin) and prolonged the thrombin time at suprapharmacologic but not on-therapy concentrations [11]. Russell viper venom is a direct activator of factor X. It is used to initiate coagulation in the DRVVT and PiCT assays. In an in vitro study, both the DRVVT and PiCT showed a curvilinear response to edoxaban with flattening of the dose–response curve at concentrations above 250 ng/mL.
Study quality
Evaluation of study quality using QUADAS-2 [8] revealed several recurrent methodological concerns among eligible studies (Table 2). All studies used spiked or ex vivo plasma from controls (mostly young healthy male volunteers) rather than patient plasma. Concern about the applicability of these studies to clinical practice within the patient selection domain was judged to be high. Because there is a paucity of data on the relationship between edoxaban levels and clinical outcomes, concern about the applicability of the reference standard (plasma drug levels measured by LC–MS/MS) was judged to be high for all eligible studies.
Table 2.
Study quality assessment using QUADAS-2 criteria
| Reference | Risk of bias |
Applicability concerns |
|||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Zafar et al. 2007 [10] | NA | U | U | L | H | L | H |
| Furugohri et al. 2008 [11] | NA | U | U | L | H | L | H |
| Ogata et al. 2010 [4] | NA | U | U | L | H | L | H |
| Mendell et al. 2011 [12] | NA | U | U | L | H | L | H |
| Wolzt et al. 2011 [13] | NA | U | U | L | H | L | H |
| Fukuda et al. 2012 [14] | NA | U | U | L | H | L | H |
| Samama et al. 2012 [15] | NA | U | U | L | H | L | H |
| Mendell et al. 2013 [16] | NA | L | L | L | H | L | H |
| Noguchi et al. 2014 [17] | NA | U | U | L | H | L | H |
H indicates high, L low, NA not applicable, U unclear
Discussion
The aim of this systematic review was to examine evidence for laboratory measurement of the anticoagulant activity of edoxaban. As yet, there are scant data on the relationship between plasma edoxaban levels and clinical outcomes [5] and no evidence to support routine monitoring and dose adjustment. Nevertheless, measurement may be useful in three circumstances: (1) to determine if any clinically relevant anticoagulant effect is present (e.g. in patients who are bleeding or awaiting an invasive procedure); (2) to determine if drug is present in typical on-therapy concentrations (e.g. in patients with suspected break-through thrombosis); and (3) to determine if drug is present in excess (e.g. in patients with suspected overdose or bioaccumulation). To be useful in all three of these circumstances, an ideal assay would need to exhibit sufficient sensitivity and linearity to permit quantification across a broad range of edoxaban levels. Aside from LC–MS/MS, no single coagulation assay satisfies these idealized criteria. Because of the limited availability of LC–MS/MS, it is important for clinicians to be familiar with how more widely accessible coagulation assays are influenced by edoxaban concentrations below, within, and above typical on-therapy levels.
Anti-Xa activity
Anti-Xa activity is the preferred alternative to LC–MS/MS for measurement of edoxaban. Anti-Xa activity measured using chromogenic substrates correlates linearly with edoxaban over a broad range of concentrations from typical trough to peak levels. The assay is more variable in measuring levels above the on-therapy range (>200–300 ng/mL). More data are needed to determine whether use of edoxaban calibrators may permit better quantitation of high drug levels compared with low molecular weight heparin standards.
Prothrombin time
The PT increases in a linear fashion with plasma edoxaban levels across a wide range of concentrations. However, it is insufficiently sensitive to edoxaban to exclude low on-therapy levels of drug. Thus, while a prolonged PT indicates on-therapy or above on-therapy edoxaban levels, a normal PT does not rule out clinically significant anticoagulant activity. The sensitivity of the PT to edoxaban varies based on thromboplastin reagent. When edoxaban calibration standards become available, it is recommended that coagulation laboratories perform dose–response studies to determine the sensitivity of their PT method.
