Abstract
Background
Cardiovascular disease and cancer increasingly coexist, yet relationships between cancer and long-term cardiovascular outcomes post–percutaneous coronary intervention (PCI) are not well studied.
Methods and Results
We examined stented PCI patients at Duke (1996–2010) using linked data from the Duke Information Systems for Cardiovascular Care and the Duke Tumor Registry (a cancer treatment registry). Our primary outcome was cardiovascular mortality. Secondary outcomes included composite cardiovascular mortality, myocardial infarction, or repeat revascularization and all-cause mortality. We used adjusted cause-specific hazard models to examine outcomes among cancer patients (cancer treatment pre-PCI) versus controls (no cancer treatment pre-PCI). Cardiovascular mortality was explored in a cancer subgroup with recent (within 1 year pre-PCI) cancer and in post-PCI cancer patients using post-PCI cancer as a time-dependent variable. Among 15 008 patients, 3.3% (n=496) were cancer patients. Observed rates of 14-year cardiovascular mortality (31.4% versus 27.7%, P=0.31) and composite cardiovascular death, myocardial infarction, or revascularization (51.1% versus 55.8%, P=0.37) were similar for cancer versus control groups; all-cause mortality rates were higher (79.7% versus 49.3%, P<0.01). Adjusted risk of cardiovascular mortality was similar for cancer patients versus controls (hazard ratio 0.95; 95% CI 0.76 to 1.20) and for patients with versus without recent cancer (hazard ratio 1.46; 95% CI 0.92 to 2.33). Post-PCI cancer, present in 4.3% (n=647) of patients, was associated with cardiovascular mortality (adjusted hazard ratio 1.51; 95% CI 1.11 to 2.03).
Conclusions
Cancer history was present in a minority of PCI patients but was not associated with worse long-term cardiovascular outcomes. Further investigation into PCI outcomes in this population is warranted.
Keywords: cancer, cardiovascular outcomes, percutaneous coronary intervention
Percutaneous coronary intervention (PCI) is the most common form of coronary revascularization. PCI can provide relief from angina and, in certain clinical scenarios, improve survival.1 Multiple prediction models have been developed to help understand outcomes after PCI and to guide clinical decision-making. Many models have examined short-term outcomes, focusing on in-hospital mortality.2–5 Several studies have assessed outcomes at 1 year or longer3,6,7; most recently, prediction models were developed for survival up to 3 years post-PCI among Medicare beneficiaries.7 Although these studies have consistently identified clinical comorbidities as significant predictors of post-PCI mortality, none have accounted for cancer history in their models.
Cardiovascular disease and cancer are the leading causes of death in developed countries worldwide, together accounting for ≈70% of disease-related mortality.8 Improved clinical outcomes in both fields have resulted from better risk factor modification, earlier disease detection, and advances in therapies.9,10 The high prevalence of these diseases puts cancer survivors and patients with new diagnoses undergoing active oncologic therapy at risk for coronary artery disease (CAD) requiring cardiovascular treatments, such as PCI.11,12 Despite the growing incidence of coexisting CAD and cancer, there is a lack of reliable data on outcomes after PCI in patients with a history of cancer. Details about cancer history are not typically collected in PCI registries, and patients with prior cancer are often excluded from PCI clinical trials, precluding examinations of the relationship between cancer and post-PCI outcomes. Therefore, we leveraged an established PCI database, the Duke Information Systems for Cardiovascular Care (DISCC), and a cancer treatment registry, the Duke Tumor Registry (DTR), at a large academic medical center to explore this issue. Using linked information from these databases, our goals were to (1) characterize the prevalence of cancer among patients undergoing PCI; and (2) examine the relationship between cancer and long-term cardiovascular outcomes after PCI.
Methods
Data Sources and Patient Population
DISCC is a database of patients undergoing cardiac catheterization and/or cardiac surgery at Duke University Medical Center.13 Patients with obstructive CAD are followed routinely for myocardial infarction (MI), coronary revascularization, and mortality at 6 months, 1 year, and annually thereafter. Cause of death is adjudicated by faculty cardiologists using hospital records and the National Death Index. Study patient demographics, clinical features, and PCI procedure characteristics were obtained from DISCC.
DTR is a registry of all patients treated for cancer at Duke. The DTR provides detailed information regarding cancer type and stage and cancer treatments administered. Data in DTR are abstracted by trained registrars and submitted to the North Carolina Central Cancer Registry in compliance with state reporting requirements. Linkage between DISCC and DTR allowed us to identify patients undergoing PCI who were also treated for cancer at Duke. This study was approved by the Duke Institutional Review Board and qualified for a waiver of informed consent.
