Abstract
Background
Little is known about the hemodynamic response to exercise in heart failure patients at various ages before and after heart transplantation (HT). This information is important because postoperative hemodynamics may be a predictor of survival. To investigate the hemodynamic response to HT and exercise, we grouped our patients based on preoperative age and examined their hemodynamics at rest and during exercise before and after HT.
Methods and Results
Ninety-four patients were evaluated at rest prior to HT with right heart catheterization at our laboratory. Of these patients, 32 were evaluated during slight supine exercise before and 1 year after HT. Postoperative evaluations were performed at rest 1 week after HT and at rest and during exercise at 4 weeks, 3 months, 6 months, and 1 year after HT. The exercise patients were divided into 2 groups based on preoperative age of ≤50 or >50 years. There were no age-dependent differences in the preoperative hemodynamic exercise responses. Hemodynamics markedly improved at rest and during exercise at 1 and 4 weeks, respectively, after HT; however, pulmonary and, in particular, ventricular filling pressures remained high during exercise at 1 year after HT, resulting in normalized pulmonary vascular resistance response but deranged total pulmonary vascular resistance response.
Conclusions
Our findings suggest that, (1) in patients with heart failure age ≤50 or >50 years may not affect the hemodynamic response to exercise to the same extent as in healthy persons, and (2) total pulmonary vascular resistance may be more adequate than pulmonary vascular resistance for evaluating the exercise response after HT.
Keywords: catheterization, exercise, heart failure, heart transplantation
Physical activity imposes prominent stress on the cardiovascular system because adequate delivery of oxygen and substrates is a necessity to sustain muscular activity.1 The provision of sufficient oxygen delivery requires both ventilatory and cardiovascular adaptations, including increases in ventilation, heart rate, and cardiac output (CO), in parallel with increased vascular conductance in active muscles and increased vascular resistance in inactive tissue.2 In severe heart failure (HF), both cardiac output and endothelial function are impaired.3 Consequently, in HF, both central and peripheral circulation may compromise sufficient oxygen delivery to active muscles, limiting exercise capacity and daily life activities.
Knowledge of systemic and pulmonary hemodynamics is important for the treatment of HF. The normal hemodynamic magnitudes have previously been clearly defined at rest4,5; however, there is a lack of understanding of the normal hemodynamic response to exercise, especially with regard to pulmonary circulation but also related to sex, body position, and different exercise intensities.6–8 This is further complicated by that the hemodynamic alterations due to age seem to be more prominent during exercise than at rest.7,8 Even though several invasive studies have investigated hemodynamics during exercise,9–13 the normal response has not been clearly defined. This lack of consensus resulted in abandoning of the “exercise criteria” in the definition of pulmonary hypertension at the World Symposium on Pulmonary Hypertension in 2008.4
Moreover, the mechanisms behind exaggerated exercise-induced increases in pulmonary artery pressures are poorly understood. For these reasons, it remains of value to further characterize hemodynamics during exercise before and after HT. Such characteristics could assist in defining an abnormal response to exercise and potentially aid in a new definition of exercise-induced pulmonary hypertension. This is important because pulmonary hypertension during exercise might be a better prognostic marker for disease severity than measurements at rest.14–19 Such a definition is also relevant because there is conflicting evidence regarding the preoperative magnitudes of different hemodynamic parameters at rest that may affect outcome after HT. This aspect illustrates the complexity in differentiating between patients with excessive vasoconstriction and those with vascular remodeling20 and highlights the need for refined criteria for defining pulmonary hypertension. Improved insight into exercise hemodynamics may also provide guidance for medical treatment, for listing for HT, and for detection of pulmonary vascular abnormalities earlier in HF and after HT.
The aim of the present study was therefore to characterize the hemodynamics of patients with severe end-stage HF at rest and during exercise in relation to age before and after HT and to relate that information to data from healthy persons in previously published systematic reviews.7,8 Our purpose was to clarify how patients with severe HF respond to slight exercise and how hemodynamics recover at rest and during exercise after HT, as well as to highlight potential abnormalities in the post-transplant response to exercise. We hypothesized that hemodynamics at rest and during exercise improve early after HT but with significant abnormalities remaining during exercise at 1 year after HT. We also hypothesized that any age-dependent differences in the hemodynamic response to exercise would be attenuated in patients with severe HF.
Methods
Study Design and Population
This retrospective single-center study reviewed the 215 HT patients followed at Skåne University Hospital in Lund, Sweden, during 1988–2010. The study was performed with informed consent and approval by the ethics board in Lund (DNR 2014/92, Dnr 2011/777, Dnr 2011/368, Dnr 2010/114) and in accordance with the Declarations of Helsinki and Istanbul. A total of 219 HTs were included, of which 214 (98%) were first-time HTs, and 72 patients (33%) received a mechanical assist prior to HT.
The study focused on adult patients evaluated at our hemodynamic laboratory prior to HT. Patients referred from and investigated at other university hospitals (n=87), children aged <18 years (n=21), repeated HTs (n=5), and other patients with incomplete hemodynamic data prior to HT (n=12) were excluded. After exclusion, 94 adults (study population) remained for analysis, of which 24 were women (25.6%). The mean age was 51.6 years, and age ranged from 19 to 69 years. The most common indications for HT were dilated (52 patients) and ischemic (24 patients) cardiomyopathy.21
Data Collection and Analysis
Right heart catheterization (RHC) was performed at rest prior to HT and at 1 and 4 weeks, 3 and 6 months, and 1 year after HT. In all patients who were capable of exercise, RHC measurements were also performed during supine bicycle exercise prior to HT and at 4 weeks, 3 and 6 months, and 1 year after HT. Men exercised at 50 W, and women exercised at 30 W. Measurements were conducted at steady state ≈5 minutes after initiation of exercise. There was no graded increase in workload.
Thirty-two of the 94 patients exercised prior to and 1 year after HT. These patients were subsequently characterized in 2 groups, 1 with patients aged ≤50 years (n=12) and 1 with patients aged >50 years (n=20) at the time of HT (Table1). This subclassification was performed to compare our findings with those presented for healthy persons published in recent systematic reviews.7,8 Overall, 21 of the 94 included HT patients were bridged to transplantation using a mechanical assist. Of these patients, 4 exercised prior to and 1 year after HT.
Table 1.
