Abstract
Background
The incremental effects of risk factor combinations for atrial fibrillation (AF) and stroke are incompletely understood. We sought to quantify the risks of incident AF and stroke for combinations of established risk factors in a large US sample.
Methods and Results
Patients with no evidence of AF or stroke in 2007 were stratified by combinations of the following risk factors: heart failure, hypertension, diabetes, age 65 to 74, age ≥75, coronary artery disease, and chronic kidney disease. Patients with ≥2 of the first 5 or ≥3 of the first 7, classified as “high-risk,” and an age-matched sample of patients with fewer risk factors, classified as “low-risk,” were followed over 2008–2010 for incident AF and stroke. Annualized incidence rates and risks were quantified for each combination of factors by using Cox regression. Annualized incidence rates for AF, stroke, and both were 3.59%, 3.27%, and 0.62% in 1 851 653 high-risk patients and 1.32%, 1.48%, and 0.18% in 1 156 221 low-risk patients, respectively. Among patients with 1 risk factor, those with age ≥75 had the highest hazards of incident AF and stroke (HR 9.2, 6.9). Among patients with 2 risk factors, those with age ≥75 and heart failure had the highest annualized incidence rates of AF and stroke (10.2%, 5.9%). The combination of age ≥75 and hypertension was prevalent and had the highest incidences of AF and stroke.
Conclusions
Adults with combinations of known risk factors are at increased risk of incident AF and stroke, but combinations of risk factors are not always additive.
Keywords: atrial fibrillation, epidemiology, risk factors/global assessment, risk stratification, stroke
Atrial fibrillation (AF), the most common cardiac arrhythmia, is projected to affect nearly 3 million people in the United States by 2015.1 AF increases stroke risk by 5-fold; this risk is further modified by the presence of other stroke risk factors.2 Although oral anticoagulant drugs significantly reduce the risk of stroke in patients diagnosed with AF, it is not uncommon for AF to be detected only after the occurrence of a stroke, even when symptoms likely indicative of arrhythmias are present.3–5
While risk factors and risk stratification schemas for AF6,7 and for stroke7–11 have been reported from epidemiologic data, the assessment of risk based on specific combinations of known risk factors have not been previously reported. The incremental risks of various factors in different combinations may not be additive; thus, different combinations with the same number of risk factors may confer very different total risks for the development of AF or stroke. However, analyzing a large number of potential risk factor combinations requires a very large sample size and thus is not feasible with most sources of clinical data. Therefore, the objective of our study was to use a large healthcare claims database to quantify and stratify the total and incremental risks associated with combinations of risk factors for AF and stroke in a large sample of the US population.
Methods
Study Design
We conducted a retrospective cohort study by using health care claims data from the Truven Health MarketScan Commercial and Medicare Supplemental Databases (January 2007 to December 2010). These databases represent the health services of >180 million employees, dependents, and retirees in the United States with primary or Medicare supplemental coverage through privately insured fee-for-service, point-of-service, or capitated health plans. These databases consist of fully integrated patient-level records that serve as the basis of >350 peer-reviewed articles since 2000 and are fully HIPAA compliant.12,13 In particular, these data have been used extensively for outcomes research related to AF and stroke risk.14–17 A protocol describing the study objectives, criteria for patient selection, data elements of interest, and statistical methods was submitted to the New England Institutional Review Board (NEIRB) and exemption was obtained (NEIRB No. 12-388).
Study Population
The study population consisted of all patients in the MarketScan databases with continuous medical and pharmacy enrollment for the entire baseline calendar year of 2007 and with no record of AF or stroke diagnoses in either inpatient or outpatient claims during that time (Appendix S1). For the purposes of this study, stroke diagnoses included both ischemic and hemorrhagic strokes, as well as transient ischemic attack (TIA).
The study cohort was classified according to the following 7 epidemiologically determined risk factors known to be associated with AF and/or stroke: (1) heart failure, (2) hypertension, (3) diabetes, (4) age ≥65 to 74 years, (5) age ≥75 years, (6) coronary artery disease (CAD), and (7) chronic kidney disease (CKD).18 Baseline risk factors were identified by using claims data from calendar year of 2007 (Appendix S2). Any patient with ≥2 of the first 5 risk factors or ≥3 of any of the 7 risk factors was defined as high risk for future AF or stroke and was assigned to the high-risk cohort. These 7 factors were selected based on epidemiologic data and previous risk prediction models for AF1,6,7,19–21 and for stroke.8,9,22,23 The threshold of 2 or 3 factors corresponds roughly with pre-2014 guidelines for oral anticoagulation of AF patients with high risk of stroke.24 To create a low-risk cohort for comparison, a random-number generator was used to draw samples of patients in a 1:1 ratio from among the patients with lower risk profiles, matched by age group to the high-risk cohort (Figure1).
Figure 1.
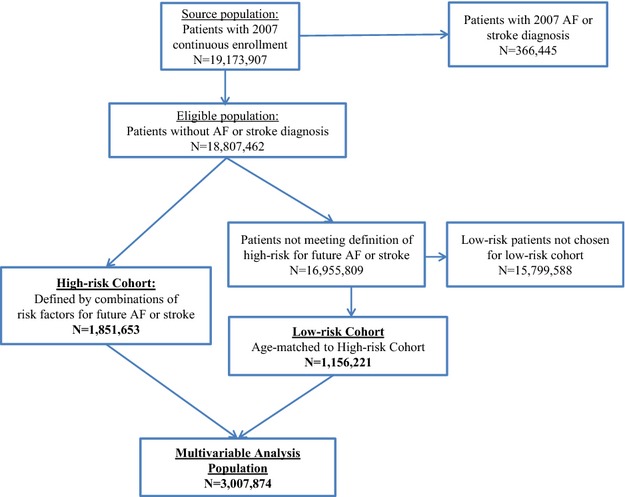
Study population. The eligible population included 18.8 million patients without AF or stroke diagnoses in 2007. From these, a high-risk cohort of 1.85 million patients and an age-matched low-risk cohort of 1.16 million patients were identified and included in our study. The multivariable analysis population included both the high-risk and the age-matched low-risk cohort, totaling ≈3.01 million patients. AF indicates atrial fibrillation.
All patients in the high-risk and age-matched low-risk cohorts were included in the multivariable analysis population (Figure1). Each patient in this population, regardless of risk cohort designation, was further classified by their distinct combination of the 7 baseline risk factors, by using a categorical variable with 96 values to represent the full spectrum of mutually exclusive and collectively exhaustive risk factor combinations.
