Abstract
Background
New guidelines for cardiovascular disease risk assessment and statin eligibility have recently been published in the United States by the American College of Cardiology and the American Heart Association (ACC-AHA). It is unknown how these guidelines compare with the Canadian Cardiovascular Society (CCS) recommendations.
Methods and Results
Using data from the Canadian Health Measures Survey 2007–2011, we estimated the cardiovascular disease risk and proportion of the Canadian population, aged 40 to 75 years without cardiovascular disease, who would theoretically be eligible for statin treatment under both the CCS and ACC-AHA guidelines. The survey sample used (n=1975) represented 13.1 million community dwelling Canadians between the ages of 40 and 75 years. In comparing the CVD risk assessment methods, we found that calculated CVD risk was higher based on the CCS guidelines compared with the ACC-AHA guidelines. Despite this, a similar proportion and number of Canadians would be eligible for statin treatment under the 2 sets of recommendations. Some discordance in recommendations was found within subgroups of the population, with the CCS guidelines recommending more treatment for individuals who are younger, with a family history of CVD, or with chronic kidney disease. The ACC-AHA recommend more treatment for people who are older (age 60+ years). These results likely overestimate the treatment rate under both guidelines because, in primary prevention, a clinician–patient discussion must occur before treatment and determines uptake.
Conclusions
Implementing the ACC-AHA lipid treatment guidelines in Canada would not result in an increase in individuals eligible for statin treatment. In fact, the proportion of the population recommended for statin treatment would decrease slightly and be targeted at different subgroups of the population.
Keywords: cardiovascular disease, risk assessment, statin eligibility, treatment guidelines
Statins are widely used in Canada to lower cholesterol levels and reduce cardiovascular risk overall.1 Guidelines for lipid treatment with statins are compiled by the Canadian Cardiovascular Society (CCS), with the last major update in 2012.2 The latest version of the CCS guidelines recommends baseline cardiovascular disease (CVD) risk measurement by using the Framingham Risk Score (FRS), developed in 2008 by D’Agostino et al.3 A modification was added for a doubling of the risk percentage in subjects between 30 and 59 with a first-degree relative with premature vascular disease. New lipid treatment guidelines have been developed by the American College of Cardiology and the American Heart Association (ACC-AHA) in the United States that use an updated risk model, redeveloped on a larger pooled cohort, which included the Framingham cohort.4,5 The ACC-AHA decided to use an updated algorithm because of concerns that the previous equation was derived in an exclusively white sample population and that the outcomes considered had limited scope.4 Therefore, the pooled cohort equations were derived from community-based cohorts that are broadly representative of the US population and focused on estimation of incident hard atherosclerotic CVD (ASCVD) events, because this outcome was more relevant to both patients and clinicians.4,6
However, this new risk algorithm has been controversial, with critics claiming that it was not appropriately calibrated and that using the pooled cohort equations to determine statin eligibility would result in many more Americans being treated with statins.7 In addition, the ACC-AHA guidelines focused on 4 patient groups most likely to benefit from statin therapy: those with existing CVD, diabetics aged 40 to 75 years with low-density lipoprotein cholesterol (LDL-C) levels <5 mmol/L, individuals with LDL-C levels >5 mmol/L, and individuals with an estimated 10-year risk of CVD of >7.5%.4,5 While the ACC-AHA and CCS guidelines essentially agree on the treatment of the first 3 groups outlined here, the ACC-AHA expand statin eligibility to all individuals with CVD risk of >7.5%, regardless of LDL-C level.4,5 To date, a direct comparison of the calculated CVD risk and resulting statin eligibility generated by the 2 sets of guidelines has yet to be completed in Canada.
The purpose of this study was to determine theoretical statin eligibility among Canadians aged 40 to 75 years, without CVD, by using both the latest Canadian and US lipid treatment guidelines. Specifically, we applied the modified FRS, recommended by the CCS, and the pooled cohort equations recommended by the ACC-AHA to data from respondents in the Canadian Health Measures Survey (CHMS). We compared CVD risk level and statin eligibility in the Canadian population under both sets of guidelines.
Methods
Data Source
We used data from 2 cycles of the CHMS, a cross-sectional population-based survey that collected physical measures including blood samples, blood pressure, weight, and height, in 11 999 Canadians aged 3 to 79 between 2007 and 2011. The CHMS, including its sampling strategy, has been described in detail elsewhere.8–13 Briefly, the CHMS has both a household and a clinic component, with data being collected at 15 pan-Canadian sites in cycle 1 and 18 sites in cycle 2. Estimates based on the combined file, therefore, reflect the average Canadian household population during the study timeframe (2007–2011). The CHMS is representative of ≈96% of the Canadian household population aged 3 to 79. However, it does not include residents of Indian Reserves, Crown lands, institutions, and certain remote regions or full-time members of the regular Canadian Forces. The response rate (calculated as the product of response fractions for the household, the household questionnaire, the mobile examination centre component, with an adjustment for the sampling strategy) for cycle 1 was 51.7% and for cycle 2 was 55.5%.9 Ethics approval for the CHMS was obtained from Health Canada’s Research Ethics Board. Written consent was requested from respondents before participation. During an in-home interview, respondents completed a wide-ranging questionnaire covering sociodemographic characteristics, medical history, current health status, prevalent conditions, health-related behaviors, and medication use.