Characteristics of the Patients Who Exercised Prior to and 1 Year After HT, Grouped According to Age at the Time of HT
| Patients Aged ≤50 Years at the Time of HT | Patients Aged >50 Years at the Time of HT | |||||
|---|---|---|---|---|---|---|
| Mean±SD | n | % | Mean±SD | n | % | |
| Recipient characteristics | ||||||
| Sex of recipient | 12 | 20 | ||||
| Female | 6 | 50.0 | 4 | 20.0 | ||
| Age of recipient, y | 42.8±7.3 | 12 | 56.6±3.7 | 20 | ||
| ≤10 | 0 | 0 | 0 | 0 | ||
| 11 to 20 | 0 | 0 | 0 | 0 | ||
| 21 to 30 | 1 | 8.3 | 0 | 0 | ||
| 31 to 40 | 2 | 16.7 | 0 | 0 | ||
| 41 to 50 | 9 | 75.0 | 0 | 0 | ||
| 51 to 60 | 0 | 0 | 16 | 80.0 | ||
| ≥61 | 0 | 0 | 4 | 20.0 | ||
| Recipient length, cm | 171.0±8.6 | 12 | 175.8±8.2 | 20 | ||
| Recipient weight, kg | 78.1±20.3 | 12 | 81.0±15.6 | 20 | ||
| Recipient blood group | 12 | 20 | ||||
| 0 | 3 | 25.0 | 7 | 35.0 | ||
| A | 8 | 66.7 | 9 | 45.0 | ||
| B | 0 | 0 | 4 | 20 | ||
| AB | 1 | 8.3 | 0 | 0 | ||
| Time on waiting list, days | 121.5±146.6 | 12 | 138.9±130.1 | 20 | ||
| Indication for HT | 12 | 20 | ||||
| DCM | 8 | 66.7 | 12 | 60.0 | ||
| HCM | 0 | 0 | 1 | 5 | ||
| Myocarditis | 2 | 16.7 | 1 | 5 | ||
| IHD | 0 | 0 | 6 | 30.0 | ||
| RCM | 2 | 16.7 | 0 | 0 | ||
| Donor characteristics | ||||||
| Sex of donor | 12 | 20 | ||||
| Female | 5 | 41.7 | 9 | 45.0 | ||
| Age of donor, y | 35.5±13.9 | 12 | 45.2±15.4 | 20 | ||
| Donor length, cm | 175.7±9.1 | 12 | 176.3±10.3 | 18 | ||
| Donor weight, kg | 76.2±18.8 | 12 | 82.5±13.4 | 20 | ||
| Ischemic time, min | 163.3±63.5 | 12 | 182.7±56.5 | 20 | ||
| Recipient–donor matching | ||||||
| Age difference, y | 13.7±9.4 | 12 | 13.3±12.6 | 20 | ||
| Sex matching | 12 | 20 | ||||
| Sex matched | 9 | 75.0 | 15 | 75.0 | ||
| AB0 matching | 12 | 20 | ||||
| Identic | 9 | 75.0 | 19 | 95.0 | ||
| Compatible | 3 | 25.0 | 1 | 5.0 | ||
| Incompatible | 0 | 0 | 0 | 0 | ||
DCM indicates dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; HT, heart transplantation; IHD, ischemic heart disease; RCM, restrictive cardiomyopathy.
Right Heart Catheterization
RHC was predominantly performed via the right internal jugular vein, using a Swan Ganz catheter (Baxter Health Care Corp). If patients had >1 RHC prior to HT, the one closest to HT or assist implantation was analyzed. Mean pulmonary artery pressure (MPAP), pulmonary artery wedge pressure (PAWP), mean right atrial pressure (MRAP), and mean arterial pressure were recorded during RHC. Heart rate was recorded from ECG. CO was measured by thermodilution. Cardiac index, stroke volume (SV), SV index, left ventricular stroke work index (LVSWI), right ventricular stroke work index (RVSWI), transpulmonary gradient (TPG), pulmonary vascular resistance (PVR), PVR index, and total PVR (TPVR) were calculated using the following formulas: cardiac index=CO/body surface area; SV=CO/heart rate; SV index=SV/body surface area; LVSWI=(mean arterial pressure−PAWP)×SV index; RVSWI=(MPAP−MRAP)×SV index; TPG=MPAP−PAWP; PVR=TPG/CO; PVR index=TPG/cardiac index; and TPVR=MPAP/CO.
Statistics
A SigmaStat system (SigmaPlot 11.0) was used for statistical analysis. Parametric or nonparametric statistics were used depending on the distribution of data. One-way repeated-measures ANOVA (Tukey test) or One-way repeated-measures ANOVA on ranks (Tukey test) were used when multiple groups were compared. The Student t test and Mann-Whitney rank-sum test were used, respectively, when 2 groups were compared. Paired Student t test and Wilcoxon signed rank test were used, respectively, when comparing 1 group before and after an intervention. Pearson and Spearman correlations were used, respectively, when measuring the association between 2 variables. A P value <0.05 was considered statistically significant. All values are mean±SD.
Results
Hemodynamics at Rest and During Exercise Prior to Heart Transplantation
Baseline hemodynamics for all 94 included patients and for the subgroup of patients who exercised (n=32) are shown in Table2.
Table 2.
Preoperative Haemodynamic Characteristics of All 94 Patients and the 32 Patients Who Exercised Prior to, and 1 Year After, HT
| Parameter | Entire Study Population (at Rest) | Patients Who Exercised (at Rest) | Patients Who Exercised (During Exercise) | Patients ≤50 Years (at Rest) | Patients >50 Years (at Rest) | Significance |
|---|---|---|---|---|---|---|
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||
| Patients, n | 94 | 32 | 32 | 12 | 20 | |
| MAP, mm Hg | 74.1±13.6 | 75.4±15.8 | 87.9±19.7* | 73.5±10.9 | 77.1±18.1 | * |
| MPAP, mm Hg | 30.1±8.8 | 27.2±9.8 | 44.4±10.5* | 29.1±9.7 | 27.9±10.1 | * |
| PAWP, mm Hg | 20.5±8.2 | 18.3±8.8 | 30.5±9.5* | 18.2±9.3 | 18.4±8.7 | * |
| TPG, mm Hg | 9.6±3.5 | 8.9±3.1 | 14.0±5.3* | 7.9±2.3 | 9.5±3.3 | * |
| MRAP, mm Hg | 7.9±6.1 | 6.1±5.0 | 16.3±7.4* | 7.6±5.5 | 5.3±4.7† | *,† |
| HR, beat × min−1 | 82.6±16.8 | 82.2±15.0 | 111.9±19.6* | 77.5±16.1 | 84.8±14.1 | * |
| CO, L × min−1 | 3.7±1.0 | 4.3±0.9† | 6.4±1.7* | 4.3±1.1 | 4.3±0.9† | *,† |
| SV, mL × beat−1 | 46.7±17.1 | 53.1±15.3† | 60.2±21.6* | 57.4±19.5 | 50.7±12.4 | *,† |
| PVR (WU) | 2.8±1.3 | 2.2±0.7† | 2.2±0.8 | 2.0±0.6† | 2.3±0.8 | † |
| TPVR (WU) | 9.1±4.3 | 6.7±2.8† | 7.4±2.6 | 6.5±3.1† | 6.8±2.7† | † |
| LVSWI, mm Hg × mL × m−2 | 1395.0±730.1 | 1615.9±717.8 | 1894.7±1174.1 | 1680.5±689.9 | 1580.4±747.8 | |
| RVSWI, mm Hg × mL × m−2 | 546.2±271.1 | 594.6±333.2 | 814.8±404.4* | 563.2±333.8 | 611.9±340.2 | * |
CO indicates cardiac output; HR, heart rate; HT, heart transplantation; LVSWI, left ventricular stroke work index; MAP, mean atrial pressure; MPAP, mean pulmonary artery pressure; MRAP, mean atrial pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RVSWI, right ventricular stroke work index; SV, stroke volume; TPG, transpulmonary gradient; TPVR, total pulmonary vascular resistance; WU, Wood units.
Indicates significance (P<0.05) between rest and exercise in patients who exercised.
Indicates a significance (P<0.05) compared to the entire study population.
During the preoperative assessment of the 32 patients who exercised during RHC, exercise increased MPAP (P<0.001), PAWP (P<0.001), TPG (P<0.001), MRAP (P<0.001), CO (P<0.001), and SV (P<0.01) compared with performance at rest, whereas PVR and TPVR remained unchanged (P value not significant) (Table2). There was no correlation between change in PAWP and MRAP (R2=0.02, P value not significant) (Figure1).
Figure 1.

Correlation between preoperative changes to exercise in PAWP and MRAP. MRAP indicates mean right atrial pressure; PAWP, pulmonary artery wedge pressure.
Exercise in patients aged ≤50 years increased MPAP by 15.7±10.0 mm Hg (58.1%, P<0.001), PAWP by 10.3±8.2 mm Hg (53.8%, P<0.001), TPG by 5.4±5.9 mm Hg (68.6%, P<0.001), MRAP by 10.2±4.0 mm Hg (125.5%, P<0.001), CO by 2.2±0.7 L×min−1 (54.2%, P<0.001), and SV by 6.3±7.8 mL×beat−1 (11.3%, P<0.04), whereas PVR and TPVR remained unaltered (P value not significant) (Figures2 and 3).