Identification of Outcomes
The outcomes for this study were incident AF and incident stroke (ischemic, hemorrhagic, and TIA), as defined with International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes (Appendix S1). Diagnoses of AF and stroke were tracked for patients with continuous enrollment extending through any portion of calendar years 2008–2010. Incidence rates during the 3 years of follow-up were calculated and annualized for the entire population by dividing the total counts of patients with each event by the sum of patient-years to the first of either the patient’s index event or enrollment censoring date. Annualized incidence rates were calculated for the high-risk and low-risk cohorts, for each combination of sex and age group (ages 0 to 17, 18 to 34, 35 to 44, 45 to 54, 55 to 64, 65 to 74, and ≥75 years), as well as for each of the 96 risk factor combinations in the multivariable analysis population.
Statistical Analysis
We calculated hazard ratios (HRs) for incident AF and incident stroke during the 3-year follow-up period from January 1, 2008, to December 31, 2010, by using Cox regression models. All patients with enrollment through December 31, 2010, were censored as of that date. For each end point (AF or stroke/TIA), 2 models were constructed. All analyses were performed with SAS Version 9.2 (SAS Institute).
The first set of Cox regression models used individual baseline risks as predictor variables, such that HRs for each risk could be calculated, with adjustment for all remaining risks and other baseline characteristics. Values for individual baseline predictor variables were obtained from the claims databases. Predictor variables were age group, sex, geographic region, comorbid conditions (heart failure, hypertension, diabetes, CAD, CKD, and sleep apnea, identified based on ICD-9 codes and prescription medications as described in Appendix S2), symptoms (chest pain, palpitations, dizziness, tachycardia, and respiratory abnormalities, identified based on ICD-9 codes as described in Appendix S3), baseline medication use, and use of internal cardiac devices (pacemaker, implantable cardioverter-defibrillator, implantable loop recorder) or external electrocardiographic monitoring (Holter, external loop monitor, mobile cardiac outpatient telemetry). In addition to these predictors, the stroke model adjusted for oral anticoagulation use >14 days before stroke event or censoring date and for the diagnosis of incident AF before or concurrent with stroke event. Patients with none of the studied risk factors were used as the reference group.
The second set of multivariable models evaluated the incremental impact of individual risks within risk factor combinations. This was accomplished by creating Cox regression models for AF and stroke that used a single categorical predictor variable denoting each patient’s mutually exclusive and collectively exhaustive risk factor combination. Patients with none of the studied risk factors were used as the reference group for this model.
Results
Patient Population
Of the 19 173 907 patients in the source population with continuous medical and pharmacy enrollment throughout 2007 in the Truven Health MarketScan Commercial and Medicare Supplemental Databases, 366 445 (1.9%) were excluded based on a diagnosis of AF and/or stroke at baseline. From the remaining population of 18 807 462 patients, a high-risk cohort of 1 851 653 (9.8%) patients was identified. A total of 1 156 221 lower-risk patients were randomly selected from within each age group to match the age distribution of the high-risk cohort. The sampling ratio was 1:1 except in age groups ≥65 years, where age itself was a predefined risk factor and thus fewer low-risk patients were available. The combined multivariable analysis population consisted of 3 007 874 patients (Figure1). Baseline characteristics of the overall analysis population and the 2 risk cohorts are summarized in Table1. Among the high-risk patients, the most prevalent risk factors were hypertension (95.3%), diabetes (52.9%), and age ≥65 years (66.0%). Only a small proportion of patients with symptoms commonly associated with cardiac arrhythmias had external cardiac monitoring, regardless of risk cohort designation (Table1). There are additional baseline characteristics in Appendix S4.
Table 1.
Baseline Characteristics of Analysis Population: High-Risk Cohort and Low-Risk Cohort as Well as Combined Population for Multivariable Analysis
| Baseline Characteristics | High-Risk Cohort (n=1 851 653), No. (%) | Low-Risk Cohort (n=1 156 221), No. (%) | Combined Population (N=3 007 874), No. (%) |
|---|---|---|---|
| Age group, y | |||
| 0 to 17 | 1304 (0.1) | 1304 (0.1) | 2608 (0.1) |
| 18 to 34 | 20 463 (1.1) | 20 463 (1.8) | 40 926 (1.4) |
| 35 to 44 | 67 962 (3.7) | 67 962 (5.9) | 135 924 (4.5) |
| 45 to 54 | 205 934 (11.1) | 205 934 (17.8) | 411 868 (13.7) |
| 55 to 64 | 333 810 (18.0) | 333 810 (28.9) | 667 620 (22.2) |
| 65 to 74 | 643 258 (34.7) | 319 014 (27.6) | 962 272 (32.0) |
| ≥75 | 578 922 (31.3) | 207 734 (18.0) | 786 656 (26.2) |
| Sex | |||
| Male | 862 486 (46.6) | 556 212 (48.1) | 1 418 698 (47.2) |
| Female | 989 167 (53.4) | 600 009 (51.9) | 1 589 176 (52.8) |
| Comorbid conditions used for risk stratification | |||
| Heart failure | 138 746 (7.5) | 505 (0.0) | 139 251 (4.6) |
| Hypertension | 1 764 318 (95.3) | 186 868 (16.2) | 1 951 186 (64.9) |
| Diabetes | 978 724 (52.9) | 28 295 (2.5) | 1 007 019 (33.5) |
| Coronary artery disease | 331 185 (17.9) | 43 649 (3.8) | 374 834 (12.5) |
| Chronic kidney disease | 93 034 (5.0) | 6058 (0.5) | 99 092 (3.3) |
| Comorbid condition not used for risk stratification | |||
| Sleep apnea | 107 000 (5.8) | 26 691 (2.3) | 133 691 (4.4) |
| Symptoms | |||
| Any symptom | 517 646 (28.0) | 153 721 (13.3) | 671 367 (22.3) |
| Chest pain | 312 300 (16.9) | 86 924 (7.5) | 399 224 (13.3) |
| Dizziness | 87 437 (4.7) | 27 982 (2.4) | 115 419 (3.8) |
| Palpitations | 49 033 (2.7) | 17 374 (1.5) | 66 407 (2.2) |
| Tachycardia unspecified | 15 641 (0.8) | 3295 (0.3) | 18 936 (0.6) |
| Shortness of breath | 165 614 (8.9) | 34 918 (3.0) | 200 532 (6.7) |
| Respiratory—other | 104 711 (5.7) | 23 084 (2.0) | 127 795 (4.2) |
| Respiratory—unspecified | 10 836 (0.6) | 2476 (0.2) | 13 312 (0.4) |
| External cardiac monitoring | |||
| Overall | 27 303 (1.5) | 6140 (0.5) | 33 443 (1.1) |
| Among patients with any symptom | 22 433 (4.3) | 5046 (3.3) | 27 479 (4.1) |
Baseline characteristics are based on records from the Truven Health MarketScan® Commercial and Medicare Supplemental Databases from the calendar year of 2007. y indicates years.