Study Sample
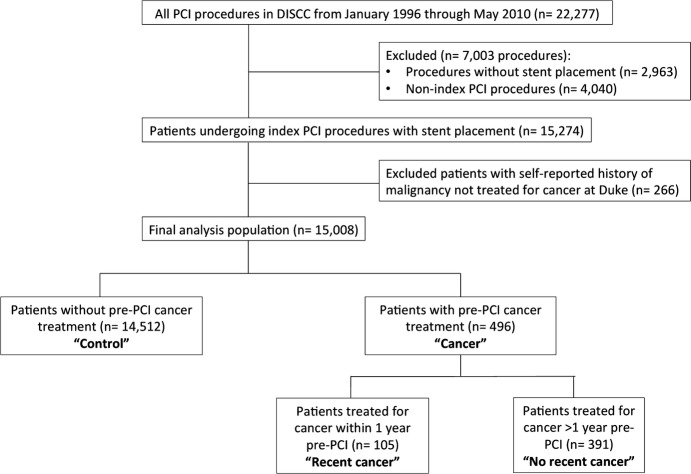
We examined all PCI procedures in DISCC from January 1996 through May 2010 (n=22 277). We excluded patients without stent placement (n=2963), since these patients were likely different, and postprocedural treatments (eg, use of dual antiplatelet therapy), which would differ after balloon angioplasty versus stent placement. For patients with multiple PCI procedures captured in DISCC, the first PCI at Duke was considered the index procedure. Patients with self-reported cancer in DISCC, but not treated at Duke per DTR data, were also excluded (n=266), as cancer treatment timing was missing. After these exclusions, the final analysis population consisted of 15 008 patients (Figure1).
Figure 1.
Patient selection. Flow diagram of patient selection from initial study population through exclusions to the final study population. DISCC indicates Duke Information Systems for Cardiovascular Care; PCI, percutaneous coronary intervention.
Outcomes and Definitions
Our primary outcome was cardiovascular mortality after index PCI through the duration of DISCC follow-up. Secondary end points included composites of cardiovascular mortality or MI and cardiovascular mortality, MI, or repeat coronary revascularization (PCI or coronary artery bypass grafting). Individual components of composite end points and all-cause mortality were also secondary end points.
As a treatment registry, the DTR provides information regarding cancer treatment (chemotherapy, radiation, and surgery) performed at Duke, but does not provide timing of malignancy diagnosis per se; therefore, we used the timing of cancer treatment to categorize patients identified in DTR as “cancer” (any cancer treatment pre-PCI) or “control” (no cancer treatment pre-PCI). We identified a subgroup of patients in the cancer group with “recent cancer” (cancer treatment within 1 year pre-PCI) and also defined “post-PCI cancer” patients as those receiving any cancer treatment after the index PCI.
Statistical Analysis
Baseline characteristics were examined by a history of cancer treatment documented in DTR prior to the index PCI. Categorical variables were presented as counts and proportions, and continuous variables were presented as medians with interquartile ranges (IQRs). We examined unadjusted cumulative incidence of post-PCI outcomes in (1) cancer versus control patients; and (2) recent cancer versus control patients. Curves of cumulative incidence versus time from index PCI were reported; groups were compared with the Gray test.
Next, we examined our primary outcome, cardiovascular mortality, after adjusting for baseline characteristics at index PCI. This was performed with a multivariable cause-specific proportional hazard model censoring patients at the time of noncardiovascular death using a published model for all-cause mortality after coronary revascularization developed from DISCC.14 The following variables were included in this model: age, sex, race, body mass index, smoking history, diabetes, hypertension, renal failure, chronic obstructive pulmonary disease, valvular heart disease, vascular disease index (1 point each for cerebrovascular disease, peripheral artery disease, or carotid bruits), liver disease, connective tissue disease, presentation with acute coronary syndrome, systolic blood pressure, duration of CAD, congestive heart failure on presentation, New York Heart Association class, ventricular gallop, number of diseased vessels, and year of index PCI. Mitral insufficiency grade and left ventricular ejection fraction (LVEF), which were variables in the original model, were not included in primary analyses due to high rates of missing data (28% each). To this model, we added pre-PCI cancer treatment and reported the adjusted cause-specific hazard ratio (HR) and associated 95% CI for this parameter. Proportional hazards assumptions with respect to cancer treatment prior to PCI and cancer treatment within 1 year prior to PCI were tested by adding a time-dependent covariate to the adjusted regression model that allowed for the association to vary as a linear function of time from PCI, and no significant violations were found.
Additional prespecified analyses were performed to explore the association of cancer with post-PCI cardiovascular outcomes. We performed sensitivity analyses including LVEF and patients with self-reported history of cancer not treated at Duke, and excluding post-PCI cancer patients (n=647). We also conducted landmark analyses beginning 30 days post-PCI to avoid potential bias related to short-term mortality. Finally, we performed exploratory analyses including postindex PCI cancer treatment as a time-dependent covariate to determine (1) unadjusted cause-specific HRs using Cox proportional hazards models for clinical outcomes; and (2) adjusted cause-specific HRs for cardiovascular mortality, which was our primary outcome.
