Abstract
Background
We examined whether type of menopause affects sex differences in coronary heart disease (CHD) events and whether the impact is similar in blacks and whites.
Methods and Results
Participants were enrolled in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort between 2003 and 2007 without CHD at baseline (n=23 086). Cox regression models were used to calculate the hazard of incident nonfatal CHD (definite or probable myocardial infarction) and acute CHD death, adjusting for age, age at last menstrual period <45 years, region, education level, income, diabetes, smoking, systolic blood pressure, lipid levels, albumin-creatinine ratio, physical activity, C-reactive protein, body mass index, waist circumference, and medication use. White women in natural menopause (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.31, 0.66) and surgical menopause (HR, 0.65; 95% CI, 0.42, 0.99) had a reduced hazard of nonfatal events, compared to white men. Black women in natural menopause (HR, 0.69; 95% CI, 0.47, 1.03), but not surgical menopause (HR, 0.81; 95% CI, 0.51, 1.29), had a marginally reduced hazard of nonfatal events, compared to black men. Women had lower risk of acute CHD death than men regardless of their menopause type and race.
Conclusions
Sex differences in the risk of incident CHD events were larger among whites than blacks and varied by type of menopause. Women consistently had a lower risk of incident CHD death than men, but the magnitude of sex differences was greater in whites than blacks for nonfatal events, regardless of menopause type.
Keywords: coronary disease, epidemiology, sex, women
Women have a lower risk of incident coronary heart disease (CHD) events, compared to men, although sex differences narrow in mid-life.1,2 As a mid-life milestone, menopause is commonly held as a risk factor for CHD,2 and the National Cholesterol Education Panel stated that women’s CHD risk is clinically significant after they enter menopause.3
Controversy exists as to whether menopause narrows sex differences in incidence of CHD events and how it might do so. Alternate explanations include decelerating rates of CHD in mid-life men or increased CHD risk factor burden in aging women.2,4,5 The mechanism through which menopause might act is not known, but may differ by menopause type. Several studies suggest that natural menopause does not increase risk of cardiovascular (CV) events (CVEs),6 but bilateral oophorectomy (BSO)7,8 and hysterectomy (with or without BSO)8–10 increase CHD events, compared to natural menopause. Sudden declines in estrogen levels with BSO have been cited,7 as well as poorer CHD risk factor profiles of women who undergo these procedures.8,11,12 However, natural menopause at <45 years of age is associated with increased CHD risk.13,14 Though explanations are speculative and include chronic disease or autoimmune disorders, this age cutpoint may identify a unique population at particular risk.
Approximately 600 000 hysterectomies are performed annually in the United States.15 Approximately half include concomitant BSO, although this proportion is declining.15 These procedures are more common in the southern United States.15,16 and may be more common in African Americans,15 although 1 recent study found no difference between blacks and whites.16 Reports from other cohorts note that sex differences in subclinical CHD are more pronounced in whites than in blacks, suggesting that sex differences should be similar by race after consideration of menopause type.17 Available literature on menopause type and CHD risk has not presented data for African Americans or examined whether risk estimates differ between nonfatal CHD and CHD death. Reports have not examined the risk of CHD in types of menopause, compared to risks in men; this would estimate the magnitude of CHD sex differences with different types of menopause and reduce confounding by age introduced by comparison of pre- and postmenopausal women.
Women and their physicians may consider CHD risk when considering whether to undergo elective hysterectomy and/or BSO.8 Although CHD risk factors may not differ by menopause type or change with gynecological surgery,18 at least 1 medical group advocates closer monitoring of blood pressure and lipids after the menopausal transition.19 Therefore, it is important to investigate the impact of menopause type upon sex differences in CHD risk. We used data from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, a large biracial cohort, to examine whether type of menopause differently increases risk of CHD events in women, compared to men, whether any associations persist after adjustment for CHD risk factors and region, whether associations differ between nonfatal MI and acute CHD death, and whether any of these relationships differ by race. We hypothesized that sex differences would be more pronounced for women who had undergone BSO than other menopause types and that these differences by menopause type would be similar in blacks and whites.
Methods
REGARDS is a prospective cohort study designed to examine regional and racial influences on stroke mortality. It oversampled blacks and residents of the southeastern stroke belt region, which includes Alabama, Arkansas, Georgia, Kentucky, Lousiana, Mississippi, North Carolina, South Carolina, Tennessee, and Virginia; the Carolinas and Georgia have the highest stroke incidence and are sometimes called the “stroke buckle.”9 Briefly, 30 239 participants at least 45 years of age were recruited from a commercially available list of residents using a combination of postal mailings and telephone and enrolled between January 2003 and October 2007. Using a computer-assisted telephone interview (CATI), trained interviewers obtained demographic information and medical history, and an in-home brief physical examination was conducted 3 to 4 weeks after the telephone interview. Participants are followed by CATI every 6 months for suspected incident CVEs and change in cognitive function. The study protocol was reviewed and approved by the participating institutions institutional review boards. All participants provided written informed consent.
For this report, we excluded participants with prevalent CHD at baseline (self-reported history of myocardial infarction [MI], coronary revascularization procedure, evidence of previous MI on the baseline electrocardiogram [ECG]) (n=5433), missing follow-up data (n=489), missing menopause status at baseline (n=338), and premenopausal women (n=1031); 24 123 participants were included. Events with complete ascertainment through December 31, 2011 were included in this analysis. The mean (SD) follow-up time was 5.7 (2.0) years.