Activated partial thromboplastin time
The APTT is less sensitive than the PT to edoxaban. The APTT should therefore not be used to measure the anticoagulant activity of edoxaban nor should a normal APTT be considered evidence of the absence of clinically significant plasma edoxaban levels.
Suggestions and limitations
Suggestions for laboratory measurement of below, within, and above on-therapy levels of edoxaban are summarized in Table 3. These suggestions must be viewed in light of several key limitations in the evidence on which they are based. First, the on-therapy range for edoxaban is derived from pharmacokinetic studies. We avoided the term “therapeutic range” because data correlating edoxaban levels with clinical outcomes are scant. Second, all published studies used plasma from healthy volunteers rather than edoxaban-treated patients. Third, most of the eligible studies that measured anti-Xa activity used low molecular weight heparin rather than edoxaban calibrators.
Table 3.
Suggestions for laboratory measurement of edoxaban
| Clinical objective | |||||
|---|---|---|---|---|---|
| Determine whether clinically relevant below on-therapy drug levels are present |
Estimate drug levels within the on-therapy range |
Determine whether above on-therapy drug levels are present |
|||
| Suggested test | Comments | Suggested test | Comments | Suggested test | Comments |
| Anti-Xa | A normal anti-Xa likely excludes clinically relevant drug levels. A normal PT or APTT cannot be assumed to exclude clinically relevant drug levels. | Anti-Xa | Measurement of anti-Xa activity permits quantification of drug levels within the on-therapy range. | Anti-Xa, PT | Drug quantification may be less reliable with anti-Xa at above on-therapy levels. A normal PT likely excludes excess drug levels. |
APTT activated partial thromboplastin time, PT prothrombin time
Conclusions
Edoxaban has variable effects on coagulation assays. Anti-Xa assays are the optimal coagulation test for measuring edoxaban levels, but may not be accurate or reliable at above on-therapy drug concentrations. If anti-Xa measurement is not available, a prolonged PT may be considered evidence of circulating edoxaban. However, a normal PT or APTT does not exclude on-therapy drug levels. Further studies are needed in edoxaban-treated patients to define the relationship between drug levels, coagulation test results, and clinical outcomes.
Acknowledgments
This work was supported by HL112903 (National Heart Lung and Blood Institute, Bethesda, MD) to AC.
Footnotes
Conflict of interest AC has provided consulting services for Bracco and Genzyme, has participated in an advisory board for CSL Behring, and has received research support from Stago and T2 Biosystems. HH has no conflicts to disclose.
Contributor Information
Adam Cuker, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; Department of Pathology & Laboratory Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; Penn Comprehensive Hemophilia and Thrombosis Program, Hospital of the University of Pennsylvania, 3400 Spruce Street, Philadelphia, PA 19143, USA.
Holleh Husseinzadeh, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
References
- 1.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM, ENGAGE AF-TIMI 48 Investigators Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 2.Investigators Hokusai-VTE. Büller HR, Décousos H, Grosso MA, Mercuri M, Middledorp S, Prins MH, Raskob GE, Schellong SM, Schwocho L, Segers A, Shi M, Verhamme P, Wells P. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–1415. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 3.Matsushima N, Lee F, Sato T, Weiss D, Mendell J. Bioavailability and safety of the factor Xa inhibitor edoxaban and the effects of quinidine in healthy subjects. Clinical Pharm in Drug Dev. 2013;2:358–366. doi: 10.1002/cpdd.53. [DOI] [PubMed] [Google Scholar]