Annualized Incidence Rates and Risk Cohorts
Risk cohort designation, based on our risk stratification scheme of baseline risk factors, differentiated patients with high-risk for incident AF, incident stroke, or both AF and stroke from low-risk patients. The annualized incidence rates for AF alone, stroke alone, and both AF and stroke were 3.59%, 3.27%, and 0.62% for the high-risk cohort and 1.32%, 1.48%, and 0.18%, respectively, for the low-risk cohort (Table2).
Table 2.
Annualized Incidence of Atrial Fibrillation, Stroke, and Both Atrial Fibrillation and Stroke
| Event Category (2008–2010) | High-Risk Cohort (n=1 851 653) | Low-Risk Cohort (n=1 156 221) | Combined Population (N=3 007 874) | |||
|---|---|---|---|---|---|---|
| Annualized Incidence | Patients | Rate/y | Patients | Rate/y | Patients | Rate/y |
| Atrial fibrillation | 134 495 | 3.59% | 31 246 | 1.32% | 165 741 | 2.71% |
| Stroke | 123 053 | 3.27% | 34 967 | 1.48% | 158 020 | 2.58% |
| Both atrial fibrillation and stroke | 23 971 | 0.62% | 4424 | 0.18% | 28 395 | 0.45% |
Annualized incidence rate is derived by dividing patient counts by total patient-years.
Annualized Incidence Rates Stratified by Age, Sex, and Risk Cohort Designation
In the multivariable analysis population, composed of both the high- and low-risk cohorts, annualized AF and stroke rates were stratified by combination of age group, sex, and high-/low-risk cohort. Among these combinations, rates of incident AF and incident stroke appeared to increase exponentially with each age group, regardless of sex or risk cohort stratification (Figure2A and 2B). Annualized incidence rates for AF were higher in men than in women in all age groups, except in the 0 to 17 age group, where the incidence rates in both men and women were very low (Figure2A). However, annualized incidence rates for stroke were similar across the sexes (Figure2B).
Figure 2.
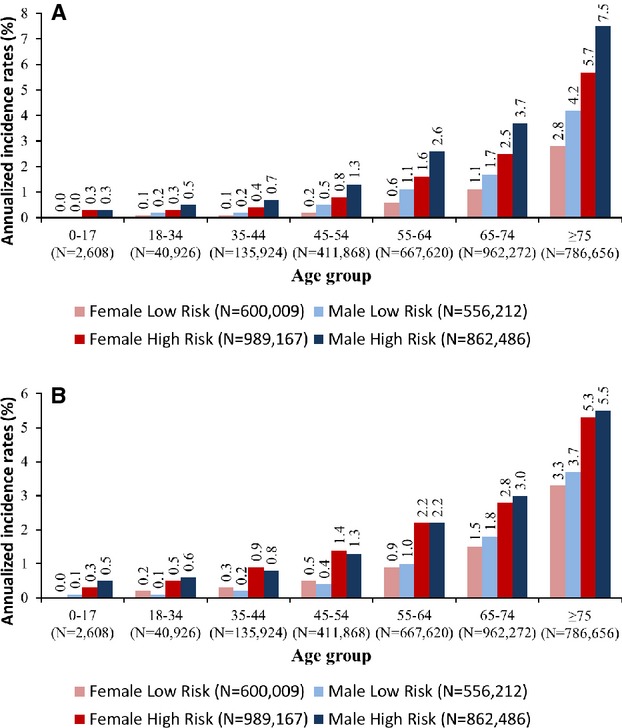
A, Annualized incidence rates of atrial fibrillation stratified by age, sex, and risk cohort, unadjusted, 2008–2011. B, Annualized incidence rates of stroke stratified by age, sex, and risk cohort, unadjusted, 2008–2011.
Relationship Between Incident AF and Incident Stroke in Patients Diagnosed With Both Incident AF and Incident Stroke in the Follow-up Period
In the combined population, 158 020 patients were diagnosed with stroke in the follow-up period. Diagnoses of incident AF were also reported for 28 395 (18%) of these patients with incident stroke. Among the patients with diagnoses of both AF and stroke in the follow-up period, diagnosis of incident AF was most commonly within 14 days of stroke diagnosis, followed by >14 days after stroke diagnosis, and was least likely to occur >14 days before stroke diagnosis (Figure3).
Figure 3.
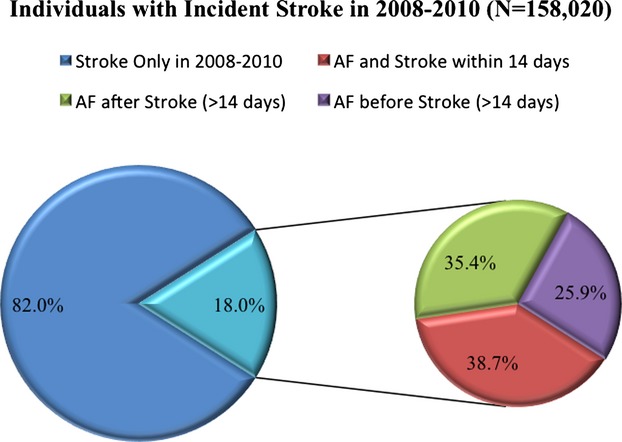
Timing of diagnosis of incident AF in relation to diagnosis of incident stroke. Of those with diagnosis of both incident AF and incident stroke in, diagnosis of AF was most commonly within 14 days of diagnosis of incident stroke. AF indicates atrial fibrillation.
Hazard Ratios of Individual Risk Factors for Incident AF and for Incident Stroke Based on Multivariable Analysis
In the first multivariable Cox regression models, HRs for incident AF and for incident stroke were calculated by using individual risk factors as independent variables. As summarized in Table3, the risk for incident AF and for incident stroke both increased exponentially with age. Male sex was a significant predictor of incident AF but not of stroke. Comorbid conditions and symptoms each individually conferred modest but significant risks for incident AF as well as stroke. In the stroke model, diagnosis of incident AF >14 days before or within 14 days of the censoring date (the first of a stroke event or end of follow-up) dramatically increased risks of stroke, whereas oral anticoagulation use >14 days before the censoring date significantly decreased risks for stroke.