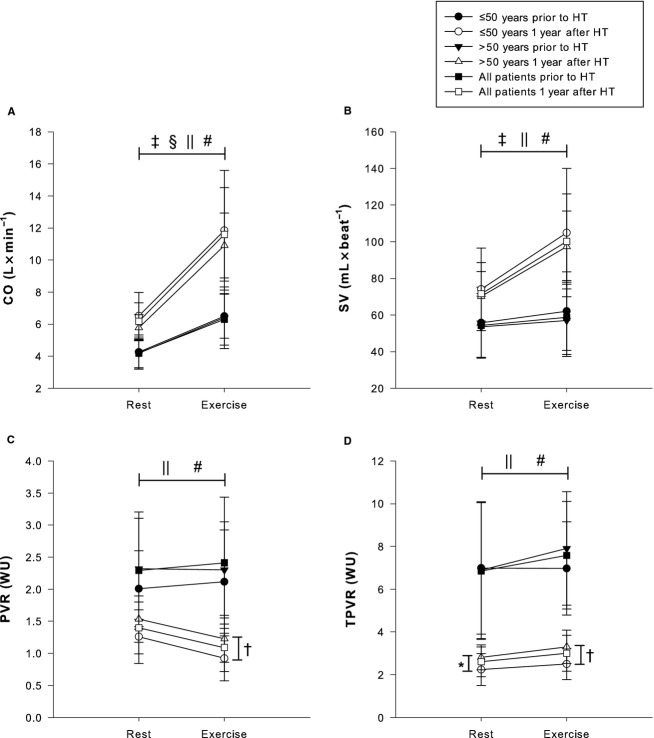
Figure 2.
Hemodynamic response to exercise with regard to pulmonary and intracardiac pressures prior to and 1 year after HT. • Patients aged ≤50 years prior to HT. ○ Patients aged ≤50 years 1 year after HT. ▾ Patients aged >50 years prior to HT; Δ Patients aged >50 years 1 year after HT. ▪ All 32 patients prior to HT. □ All 32 patients 1 year after HT. A, MPAP. B, PAWP. C, MRAP. D, TPG. †A statistically significant difference for MPAP during exercise between patients aged ≤50 and >50 years. ‡A statistically significant difference for MPAP, PAWP, MRAP, and TPG between rest and exercise prior to HT for patients aged ≤50 years. §A statistically significant difference for MPAP, PAWP, MRAP, and TPG between rest and exercise prior to HT for patients aged >50 years. ‖A statistically significant difference for MPAP, PAWP, MRAP, and TPG between rest and exercise at 1 year after HT for patients aged ≤50 years. #A statistically significant difference for MPAP, PAWP, MRAP, and TPG between rest and exercise at 1 year after HT for patients aged >50 years. HT indicates heart transplantation; MPAP, mean pulmonary artery pressure; MRAP, mean right atrial pressure; PAWP, pulmonary artery wedge pressure; TPG, transpulmonary gradient.
Figure 3.
Hemodynamic response to exercise with regard to cardiac output, stroke volume, and pulmonary vascular resistances prior to and 1 year after HT. • Patients aged ≤50 years prior to HT. ○ Patients aged ≤50 years 1 year after HT. ▾ Patients aged >50 years prior to HT. Δ Patients aged >50 years 1 year after HT. ▪ All 32 patients prior to HT. □ All 32 patients 1 year after HT. A, CO. B, SV. C, PVR. D, TPVR. *A statistically significant difference for TPVR at rest between patients aged ≤50 and >50 years 1 year after HT. †A statistically significant difference for PVR and TPVR during exercise between patients aged ≤50 and >50 years. ‡A statistically significant difference for CO and SV between rest and exercise prior to HT for patients aged ≤50 years. §A statistically significant difference for CO between rest and exercise prior to HT for patients aged >50 years. ‖A statistically significant difference for CO, SV, PVR, and TPVR between rest and exercise at 1 year after HT for patients aged ≤50 years. #A statistically significant difference for CO, SV, PVR, and TPVR between rest and exercise at 1 year after HT for patients aged >50 years. CO indicates cardiac output; HT, heart transplantation; PVR, pulmonary vascular resistance; SV, stroke volume; TPVR, total pulmonary vascular resistance; WU, Wood units.
Similarly, in patients aged >50 years, exercise increased MPAP by 18.8±11.5 mm Hg (69.4%, P<0.001), PAWP by 13.5±11.3 mm Hg (77.2%, P<0.001), TPG by 5.4±4.2 mm Hg (55.3%, P<0.001), MRAP by 10.8±3.8 mm Hg (259.0%, P<0.001), and CO by 2.0±1.7 L×min−1 (48.1%, P<0.001), whereas SV, PVR, and TPVR remained unaltered (P value not significant) (Figures2 and 3).
With regard to pre-HT age-dependent differences, none of these parameters differed for patients aged ≤50 or >50 years (P value not significant) at rest and during exercise. The percentage change did neither differ between the groups (P value not significant) (Figures2 and 3).
Hemodynamic Improvement After Heart Transplantation
The hemodynamic response and improvement in relation to HT are shown in Figures4 and 5 for all 94 patients at rest and for 32 patients during slight supine exercise. For the 94 patients at rest 1 week after HT, there was a decrease in MPAP (P<0.001), PAWP (P<0.001), PVR (P<0.001), and TPVR (P<0.001) along with an increase in mean arterial pressure (P<0.001), CO (P<0.001), and SV (P<0.001) compared with performance at rest prior to HT (Figures4 and 5). TPG and MRAP were unaltered (P value not significant) (Figures4 and 5).
Figure 4.

Hemodynamic characteristics with regard to pulmonary and intracardiac pressures in the 94 patients at rest prior to and after HT. • Patients at rest. ○ Patients during exercise. Graphs show (A) MPAP, (B) PAWP, (C) MRAP (D) TPG, (E) PVR, and (F) TPVR at rest and during exercise before and after HT. *A statistically significant difference at rest compared with prior to HT. †A statistically significant difference at rest compared with 4 weeks after HT. ‡A statistically significant difference at rest compared with 4 weeks after HT. §A statistically significant difference during exercise compared with prior to HT. ‖A statistically significant difference during exercise compared with 4 weeks after HT. Of the 32 patients who exercised prior to HT, 16 exercised at 4 weeks, 22 at 3 months, 22 at 6 months, and 32 at 1 year after HT. HT indicates heart transplantation; MPAP, mean pulmonary artery pressure; MRAP, mean right atrial pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; TPG, transpulmonary gradient; TPVR, total pulmonary vascular resistance; WU, Wood units.
Figure 5.