Statistical tests were 2-sided, and a P-value <0.05 was considered statistically significant. Analyses were performed at the Duke Clinical Research Institute using SAS (version 9.2; SAS Institute, Cary, NC) and STATA (Release 11; StataCorp, College Station, TX).
Results
Baseline Characteristics
Of 15 008 patients, 3.3% (n=496) were cancer patients, and 96.7% (n=14 512) were controls (Figure1). Among the cancer group, 21.1% (n=105) had recent cancer. The distribution of malignancy types among the 496 cancer patients is shown in Table S1. The 3 most commonly treated malignancies were prostate, lung, and breast cancer. Cancer patients were older, more often male, and more often of white race compared with control patients (Table1). There was a higher proportion of prior cerebrovascular disease and heart failure in the cancer group, although LVEF was not markedly different between groups. Compared with controls, cancer patients were more likely to have 3-vessel CAD. The proportion of cancer patients undergoing PCI increased during the latter two thirds of the study. From 2003 to 2010, when drug-eluting stents were available, a greater proportion of cancer versus control patients received bare metal stents.
Table 1.
Baseline Patient and Procedural Characteristics
| No Pre-PCI Cancer (“Control”) n=14 512 | Pre-PCI Cancer (“Cancer”) n=496 | |
|---|---|---|
| Demographics | ||
| Median age, y | 62 (53, 71) | 68 (61, 75) |
| Female sex, % | 4926 (33.9) | 142 (28.6) |
| White race, % | 10 754 (75.7) | 397 (80.4) |
| Median BMI, kg/m2 | 28 (25, 32) | 28 (25, 31) |
| Medical history | ||
| Prior congestive heart failure, % | 2191 (15.4) | 99 (20.2) |
| Prior myocardial infarction, % | 7414 (51.1) | 246 (49.6) |
| Diabetes mellitus, % | 4013 (27.7) | 129 (26.0) |
| Hypertension, % | 9478 (65.3) | 334 (67.3) |
| Renal insufficiency, % | 254 (1.8) | 12 (2.4) |
| Prior chronic obstructive lung disease, % | 793 (5.5) | 29 (5.8) |
| Prior cerebrovascular disease, % | 1196 (8.2) | 66 (13.3) |
| Prior peripheral artery disease, % | 1238 (8.5) | 46 (9.3) |
| Vascular index* | ||
| 0 | 11 918 (82.1) | 386 (77.8) |
| 1 | 1995 (13.7) | 87 (17.5) |
| 2 | 475 (3.3) | 19 (3.8) |
| 3 | 124 (0.9) | 4 (0.8) |
| History of smoking, % | 7712 (53.1) | 238 (48.0) |
| Liver disease, % | 68 (0.5) | 2 (0.4) |
| Connective tissue disease, % | 80 (0.6) | 2 (0.4) |
| Valvular heart disease, % | 31 (0.2) | 2 (0.4) |
| Presentation features | ||
| Acute coronary syndrome, %† | 10 481 (72.4) | 329 (66.6) |
| Median ejection fraction (expressed as %) | 56 (45, 62) | 56 (45, 62) |
| Median SBP, mm Hg | 142 (126, 161) | 145 (128, 166) |
| NYHA functional class, % | ||
| 0 to 1 | 12 787 (90.8) | 420 (85.9) |
| 2 | 438 (3.1) | 25 (5.1) |
| 3 | 554 (3.9) | 31 (6.3) |
| 4 | 300 (2.1) | 13 (2.7) |
| Procedure features | ||
| Year of index PCI, % | ||
| 1996–2000 | 5504 (37.9) | 104 (21.0) |
| 2001–2005 | 5721 (39.4) | 226 (45.6) |
| 2006–2010 | 3287 (22.7) | 166 (33.5) |
| Number of diseased vessels, % | ||
| 1 | 8839 (65.4) | 255 (55.8) |
| 2 | 3095 (22.9) | 131 (28.7) |
| 3 | 1574 (11.7) | 71 (15.5) |
| Drug-eluting stent use, %‡ | 4209 (62.1) | 164 (54.5) |
| Number of stents used during index PCI, % | ||
| 1 | 8798 (61.2) | 292 (59.8) |
| 2 | 3718 (25.8) | 131 (26.8) |
| ≥3 | 1278 (8.9) | 47 (9.6) |
| Number of vessels treated during index PCI, % | ||
| 1 | 12 294 (84.7) | 411 (82.9) |
| 2 | 2025 (14.0) | 77 (15.5) |
| 3 | 117 (0.8) | 4 (0.8) |
| Target vessel treated during index PCI, % | ||
| Right coronary artery | 6060 (41.8) | 223 (45.0) |
| Left circumflex artery | 4482 (30.9) | 147 (29.6) |
| Left anterior descending artery | 5759 (39.7) | 194 (39.1) |
| Left main artery | 284 (2.0) | 10 (2.0) |
| Discharge medications, % | ||
| ACE-I | 9803 (67.6) | 350 (70.6) |
| ARB | 1189 (8.2) | 68 (13.7) |
| Aspirin | 14 355 (98.9) | 489 (98.6) |
| β-Bblocker | 13 077 (90.1) | 447 (90.1) |
| Clopidogrel§ | 11 023 (76.0) | 437 (88.1) |
| Warfarin | 569 (3.9) | 26 (5.2) |
ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SBP, systolic blood pressure.