Table 3.
Multivariable Hazard Ratios of Individual Risk Factors for Incident Atrial Fibrillation (AF) or Incident Stroke Based on Cox Regression Models
| AF* | Stroke*† | |||
|---|---|---|---|---|
| Hazard Ratio | P Value | Hazard Ratio | P Value | |
| Predictor variable | ||||
| Age group, y (reference = 18 to 34 years) | ||||
| 35 to 44 | 1.30 | 0.0010 | 1.54 | <0.0001 |
| 45 to 54 | 2.40 | <0.0001 | 2.41 | <0.0001 |
| 55 to 64 | 4.65 | <0.0001 | 3.99 | <0.0001 |
| 65 to 74 | 8.19 | <0.0001 | 6.56 | <0.0001 |
| ≥75 | 16.37 | <0.0001 | 10.82 | <0.0001 |
| Sex (reference = Female) | ||||
| Male | 1.32 | <0.0001 | 0.99 | 0.0765 |
| Comorbid conditions used for risk stratification of cohorts, 2007 (reference=no) | ||||
| Heart failure | 1.72 | <0.0001 | 1.23 | <0.0001 |
| Hypertension | 1.31 | <0.0001 | 1.24 | <0.0001 |
| Diabetes | 1.11 | <0.0001 | 1.25 | <0.0001 |
| Coronary artery disease | 1.21 | <0.0001 | 1.04 | <0.0001 |
| Chronic kidney disease | 1.23 | <0.0001 | 1.26 | <0.0001 |
| Comorbid condition not used for risk stratification of cohorts, 2007 (reference=no) | ||||
| Sleep apnea | 1.21 | <0.0001 | 1.14 | <0.0001 |
| Symptoms | ||||
| Chest pain | 1.01 | 0.0411 | 1.27 | <0.0001 |
| Dizziness | 1.07 | <0.0001 | 1.40 | <0.0001 |
| Palpitations | 1.46 | <0.0001 | 1.07 | <0.0001 |
| Tachycardia unspecified | 1.19 | <0.0001 | 1.13 | <0.0001 |
| Shortness of breath | 1.16 | <0.0001 | 1.13 | <0.0001 |
| Respiratory—other | 1.01 | <0.0001 | 1.12 | <0.0001 |
| Respiratory—unspecified | 1.09 | 0.6292 | 1.09 | 0.0033 |
| Indicators of stroke timing† (reference=no) | ||||
| AF event >14 d before stroke event | — | — | 16.2 | <0.0001 |
| AF event within 14 d of stroke event | — | — | 21.9 | <0.0001 |
| Oral anticoagulation use >14 d before stroke event | — | — | 0.6 | <0.0001 |
d indicates days; y, years.
Estimates for each risk factor were adjusted for geographic region, medications, and use of internal/external electrocardiographic recording devices, in addition to all other risk factors shown in the table.
Stroke model also included indicators of stroke timing.
Annualized Incidence Rates and Hazard Ratios of Unique Risk Combinations for Incident AF and for Incident Stroke
Annualized risks were calculated for each mutually exclusive and collectively exhaustive combination of risk factors. Hazard ratios were calculated separately for incident AF and stroke for each of the 96 unique risk factor combinations by using Cox regression models with a single predictor variable that categorized patients according to their baseline combination. Patients with none of the studied risk factors were used as the reference group. Annualized incidence rates, HRs, p-value levels, event counts, and sample sizes for all 96 risk factor combinations are summarized in Table4. The most prevalent high-risk combinations were hypertension plus age 65 to 74 and hypertension plus diabetes.
Table 4.
Annualized Incidence Rates and HRs of All Unique Risk Combinations for Incident AF and for Incident Stroke
| Risk Factors | Sample Size (2007) | Stroke Incidence | AF Incidence | ||||
|---|---|---|---|---|---|---|---|
| Diagnoses (2008–10) | Annualized Rate* (%/year) | HR† | Diagnoses (2008–10) | Annualized Rate* (%/year) | HR† | ||
| Cohort with no risk factors | |||||||
| None | 409 074 | 3969 | 0.5 | Ref | 2900 | 0.4 | Ref |
| Cohorts with single risk factors | |||||||
| Age 65 to 74 | 305 984 | 10 898 | 1.6 | 3.3**** | 8840 | 1.3 | 3.7**** |
| Age 75+ | 195 120 | 13 294 | 3.3 | 6.9**** | 12 958 | 3.2 | 9.2**** |
| HTN | 168 932 | 3336 | 1.0 | 2.1**** | 3114 | 0.9 | 2.6**** |
| Diabetes | 27 025 | 525 | 1.0 | 2.0**** | 346 | 0.6 | 1.8**** |
| CAD | 4134 | 123 | 1.6 | 3.3**** | 89 | 1.1 | 3.2**** |
| CKD | 563 | 19 | 1.8 | 3.6**** | 9 | 0.8 | 2.3* |
| HF | 414 | 17 | 2.2 | 4.5**** | 13 | 1.7 | 4.7**** |
| Cohorts with 2 risk factors | |||||||
| HTN+diabetes | 490 924 | 13 036 | 1.3 | 2.8**** | 9640 | 1.0 | 2.8**** |
| HTN+age 65 to 74 | 338 402 | 17 071 | 2.3 | 4.7**** | 17 666 | 2.4 | 6.8**** |
| HTN+age 75+ | 298 560 | 28 968 | 4.7 | 9.6**** | 32 268 | 5.3 | 14.8**** |