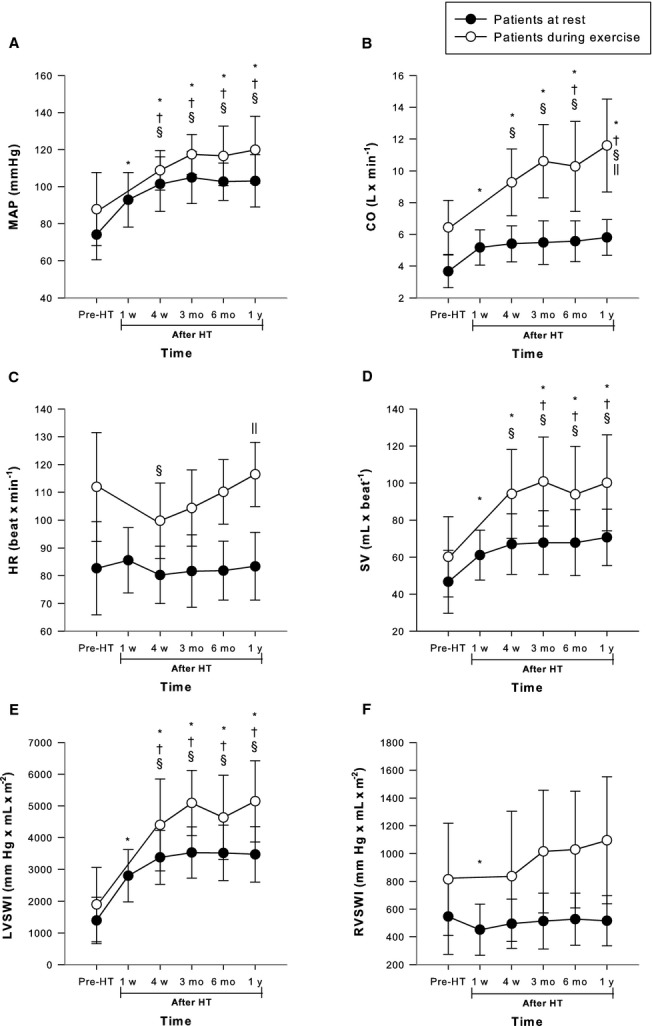
Hemodynamic characteristics with regard to heart rate, arterial pressure cardiac output, and stroke volume in the 94 patients at rest prior to and after HT. • Patients at rest. ○ Patients during exercise. Graphs show (A) MAP, (B) CO, (C) HR, (D) SV, (E) LVSWI, and (F) RVSWI at rest and during exercise before and after HT. *A statistically significant difference compared with examination prior to HT. †A statistically significant difference compared with 1 week after HT. §A statistically significant difference during exercise compared to prior to HT. ‖A statistically significant difference during exercise compared with 4 weeks after HT. CO indicates cardiac output; HR, heart rate; HT, heart transplantation; LVSWI, left ventricular stroke work index; MAP, mean arterial pressure; RVSWI, right ventricular stroke work index; SV, stroke volume; WU, Wood units.
Of these parameters, PVR remained constant (P value not significant) throughout the first year after HT, whereas MPAP further decreased (P<0.009) and mean arterial pressure further increased (P<0.02) at 4 weeks versus 1 week after HT and remained constant (P value not significant) thereafter. MRAP also decreased (P<0.001) at 4 weeks as compared to both prior to HT and one week after HT, and then temporarily decreased (P<0.04) at 6 months compared with 4 weeks after HT. SV further increased (P<0.01) at 3 months compared with 1 week after HT and remained constant (P value not significant) thereafter. Finally, PAWP and TPVR further decreased (P<0.001 and P<0.02, respectively) and CO further increased (P<0.03) at 6 months compared with 1 week after HT and remained constant (P value not significant) thereafter.
Compared with performance during exercise prior to HT, at 4 weeks after HT, there was a decrease in MPAP (P<0.001), PAWP (P<0.001), PVR (P<0.003), and TPVR (P<0.001) along with an increase in mean arterial pressure (P<0.001), CO (P<0.001), and SV (P<0.001). TPG and MRAP were unaltered (P value not significant) (Figures4 and 5). All of these parameters remained stable (P value not significant) throughout the first year after HT except for CO, which further increased (P<0.004), and for MRAP and PVR, which decreased (P<0.005 and P<0.03, respectively), at 1 year after HT (Figures4 and 5).
Hemodynamic Response to Exercise at 1 Year After Heart Transplantation
For the 32 patients who performed RHC at rest and during exercise 1 year after HT, exercise increased (P<0.001 for all parameters) MPAP by 17.8±6.1 mm Hg (114.6%), PAWP by 14.1±5.5 mm Hg (193.1%), TPG by 3.7±3.1 mm Hg (44.4%), MRAP by 9.8±4.3 mm Hg (371.4%), CO by 5.4±2.4 L×min−1 (88.1%), SV by 28.5±15.6 mL×beat−1 (39.8%), and TPVR by 0.4±0.7 Wood units (WU; 15.4%) compared with performance at rest, whereas PVR decreased by 0.3±0.3 WU (22.1%, P<0.001) (Figures2 and 3). Change in MPAP from rest to exercise correlated to change in PAWP (R2=0.75, P<0.001) (Figure6).
Figure 6.

Correlation between changes to exercise in PAWP and MPAP at 1 year after HT. HT indicates heart transplantation; PAWP, pulmonary artery wedge pressure; MPAP, mean pulmonary artery pressure.
Exercise in patients aged ≤50 years increased MPAP by 14.8±4.6 mm Hg (104.1%, P<0.001), PAWP by 12.1±4.3mm Hg (190.8%, P<0.001), TPG by 2.8±3.3 mm Hg (34.7%, P<0.016), MRAP by 8.6±5.3 mm Hg (412.0%, P<0.001), CO by 5.8±3.4 L×min−1 (88.7%, P<0.001), SV by 30.9±19.3mL×beat−1 (41.7%, P<0.001), and TPVR by 0.2±0.4 WU (11.1%, P<0.05), whereas PVR decreased by 0.3±0.3 WU (26.7%, P<0.004) (Figures2 and 3).
Similarly, exercise in patients aged >50 years increased MPAP by 19.6±6.4 mm Hg (118.5%, P<0.001), PAWP by 15.3±6.1 mm Hg (194.3%, P<0.001), TPG by 4.3±2.8mm Hg (49.7%, P<0.001), MRAP by 10.5±4.0 mm Hg (354.2%, P<0.001), CO by 5.2±1.6 L×min−1 (87.7%, P<0.001), SV by 27.1±16.0 mL×beat−1 (38.6%, P<0.001), and TPVR by 0.5±0.8 WU (17.5%, P<0.008), whereas PVR decreased by 0.3±0.3 WU (19.7%, P<0.001) (Figures2 and 3).
Resting TPVR was generally higher in patients aged >50 years compared with patients aged ≤50 years (P<0.03). In contrast, during exercise, not only TPVR but also MPAP and PVR were higher (P<0.05) in patients aged >50 years compared with patients aged ≤50 years; however, the percentage change in these parameters did not differ between groups (Figures2 and 3).
Discussion
The present study evaluated the hemodynamics at rest and during slight supine exercise in patients with severe HF before and after HT. Our results showed that already at 1 week after HT, hemodynamics markedly improved at rest and remained constant or further improved during the first year of follow-up (Figures4 and 5). The functional response to exercise was also substantially improved after HT, represented by an adequate increase in CO and SV (Figure3); however, MPAP and particularly PAWP and MRAP remained high (Figures2 and 5). The absolute response to exercise of these pressures was similar before and after HT, resulting in a higher relative response to exercise after HT (Figure2). Moreover, the age-dependent differences in the hemodynamic response to exercise previously reported in healthy persons7,8 were not observed in our patients with severe end-stage HF prior to HT. Together, these findings suggest that in patients with severe HF, age may not be as dominant in affecting hemodynamic response to exercise as in healthy persons. The findings also indicate that despite normalized hemodynamics at rest, the response to even slight exercise after HT differed compared with the normal innervated heart.