Study Sample
Of the total number of respondents (N=11 999) in the combined cycles of the CHMS, a subsample of participants (n=5427) provided fasting blood samples during their clinic visit. This enabled a full lipid profile that was necessary to assess CVD risk. Of this subsample, only adult respondents aged 40 to 75 years who were not pregnant and were without established CVD were selected for study (n=1975). The age range of our analysis was limited to ages 40 to 75 because 40 coincides with the age at which lipid screening usually begins and the ACC-AHA guidelines do not make primary prevention recommendations for those aged >75 years.2,3 Survey weights calculated for the fasting subsample were applied in this analysis to represent the Canadian population.9
Analysis―CVD Risk Calculation
CCS guidelines
Absolute CVD risk was first calculated by using the FRS algorithm for 10-year risk of total cardiovascular events (including coronary death, myocardial infarction, coronary insufficiency, angina), cerebrovascular events (including ischemic stroke, hemorrhagic stroke, and transient ischemic attack), peripheral artery disease (intermittent claudication), and heart failure over 10 years.3 The estimated 10-year risk of a CVD event is calculated as 1 minus the baseline survival raised to the power of the exponent of the sum of the coefficients by the values, minus the sum of the coefficients by the population mean values.3 This algorithm has been recommended by the CCS to help primary care providers to identify patients most likely to benefit from statin treatment.2 The CVD risk factors included are age, total cholesterol, high-density lipoprotein cholesterol (HDL-C), systolic blood pressure, treatment for high blood pressure, smoking, and diabetes status. Separate risk scores were calculated for male and female respondents (Table1. The CCS guidelines recommend doubling the calculated CVD risk for people aged 30 to 59 years with a family history of premature CVD.2,14 CHMS respondents were asked whether they had a first-degree relative who had had a heart attack (or heart disease) or stroke and what age that relative was when he or she was diagnosed. By using responses to these 2 questions, we defined family history of premature CVD as having a first-degree relative who had had a heart attack or stroke before age 60.
Table 1.
Contrasting the FRS and Pooled Cohort Equations
| FRS | Pooled Cohort Equations | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes modeled* | Outcomes modeled† | |||||||
| Terms | Male | Female | Male Terms | White Man | African American Man | Female Terms | White Woman | African American Woman |
| Ln age | 3.06117 | 2.32888 | Ln age | 12.344 | 2.469 | Ln age | −29.799 | 17.114 |
| Ln TC | 1.12370 | 1.20904 | Ln TC | 11.853 | 0.302 | Ln TC | 13.540 | 0.940 |
| Ln HDL-C | −0.93263 | −0.70833 | Ln HDL-C | −7.990 | −0.307 | Ln HDL-C | −13.578 | −18.920 |
| Ln SBP treated | 1.99881 | 2.82263 | Ln SBP treated | 1.797 | 1.916 | Ln SBP treated | 2.019 | 29.291 |
| Ln SBP not treated | 1.93303 | 2.76157 | Ln SBP not treated | 1.764 | 1.809 | Ln SBP not treated | 1.857 | 27.820 |
| Smoking | 0.65451 | 0.52873 | Smoking | 7.837 | 0.549 | Smoking | 7.574 | 0.691 |
| Diabetes | 0.57367 | 0.69154 | Diabetes | 0.658 | 0.645 | Diabetes | 0.661 | 0.874 |
| Ln age×Ln TC | −2.664 | n/a | Ln age×Ln TC | −3.114 | n/a | |||
| Ln age×Ln HDL-C | 1.767 | n/a | Ln age×Ln HDL-C | 3.149 | 4.475 | |||
| Ln age×smoking | −1.795 | n/a | Ln age×Ln SBP treated | n/a | −6.432 | |||
| Ln age×Ln SBP not treated | n/a | −6.087 | ||||||
| Ln age×current smoker | −1.665 | n/a | ||||||
| Ln age squared | 4.884 | n/a | ||||||
FRS indicates Framingham Risk Score; HDL-C, high-density lipoprotein cholesterol; Ln, natural log; SBP, systolic blood pressure; TC, total cholesterol.
Coronary death, myocardial infarction, coronary insufficiency, angina, cerebrovascular events (including ischemic stroke, hemorrhagic stroke, and transient ischemic attack), peripheral artery disease (intermittent claudication), and heart failure (see reference 3 for more details).
Coronary heart disease death, nonfatal myocardial infarction, and fatal and nonfatal stroke (see reference 4 for more details).
ACC-AHA guidelines
Absolute CVD risk was also calculated by using the pooled cohort equations, which were designed to predict 10-year risk of hard ASCVD events including coronary heart disease death, nonfatal myocardial infarction, and fatal and nonfatal stroke.4 The risk factors included in the pooled cohort equations were similar to those contained in the FRS, namely age, total cholesterol, HDL-C, systolic blood pressure, treatment for high blood pressure, smoking, and diabetes status. Compared with the FRS, both the outcomes modeled and the form of the equation were different; Table1 contrasts the 2 algorithms. In addition, separate risks were calculated for white and African American and for male and female respondents. When the algorithm was applied to the Canadian population, CVD risk for nonwhite but non African American individuals was calculated by using the white equation. Unlike the FRS, the risk scores resulting from the pooled cohort equations were not modified for premature family history of CVD.3 The estimated 10-year risk of a first hard ASCVD event is calculated as 1 minus the baseline survival rate, raised to the power of the exponent of the sum of the coefficients by the values, minus the sum of the coefficients by the race- and sex-specific overall mean values.4
Analysis―Determining Statin Eligibility
CCS guidelines
It is important to note that in this study, we determined “theoretical” statin eligibility―that is, the number of individuals that would be recommended for treatment based purely on the treatment guidelines in the absence of a “risk” discussion with the treating physician. To determine statin eligibility, the Canadian population was risk stratified as being of high, intermediate, or low risk. First, high-risk individuals were identified based on self-reported and measured diagnosis of diabetes, chronic kidney disease, and high-risk hypertension (see detailed covariate definitions later). For individuals who did not automatically fall into the high-risk category, their FRS CVD risk was assessed.2 Based on this score, people were further stratified into explicit high-risk (≥20%), intermediate-risk (10% to 19%), and low-risk (<10%) categories. Individuals were considered to be eligible for treatment based on the calculation of their baseline risk as just described and their LDL-C levels. For those individuals in the sample who were already taking statins, eligibility was determined based on adjusted levels of LDL-C and non-HDL-C. LDL-C and non-HDL-C levels were adjusted back to pretreatment levels by using results from a meta-analysis that provided estimates of statin effectiveness by statin type and dose (Table2.15 Low-risk individuals were recommended for treatment if their LDL-C level was ≥5.0 mmol/L. Intermediate-risk individuals were recommended for treatment if their LDL- C level was ≥3.5 mmol/L or their non-HDL-C level was ≥4.3 mmol/L. All high-risk (those previously determined as high-risk according to the self-reported conditions listed above and those with FRS ≥20%) individuals were recommended for treatment.