| Diabetes+age 65 to 74 | 41 697 | 2243 | 2.4 | 5.0**** | 1600 | 1.7 | 4.9**** |
| Diabetes+age 75+ | 25 025 | 2480 | 4.8 | 9.9**** | 2138 | 4.1 | 11.6**** |
| HF+HTN | 17 427 | 682 | 2.1 | 4.4**** | 1181 | 3.8 | 10.6**** |
| HTN+CAD | 15 835 | 555 | 1.9 | 4.0**** | 594 | 2.1 | 5.8**** |
| CAD+age 65 to 74 | 11 679 | 657 | 2.7 | 5.5**** | 693 | 2.8 | 8.0**** |
| CAD+age 75+ | 10 885 | 1147 | 5.3 | 10.8**** | 1281 | 5.9 | 16.7**** |
| HF+age 75+ | 3672 | 342 | 5.9 | 12.1**** | 565 | 10.2 | 28.6**** |
| HTN+CKD | 2101 | 93 | 2.4 | 4.8**** | 63 | 1.6 | 4.5**** |
| CKD+age 75+ | 1729 | 174 | 5.4 | 11.1**** | 206 | 6.5 | 18.2**** |
| HF+diabetes | 1443 | 57 | 2.2 | 4.5**** | 62 | 2.4 | 6.8**** |
| CKD+age 65 to 74 | 1351 | 90 | 3.2 | 6.6**** | 95 | 3.4 | 9.5**** |
| HF+age 65 to 74 | 1279 | 92 | 3.7 | 7.6**** | 133 | 5.5 | 15.4**** |
| Diabetes+CAD | 1005 | 54 | 3.0 | 6.2**** | 30 | 1.6 | 4.6**** |
| Diabetes+CKD | 265 | 14 | 3.0 | 6.2**** | 12 | 2.6 | 7.2**** |
| HF+CAD | 76 | 2 | 1.5 | 3.0 | 2 | 1.5 | 4.2* |
| CAD+CKD | 35 | 0 | 0.0 | 0.0 | 1 | 1.7 | 4.7 |
| HF+CKD | 14 | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 |
| Cohorts with 3 risk factors | |||||||
| HTN+diabetes+age 65 to 74 | 114 730 | 7711 | 3.1 | 6.4**** | 7270 | 2.9 | 8.2**** |
| HTN+diabetes+age 75+ | 79 597 | 8805 | 5.4 | 11.1**** | 9217 | 5.7 | 16.0**** |
| HTN+CAD+age 75+ | 65 200 | 7660 | 5.8 | 11.9**** | 9995 | 7.8 | 21.8**** |
| HTN+diabetes+CAD | 62 433 | 2976 | 2.6 | 5.4**** | 2779 | 2.4 | 6.9**** |
| HTN+CAD+age 65 to 74 | 61 478 | 4471 | 3.4 | 7.1**** | 5490 | 4.3 | 12.0**** |
| HTN+diabetes+CKD | 16 380 | 942 | 3.1 | 6.4**** | 733 | 2.4 | 6.8**** |
| HF+HTN+age 75+ | 16 339 | 1961 | 7.0 | 14.4**** | 3255 | 12.4 | 34.8**** |
| HTN+CKD+age 75+ | 11 641 | 1337 | 5.9 | 12.2**** | 1545 | 6.9 | 19.5**** |
| HF+HTN+diabetes | 9708 | 618 | 3.6 | 7.5**** | 787 | 4.7 | 13.1**** |
| HF+HTN+CAD | 8643 | 446 | 3.0 | 6.2**** | 782 | 5.4 | 15.1**** |
| HTN+CKD+age 65 to 74 | 7918 | 637 | 3.8 | 7.9**** | 583 | 3.5 | 9.8**** |
| HF+HTN+age 65 to 74 | 6657 | 568 | 4.3 | 8.8**** | 988 | 7.8 | 21.8**** |
| Diabetes+CAD+age 65 to 74 | 3551 | 293 | 3.9 | 8.1**** | 261 | 3.5 | 9.9**** |
| Diabetes+CAD+age 75+ | 2889 | 350 | 6.1 | 12.5**** | 411 | 7.2 | 20.3**** |
| HTN+CAD+CKD | 2675 | 170 | 3.7 | 7.7**** | 166 | 3.6 | 10.2**** |
| HF+HTN+CKD | 1367 | 115 | 5.2 | 10.7**** | 118 | 5.3 | 14.9**** |
| HF+diabetes+age 75+ | 1135 | 119 | 6.4 | 13.2**** | 176 | 9.8 | 27.5**** |
| HF+CAD+age 75+ | 1029 | 131 | 8.0 | 16.4**** | 206 | 13.4 | 37.5**** |
| Diabetes+CKD+age 65 to 74 | 830 | 82 | 4.9 | 10.1**** | 77 | 4.6 | 13.0**** |
| Diabetes+CKD+age 75+ | 792 | 102 | 6.9 | 14.2**** | 91 | 6.1 | 17.1**** |
| HF+diabetes+age 65 to 74 | 567 | 59 | 5.5 | 11.3**** | 90 | 8.8 | 24.8**** |
| HF+CAD+age 65 to 74 | 379 | 22 | 3.0 | 6.2**** | 49 | 7.1 | 20.0**** |
| HF+diabetes+CAD | 353 | 28 | 4.7 | 9.6**** | 29 | 4.7 | 13.3**** |
| CAD+CKD+age 75+ | 289 | 33 | 6.4 | 13.2**** | 41 | 8.2 | 23.2**** |
| HF+CKD+age 75+ | 233 | 31 | 10.5 | 21.7**** | 38 | 13.4 | 37.2**** |
| Diabetes+CAD+CKD | 222 | 15 | 4.1 | 8.4**** | 12 | 3.2 | 9.1**** |
| CAD+CKD+age 65 to 74 | 139 | 9 | 3.1 | 6.3**** | 15 | 5.4 | 15.1**** |
| HF+diabetes+CKD | 114 | 8 | 4.7 | 9.6**** | 15 | 8.7 | 24.3**** |
| HF+CKD+age 65 to 74 | 48 | 3 | 4.0 | 8.2*** | 4 | 5.5 | 15.3**** |
| HF+CAD+CKD | 33 | 4 | 7.4 | 15.2**** | 7 | 15.0 | 41.9**** |
| Cohorts with 4 risk factors | |||||||
| HTN+diabetes+CAD+age 65 to 74 | 31 774 | 2940 | 4.4 | 9.1**** | 3273 | 4.9 | 13.9**** |
| HTN+diabetes+CAD+age 75+ | 24 614 | 3271 | 6.7 | 13.7**** | 3871 | 8.0 | 22.6**** |
| HF+HTN+CAD+age 75+ | 11 270 | 1538 | 7.8 | 16.0**** | 2640 | 14.4 | 40.5**** |
| HF+HTN+diabetes+CAD | 7886 | 608 | 4.7 | 9.6**** | 870 | 6.8 | 19.1**** |
| HF+HTN+diabetes+age 75+ | 7243 | 1026 | 8.4 | 17.2**** | 1494 | 12.7 | 35.7**** |
| HTN+diabetes+CKD+age 65 to 74 | 7161 | 732 | 5.0 | 10.2**** | 692 | 4.7 | 13.2**** |
| HTN+diabetes+CKD+age 75+ | 6189 | 844 | 7.1 | 14.7**** | 868 | 7.4 | 20.8**** |