Exercise Hemodynamics Prior to Heart Transplantation
The patients in our study who exercised prior to HT generally responded with a marked increase in PAWP and MRAP, suggesting enhanced biventricular failure on exertion (Table2). Previous reports in healthy volunteers found a close relationship between exercise-induced increases in PAWP and MRAP.22,23 Such a correlation was not observed in our patients (Figure1), suggesting that the increased load on the right ventricle is not solely due to increased left ventricular filling pressures and subsequent pulmonary venous hypertension or ventricular interdependence but also is due to an exaggerated increase in MPAP. Indeed, the TPG in our patients, which increased by 5.4 mm Hg, is in line with the findings by Lewis and colleagues, who investigated 60 patients with stable New York Heart Association class II to IV during slight upright exercise.19 In contrast to the findings by Lewis et al19 and others24,25 investigating slight upright exercise in HF patients, in our study, PVR did not significantly decrease during slight supine exercise. This finding is also in contrast to the findings in healthy persons reported previously8 and further supports an exaggerated increase in MPAP compared with the increase in PAWP and CO (Table2). The increase in MPAP could be due to several factors. One such factor that may explain a part of the increase is hypoxic pulmonary vasoconstriction26 related to decreased mixed venous Po2. In support of this hypothesis, the mixed venous saturation decreased from 67% at rest to 26% during exercise in the 9 patients for whom these data were available (data not shown).
It is well known that during exercise in healthy persons, there is great interindividual variation in TPVR.6 Despite this knowledge, exercise-induced pulmonary hypertension was recently suggested to be defined as TPVR >3 WU.27,28 With a mean TPVR of 7.4 WU in our patients, the preoperative exercise TPVR was higher than in healthy persons27,28 but in line with previous results in patients with HF.19 This finding, together with the increase in TPG, suggests that although our study exclusively included patients referred for HT, the results with regard to hemodynamics are representative for patients with severe HF.
Hemodynamic Response to Heart Transplantation
Previous investigations of exercise after HT have used mainly noninvasive methods and focused primarily on exercise capacity and/or reinnervation of the transplanted heart29–38; however, a few studies have evaluated hemodynamics obtained from RHC during exercise after HT.26,39–44 In 1980, Pope and colleagues presented a report on the hemodynamic and catecholamine response to exercise in 9 long-term HT survivors. They demonstrated that in denervated hearts, CO is increased by augmented preload and the Frank Starling mechanism early in exercise and later by the effects of increased levels of catecholamines.40 This report40 did not examine patients early after HT; however, this work was performed in a later study of 20 patients.26 The authors then found that MPAP and PAWP decreased early after HT. In contrast to these reports, we illustrated the complete hemodynamic picture, at rest and during exercise prior to HT, in which the same patients were followed repeatedly during the first year after HT, enabling the study of hemodynamic changes over time.
In contrast to exercise hemodynamics after HT, several studies have evaluated hemodynamics at rest early and late after HT.41–43,45–56 In line with those reports, we showed that even at 1 week after HT, resting hemodynamics improved dramatically and were maintained throughout the first year after HT. Pulmonary artery pressures as well as CO, SV, TPVR, and LVSWI continued to improve during the first year after HT (Figures4 and 5). In contrast to what is expected in healthy persons based on previous systematic reviews,7,8 the pulmonary artery pressure, PAWP, and MRAP responses to exercise were exaggerated (Figures2 and 4). The absolute increase was, in fact, similar to that seen prior to HT (Figure2), resulting in a greater percentage increase after transplantation. Moreover, there was a correlation between the increase in MPAP and PAWP (Figure6), suggesting that the MPAP response during exercise is caused, at least in part, by the elevated PAWP. This exaggerated increase in ventricular filling pressures has been described previously.42,44 The reason for similar pressure increases during exercise after versus before HT, despite lower resting values, needs further investigation. It could be hypothesized that, in our population, these findings were due to a prominent decrease in diastolic compliance in denervated hearts that reaches its maximum during slight to moderate exercise.57 This is supported in that the denervated heart, due to lack of sympathetic stimulation, is more dependent on the Frank Starling mechanism than is the innervated heart.40,58,59 Factors such as lack of heart rate reserve and Bainbridge reflex, rejections, fluid retention, hypertension, myocardial ischemia, and diastolic dysfunction have also been suggested to account for post-transplant abnormalities.42–44,47,48 These studies, however, show diverging results. The ultimate cause remains unclear and possibly is multifactorial.42 In 1994, Kao and colleagues performed invasive hemodynamic measurements during upright graded exercise in 30 HT patients with normal left ventricular ejection fraction at 3 to 16 months after HT and compared their findings with healthy controls.44 They showed that in their population, heart rate reserve and SV index were impaired after HT, suggesting that during maximal exercise, chronotropic insufficiency and diastolic dysfunction explained the increased filling pressures.44 These findings, however, differ from ours. Based on what has been described previously in healthy persons,8 the patients in our study at 1 year after HT responded during slight supine exercise with adequate increase in heart rate and SV. In fact, the SV in our patients was in line with that of the healthy persons in the study by Kao et al during submaximal upright exercise.44 Moreover, heart rate increased to 116.4±11.6 beats×min−1, which is in the upper limit of what is expected during slight exercise in healthy persons8 and in line with other previous reports in HT patients.40 The difference between our findings and those presented by Kao et al could potentially be explained by the difference in body positions in the studies; however, they may also, at least in part, be due to less healthy patients in the study by Kao et al, with low maximum oxygen consumption (12.3±4.0 mL × Kg−1 × min−1) despite normal ejection fraction.44 Moreover, in contrast to all previous reports, in the present study, we evaluated the age-dependent differences in exercise response after HT and investigated the impact of the elevated filling pressures on pulmonary resistance.
Age-Dependent Exercise Response
It has been shown previously that the hemodynamic response to exercise differs for healthy persons aged ≤50 versus >50 years.7,8 In the most recent of these reports, Kovacs and colleagues analyzed the hemodynamic response to exercise in 222 healthy persons from 24 different studies in a systematic review and stratified the participants according to age.8 The results confirmed earlier reports and showed that slight supine exercise results in an increase in CO by 85% in participants aged ≤50 years and is accompanied by an increase in MPAP by 41% and a decrease in PVR and TPVR by 12% and 25%, respectively. In contrast, in participants aged >50 years, an increase in CO by 71% is accompanied by an increase in MPAP by 66% and a decrease in PVR by 19%, whereas TPVR remained virtually unchanged.8 These findings differ from those in our HF population both before and 1 year after HT. The MPAP response prior to HT was exaggerated in both groups when related to the corresponding increase in CO, whereas the PVR and TPVR responses were attenuated (Figures2 and 3). After HT, the CO response to exercise was adequate, but the MPAP response was still increased when related to the increase in CO (Figures2 and 3). Because the exercise increase in PAWP was even further exaggerated, PVR decreased in patients aged ≤50 and >50 years, comparable to expectations for healthy persons.7,8 TPVR, which is not directly affected by PAWP, was still deranged at 1 year after HT and increased during exercise in patients aged ≤50 and >50 years (Figures2 and 3).
In contrast to previous findings in healthy persons,7,8 MPAP, PVR, and TPVR of our patients prior to HT did not differ significantly between patients aged ≤50 and >50 years at rest (Figures2 and 3). Furthermore, no difference was noted between patients aged ≤50 and >50 years during exercise prior to HT (Figures2 and 3). Considering the low number of patients in our trial, these findings have to be interpreted with caution. Indeed, MRAP tended to be higher in patients aged ≤50 years at rest prior to HT and could indicate that these patients had more pronounced biventricular heart failure, which may affect the results. PAWP, CO, and SV were however similar in both groups, and TPG and PVR tended to be even lower in the younger patients. Consequently, differences in disease severity between patients aged ≤50 and >50 years are unlikely to play a major part in hemodynamic response. In contrast, at 1 year after transplantation, TPVR at rest and MPAP, PVR, and TPVR during exercise were higher among patients aged >50 years (Figures2 and 3). Although the absolute and percentage responses did not differ significantly for any of the parameters, there was a tendency toward greater changes in pressures in the patients aged >50 years. Together, these observations suggest that in patients with end-stage HF, the age-dependent response to exercise may be attenuated due to the severity of the disease, and that parts of this response may be restored after HT. Because MPAP and particularly PAWP remained deranged at 1 year after HT, the findings also suggest that although the PVR response to exercise is normalized, PVR may not adequately reflect pulmonary circulation after HT. In this setting, TPVR may be a better marker because it reflects the increase in MPAP in relation to CO, independent of PAWP.