Vascular index=1 point each for peripheral artery disease+cerebrovascular disease+carotid bruit.
Acute coronary syndrome=unstable angina, non–ST-segment elevation myocardial infarction, ST-segment elevation myocardial infarction.
Calculated among procedures performed from 2003 to 2010, when drug-eluting stents were available.
No clopidogrel use prior to Food and Drug Administration approval in November 17, 1997.
Numbers in parentheses represent the 25th and 75th percentiles for continuous variables and percentages for categorical variables.
Outcomes in Cancer Versus Control Groups
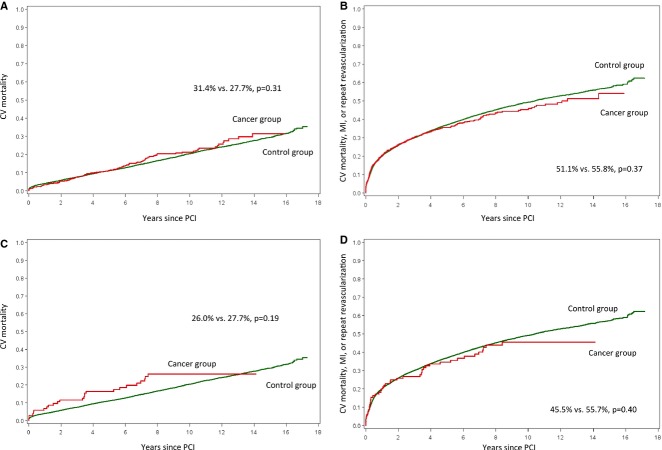
We examined 14-year clinical outcomes, with median follow-up times (interquartile range) to death or last contact in cancer and control groups of 5.3 years (2.6, 8.2) and 7.7 years (4.3, 11.1), respectively. The observed cumulative incidence of the primary outcome, cardiovascular mortality, for cancer versus control patients was similar (31.4% versus 27.7%, P=0.31, Figure2A). As shown in Table2, cumulative incidences of the composites of cardiovascular death or MI and cardiovascular death, MI, or repeat revascularization (Figure2B), and of repeat revascularization alone, were not significantly different. The observed cumulative incidence of MI was significantly lower for cancer versus control patients (8.2% versus 14.1%, P=0.01). Cumulative all-cause mortality was substantially higher than cardiovascular mortality overall and was significantly higher for cancer patients compared with controls (79.7% versus 49.3%, P<0.01). Using multivariable analysis to examine our primary outcome, we found no significant difference in the adjusted cause-specific risk of cardiovascular mortality between cancer and control groups (adjusted HR 0.95; 95% CI: 0.76 to 1.20).
Figure 2.
Clinical outcomes among cancer patients. Displayed are the cumulative incidence curves for (A) CV mortality; and (B) composite CV mortality, MI, or repeat revascularization for cancer patients vs controls. Cumulative incidence curves for (C) CV mortality; and (D) composite CV mortality, MI, or repeat revascularization for patients with vs without recent cancer are also shown. CV indicates cardiovascular; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Table 2.
Unadjusted 14-Year Cumulative Incidence of Outcomes According to Cancer Status
| No Pre-PCI Cancer (“Control”), % n=14 512 | Pre-PCI Cancer (“Cancer”), % n=496 | P Value | No Pre-PCI Cancer Within 1 Year (“No Recent Cancer”), % n=14 903 | Pre-PCI Cancer Within 1 Year (“Recent Cancer”), % n=105 | P Value | |
|---|---|---|---|---|---|---|
| CV mortality | 27.7 | 31.4 | 0.31 | 27.7 | 26.0 | 0.19 |
| MI | 14.1 | 8.2 | 0.01 | 14.0 | 5.7 | 0.06 |
| Repeat revascularization | 34.8 | 29.9 | 0.25 | 34.8 | 21.0 | 0.02 |
| CV mortality or MI | 36.6 | 36.7 | 0.55 | 36.6 | 30.6 | 0.80 |
| CV mortality, MI, or repeat revascularization | 55.8 | 51.1 | 0.37 | 55.7 | 45.5 | 0.40 |
| All-cause mortality | 49.3 | 79.7 | <0.01 | 49.9 | 86.5 | <0.01 |
CV indicates cardiovascular; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Outcomes Among Recent Cancer Patients
Clinical outcomes in patients with recent cancer are shown in Table2 and Figure2. The cumulative incidence of cardiovascular mortality was not statistically different among patients with versus without recent cancer (26.0% versus 27.7%, P=0.19, Figure2C). Cumulative incidences of the composite outcomes of cardiovascular death or MI and cardiovascular death, MI, or repeat revascularization (Figure2D) were also not statistically different between recent cancer and no recent cancer groups. Recent cancer patients had significantly lower cumulative incidence of 14-year repeat revascularization, while cumulative incidence of MI was similar between groups. All-cause mortality was significantly higher for recent cancer patients (86.5% versus 49.9%, P<0.01). We examined our primary outcome, cardiovascular mortality, after multivariable adjustment and found a numerical increase, though not statistically significant, in cardiovascular mortality risk for patients with versus without recent cancer (adjusted HR 1.46; 95% CI 0.92 to 2.33; P=0.11).