Table 2.
Adjustment Factors Used to Convert Cholesterol Values to Pretreatment Levels15
| Statin | Total Cholesterol | LDL | HDL-C | Triglycerides |
|---|---|---|---|---|
| Atorvastatin (all doses) | +2.0 | +1.8 | −0.1 | +0.3 |
| Fluvastatin (all doses) | +1.6 | +1.6 | −0.1 | +0.2 |
| Lovastatin (all doses) | +1.2 | +1.5 | −0.1 | +0.3 |
| Pravastatin (all doses) | +1.3 | +1.2 | −0.1 | +0.2 |
| Rosuvastatin (all doses) | +2.2 | +2.2 | −0.1 | +0.4 |
| Simvastatin (all doses) | +1.6 | +1.4 | −0.1 | +0.4 |
HDL-C indicates high-density lipoprotein; LDL, low-density lipoprotein.
ACC-AHA guidelines
Determining statin eligibility worked differently under the ACC-AHA guidelines. Individuals were recommended for treatment if their LDL-C level was ≥5.0 mmol/L or if they had diabetes. This group of individuals was a subset of the high-risk group identified under the CCS guidelines. As with the CCS guidelines, for those individuals in the sample who were already taking statins, eligibility was determined based on adjusted levels of LDL-C and non-HDL-C. For all other individuals, their pooled cohort equation ASCVD risk was assessed.4 Based on this score, people were further stratified into 2 risk categories: CVD risk <7.5% and CVD risk ≥7.5%. While these categories were not explicitly labeled low and high-risk in the ACC-AHA guidelines, we applied these labels to the categories to facilitate comparison. All individuals with CVD risk ≥7.5% were recommended for treatment.
Analysis―Risk Stratification and Comparison of Canadian and US Recommendations
Descriptive analysis of sociodemographic and CVD risk factor variables was performed. The risk factors were measured and derived as detailed next. The proportion of the Canadian population aged 40 to 75 classified into risk groups according to the CCS and ACC-AHA guidelines, and the proportion that would be considered eligible for statin treatment in each risk group was tabulated and graphed. Finally, to highlight the difference between the 2 guidelines, we examined sociodemographic and CVD risk factor characteristics of 4 mutually exclusive groups of individuals: (1) those not recommended for statin therapy by either guideline; (2) those recommended for therapy by both guidelines; (3) those recommended for therapy by the CCS guidelines but not the ACC-AHA guidelines; and (4) those recommended for therapy by the ACC-AHA guidelines but not the CCS guidelines.
Analysis―Detailed Covariate Definitions
Blood pressure
Blood pressure was measured with use of the BpTRU BP-300 device (BpTRU Medical Devices Ltd) at the mobile examination center. The BpTRU™ is an automated electronic monitor that has been validated and is recommended for use in the Canadian Hypertension Education Program.16,17 Six BpTRU readings were taken for each participant, with the last 5 averaged to determine the systolic and diastolic blood pressure reading.18 During the home interview, 39 respondents who could not visit the mobile examination centre had their blood pressure measured with use of the BpTRU BP-100 device.
Antihypertensive medication use
During data processing, audited medications in current use by respondents were assigned codes from the Anatomical Therapeutic Chemical (ATC) Classification System. The following categories of antihypertensive medications were specified: β-blockers (ATC codes C07, excluding C07AA07, C07AA12, and C07AG02); agents acting on the renin-angiotensin system (ATC codes C09); thiazide diuretics (ATC codes C03, excluding C03BA08 and C03CA01); calcium channel antagonists (ATC codes C08); and miscellaneous antihypertensives (ATC codes C02, excluding C02KX01). Respondents were categorized as using antihypertensive medication if an ATC code corresponded to this list and/or they self-reported the use of blood pressure–lowering medication.
Statin medication use
Respondents were categorized as using statin medication by using 2 ATC codes: C10A and C10B. These codes also identified users of nonstatin lipid-lowering medications like fenofibrate. If respondents only reported using nonstatin medications, they were classified as nonstatin users.
Hypertension
Respondents were categorized as hypertensive if they had an average systolic blood pressure ≥140 mm Hg and/or were using antihypertensive medication and/or reported a health care provider diagnosis of hypertension.
High-risk hypertension
Respondents were categorized as having high-risk hypertension if they were hypertensive and had ≥3 of the following risk factors: were male, aged >55 years, a smoker, total cholesterol–to–HDL-C ratio >6, or a family history of premature CVD.
Smoker
Respondents who reported smoking daily or occasionally were categorized as smokers.
Diabetes
Respondents were categorized as having diabetes if their measured blood glucose was ≥126 mg/dL and/or had an audited use of glucose-lowering medication (ATC code A10) and/or a self-reported health care provider–assigned diagnosis of diabetes.
Chronic kidney disease
Chronic kidney disease was defined as an estimated glomerular filtration rate of <60 mL/min per 1.73 m2. Estimated glomerular filtration rate=175×(serum creatinine in mg/dL)−1.154×(age)−0.203×(0.742 if female)×(1.212 if cultural or racial background is African American).19
Results
The sociodemographic and CVD risk factor characteristics of the study population are shown in Table3. The Canadian population assessed for statin treatment was composed of slightly more women (52.1%) and more individuals overall aged 40 to 54 years (71.4%). The population was predominantly educated to a secondary school level and higher (83.6%). The most prevalent CVD risk factor in the population was elevated LDL-C (47.3%), followed by hypertension (29.0%), antihypertensive medication use (25.9%), and smoking (21.5%).
Table 3.