Limitations
Although a retrospective study such as the present has disadvantages compared with a prospective ditto, and that the long time span of our study could influence the results, the findings are of interest. This is supported by the fact that our study population was representative with regard to similar demographic parameters and survival compared with our entire HT population.21 However, the small sample size could mask age-dependent differences in the response to exercise; therefore, these results must be interpreted with caution. This is particularly true after HT, where patients aged >50 years seem to have a greater increase in pulmonary and ventricular filling pressures, whereas the response to exercise prior to HT seems to be similar between the groups, despite higher resting MRAP in the younger patients. When discussing the normal response to exercise, one must also keep in mind that we did not study healthy controls in the present report. Instead, we evaluated our results based on previously published systematic reviews of healthy persons. Although this approach may not be optimal, given the ethical issues of performing heart catheterizations on healthy persons, we find our approach to be a valid alternative. Furthermore, our study included only patients evaluated for HT; therefore, the findings prior to HT may not be applicable to all patients with severe HF. Nonetheless, considering that the TPG and TPVR responses to exercise in our patients were comparable to previous results in patients with HF,19 we believe that our observations are representative with regard to hemodynamics for patients with severe HF. Finally, previous studies have shown that rejections,43,44,48,60 ischemic time of the donor heart,43,44,48,60 and systemic hypertension44,60 do not account for the hemodynamic abnormalities seen after HT. It is possible, however, that other donor characteristics and donor–recipient mismatches as well as patients’ medical therapies may influence the findings. This possibility was not investigated in the present study, apart from what is shown in Table1, and further research is encouraged.
Conclusion
The present study illustrates the hemodynamic response to exercise in patients with severe end-stage HF prior to and after HT. Our findings reveal that resting hemodynamics recover within the first week after HT and are maintained or even improved throughout the first year after transplantation. Moreover, the age-dependent differences in response to exercise in healthy persons were not observed in our patients with severe end-stage HF prior to HT. This suggests that the hemodynamic response to exercise may be less age dependent in HF patients than in the normal population. Finally, although the hemodynamics at rest and during exercise were greatly improved after HT, and the functional response to exercise with regard to CO and SV is adequate at 1 year after HT, there is an exaggerated increase in MPAP, PAWP, and MRAP in response to exercise. This is evident for patients aged ≤50 and >50 years and, at least in part, is likely due to increased PAWP. As a result of the exaggerated increase in PAWP, the PVR response to exercise is normalized after HT, masking the increased MPAP. Consequently, TPVR, which is independent of PAWP, may be a better marker than PVR for hemodynamic evaluations after HT. Larger studies are encouraged to confirm these findings and to define the normal hemodynamic magnitudes during exercise prior to and after HT to explain the exaggerated increase in PAWP to exercise.
Acknowledgments
This work is dedicated to Professor Bengt Saltin. We acknowledge the support of the staff at the Hemodynamics Laboratory, Clinic for Heart Failure and Valvular Disease, Skåne University Hospital, and the Department of Cardiology, Clinical Sciences, Lund University, Lund, Sweden.
Sources of Funding
This work was supported by unrestricted research grants from Anna-Lisa and Sven-Erik Lundgren’s and ALF’s Foundations, the Swedish Society of Pulmonary Hypertension and Actelion Pharmaceuticals Sweden AB. The funding organizations played no role in the collection, analysis or interpretation of the data and have no right to restrict the publishing of the article.
Disclosures
Mr Lundgren reports an unrestricted research grant from The Swedish Society of Pulmonary Hypertension, and Dr Rådegran reports unrestricted research grants from Anna-Lisa and Sven-Erik Lundgren’s, Maggie Stephen’s, ALF’s and Actelion Pharmaceuticals Sweden AB, during the conduct of the study. Mr Lundgren reports personal lecture fees from Actelion Pharmaceuticals Sweden AB and GlaxoSmithKline outside the submitted work. Dr Rådegran reports personal lecture fees from Actelion Pharmaceuticals Sweden AB, GlaxoSmithKline, Bayer and Sandoz/Novartis outside the submitted work. Dr Rådegran is, and has been primary-, or co-, investigator in; clinical PAH trials for GlaxoSmithKline, Actelion Pharmaceuticals Sweden AB, Pfizer, Bayer and United Therapeutics, and in clinical heart transplantation immuno-suppression trials for Novartis. The companies had no role in the data collection, analysis, or interpretation; or in the preparation or approval of the manuscript.
References
- Saltin B. Hemodynamic adaptations to exercise. Am J Cardiol. 1985;55:42D–47D. doi: 10.1016/0002-9149(85)91054-9. [DOI] [PubMed] [Google Scholar]
- Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- Shantsila E, Wrigley BJ, Blann AD, Gill PS, Lip GY. A contemporary view on endothelial function in heart failure. Eur J Heart Fail. 2012;14:873–881. doi: 10.1093/eurjhf/hfs066. [DOI] [PubMed] [Google Scholar]
- Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G ESC Committee for Practice Guidelines (CPG) Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galie N, Ghio S, Gibbs JS, Martinez F, Semigran M, Simonneau G, Wells A, Seeger W. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62:D100–D108. doi: 10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- Naeije R, Chesler N. Pulmonary circulation at exercise. Compr Physiol. 2012;2:711–741. doi: 10.1002/cphy.c100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34:888–894. doi: 10.1183/09031936.00145608. [DOI] [PubMed] [Google Scholar]
- Kovacs G, Olschewski A, Berghold A, Olschewski H. Pulmonary vascular resistances during exercise in normal subjects: a systematic review. Eur Respir J. 2012;39:319–328. doi: 10.1183/09031936.00008611. [DOI] [PubMed] [Google Scholar]
- Riley RL, Himmelstein A. Studies of the pulmonary circulation at rest and during exercise in normal individuals and in patients with chronic pulmonary disease. Am J Physiol. 1948;152:372–382. doi: 10.1152/ajplegacy.1948.152.2.372. [DOI] [PubMed] [Google Scholar]
- Holmgren A, Jonsson B, Sjostrand T. Circulatory data in normal subjects at rest and during exercise in recumbent position, with special reference to the stroke volume at different work intensities. Acta Physiol Scand. 1960;49:343–363. doi: 10.1111/j.1748-1716.1960.tb01957.x. [DOI] [PubMed] [Google Scholar]