Sensitivity Analyses
In sensitivity analyses, adjustment for LVEF did not change the similarity in cardiovascular mortality risk for cancer versus control patients (adjusted HR 1.01; 95% CI 0.79 to 1.29) or patients with versus without recent cancer (adjusted HR 1.31; 95% CI 0.78 to 2.23). We added patients with a self-reported history of cancer not treated at Duke to the cancer group and found no impact on adjusted cardiovascular mortality (adjusted HR 0.92; 95% CI 0.77 to 1.10). Next, we excluded post-PCI cancer patients from the control group without significant change to cardiovascular mortality risk (cancer versus control: adjusted HR 0.94, 95% CI 0.75 to 1.18; recent cancer versus control: adjusted HR 1.44, 95% CI 0.91 to 2.29). Finally, landmark analyses starting 30 days post-PCI in the overall cohort did not change our primary results for cardiovascular mortality (cancer versus control: adjusted HR 0.98, 95% CI 0.78 to 1.24; recent cancer versus control: adjusted HR 1.45, 95% CI 0.87 to 2.40).
Association Between Post-PCI Cancer and Outcomes
In a prespecified exploratory analysis including post-PCI cancer as a time-dependent variable, we examined the association between post-PCI cancer and outcomes. There were 647 post-PCI cancer treatment patients, 6.3% (n=41) of whom had also received cancer treatment prior to index PCI. The median time from PCI to first post-PCI cancer treatment was 2.8 years (interquartile range 0.9, 5.7). Table3 shows unadjusted cause-specific HRs for outcomes for pre-PCI cancer patients and post-PCI cancer patients compared with patients without any cancer. Compared with patients without cancer, both pre-PCI cancer and post-PCI cancer patients had significantly higher observed risk of cardiovascular mortality and all-cause mortality. In a multivariable adjusted examination of our primary outcome, only post-PCI cancer remained significantly associated with higher risk of cardiovascular mortality (adjusted HR 1.51; 95% CI 1.11 to 2.03). Similar results were obtained after adding available baseline LVEF data.
Table 3.
Cancer Treatment Effect Estimates From Unadjusted Cox Proportional Cause-Specific Hazards Models
| End Point | Parameter* | HR (95% CI) | P Value |
|---|---|---|---|
| CV mortality | Pre-PCI cancer | 1.36 (1.09 to 1.68) | 0.01 |
| Post-PCI cancer | 1.69 (1.30 to 2.20) | <0.001 | |
| MI | Pre-PCI cancer | 0.82 (0.60 to 1.13) | 0.22 |
| Post-PCI cancer | 1.33 (0.90 to 1.96) | 0.15 | |
| Repeat revascularization | Pre-PCI cancer | 1.02 (0.85 to 1.21) | 0.86 |
| Post-PCI cancer | 0.99 (0.73 to 1.34) | 0.96 | |
| CV mortality or MI | Pre-PCI cancer | 1.12 (0.93 to 1.35) | 0.24 |
| Post-PCI cancer | 1.47 (1.16 to 1.86) | <0.01 | |
| CV mortality/MI/repeat revascularization | Pre-PCI cancer | 1.08 (0.94 to 1.25) | 0.27 |
| Post-PCI cancer | 1.20 (0.97 to 1.49) | 0.10 | |
| All-cause mortality | Pre-PCI cancer | 2.08 (1.80 to 2.39) | <0.001 |
| Post-PCI cancer | 5.28 (4.68 to 5.95) | <0.001 |
CV indicates cardiovascular; HR, hazard ratio; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Post-PCI cancer was included in these models as a time-dependent covariate.