- 4.Ogata K, Mendell-Harary J, Tachibana M, Masumoto H, Oguma T, Kojima M, Kunitada S. Clinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel factor Xa inhibitor edoxaban in healthy volunteers. J Clin Pharmacol. 2010;50:743–753. doi: 10.1177/0091270009351883. [DOI] [PubMed] [Google Scholar]
- 5.Weitz JI, Connolly SJ, Patel I, Salazar D, Rohatagi S, Mendell J, Kastrissios H, Jin J, Kunitada S. Randomised, parallel-group, multicentre, multinational phase 2 study comparing edoxaban, an oral factor Xa inhibitor, with warfarin for stroke prevention in patients with atrial fibrillation. Thromb Haemost. 2010;104:633–641. doi: 10.1160/TH10-01-0066. [DOI] [PubMed] [Google Scholar]
- 6.Chung N, Jeon HK, Lien LM, Lai WT, Tse HF, Chung WS, Lee TH, Chen SA. Safety of edoxaban, an oral factor Xa inhibitor, in Asian patients with non-valvular atrial fibrillation. Thromb Haemost. 2010;105:535–544. doi: 10.1160/TH10-07-0451. [DOI] [PubMed] [Google Scholar]
- 7.Cuker A, Siegal DM, Crowther MA, Garcia DA. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol. 2014;64:1128–1139. doi: 10.1016/j.jacc.2014.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM, QUADAS-2 Group QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 10.Zafar MU, Vorchheimer DA, Gaztanaga J, Velez M, Yadegar D, Moreno PR, Kunitada S, Pagan J, Fuster V, Badimon JJ. Antithrombotic effects of factor Xa inhibition with DU-176b: phase-I study of an oral, direct factor Xa inhibitor using an ex vivo flow chamber. Thromb Haemost. 2007;98:883–888. doi: 10.1160/th07-04-0312. [DOI] [PubMed] [Google Scholar]
- 11.Furugohri T, Isobe K, Honda Y, Kamisato-Matsumoto C, Sugiyama N, Nagahara T, Morishima Y, Shibano T. DU-176b, a potent and orally active factor Xa inhibitor: in vitro and in vivo pharmacological profiles. J Thromb Haemost. 2008;6:1542–1549. doi: 10.1111/j.1538-7836.2008.03064.x. [DOI] [PubMed] [Google Scholar]
- 12.Mendell J, Tachibana M, Shi M, Kunitada S. Effects of food on the pharmacokinetics of edoxaban, an oral direct factor Xa inhibitor, in healthy volunteers. J Clin Pharmacol. 2011;51:687–694. doi: 10.1177/0091270010370974. [DOI] [PubMed] [Google Scholar]
- 13.Wolzt M, Samama MM, Kapiotis S, Ogata K, Mendell J, Kunitada S. Effect of edoxaban on markers of coagulation in venous and she blood compared with fondaparinux. Thromb Haemost. 2011;105:1080–1090. doi: 10.1160/TH10-11-0705. [DOI] [PubMed] [Google Scholar]
- 14.Fukada T, Honda Y, Kamisato C, Morishima Y, Shibano T. Reversal of anticoagulant effects of edoxaban, an oral, direct factor Xa inhibitor, with haemostatic agents. Thromb Haemost. 2012;107:253–259. doi: 10.1160/TH11-09-0668. [DOI] [PubMed] [Google Scholar]
- 15.Samama MM, Mendell J, Guinet C, Le Flem L, Kunitada S. In vitro study of the anticoagulant effects of edoxaban and its effect on thrombin generation in comparison to fondaparinux. Thromb Res. 2012;129:e77–e82. doi: 10.1016/j.thromres.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 16.Mendell J, Noveck RJ, Shi M. A randomized trial of the safety, pharmacokinetics and pharmacodynamics of edoxaban, an oral factor Xa inhibitor, following a switch from warfarin. Br J Clin Pharmacol. 2013;75:966–978. doi: 10.1111/j.1365-2125.2012.04409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noguchi K, Morishima Y, Takahashi S, Ishihara H, Shibano T, Murata M. Impact of nonsynonymous mutations of factor X on the functions of factor X and anticoagulant activity of edoxaban. Blood Coagul Fibrinolysis. 2015;26:117–122. doi: 10.1097/MBC.0000000000000147. [DOI] [PubMed] [Google Scholar]