Characteristics of Analytical Population 40 to 75 Years Old, by Sex
| Total | Men | Women | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size | % | 95% CI | Sample Size | % | 95% CI | Sample Size | % | 95% CI | ||||
| Total | 1975 | 100.0 | — | — | 950 | 47.9 | 46.8 | 49.0 | 1025 | 52.1 | 51.0 | 53.2 |
| Age group | ||||||||||||
| 40 to 59 y | 1175 | 71.4 | 69.8 | 73.0 | 566 | 73.0 | 71.1 | 74.9 | 609 | 69.9 | 67.9 | 71.8 |
| 60 to 75 y | 905 | 28.6 | 27.0 | 30.2 | 384 | 27.0 | 25.1 | 28.9 | 416 | 30.1 | 28.2 | 32.1 |
| Secondary school graduation and higher | 1692 | 83.6 | 80.5 | 86.3 | 806 | 80.8 | 75.4 | 85.2 | 886 | 86.3 | 82.6 | 89.3 |
| Daily or occasional smoker | 347 | 21.5 | 18.3 | 25.0 | 171 | 21.2 | 16.5 | 26.8 | 176 | 21.7 | 17.6 | 26.4 |
| Diabetes | 172 | 8.7 | 6.6 | 11.5 | 88 | 10.3* | 7.2 | 14.5 | 84 | 7.3 | 5.2 | 10.1 |
| Chronic kidney disease | 117 | 4.5 | 3.4 | 5.9 | 44 | 3.3* | 2.3 | 4.8 | 73 | 5.5* | 3.9 | 7.9 |
| Hypertensive | 677 | 29.0 | 26.3 | 32.0 | 341 | 30.2 | 25.3 | 35.7 | 336 | 28.0 | 24.7 | 31.5 |
| Antihypertensive medication use | 582 | 25.9 | 23.2 | 28.8 | 286 | 26.7 | 21.9 | 32.2 | 296 | 25.2 | 21.9 | 28.7 |
| Family history of CVD at age ≤60 y | 431 | 20.4 | 17.8 | 23.2 | 186 | 17.4 | 13.9 | 21.5 | 245 | 23.1 | 19.9 | 26.7 |
| LDL cholesterol level ≥3.5 mmol/L | 980 | 47.3 | 43.2 | 51.3 | 520 | 50.9 | 45.0 | 56.8 | 460 | 43.9 | 40.2 | 47.6 |
| Statin medication use | 301 | 13.0 | 10.7 | 15.6 | 167 | 15.0 | 11.5 | 19.4 | 134 | 11.1 | 9.0 | 13.8 |
Source: Combined 2007–2009 and 2009–2011 Canadian Health Measures Survey.
CI indicates confidence interval; CVD, cardiovascular disease; LDL, low-density lipoprotein.
Use with caution (coefficient of variation 16.6% to 33.3%).
Mean CVD Risk and Risk Stratification
With use of the modified FRS and US pooled cohort equations, the baseline risk of CVD was calculated for Canadians and the population was risk stratified as described in the Methods section. Table4 shows mean CVD risk scores and the risk stratification for both algorithms by sex. Compared with the modified FRS, the mean 10-year CVD risk calculated by using the pooled cohort equations was lower (6.6% versus 12.4%). In addition, the pooled cohort equations classified more individuals as high risk (28.6% versus 24.6%). Table4 shows that the risk classification for the 2 algorithms also differs among men. With use of the pooled cohort equations, a greater proportion of men would be classified as high risk (42.4% versus 34.3%) and a similar proportion of women would be classified as high-risk (15.9% versus 15.6%).
Table 4.
Average Risk Scores and Percent Distribution Across Risk Categories by Modified Framingham and Pooled Cohort Risk Categories, by Sex
| Total | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | 95% CI | |||||||
| Modified Framingham Risk Score | |||||||||
| Mean score | 12.4 | 11.5 | 13.3 | 17.1 | 15.6 | 18.7 | 8.0* | 7.3 | 8.7 |
| Risk categories | |||||||||
| Low risk (<10%) | 56.9 | 52.8 | 60.8 | 42.1 | 36.4 | 48.0 | 70.5* | 66.1 | 74.5 |
| Medium risk (10<20%) | 18.5 | 15.9 | 21.6 | 23.6 | 19.1 | 28.8 | 13.9* | 10.8 | 17.7 |
| High risk (≥20%) | 24.6 | 22.0 | 27.4 | 34.3 | 30.3 | 38.9 | 15.6* | 13.2 | 18.4 |
| Pooled Cohort Equations | |||||||||
| Mean score | 6.6 | 6.1 | 7.1 | 9.0 | 8.2 | 9.9 | 4.3* | 3.9 | 4.8 |
| Risk categories | |||||||||
| Low risk (<7.5%) | 71.4 | 68.8 | 74.0 | 57.6 | 52.6 | 62.5 | 84.1* | 81.5 | 86.3 |
| High risk (≥7.5%) | 28.6 | 26.0 | 31.4 | 42.4 | 37.5 | 47.4 | 15.9* | 13.7 | 18.5 |
Source: Combined 2007–2009 and 2009–2011 Canadian Health Measures Survey.
CI indicates confidence interval.
Significantly different from estimate for men (P<0.05).
Statin Eligibility
Overall, 37.7% (or 4.9 million [95% CI 4.4 to 5.5 million]) of Canadians aged 40 to 75 years were eligible for statin therapy under the CCS guidelines compared with 33.0% (4.3 million [95% CI 3.9 to 4.8 million]) under the ACC-AHA guidelines (Table5). By risk group, 2.0%, 64.3%, and 100.0% of low-, intermediate-, and high-risk individuals would be recommended for treatment, respectively, under the CCS guidelines. According to the ACC-AHA guidelines, 6.2% of low-risk and 100.0% of high-risk individuals would be recommended for treatment (Table5). Figure1 shows statin eligibility overall and by sex.
Table 5.