Discussion
Whether cancer history is associated with post-PCI outcomes has not been well studied but is an important consideration given the growing population of patients with cancer and CAD. In this single-center study, we linked longitudinal databases to examine more than 15 000 PCI procedures performed at Duke from 1996 to 2010. We identified 3.3% of patients with pre-PCI cancer and 4.3% with post-PCI cancer. In an adjusted comparison, 14-year cardiovascular mortality in patients receiving oncologic treatment prior to PCI was not significantly different from controls, though we observed higher overall mortality in cancer patients. These preliminary data suggest that in this population, pre-PCI cancer does not appear to alter cardiovascular outcomes, and post-PCI mortality is largely determined by noncardiovascular causes. Although this is the largest analysis to date on this topic, whether our observations are generalizable to different malignancy types or stages is unknown, and these findings should be further investigated in larger databases and with prospective studies.
There has been great interest in examining PCI outcomes, and multiple models have been developed to predict post-PCI mortality.2,4,6,7 In general, models predicting in-hospital and short-term mortality have performed better than those predicting long-term outcomes. These results suggest that longer-term outcomes may be associated with factors not currently captured in established data sets.
One such factor with the potential to influence post-PCI cardiovascular outcomes that has not been well studied is cancer. The rising prevalence of concurrent cancer and CAD may be due to an overlap in risk factors (eg, age, smoking, etc), as well as a predisposition to early atherosclerosis due to certain oncologic treatments, such as radiation or tyrosine kinase inhibitors.15 In general, patients undergoing PCI are at risk of both thrombosis from PCI-induced vascular injury and stent thrombosis and bleeding from procedural instrumentation and use of antithrombotic agents. Malignancy is associated with a hypercoagulable state due to the ability of tumor cells to activate the coagulation cascade and generate acute phase reactants.16 Consequently, cancer patients are at high baseline thrombotic risk, which may be exacerbated by interrupting antiplatelet therapy for invasive biopsies or surgical treatment. In contrast, chemotherapy-related thrombocytopenia, malignant gastrointestinal or cerebral disease, and need for surgical treatment may all increase bleeding risk in cancer patients.
Despite potential associations between cancer and PCI outcomes, there have been few opportunities to study this topic. Literature in this area has been limited to reports of stent thrombosis following PCI in cancer patients17,18 and a recent study of 3423 Dutch patients undergoing primary PCI for ST-segment elevation myocardial infarction from 2006 to 2009, which demonstrated that pre-PCI cancer was associated with increased short-term mortality.19 Several reasons account for the paucity of data in this field. First, cancer patients are routinely excluded from randomized clinical trials of therapies for CAD, and vice versa.20,21 Second, cardiovascular events may be under-reported in prospective oncology clinical trials.22 Third, malignancy history is either not captured or minimally reported in most cardiovascular registries, including the world’s largest PCI registry, the CathPCI Registry, while oncology registries collect nominal details regarding cardiovascular history and risk factors. Finally, even when data are available, the focus of traditional cardio-oncologic studies has been on cardiac toxicities of cancer treatment.23–26
To our knowledge, this is the first large-scale systematic investigation of the association between cancer and long-term cardiovascular outcomes after PCI for all indications. Linking PCI and cancer treatment registries at a single center with experienced cardiology and oncology specialists provided us access to patient-level clinical and procedural details and prospectively collected outcomes with adjudicated cause of death. We found that cancer treatment prior to PCI was not associated with increased long-term cardiovascular mortality. Thus, concern for worse cardiovascular outcomes in cancer versus noncancer patients undergoing PCI should not necessarily deter providers from offering this procedure to select cancer patients. Accounting for cancer history may also improve performance of models to predict long-term post-PCI all-cause mortality. We also found an association between worse cardiovascular outcomes and post-PCI cancer; although one cannot predict future cancer at the time of PCI, strategies for managing stent-related risk in patients subsequently treated for cancer or for integrating cancer screening in CAD patients might be important considerations. Our work highlights an unexplored aspect of cardio-oncology and should be followed with prospective studies. In the absence of randomized trials, however, our use of linked clinical registry data may represent a strategy for further investigations in the growing CAD and cancer population.
These data have several important limitations. Our study was single-center, and the patient population, provider practice patterns, and care systems at Duke may be different from other centers, limiting the generalizability of our findings. Our use of observational data may also have resulted in unmeasured confounding, and despite adjustment for baseline factors, potentially confounding postbaseline factors were also unaccounted for. Due to the nature of the DTR, we used cancer treatment timing as a proxy for cancer diagnosis. Control patients may have had untreated cancer or cancer treated outside of Duke, although including self-reported cancer patients did not change our results. Additionally, there was a high rate of missing LVEF data, a variable that could be related to cancer treatment and could drive cardiovascular outcomes; sensitivity analyses including available LVEF data did not change our primary results. We were missing data regarding long-term cardiovascular medication compliance and temporary interruptions, and our population was too small to stratify outcomes by stent type. Our data did not allow for mechanistic insight underlying safety concerns and outcomes (eg, bleeding, stent thrombosis, heart failure, etc). Due to sample size, we were also not able to stratify by cancer type, stage, or treatment modalities, nor could we account for temporal changes in staging definitions and treatments. We had no data regarding provider rationale for selecting patients for PCI, and provider selection bias may have affected outcomes; for example, cancer patients selected for PCI might have more severe CAD, potentially biasing cardiovascular outcomes in this group. Finally, the overall cancer population was small, and included subgroups relevant to cardiovascular safety concerns are potentially underpowered. Overall, these limitations highlight the need for improved “crosstalk” in cardiac and oncologic data collection and strategies to include these patients in clinical trials.