- Granath A, Jonsson B, Strandell T. Circulation, in healthy, old men, studied by right heart catheterization at rest and during exercise in supine and sitting position. Acta Med Scand. 1964;176:425–446. doi: 10.1111/j.0954-6820.1964.tb00949.x. [DOI] [PubMed] [Google Scholar]
- Damato AN, Galante JG, Smith WM. Hemodynamic response to treadmill exercise in normal subjects. J Appl Physiol. 1966;21:959–966. doi: 10.1152/jappl.1966.21.3.959. [DOI] [PubMed] [Google Scholar]
- Ehrsam RE, Perruchoud A, Oberholzer M, Burkart F, Herzog H. Influence of age on pulmonary haemodynamics at rest and during supine exercise. Clin Sci (Lond) 1983;65:653–660. doi: 10.1042/cs0650653. [DOI] [PubMed] [Google Scholar]
- Tolle JJ, Waxman AB, Van Horn TL, Pappagianopoulos PP, Systrom DM. Exercise-induced pulmonary arterial hypertension. Circulation. 2008;118:2183–2189. doi: 10.1161/CIRCULATIONAHA.108.787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudiz RJ, Rubin LJ. Exercise-induced pulmonary arterial hypertension: a new addition to the spectrum of pulmonary vascular diseases. Circulation. 2008;118:2120–2121. doi: 10.1161/CIRCULATIONAHA.108.819573. [DOI] [PubMed] [Google Scholar]
- Kovacs G, Maier R, Aberer E, Brodmann M, Scheidl S, Tröster N, Hesse C, Salmhofer W, Graninger W, Gruenig E, Rubin LJ, Olschewski H. Borderline pulmonary arterial pressure is associated with decreased exercise capacity in scleroderma. Am J Respir Crit Care Med. 2009;180:881–886. doi: 10.1164/rccm.200904-0563OC. [DOI] [PubMed] [Google Scholar]
- Naeije R. In defence of exercise stress tests for the diagnosis of pulmonary hypertension. Heart. 2011;97:94–95. doi: 10.1136/hrt.2010.212126. [DOI] [PubMed] [Google Scholar]
- Whyte K, Hoette S, Herve P, Montani D, Jaïs X, Parent F, Savale L, Natali D, O’Callaghan DS, Garcia G, Sitbon O, Simonneau G, Humbert M, Chemla D. The association between resting and mild-to-moderate exercise pulmonary artery pressure. Eur Respir J. 2012;39:313–318. doi: 10.1183/09031936.00019911. [DOI] [PubMed] [Google Scholar]
- Lewis GD, Murphy RM, Shah RV, Pappagianopoulos PP, Malhotra R, Bloch KD, Systrom DM, Semigran MJ. Pulmonary vascular response patterns during exercise in left ventricular systolic dysfunction predict exercise capacity and outcomes. Circ Heart Fail. 2011;4:276–285. doi: 10.1161/CIRCHEARTFAILURE.110.959437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren J, Radegran G. Pathophysiology and potential treatments of pulmonary hypertension due to systolic left heart failure. Acta Physiol (Oxf) 2014;211:314–333. doi: 10.1111/apha.12295. [DOI] [PubMed] [Google Scholar]
- Lundgren J, Algotsson L, Kornhall B, Radegran G. Preoperative pulmonary hypertension and its impact on survival after heart transplantation. Scand Cardiovasc J. 2014;48:47–58. doi: 10.3109/14017431.2013.877153. [DOI] [PubMed] [Google Scholar]
- Reeves JT, Groves BM, Cymerman A, Sutton JR, Wagner PD, Turkevich D, Houston CS. Operation Everest II: cardiac filling pressures during cycle exercise at sea level. Respir Physiol. 1990;80:147–154. doi: 10.1016/0034-5687(90)90078-d. [DOI] [PubMed] [Google Scholar]
- Reeves JT, Groves BM, Sutton JR, Wagner PD, Cymerman A, Malconian MK, Rock PB, Young PM, Houston CS. Operation Everest II: preservation of cardiac function at extreme altitude. J Appl Physiol. 1985;1987:531–539. doi: 10.1152/jappl.1987.63.2.531. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. 1989;80:769–781. doi: 10.1161/01.cir.80.4.769. [DOI] [PubMed] [Google Scholar]
- Janicki JS, Weber KT, Likoff MJ, Fishman AP. The pressure-flow response of the pulmonary circulation in patients with heart failure and pulmonary vascular disease. Circulation. 1985;72:1270–1278. doi: 10.1161/01.cir.72.6.1270. [DOI] [PubMed] [Google Scholar]
- Naeije R, Lipski A, Abramowicz M, Lejeune P, Melot C, Antoine M, De SmetJM, Leclerc JL, Primo G. Nature of pulmonary hypertension in congestive heart failure. Effects of cardiac transplantation. Am J Respir Crit Care Med. 1994;149:881–887. doi: 10.1164/ajrccm.149.4.8143050. [DOI] [PubMed] [Google Scholar]
- Naeije R, Vanderpool R, Dhakal BP, Saggar R, Saggar R, Vachiery JL, Lewis GD. Exercise-induced pulmonary hypertension: physiological basis and methodological concerns. Am J Respir Crit Care Med. 2013;187:576–583. doi: 10.1164/rccm.201211-2090CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GD, Bossone E, Naeije R, Grunig E, Saggar R, Lancellotti P, Ghio S, Varga J, Rajagopalan S, Oudiz R, Rubenfire M. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation. 2013;128:1470–1479. doi: 10.1161/CIRCULATIONAHA.112.000667. [DOI] [PubMed] [Google Scholar]
- Renlund DG, Taylor DO, Ensley RD, O’Connell JB, Gilbert EM, Bristow MR, Ma H, Yanowitz FG. Exercise capacity after heart transplantation: influence of donor and recipient characteristics. J Heart Lung Transplant. 1996;15:16–24. [PubMed] [Google Scholar]
- Osada N, Chaitman BR, Donohue TJ, Wolford TL, Stelken AM, Miller LW. Long-term cardiopulmonary exercise performance after heart transplantation. Am J Cardiol. 1997;79:451–456. doi: 10.1016/s0002-9149(96)00785-0. [DOI] [PubMed] [Google Scholar]
- Givertz MM, Hartley LH, Colucci WS. Long-term sequential changes in exercise capacity and chronotropic responsiveness after cardiac transplantation. Circulation. 1997;96:232–237. doi: 10.1161/01.cir.96.1.232. [DOI] [PubMed] [Google Scholar]
- Savin WM, Haskell WL, Schroeder JS, Stinson EB. Cardiorespiratory responses of cardiac transplant patients to graded, symptom-limited exercise. Circulation. 1980;62:55–60. doi: 10.1161/01.cir.62.1.55. [DOI] [PubMed] [Google Scholar]
- Kavanagh T, Yacoub MH, Mertens DJ, Kennedy J, Campbell RB, Sawyer P. Cardiorespiratory responses to exercise training after orthotopic cardiac transplantation. Circulation. 1988;77:162–171. doi: 10.1161/01.cir.77.1.162. [DOI] [PubMed] [Google Scholar]
- Kavanagh T, Mertens DJ, Shephard RJ, Beyene J, Kennedy J, Campbell R, Sawyer P, Yacoub M. Long-term cardiorespiratory results of exercise training following cardiac transplantation. Am J Cardiol. 2003;91:190–194. doi: 10.1016/s0002-9149(02)03108-9. [DOI] [PubMed] [Google Scholar]
- Lord SW, Brady S, Holt ND, Mitchell L, Dark JH, McComb JM. Exercise response after cardiac transplantation: correlation with sympathetic reinnervation. Heart. 1996;75:40–43. doi: 10.1136/hrt.75.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires RW, Leung TC, Cyr NS, Allison TG, Johnson BD, Ballman KV, Wagner JA, Olson LJ, Frantz RP, Edwards BS, Kushwaha SS, Dearani JA, Daly RC, McGregor CG, Rodeheffer RJ. Partial normalization of the heart rate response to exercise after cardiac transplantation: frequency and relationship to exercise capacity. Mayo Clin Proc. 2002;77:1295–1300. doi: 10.4065/77.12.1295. [DOI] [PubMed] [Google Scholar]