Proportion of Individuals Recommended for Statin Therapy According to CSS and ACC-AHA Guidelines and 10-Year CVD Risk Categories, by Sex
| All, 40 to 75 y | Men, 40 to 75 y | Women, 40 to 75 y | |||||||
|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | ||||
| CSS guidelines | |||||||||
| Overall | 37.7 | 34.2 | 41.3 | 48.4 | 43.2 | 53.6 | 27.8 | 23.9 | 32.0 |
| Low risk (<10%) | 2.0* | 1.1 | 3.7 | † | NA | NA | <4.8‡ | NA | NA |
| Medium risk (10%<20%) | 64.3 | 56.1 | 71.7 | 57.2 | 46.9 | 66.9 | 75.2 | 63.8 | 84.0 |
| High risk (≥20%) | 100.0 | NA | NA | 100.0 | NA | NA | 100.0 | NA | NA |
| ACC-AHA guidelines | |||||||||
| Overall | 33.0 | 29.8 | 36.4 | 44.5 | 39.3 | 49.8 | 22.5 | 19.4 | 25.9 |
| Low risk (<7.5%) | 6.2 | 4.4 | 8.7 | <8.0‡ | NA | NA | 7.8* | 5.0 | 11.7 |
| High risk (≥7.5%) | 100.0 | NA | NA | 100.0 | NA | NA | 100.0 | NA | NA |
Source: Combined 2007–2009 and 2009–2011 Canadian Health Measures Survey.
ACC-AHA indicates American College of Cardiology and American Heart Association; CI, confidence interval; CSS, Canadian Cardiovascular Society; CVD, cardiovascular disease; NA; not applicable.
Use with caution (coefficient of variation 16.6% to 33.3%). †Estimate suppressed due to small sample size. ‡If coefficient of variation of estimate exceeds 33%, estimate is indicated as being less than upper limit of 95% CI. The total number of people aged 40 to 75 years old recommended for statin use are 4.9 million, 95% CI 4.4 to 5.5 million, according to the Modified Framingham risk algorithm and CCS guidelines and 4.3 million, 95% CI 3.9 to 4.8 million, according to the US pooled risk algorithm and ACC-AHA guidelines.
Figure 1.
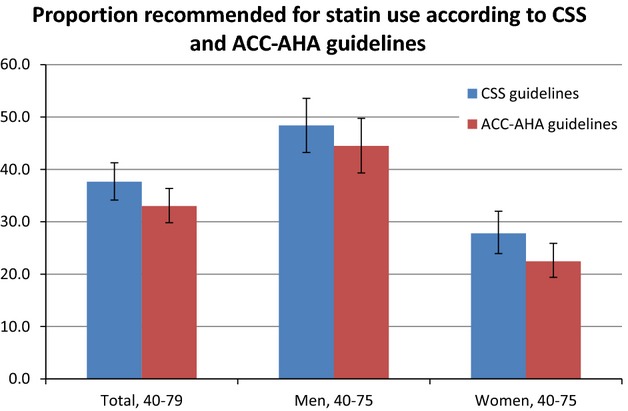
The proportion of Canadians without cardiovascular disease aged 40 to 75 years who would be recommended for statin therapy according to the CSS (blue bar) and ACC-AHA (red bar) guidelines, by sex. ACC-AHA indicates American College of Cardiology and the American Heart Association; CSS, Canadian Cardiovascular Society.
Discordance Between the CCS and ACC-AHA Guidelines
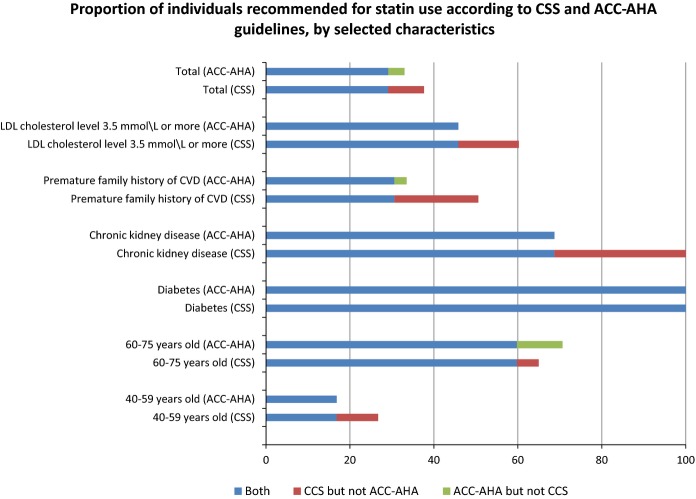
Despite the fact that a similar proportion of Canadians would be treated under both guidelines, further analysis revealed that there was discordance between certain subgroups for whom treatment would be recommended. Table6 shows the sociodemographic and CVD risk factor characteristics of 4 subgroups of individuals. While the majority of individuals recommended for treatment would be treated under both guidelines (column B), a small proportion of those identified in the CCS guidelines would not be treated under the ACC-AHA guidelines (column C), and vice versa (column D). Specifically, 29.1% of the Canadian population aged 40 to 75 without CVD would be treated under both sets of recommendations. An additional 8.5% would be treated under the CCS guidelines only, while an additional 3.9% would be treated under the ACC-AHA guidelines only. Figure2 summarizes important differences between the guidelines. Where there is discordance, older individuals are more likely to be recommended for treatment by ACC-AHA guidelines, while individuals with LDL ≥3.5 mmol/L, premature family history of CVD, or chronic kidney disease are more likely to be recommended for treatment by CCS guidelines.
Table 6.