In conclusion, there is a growing population of patients with CAD and cancer, yet models for long-term post-PCI outcomes do not account for cancer, and available data to study the relationship between cancer and PCI outcomes are sparse. We found the adjusted risk of long-term cardiovascular mortality was not significantly different in cancer versus noncancer PCI patients. In patients with post-PCI cancer, some of whom may have had occult cancer at the time of PCI, adjusted risk of cardiovascular mortality was significantly greater than for controls. These data provide the first large-scale exploration of the association between cancer and cardiovascular outcomes after PCI and signal the need for better cardio-oncology data integration and insight through future work.
Acknowledgments
We thank Linda K. Shaw, MS for her advice and knowledge of DISCC and Michael Mackenzie, MS for his help merging the DISCC and DTR databases. We thank Erin Hanley for her editorial contributions to this article. Shaw, Mackenzie, and Hanley did not receive compensation for their contributions, apart from their employment at the institution where this study was conducted.
Sources of Funding
Hess received support from the National Institutes of Health (grant number 5T32HL069749-09).
Disclosures
Hess, Clare, Chiswell, Kelly, Tcheng, Hagstrom, James, Khouri, Hirsch, and Kong have no relevant disclosures to report. Dr Roe reports research funding from Eli Lilly & Company, Sanofi-Aventis, Daiichi-Sankyo, Amgen, and the FH Foundation (all significant); educational activities from Astra Zeneca and Bristol Myers Squibb (both modest); consulting (including CME) from Eli Lilly & Company, Janssen Pharmaceuticals, Elsevier Publishers (all modest), Astra Zeneca, Merck & Co, and Amgen (all significant). Dr Abernethy reports research funding from Alexion, Amgen Inc, Pfizer, Biovex, DARA, MICO, and Helsinn (all significant); consulting (including CME) from Novartis Pharmaceutical Company, Pfizer, and Proventys (all modest). Dr Krucoff reports research funding from Abbott Vascular Business, Angel Medical Systems Inc, Cappella, Eli Lilly & Company, EnteroMedics Inc, Idera Pharmaceutical Inc, KAI Pharmaceuticals, Medtronic Inc, OrbusNeich, and Terumo (all significant); consulting (including CME) from Angel Medical Systems Inc, Cappella, Ischemix Inc, KAI Pharmaceuticals, Medtronic Inc, Merck & Co, OrbusNeich, Svelte Medical, St. Jude Medical, and Terumo (all modest), and Abbott Vascular Business (significant).
Supporting Information
Table S1. Distribution of malignancy types among cancer patients (n=496).
References
- Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–e651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- Peterson ED, Dai D, DeLong ER, Brennan JM, Singh M, Rao SV, Shaw RE, Roe MT, Ho KK, Klein LW, Krone RJ, Weintraub WS, Brindis RG, Rumsfeld JS, Spertus JA NCDR Registry Participants. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol. 2010;55:1923–1932. doi: 10.1016/j.jacc.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addala S, Grines CL, Dixon SR, Stone GW, Boura JA, Ochoa AB, Pellizzon G, O’Neill WW, Kahn JK. Predicting mortality in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention (PAMI risk score) Am J Cardiol. 2004;93:629–632. doi: 10.1016/j.amjcard.2003.11.036. [DOI] [PubMed] [Google Scholar]
- Chowdhary S, Ivanov J, Mackie K, Seidelin PH, Dzavik V. The Toronto score for in-hospital mortality after percutaneous coronary interventions. Am Heart J. 2009;157:156–163. doi: 10.1016/j.ahj.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Moscucci M, Kline-Rogers E, Share D, O’Donnell M, Maxwell-Eward A, Meengs WL, Kraft P, DeFranco AC, Chambers JL, Patel K, McGinnity JG, Eagle KA. Simple bedside additive tool for prediction of in-hospital mortality after percutaneous coronary interventions. Circulation. 2001;104:263–268. doi: 10.1161/01.cir.104.3.263. [DOI] [PubMed] [Google Scholar]
- MacKenzie TA, Malenka DJ, Olmstead EM, Piper WD, Langner C, Ross CS, O’Connor GT Northern New England Cardiovascular Disease Study Group. Prediction of survival after coronary revascularization: modeling short-term, mid-term, and long-term survival. Ann Thorac Surg. 2009;87:463–472. doi: 10.1016/j.athoracsur.2008.09.042. [DOI] [PubMed] [Google Scholar]
- Weintraub WS, Grau-Sepulveda MV, Weiss JM, Delong ER, Peterson ED, O’Brien SM, Kolm P, Klein LW, Shaw RE, McKay C, Ritzenthaler LL, Popma JJ, Messenger JC, Shahian DM, Grover FL, Mayer JE, Garratt KN, Moussa ID, Edwards FH, Dangas GD. Prediction of long-term mortality after percutaneous coronary intervention in older adults: results from the National Cardiovascular Data Registry. Circulation. 2012;125:1501–1510. doi: 10.1161/CIRCULATIONAHA.111.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global status report on noncommunicable diseases 2010. Available at: http://whqlibdoc.who.int/publications/2011/9789240686458_eng.pdf?ua=1. Accessed October 30, 2014.