| HF+HTN+CAD+age 65 to 74 | 5399 | 556 | 5.2 | 10.8**** | 948 | 9.4 | 26.5**** |
| HF+HTN+diabetes+age 65 to 74 | 4801 | 523 | 5.6 | 11.5**** | 769 | 8.5 | 24.0**** |
| HTN+diabetes+CAD+CKD | 4384 | 396 | 5.3 | 10.9**** | 350 | 4.6 | 13.0**** |
| HTN+CAD+CKD+age 75+ | 4212 | 574 | 7.2 | 14.8**** | 745 | 9.6 | 27.0**** |
| HTN+CAD+CKD+age 65 to 74 | 2333 | 250 | 5.2 | 10.7**** | 287 | 6.1 | 17.1**** |
| HF+HTN+diabetes+CKD | 2304 | 212 | 5.9 | 12.1**** | 237 | 6.6 | 18.6**** |
| HF+HTN+CKD+age 75+ | 2059 | 250 | 7.9 | 16.3**** | 430 | 14.5 | 40.5**** |
| HF+HTN+CKD+age 65 to 74 | 697 | 82 | 6.7 | 13.7**** | 118 | 10.0 | 28.0**** |
| HF+HTN+CAD+CKD | 693 | 59 | 5.7 | 11.7**** | 102 | 10.5 | 29.5**** |
| HF+diabetes+CAD+age 75+ | 421 | 68 | 10.8 | 22.2**** | 84 | 13.4 | 37.4**** |
| HF+diabetes+CAD+age 65 to 74 | 260 | 31 | 6.5 | 13.4**** | 41 | 8.9 | 24.9**** |
| Diabetes+CAD+CKD+age 75+ | 188 | 29 | 7.9 | 16.3**** | 24 | 6.6 | 18.6**** |
| Diabetes+CAD+CKD+age 65 to 74 | 178 | 17 | 5.1 | 10.5**** | 24 | 7.5 | 21.1**** |
| HF+diabetes+CKD+age 75+ | 145 | 12 | 6.0 | 12.4**** | 29 | 15.3 | 42.8**** |
| HF+CAD+CKD+age 75+ | 97 | 2 | 1.5 | 3.0 | 25 | 21.5 | 60.0**** |
| HF+diabetes+CKD+age 65 to 74 | 80 | 5 | 3.9 | 8.0**** | 21 | 18.2 | 51.0**** |
| HF+diabetes+CAD+CKD | 69 | 5 | 5.2 | 10.8**** | 9 | 9.9 | 27.7**** |
| HF+CAD+CKD+age 65 to 74 | 29 | 0 | 0.0 | 0.0 | 5 | 12.6 | 35.2**** |
| Cohorts with 5 risk factors | |||||||
| HF+HTN+diabetes+CAD+age 75+ | 6526 | 1000 | 8.9 | 18.4**** | 1434 | 13.4 | 37.7**** |
| HF+HTN+diabetes+CAD+age 65 to 74 | 5308 | 675 | 6.7 | 13.8**** | 995 | 10.3 | 29.0**** |
| HTN+diabetes+CAD+CKD+age 65 to 74 | 3330 | 408 | 6.2 | 12.7**** | 450 | 6.8 | 19.2**** |
| HTN+diabetes+CAD+CKD+age 75+ | 3172 | 443 | 7.4 | 15.3**** | 647 | 11.3 | 31.8**** |
| HF+HTN+diabetes+CAD+CKD | 2415 | 272 | 8.0 | 16.4**** | 314 | 9.2 | 25.7**** |
| HF+HTN+CAD+CKD+age 75+ | 2038 | 295 | 9.5 | 19.5**** | 473 | 16.3 | 45.7**** |
| HF+HTN+diabetes+CKD+age 75+ | 1875 | 278 | 9.7 | 20.0**** | 404 | 14.9 | 41.7**** |
| HF+HTN+diabetes+CKD+age 65 to 74 | 1429 | 195 | 7.9 | 16.2**** | 250 | 10.3 | 28.8**** |
| HF+HTN+CAD+CKD+age 65 to 74 | 694 | 81 | 6.8 | 14.0**** | 145 | 13.1 | 36.7**** |
| HF+diabetes+CAD+CKD+age 75+ | 90 | 14 | 10.6 | 21.9**** | 24 | 19.1 | 53.4**** |
| HF+diabetes+CAD+CKD+age 65 to 74 | 59 | 5 | 6.0 | 12.3**** | 10 | 14.2 | 39.5**** |
| Cohort with 6 risk factors | |||||||
| HF+HTN+diabetes+CAD+CKD+age 75+ | 2382 | 369 | 10.3 | 21.3**** | 510 | 15.0 | 41.9**** |
| HF+HTN+diabetes+CAD+CKD+age 65 to 74 | 2051 | 311 | 9.2 | 18.9**** | 400 | 12.1 | 34.0**** |
Each section is displayed in descending order of HR for AF or stroke. HR indicates hazard ratio; AF, atrial fibrillation; HTN, hypertension; CAD, coronary artery disease; CKD, chronic kidney disease; HF, heart failure.
Unadjusted annualized incidence rate.
HRs were calculated by using the second Cox regression model with mutually exclusive and collectively exhaustive risk factor combination as a predictor and patients with none of the studied risk factors were used as the reference group.
P<0.05
P<0.01
P<0.001
P<0.0001.
Annualized rates of AF and stroke diagnoses among patients with no risk factors were 0.35% and 0.49%, respectively, while rates ranged from 0.65% to 3.34% for those with single risk factors (Figure4). Among patients with a single risk factor, patients with age ≥75 had the highest risks of AF (annualized incidence rate of 3.25%, HR 9.2) and highest risks of stroke (annualized incidence rate of 3.34%, HR 6.9) (Figure4 and Table4). Among patients with 2 risk factors, those with age ≥75 in combination with HF had the highest annualized incidence rate for AF and for stroke (10.2% and 5.9%, respectively), and the annualized incidence rates for AF were supra-additive (exceeding summation of 3.2% for age ≥75 alone and 1.7% for heart failure alone) (Table4). The estimate of excess risk attributable to the interaction of these 2 risk factors is 5.3%, which is highly statistically significant due to the large sample sizes within each of these risk factor cohorts.