Proportion of Individuals Recommended Statin Therapy According to Concordant and Discordant Recommendations, by Selected Characteristics
| Concordant Recommendations | Discordant Recommendations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neither Recommended Statin Therapy | Both Recommended Statin Therapy | Recommended by CCS Guidelines but Not ACC-AHA | Recommended by ACC-AHA Guidelines but Not CCS | |||||||||
| Column | A | 95% CI | B | 95% CI | C | 95% CI | D | 95% CI | ||||
| Population (millions) | 7.6 | 7.1 | 8.1 | 3.8 | 3.3 | 4.3 | 1.1 | 0.8 | 1.3 | 0.5 | 0.3 | 0.6 |
| Proportion, % | 58.5 | 54.8 | 62.0 | 29.1 | 26.0 | 32.5 | 8.5 | 7.0 | 10.3 | 3.9 | 2.9 | 5.1 |
| Age group, % | ||||||||||||
| 40 to 59 y | 88.2 | 85.7 | 90.2 | 41.3 | 35.4 | 47.4 | 82.6 | 76.0 | 87.8 | † | NA | NA |
| 60 to 75 y | 11.8 | 9.8 | 14.3 | 58.7 | 52.6 | 64.6 | 17.4 | 12.2 | 24.0 | 80.1 | 61.9 | 90.9 |
| Sex, % | ||||||||||||
| Men | 37.9 | 35.0 | 41.0 | 64.4 | 60.0 | 68.5 | 51.8 | 40.7 | 62.8 | 65.8 | 54.2 | 75.8 |
| Women | 62.1 | 59.0 | 65.0 | 35.6 | 31.5 | 40.0 | 48.2 | 37.2 | 59.3 | 34.2 | 24.2 | 45.8 |
| Daily or occasional smoker, % | 19.9 | 15.9 | 24.6 | 24.2 | 18.0 | 31.7 | 19.7* | 12.4 | 30.0 | 28.2* | 16.1 | 44.6 |
| Diabetes, % | 0.0 | NA | NA | 29.9 | 23.8 | 36.9 | 0.0 | NA | NA | 0.0 | NA | NA |
| Chronic kidney disease, % | 0.0 | NA | NA | 10.6* | 7.3 | 15.2 | 16.5* | 9.5 | 27.0 | 0.0 | NA | NA |
| Hypertensive, % | 9.0 | 6.9 | 11.6 | 67.6 | 60.8 | 73.7 | 28.7 | 20.6 | 38.5 | 42.0 | 29.5 | 55.6 |
| Antihypertensive medication use, % | 7.9 | 5.9 | 10.5 | 62.6 | 54.4 | 68.3 | 24.3* | 16.6 | 34.0 | 32.9* | 20.7 | 47.9 |
| Family history of CVD at age ≤60 y, % | 16.2 | 13.6 | 19.2 | 21.4 | 17.1 | 26.4 | 47.8 | 37.2 | 58.6 | 15.2* | 7.9 | 27.3 |
| LDL cholesterol level ≥3.5 mmol/L, % | 32.1 | 28.1 | 36.4 | 74.0 | 65.7 | 81.4 | 79.9 | 70.2 | 87.1 | † | — | — |
Source: Combined 2007–2009 and 2009–2011 Canadian Health Measures Survey.
ACC-AHA, American College of Cardiology and American Heart Association; CI, confidence interval; CSS, Canadian Cardiovascular Society; CVD, cardiovascular disease; LDL, low-density lipoprotein; NA, not applicable.
Use with caution (coefficient of variation 16.6% to 33.3%). †Estimate suppressed due to small sample size.
Figure 2.
The proportion of Canadians without cardiovascular disease aged 40 to 75 years who would be recommended for statin therapy by both guidelines (blue bar), who would be recommended by the ACC-AHA guidelines but not the CCS guidelines (green bar) and who would be recommended by the CCS guidelines but not the ACC-AHA guidelines (red bar), by selected characteristics. ACC-AHA indicates American College of Cardiology and the American Heart Association; CSS, Canadian Cardiovascular Society; CVD, cardiovascular disease; LDL, low-density lipoprotein.
Discussion
In comparing the CCS and ACC-AHA lipid treatment guidelines, we found that a similar proportion and number of Canadians would be eligible for statin treatment under the 2 sets of recommendations. In fact, the current CCS guidelines recommend that a slightly higher proportion of Canadians receive statin therapy. For the most part, a similar proportion are recommended statins within subgroups; however, under the CCS guidelines, more people are recommended statins who are younger, have LDL levels of ≥3.5 mmol/L, have a family history of CVD, or have chronic kidney disease. ACC-AHA guidelines recommend more treatment for people who are older (aged ≥60 years).
Despite the overall similar treatment rates determined by the 2 sets of guidelines, there are several major differences between the CCS and ACC-AHA: risk calculation, statin recommendations based on lipid thresholds (triggers), and treatment targets. For risk calculation, the average 10-year risk of CVD was almost twice as high under the CCS guidelines using the modified FRS compared with the ACC-AHA guidelines using the pooled cohort equations. It appears that there are several factors contributing to the increased risk estimation. First and perhaps most important, the modified FRS includes a broader range of outcomes than the pooled cohort equations. The focus on hard ASCVD rather than total CVD events is a departure from the previous version of the American guidelines, which also used the modified FRS.20 In the ACC-AHA guideline document, Goff et al reasoned that ASCVD was of greater relevance to both patients and providers.4 More specifically, the Systematic Evidence Review document from the Risk Assessment Working Group stated that scores using composite end points that included CVD events that are less severe, difficult to diagnose reliably, or subject to significant variability depending on practice patterns were considered suboptimal.21 The doubling of risk among eligible patients with a positive family history of CVD also likely contributes to higher risk estimation with the FRS. Also, differences in the cohorts used to derive the risk algorithms may influence overall risk estimation. While the FRS (not modified for family history) has been extensively validated,22–24 to date only 2 studies have attempted to validate the pooled cohort equations.25,26 Importantly, neither risk assessment method has been explicitly calibrated in the Canadian population.
Lack of LDL-C treatment triggers is the second major difference between the CCS and ACC-AHA guidelines. In the ACC-AHA guidelines, the LDL-C triggers were eliminated, resulting in everybody with a calculated risk ≥7.5% being recommended statins regardless of their LDL-C level. This is analogous to treating all the high-risk patients and many of the intermediate-risk patients identified under the CCS guidelines.