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–1441. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Rosati RA, McNeer JF, Starmer CF, Mittler BS, Morris JJ, Jr, Wallace AG. A new information system for medical practice. Arch Intern Med. 1975;135:1017–1024. [PubMed] [Google Scholar]
- Smith PK, Califf RM, Tuttle RH, Shaw LK, Lee KL, Delong ER, Lilly RE, Sketch MH, Jr, Peterson ED, Jones RH. Selection of surgical or percutaneous coronary intervention provides differential longevity benefit. Ann Thorac Surg. 2006;82:1420–1428. doi: 10.1016/j.athoracsur.2006.04.044. discussion 1428–1429. [DOI] [PubMed] [Google Scholar]
- Khakoo AY, Yeh ET. Therapy insight: management of cardiovascular disease in patients with cancer and cardiac complications of cancer therapy. Nat Clin Pract Oncol. 2008;5:655–667. doi: 10.1038/ncponc1225. [DOI] [PubMed] [Google Scholar]
- Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4:465–473. doi: 10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Yoon CH. Acute coronary stent thrombosis in cancer patients: a case series report. Korean Circ J. 2012;42:487–491. doi: 10.4070/kcj.2012.42.7.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalchi M, Chengot T, Marzo K. Very late stent thrombosis and antineoplastic therapy. J Invasive Cardiol. 2010;22:E216–E219. [PubMed] [Google Scholar]
- Velders MA, Boden H, Hofma SH, Osanto S, van der Hoeven BL, Heestermans AA, Cannegieter SC, Jukema JW, Umans VA, Schalij MJ, van Boven AJ. Outcome after ST elevation myocardial infarction in patients with cancer treated with primary percutaneous coronary intervention. Am J Cardiol. 2013;112:1867–1872. doi: 10.1016/j.amjcard.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Cherubini A, Oristrell J, Pla X, Ruggiero C, Ferretti R, Diestre G, Clarfield AM, Crome P, Hertogh C, Lesauskaite V, Prada GI, Szczerbinska K, Topinkova E, Sinclair-Cohen J, Edbrooke D, Mills GH. The persistent exclusion of older patients from ongoing clinical trials regarding heart failure. Arch Intern Med. 2011;171:550–556. doi: 10.1001/archinternmed.2011.31. [DOI] [PubMed] [Google Scholar]
- Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, Housman MG, Escarce JJ. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Witteles RM, Telli M. Underestimating cardiac toxicity in cancer trials: lessons learned? J Clin Oncol. 2012;30:1916–1918. doi: 10.1200/JCO.2011.40.4012. [DOI] [PubMed] [Google Scholar]
- Bovelli D, Plataniotis G, Roila F ESMO Guidelines Working Group. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO Clinical Practice Guidelines. Ann Oncol. 2010;21:v277–v282. doi: 10.1093/annonc/mdq200. [DOI] [PubMed] [Google Scholar]
- Curigliano G, Mayer EL, Burstein HJ, Winer EP, Goldhirsch A. Cardiac toxicity from systemic cancer therapy: a comprehensive review. Prog Cardiovasc Dis. 2010;53:94–104. doi: 10.1016/j.pcad.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Eschenhagen T, Force T, Ewer MS, de Keulenaer GW, Suter TM, Anker SD, Avkiran M, de Azambuja E, Balligand JL, Brutsaert DL, Condorelli G, Hansen A, Heymans S, Hill JA, Hirsch E, Hilfiker-Kleiner D, Janssens S, de Jong S, Neubauer G, Pieske B, Ponikowski P, Pirmohamed M, Rauchhaus M, Sawyer D, Sugden PH, Wojta J, Zannad F, Shah AM. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011;13:1–10. doi: 10.1093/eurjhf/hfq213. [DOI] [PubMed] [Google Scholar]
- Groarke JD, Cheng S, Moslehi J. Cancer-drug discovery and cardiovascular surveillance. N Engl J Med. 2013;369:1779–1781. doi: 10.1056/NEJMp1313140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Distribution of malignancy types among cancer patients (n=496).