Figure 4.
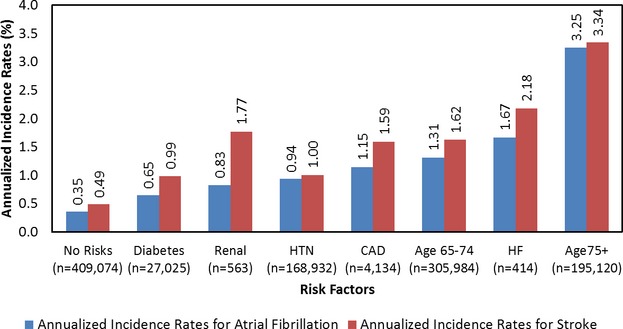
Annualized incidence rates of AF and stroke diagnoses for patients with no risk factors or a single risk factor. AF indicates atrial fibrillation; CAD, coronary artery disease; HF, heart failure; HTN, hypertension.
As illustrated in Table4, risks for incident AF and for incident stroke did not increase linearly with the number of risk factors, and not all risk factor combinations conferred equal risks. Combinations inclusive of heart failure, hypertension, or age ≥75 appear to confer the highest risks for incident AF or incident stroke among 2–, 3–, 4–, 5–, or 6–risk factor combinations. The single risk factor of age ≥75 conferred greater risks for incident AF and for incident stroke than did 2–risk factor combinations of hypertension with age 65 to 74, or diabetes with age, or hypertension with diabetes. Similarly, among the 2–risk factor combinations, the combination group with age ≥75 and hypertension had greater risks for incident AF and for incident stroke than 3–risk factor combinations of hypertension with CAD and age 65 to 74 or hypertension with diabetes and age 65 to 74, or hypertension with diabetes and CAD.
Furthermore, the prevalence of risk factor combinations in the population at baseline contributes to overall incidences of AF and stroke. Because age ≥75 was a prevalent risk factor, patients with age ≥75, as a single risk factor or in combination with additional risk factors, accounted for large numbers of AF and stroke events. In particular, the combination of age ≥75 and hypertension (298 560 patients) had the highest absolute numbers of incident AF (32 268 diagnoses, HR 14.83) and incident stroke (28 968 diagnoses, HR 9.64) events.
The HRs computed in this analysis used the healthiest cohort (ie, patients with no risk factors) as the reference group. However, because all of these ratios were computed by using the same reference, the HR for any risk cohort compared with any other risk cohort can be estimated from the results in Table4 by simply dividing the HR for the cohort of interest by the HR for the reference group of interest. For instance, if it is more desirable to compare the stroke risk for a patient of age 65 to 74 with diabetes to a patient of the same age without diabetes, the HR associated with the added risk of diabetes could be calculated as 5.0/3.3=1.5.
Discussion
In this study of >3 million US patients, for the first time, we quantified the separate and combinatorial effects of individual risk factors for incident AF and incident stroke by using a large claims database. In our stratification system, patients classified as high risk based on the presence of a combination of heart failure, hypertension, diabetes, advanced age (65 to 74 or ≥75 years), CAD, and CKD were indeed at higher risk for incident AF and incident stroke. These findings are consistent with epidemiologic data and previous risk prediction models for AF1,6,7,19–21 and for stroke,8,9,22,23 validating the concept that comorbidities determined from a claims database may provide reasonably accurate risk prediction.
Male sex was a significant predictor of incident AF in our study. This is consistent with published data from the Framingham Heart Study,6 the Atherosclerosis Risk in Communities Study,23 and the Cardiovascular Health Study.20 Sex was not, however, a significant predictor of all-cause stroke in our study. This is not inconsistent with published data; although existing models (CHA2DS2-VASc, Framingham Heart Study) have demonstrated an independent association between female sex and stroke in the particular setting of AF, sex is not an independent predictor of all-cause stroke in the Framingham Heart Study model.22
The large size of our sample provided statistical power to compare risks associated with specific factors, not only in isolation but also in various combinations. With this approach, we demonstrated that not all risk factors—or risk factor combinations—are “created equal.” Age ≥75 was the single most important risk factor for both incident AF and incident stroke, whether in isolation or in combination with other factors. Combinations of risk factors that included heart failure, hypertension, or age ≥75 conferred the highest risk for incident AF and incident stroke. Furthermore, we showed that risk factors are not simply additive—despite the convenience of certain existing models, 1 plus 1 does not always equal 2. For example, when combined, the risks of age ≥75 and heart failure were supra-additive, exceeding the sum of the risks associated with each factor in isolation.
The most recently revised AF guideline from the American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society highlights clinical risk factors associated with increased risks for AF but has not incorporated risk assessment schema for the identification and management of patients at risk for AF.25 As the management of common cardiovascular conditions (eg, heart failure and ischemic heart disease) has moved to a stratified approach, based on risk level, we anticipate the management of AF and stroke to follow this similarly expanded framework. Observations from our study have relevant implications with respect to risk stratification of the population.
First, our data underscore the importance of age ≥75, especially when combined with hypertension, as a prevalent and high-risk combination for both incident AF and incident stroke. In this particularly vulnerable and prevalent cohort, more proactive measures to screen for incident AF and other modifiable risk factors may be warranted. The use of longer-term, external electrocardiographic monitoring devices could provide a particular benefit in this population, especially if symptoms are present. Given that AF is underdiagnosed and may even present as incident stroke,26 earlier identification of subclinical AF in this high-risk cohort may permit earlier introduction of preventive measures, such as thromboprophylaxis where indicated. The value and optimal methodology for screening for AF in this cohort are incompletely understood and require further study.
Second, our data challenge the present paradigm of risk prediction by addition, rather than combination. There is a need to develop new risk predictive models that account for the observed differential effects of specific combinations of risk factors. The ubiquity of computing and, increasingly, smartphone-based applications in modern medicine lowers the bar for clinical application of a more sophisticated combinatorial model. Such a combinatorial model could potentially offer a more individualized and accurate risk prediction for a given patient, with direct implications for consideration of more intensive monitoring and the use of preventive interventions.