The third major difference between the CCS and ACC-AHA guidelines is a lack of LDL-C treatment targets. Treatment targets were eliminated by the ACC-AHA because the evidence to support their use was not of the highest quality (ie, not gleaned from clinical trials).6 In addition, this approach simplifies treatment for primary care physicians and patients. While the CCS considered eliminating targets in the latest version of the guidelines, ultimately they were retained for a number of reasons.6
These differences have important implications for guideline writers in Canada, and although the treatment rates would not be dramatically different under the ACC-AHA guidelines, the risk assessment/risk stratification method, (lack of) treatment triggers and targets, and the subpopulations of patients eligible for treatment would differ. Translating these changes for primary care physicians would be challenging given that the FRS has been in use for many years and has been integrated into many electronic medical records across the country. Moreover, risk assessment tools are likely underused, and the existing CCS guidelines are not yet optimally implemented in Canada, resulting in significant treatment gaps still existing for high- and intermediate-risk Canadians.27,28 A recent review by Morris et al suggests that this is not a uniquely Canadian problem; she concluded that confusion regarding multiple published guidelines in the United States has contributed to an inadequate number of patients receiving potentially beneficial statin therapy.29 While guidelines for CVD risk assessment and statin treatment are certainly necessary, so, too, is knowledge translation related to the benefits of risk assessment and the framing of risk discussions with patients.
Other studies have compared risk calculation/stratification and statin eligibility under the ACC-AHA guidelines to other national recommendations and previous US guidelines (the Third Adult Treatment Panel).30,31 Specifically, Vaucher and colleagues showed, by using a population-based sample, that, compared with guidelines from the European Society of Cardiology, the ACC-AHA recommendations would lead to a considerable increase in the number of individuals recommended statins.30 Also using a population-based sample, Pencina et al showed that the updated AHA-ACC guidelines would increase the number of Americans eligible for statins, by ≈13 million, to 48.6% of the population.30,31 Our results differ from those of Vaucher and Pencina and colleagues, suggesting that the CCS guidelines are more similar to the updated ACC-AHA than to the European Society of Cardiology and Third Adult Treatment Panel recommendations. The CCS guidelines likely increased the number of patients treated in Canada when they changed from the earlier version of the FRS32 to the total cardiovascular risk model of D’Agostino et al3 with the 2009 iteration of the guidelines.33 However, the application of the ACC-AHA guidelines to the Canadian population results in a lower overall proportion eligible for treatment (33.0%) compared with the US population (48.6%). This difference is likely due to the higher prevalence of CVD risk factors in the United States, as well as differences in the ethnic profile between the United States and Canada. Pencina et al showed that the US adult population aged 40 to 75 years had a substantially higher proportion with diabetes (20.6% versus 8.7%) and hypertension (46.0% versus 29.0%), compared with the Canadian population aged 40 to 75 years assessed in this study.31
Limitations
This study has limitations. First, this was not a study of CVD outcomes or an attempt to calibrate either risk algorithm in the Canadian population; rather, we were interested in determining the theoretical statin eligibility among Canadians aged 40 to 75, without CVD, by using both the latest Canadian and US lipid treatment guidelines. For the purposes of this comparison, we assumed that statin eligibility equaled statin treatment; however, both the CCS and ACC-AHA recommend that physicians and patients discuss the risks and benefits before treatment initiation. Without taking this risk discussion into account, we overestimated the “real” treatment rate.
Even though we combined 2 cycles of the CHMS survey, some of the estimates of interest were not reportable due to small sample sizes, and because the CHMS was designed to produce national estimates, it was not possible to examine cardiovascular risk and statin eligibility by province. In addition, the combined cycle 1-cycle 2 overall response rate to the CHMS was 53.5%,9 and although applying the survey weights ensured that the sample was representative of the target population, bias might exist if nonrespondents differed systematically from respondents.9 CHMS collection is ongoing, and as these data accumulate it will be possible to monitor eligibility for commonly used medications in an ongoing way. Because some of the variables used in this analysis depended on self-report, such as family history of CVD, the prevalence of family history of CVD in the Canadian population was likely underestimated.
Conclusions
This study has several implications. Although no change to the CCS guidelines is currently contemplated,6 this analysis provides information for clinicians and guideline writers as to the impacts of adopting the updated ACC-AHA guidelines in Canada. Implementing the ACC-AHA lipid treatment guidelines in Canada would not result in an increase in individuals eligible for statin treatment. In fact, the proportion of the population recommended for statin treatment would decrease slightly and be targeted at different subgroups of the population. Another important consideration is the ongoing validation of the pooled cohort equations, especially in the Canadian population, which has a different risk factor and ethnic profile compared with the United States. In this study, we produced estimates of the percentage of the Canadian population aged 40 to 75 years eligible for statin therapy; however, in reality, treatment guidelines do not reflect current treatment practices. An opportunity exists to make gains in population cardiovascular health by increasing uptake of statin treatment among intermediate- and higher-risk Canadians, whether that risk is calculated via the modified FRS or the pooled cohort equations.
Acknowledgments
The authors would like to acknowledge Margot Shields for providing the SAS code.
Disclosures
Drs Hennessy, Bushnik, and Manuel have no disclosures. Dr Anderson has been involved in lipid lowering research studies funded by Merck and AMGEN.
References
- Rotermann M, Sanmartin C, Hennessy D, Arthur M. Prescription medication use by Canadians aged 6 to 79. Health Rep. 2014;25:3–9. [PubMed] [Google Scholar]
- Anderson TJ, Grégoire J, Hegele RA, Couture P, Mancini GB, McPherson R, Francis GA, Poirier P, Lau DC, Grover S, Genest J, Jr, Carpentier AC, Dufour R, Gupta M, Ward R, Leiter LA, Lonn E, Ng DS, Pearson GJ, Yates GM, Stone JA, Ur E. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29:151–167. doi: 10.1016/j.cjca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Grégoire J, Hegele RA, Couture P, Mancini GB, McPherson R, Francis GA, Poirier P, Lau DC, Grover S, Genest J, Jr, Carpentier AC, Dufour R, Gupta M, Ward R, Leiter LA, Lonn E, Ng DS, Pearson GJ, Yates GM, Stone JA, Ur E. Are the ACC/AHA guidelines on the treatment of blood cholesterol a game changer? A perspective from the Canadian Cardiovascular Society Dyslipidemia Panel. Can J Cardiol. 2014;30:377–380. doi: 10.1016/j.cjca.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762–1765. doi: 10.1016/S0140-6736(13)62388-0. [DOI] [PubMed] [Google Scholar]
- Day B, Langlois R, Tremblay M, Knoppers BM. Canadian Health Measures Survey: ethical, legal and social issues. Health Rep. 2007;18:37–51. [PubMed] [Google Scholar]
- Statistics Canada. 2013. Ottawa CHMS documentation. Available at: http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=5071&lang=en&db=imdb&adm=8&dis=2. Accessed March 15, 2014.