- Richard R, Verdier JC, Duvallet A, Rosier SP, Leger P, Nignan A, Rieu M. Chronotropic competence in endurance trained heart transplant recipients: heart rate is not a limiting factor for exercise capacity. J Am Coll Cardiol. 1999;33:192–197. doi: 10.1016/s0735-1097(98)00513-0. [DOI] [PubMed] [Google Scholar]
- Labovitz AJ, Drimmer AM, McBride LR, Pennington DG, Willman VL, Miller LW. Exercise capacity during the first year after cardiac transplantation. Am J Cardiol. 1989;64:642–645. doi: 10.1016/0002-9149(89)90494-3. [DOI] [PubMed] [Google Scholar]
- Campeau L, Pospisil L, Grondin P, Dyrda I, Lepage G. Cardiac catheterization findings at rest and after exercise in patients following cardiac transplantation. Am J Cardiol. 1970;25:523–528. doi: 10.1016/0002-9149(70)90590-4. [DOI] [PubMed] [Google Scholar]
- Pope SE, Stinson EB, Daughters GT, Schroeder JS, Ingels NB, Jr, Alderman EL. Exercise response of the denervated heart in long-term cardiac transplant recipients. Am J Cardiol. 1980;46:213–218. doi: 10.1016/0002-9149(80)90060-0. [DOI] [PubMed] [Google Scholar]
- Lindelow B, Andersson B, Waagstein F, Bergh CH. High and low pulmonary vascular resistance in heart transplant candidates. A 5-year follow-up after heart transplantation shows continuous reduction in resistance and no difference in complication rate. Eur Heart J. 1999;20:148–156. doi: 10.1053/euhj.1998.1155. [DOI] [PubMed] [Google Scholar]
- Pflugfelder PW, McKenzie FN, Kostuk WJ. Hemodynamic profiles at rest and during supine exercise after orthotopic cardiac transplantation. Am J Cardiol. 1988;61:1328–1333. doi: 10.1016/0002-9149(88)91178-2. [DOI] [PubMed] [Google Scholar]
- Hosenpud JD, Pantely GA, Morton MJ, Wilson RA, Norman DJ, Cobanoglu AM, Starr A. Lack of progressive “restrictive” physiology after heart transplantation despite intervening episodes of allograft rejection: comparison of serial rest and exercise hemodynamics one and 2 years after transplantation. J Heart Transplant. 1990;9:119–123. [PubMed] [Google Scholar]
- Kao AC, Van TP, III, Shaeffer-McCall GS, Shaw JP, Kuzil BB, Page RD, Higginbotham MB. Central and peripheral limitations to upright exercise in untrained cardiac transplant recipients. Circulation. 1994;89:2605–2615. doi: 10.1161/01.cir.89.6.2605. [DOI] [PubMed] [Google Scholar]
- Young JB, Leon CA, Short HD, III, Noon GP, Lawrence EC, Whisennand HH, Pratt CM, Goodman DA, Weilbaecher D, Quinones MA. Evolution of hemodynamics after orthotopic heart and heart-lung transplantation: early restrictive patterns persisting in occult fashion. J Heart Transplant. 1987;6:34–43. [PubMed] [Google Scholar]
- Bhatia SJ, Kirshenbaum JM, Shemin RJ, Cohn LH, Collins JJ, Di Sesa VJ, Young PJ, Mudge GH, Jr, Sutton MG. Time course of resolution of pulmonary hypertension and right ventricular remodeling after orthotopic cardiac transplantation. Circulation. 1987;76:819–826. doi: 10.1161/01.cir.76.4.819. [DOI] [PubMed] [Google Scholar]
- Corcos T, Tamburino C, Leger P, Vaissier E, Rossant P, Mattei MF, Daudon P, Gandjbakhch I, Pavie A, Cabrol A, Cabrol C. Early and late hemodynamic evaluation after cardiac transplantation: a study of 28 cases. J Am Coll Cardiol. 1988;11:264–269. doi: 10.1016/0735-1097(88)90090-3. [DOI] [PubMed] [Google Scholar]
- Tamburino C, Corcos T, Feraco E, Leger P, Desruennes M, Vaissier E, Gandjbakhch I, Pavie A, Cabrol A, Cabrol C. Hemodynamic parameters one and 4 weeks after cardiac transplantation. Am J Cardiol. 1989;63:635–637. doi: 10.1016/0002-9149(89)90917-x. [DOI] [PubMed] [Google Scholar]
- Bourge RC, Kirklin JK, Naftel DC, White C, Mason DA, Epstein AE. Analysis and predictors of pulmonary vascular resistance after cardiac transplantation. J Thorac Cardiovasc Surg. 1991;101:432–444. [PubMed] [Google Scholar]
- von Scheidt W, Ziegler U, Kemkes BM, Erdmann E. Heart transplantation: hemodynamics over a 5-year period. J Heart Lung Transplant. 1991;10:342–350. [PubMed] [Google Scholar]
- Tenderich G, Koerner MM, Stuettgen B, Mirow N, Arusoglu L, Morshuis M, Bairaktaris A, Minami K, Koerfer R. Pre-existing elevated pulmonary vascular resistance: long-term hemodynamic follow-up and outcome of recipients after orthotopic heart transplantation. J Cardiovasc Surg (Torino) 2000;41:215–219. [PubMed] [Google Scholar]
- Delgado JF, Gomez-Sanchez MA, Saenz de la Calzada C, Sanchez V, Escribano P, Hernandez-Afonso J, Tello R, Gomez de la Camara A, Rodriguez E, Rufilanchas JJ. Impact of mild pulmonary hypertension on mortality and pulmonary artery pressure profile after heart transplantation. J Heart Lung Transplant. 2001;20:942–948. doi: 10.1016/s1053-2498(01)00286-8. [DOI] [PubMed] [Google Scholar]
- Chang PP, Longenecker JC, Wang NY, Baughman KL, Conte JV, Hare JM, Kasper EK. Mild vs severe pulmonary hypertension before heart transplantation: different effects on posttransplantation pulmonary hypertension and mortality. J Heart Lung Transplant. 2005;24:998–1007. doi: 10.1016/j.healun.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Goland S, Czer LS, Kass RM, De Robertis MA, Mirocha J, Coleman B, Capelli C, Raissi S, Cheng W, Fontana G, Trento A. Pre-existing pulmonary hypertension in patients with end-stage heart failure: impact on clinical outcome and hemodynamic follow-up after orthotopic heart transplantation. J Heart Lung Transplant. 2007;26:312–318. doi: 10.1016/j.healun.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Wahl A, Feller M, Wigger E, Tanner H, Stoupis C, Carrel T, Mohacsi P, Hullin R. Pretransplant pulmonary hypertension and long-term allograft right ventricular function. Eur J Cardiothorac Surg. 2010;37:61–67. doi: 10.1016/j.ejcts.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Gude E, Simonsen S, Geiran OR, Fiane AE, Gullestad L, Arora S, Relbo A, Andreassen AK. Pulmonary hypertension in heart transplantation: discrepant prognostic impact of pre-operative compared with 1-year post-operative right heart hemodynamics. J Heart Lung Transplant. 2010;29:216–223. doi: 10.1016/j.healun.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Cobb FR, Higginbotham MB. Stroke volume increases by similar mechanisms during upright exercise in normal men and women. Am J Cardiol. 1991;67:1405–1412. doi: 10.1016/0002-9149(91)90472-w. [DOI] [PubMed] [Google Scholar]
- Donald DE. Capacity for exercise after denervation of the heart. Circulation. 1968;38:225–226. doi: 10.1161/01.cir.38.2.225. [DOI] [PubMed] [Google Scholar]
- Carleton RA, Heller SJ, Najafi H, Clark JG. Hemodynamic performance of a transplanted human heart. Circulation. 1969;40:447–452. doi: 10.1161/01.cir.40.4.447. [DOI] [PubMed] [Google Scholar]
- Greenberg ML, Uretsky BF, Reddy PS, Bernstein RL, Griffith BP, Hardesty RL, Thompson ME, Bahnson HT. Long-term hemodynamic follow-up of cardiac transplant patients treated with cyclosporine and prednisone. Circulation. 1985;71:487–494. doi: 10.1161/01.cir.71.3.487. [DOI] [PubMed] [Google Scholar]