Limitations to our study include its retrospective design and its reliance on claims data. ICD-9 codes were used to ascertain diagnoses of AF and stroke, as well as risk factors and symptoms. There was likely variation in levels of arrhythmia monitoring and follow-up. In view of the acknowledged underdiagnosis of AF in the literature,26 ICD-9 coding used in our study may have underestimated the true incidence of AF. Similarly, patient risks could be underestimated, though this would have a conservative dampening effect on the risk differentials. Finally, MarketScan data consist only of insured patients and so are not representative of the uninsured population in the United States. Acknowledging these limitations, our study had multiple strengths, including its large, real-world sample and novel combinatorial approach. These advantages permit an important contribution to the body of literature on the prediction of AF and stroke and prompt new directions in public health strategies and research. Further, the annualized risks of AF and stroke associated with specific combinations of risk factors in our study provide clinicians with concrete estimates for individual patients in the clinic.
Conclusion
Adults with combinations of heart failure, hypertension, diabetes, advanced age (65 to 74 or ≥75), CAD, and CKD are at increased risks of incident AF and incident stroke. Risks associated with combinations of risk factors are not always additive. Rather, certain combinations of risk factors are associated with substantially higher risks of incident AF and incident stroke. The combination of age ≥75 and hypertension in particular is both prevalent and high risk, contributing substantially to the incidence of AF and the incidence of stroke, thus constituting an important target for more active monitoring and prevention.
Sources of Funding
This study was supported by Medtronic, Inc.
Disclosures
Dr. Chyou has no relationships relevant to the contents of this article to disclose. Dr. Hunter is an employee of CTI Clinical Trial and Consulting Services, which provides consulting services to Medtronic, plc. Ms. Mollenkopf is an employee of Medtronic, plc. Dr. Turakhia is a consultant to Medtronic, plc and St. Jude Medical, Inc and has received honoraria for speaking from St. Jude Medical, Inc and Boston Scientific. Dr. Reynolds is a consultant to Medtronic, plc.
Supporting Information
Appendix S1. ICD-9 Codes Used for the Diagnosis of AF or Stroke.
Appendix S2. ICD-9 Codes and Drug Classes Used for Risk Factor Identification.
Appendix S3. Codes Used for the Identification of Symptoms, Additional Comorbid Condition, and External Cardiac Monitoring.
Appendix S4. Additional Baseline Patient Characteristics.
References
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- Lip GY, Lim HS. Atrial fibrillation and stroke prevention. Lancet Neurol. 2007;6:981–993. doi: 10.1016/S1474-4422(07)70264-8. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Wann LS, Curtis AB, Ellenbogen KA, Estes NA, III, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Le Heuzey JY, Kay GN, Olsson SB, Prystowsky EN, Tamargo JL, Wann S. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:1935–1944. doi: 10.1016/j.jacc.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, Lau CP, Van Gelder IC, Hohnloser SH, Carlson M, Fain E, Nakamya J, Mairesse GH, Halytska M, Deng WQ, Israel CW, Healey JS. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. doi: 10.1161/CIRCULATIONAHA.113.007825. [DOI] [PubMed] [Google Scholar]
- Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D’Agostino RB, Sr, Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, Soliman EZ, Astor BC, Coresh J. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:2946–2953. doi: 10.1161/CIRCULATIONAHA.111.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- Piccini JP, Stevens SR, Chang Y, Singer DE, Lokhnygina Y, Go AS, Patel MR, Mahaffey KW, Halperin JL, Breithardt G, Hankey GJ, Hacke W, Becker RC, Nessel CC, Fox KA, Califf RM. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation. 2013;127:224–232. doi: 10.1161/CIRCULATIONAHA.112.107128. [DOI] [PubMed] [Google Scholar]
- Singer DE, Chang Y, Borowsky LH, Fang MC, Pomernacki NK, Udaltsova N, Reynolds K, Go AS. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc. 2013;2:e000250. doi: 10.1161/JAHA.113.000250. doi: 10.1161/JAHA.113.000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truven Health MarketScan® Commercial and Medicare Supplemental Databases. Available at: http://img.en25.com/Web/TruvenHealthAnalytics/PH_11609_0213_MarketScanResearchDatabases_SS_web.pdf. Accessed 08/12/2013.
- Truven Health MarketScan® Commercial and Medicare Supplemental Databases. Available at: http://img.en25.com/Web/TruvenHealthAnalytics/PH_12315_0513_DataStoryboard_17x11_PRINT.pdf. Accessed 08/12/2013.
- Reynolds MR, Gunnarsson CL, Hunter TD, Ladapo JA, March JL, Zhang M, Hao SC. Health outcomes with catheter ablation or antiarrhythmic drug therapy in atrial fibrillation: results of a propensity-matched analysis. Circ Cardiovasc Qual Outcomes. 2012;5:171–181. doi: 10.1161/CIRCOUTCOMES.111.963108. [DOI] [PubMed] [Google Scholar]
- Hao SC, Hunter TD, Gunnarsson C, March JL, White SA, Ladapo JA, Reynolds MR. Acute safety outcomes in younger and older patients with atrial fibrillation treated with catheter ablation. J Interv Card Electrophysiol. 2012;35:173–182. doi: 10.1007/s10840-012-9690-5. [DOI] [PubMed] [Google Scholar]
- Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- Naccarelli GV, Johnston SS, Lin J, Patel PP, Schulman KL. Cost burden of cardiovascular hospitalization and mortality in ATHENA-like patients with atrial fibrillation/atrial flutter in the United States. Clin Cardiol. 2010;33:270–279. doi: 10.1002/clc.20759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman EZ, Prineas RJ, Go AS, Xie D, Lash JP, Rahman M, Ojo A, Teal VL, Jensvold NG, Robinson NL, Dries DL, Bazzano L, Mohler ER, Wright JT, Feldman HI. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC) Am Heart J. 2010;159:1102–1107. doi: 10.1016/j.ahj.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, Ambrose M, Alonso A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2011;107:85–91. doi: 10.1016/j.amjcard.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study) Am J Cardiol. 1994;74:236–241. doi: 10.1016/0002-9149(94)90363-8. [DOI] [PubMed] [Google Scholar]
- Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- Chambless LE, Heiss G, Shahar E, Earp MJ, Toole J. Prediction of ischemic stroke risk in the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2004;160:259–269. doi: 10.1093/aje/kwh189. [DOI] [PubMed] [Google Scholar]
- Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:700–752. [Google Scholar]
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:2246–2280. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. ICD-9 Codes Used for the Diagnosis of AF or Stroke.
Appendix S2. ICD-9 Codes and Drug Classes Used for Risk Factor Identification.
Appendix S3. Codes Used for the Identification of Symptoms, Additional Comorbid Condition, and External Cardiac Monitoring.
Appendix S4. Additional Baseline Patient Characteristics.