- Tremblay M, Langlois R, Bryan S, Esliger D, Patterson J. Canadian Health Measures Survey pre-test: design, methods, results. Health Rep. 2007;18:21–30. [PubMed] [Google Scholar]
- Tremblay M, Wolfson M, Connor Gorber S. Canadian Health Measures Survey: rationale, background and overview. Health Rep. 2007;18:7–20. [PubMed] [Google Scholar]
- Tremblay MS, Connor Gorber S. Canadian Health Measures Survey: brief overview. Can J Public Health. 2007;98:453–456. doi: 10.1007/BF03405437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux S, Labrecque F, Quigley A. 2013. Ottawa Statistics Canada Sampling documentation for cycle 2 of the Canadian Health Measures Survey. Methodology Branch Working Paper, HSM –2013–002E/F.
- Lloyd-Jones DM, Nam BH, D’Agostino RB, Sr, Levy D, Murabito JM, Wang TJ, Wilson PW, O’Donnell CJ. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA. 2004;291:2204–2211. doi: 10.1001/jama.291.18.2204. [DOI] [PubMed] [Google Scholar]
- Edwards JE, Moore RA. Statins in hypercholesterolaemia: a dose-specific meta-analysis of lipid changes in randomised, double blind trials. BMC Fam Pract. 2003;4:18. doi: 10.1186/1471-2296-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattu GS, Perry TL, Jr, Wright JM. Comparison of the oscillometric blood pressure monitor (BPM-100(Beta)) with the auscultatory mercury sphygmomanometer. Blood Press Monit. 2001;6:153–159. doi: 10.1097/00126097-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Wright JM, Mattu GS, Perry TL, Jr, Gelferc ME, Strange KD, Zorn A, Chen Y. Validation of a new algorithm for the BPM-100 electronic oscillometric office blood pressure monitor. Blood Press Monit. 2001;6:161–165. doi: 10.1097/00126097-200106000-00008. [DOI] [PubMed] [Google Scholar]
- Bryan S, Saint-Pierre Larose M, Campbell N, Clarke J, Tremblay MS. Resting blood pressure and heart rate measurement in the Canadian Health Measures Survey, cycle 1. Health Rep. 2010;21:71–78. [PubMed] [Google Scholar]
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- ACC-AHA Risk Assessment Working Group. 2013. Report on the assessment of cardiovascular risk: full work group report supplement. Available at://jaccjacc.cardiosource.com/acc_documents/2013_FPR_S5_Risk_Assesment.pdf. Accessed December 19, 2014.
- Eichler K, Puhan MA, Steurer J, Bachmann LM. Prediction of first coronary events with the Framingham score: a systematic review. Am Heart J. 2007;153:722–731. doi: 10.1016/j.ahj.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Hemann BA, Bimson WF, Taylor AJ. The Framingham Risk Score: an appraisal of its benefits and limitations. Am Heart Hosp J. 2007;5:91–96. doi: 10.1111/j.1541-9215.2007.06350.x. [DOI] [PubMed] [Google Scholar]
- D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- Muntner P, Colantonio LD, Cushman M, Goff DC, Jr, Howard G, Howard VJ, Kissela B, Levitan EB, Lloyd-Jones DM, Safford MM. Validation of the atherosclerotic cardiovascualr disease Pooled Cohort risk equations. JAMA. 2014;311:1406–1415. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavousi M, Leening MJG, Nanchen D, Greenland P, Graham IM, Streyerberg EW, Ikram MA, Stricker BH, Hofman A, Franco OH. Comparison of application of the ACC/AHA guidelines, Adult Treatment Panel III Guidelines, and European Society of Cardiology guidelines for cardiovascular disease prevention in a European Cohort. JAMA. 2014;311:1416–1423. doi: 10.1001/jama.2014.2632. [DOI] [PubMed] [Google Scholar]
- Müller-Riemenschneider F, Holmberg C, Rieckmann N, Kliems H, Rufer V, Müller-Nordhorn J, Willich SN. Barriers to routine risk-score use for healthy primary care patients: survey and qualitative study. Arch Intern Med. 2010;170:719–724. doi: 10.1001/archinternmed.2010.66. [DOI] [PubMed] [Google Scholar]
- Joffres M, Shields M, Tremblay MS, Connor Gorber S. Dyslipidemia prevalence, treatment, control, and awareness in the Canadian Health Measures Survey. Can J Public Health. 2013;104:e252–e257. doi: 10.17269/cjph.104.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris PB, Ballantyne CM, Birtcher KK, Dunn SP, Urbina E. Review of clinical practice guidelines for the management of LDL-related risk. J Am Coll Cardiol. 2014;64:196–206. doi: 10.1016/j.jacc.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Vaucher J, Marques-Vidal P, Preisig M, Waeber G, Vollenweider P. Population and economic impact of the 2013 ACC/AHA guidelines compared with European guidelines to prevent cardiovascular disease. Eur Heart J. 2014;35:958–959. doi: 10.1093/eurheartj/ehu064. [DOI] [PubMed] [Google Scholar]
- Pencina MJ, Navar-Boggan AM, D’Agostino RB, Sr, Williams K, Neely B, Sniderman AD, Peterson ED. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370:1422–1431. doi: 10.1056/NEJMoa1315665. [DOI] [PubMed] [Google Scholar]
- Wilson PWF, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- Armstrong DW, Brouillard D, Matangi MF. The effect of the change in the Framingham Risk Score calculator between the 2006 and 2009 Canadian lipid guidelines. Can J Cardiol. 2011;27:167–170. doi: 10.1016/j.cjca.2010.12.025. [DOI] [PubMed] [Google Scholar]