Acute CHD Events
Suspected CHD events were adjudicated by a team of experts following published guidelines.20 For MI, medical records were examined for the presence of signs or symptoms suggestive of ischemia; a rising and/or falling pattern in cardiac troponin level or creatine phosphokinase–myocardial band level over 6 or more hours with a peak level greater than twice the upper limit of normal (diagnostic cardiac enzymes); and ECG changes consistent with ischemia or MI, guided by the Minnesota code and classified as evolving diagnostic, positive, nonspecific, or not consistent with ischemia.21 Definite MIs were those with diagnostic enzymes or ECG. Probable MIs were those with elevated, but not diagnostic (ie, equivocal), enzymes with a positive, but not diagnostic, ECG; or, if enzymes were missing, with a positive ECG in the presence of ischemic signs or symptoms. Only definite or probable MIs were included in this study. For acute CHD death, the medical history, hospital records, autopsy reports, interviews with next of kin or proxies, and death certificate or National Death Index data were reviewed to adjudicate the cause of death, with definite or probable CHD death included in this analysis. Cases were assigned to 2 adjudicators and disagreements were adjudicated by committee, with a κ>0.80 for the presence of definite or probable MI or definite or probable acute CHD death. Primary outcomes were total CHD events, consisting of definite or probable CHD deaths and nonfatal definite or probable MIs, as well as acute CHD death and nonfatal MI separately.
Type of Menopause
At baseline, women were asked, “Have you gone through the change of life?” and “How old were you at the time of your last menstrual period?” To identify those with surgical menopause, participants were asked, “Have you ever had an ovary removed?” If they answered “yes,” participants were asked, “How many ovaries were removed?” Women were also asked, “Have you ever had a hysterectomy, that is, surgery to remove your uterus or womb?” Women were classified as having menopause resulting from BSO, with or without hysterectomy; menopause resulting from hysterectomy alone; or natural menopause. Women were further categorized as being in natural or surgical menopause. Women with menopause resulting from BSO with or without hysterectomy were defined as such owing to the fact that the ovaries continue to make androgens in postmenopause, even as estrogen production ceases,22 and androgens have been postulated to be influential in atherosclerotic disease in women.23 Natural menopause was defined as a last menstrual period occurring ≥12 months before the interview, in the absence of hysterectomy or BSO. If women had stopped menstruating before hysterectomy or BSO, they were defined as being in natural menopause.
Covariates
Age, sex, race, income, education, and smoking status were self-reported. Annual household income was dichotomized at $35 000 based on previous analyses in this cohort,24 and education was dichotomized at high school diploma. Smokers were defined as having smoked at least 100 cigarettes in their lifetime and smoking now, even occasionally. Participants were asked, “How many times per week do you engage in intense physical activity, enough to work up a sweat?” Data on antihypertensive drugs and statin use were based on self-report and in-home pill bottle review, respectively, and data on estrogen therapy were based on in-home reviews conducted by trained interviewers. Anthropometrics and blood pressure were ascertained during an in-home visit using a standardized protocol; body mass index (BMI) and waist circumference have previously been demonstrated to predict mortality risk independently in REGARDS.25 Fasting blood and urine markers included total cholesterol, high-density lipoprotein (HDL) cholesterol, glucose, high-sensitivity C-reactive protein (CRP), serum creatinine, and urinary albumin and creatinine from a spot urine specimen.26 CRP was log-transformed and modeled as a continuous variable. Diabetes was classified as present if the fasting glucose level was 126 mg/dL or higher (nonfasting glucose, ≥200 mg/dL) or if the participant was taking diabetes medications (to convert glucose to millimoles per liter, multiply by 0.0555). Urinary albumin and creatinine were used to define the urinary albumin-creatinine ratio (ACR).
Statistical Analysis
Primary analyses were stratified by race/ethnicity. Baseline participant characteristics were tabulated by the categories of natural menopause, BSO with or without hysterectomy, hysterectomy alone, and men, using chi-square and ANOVA tests. Cox proportional hazards regression modeling was used to examine the association between menopause type and incident CHD with men as a reference for each menopause type. A parallel set of models was generated that categorized women as being in natural or surgical menopause. Models were adjusted incrementally for age, region (Model 1), and further for education and income (Model 2); model 2 covariates and total cholesterol, HDL, triglycerides, smoking, exercise, systolic blood pressure, diabetes, ACR, use of antihypertensive medications and statins, and CRP (Model 3); model 3 covariates and estrogen therapy at baseline (Model 4); model 4 covariates and waist circumference and BMI (Model 5); and model 5 covariates and menopause age dichotomized at 45 years (Model 6). Missing data in covariates were imputed using chained equations with 20 data sets, in STATA 12 (StataCorp LP, College Station, TX).27
To determine whether the relationship between menopause type and CHD incidence differed significantly by race, Cox models were created that pooled whites and blacks and menopause types and included an interaction term between race and menopause category in the fully adjusted model. Race-stratified models were also created that evaluated an interaction term between age at last menstrual period and type of menopause to determine whether the association between menopause type and incident CHD differed in women who were <45 years versus ≥45 years of age. The assumptions of proportionality were met. Individuals were censored at the time of their event, death, or the end of follow-up. Statistical significance for all analyses was considered as P<0.05 and for interactions as P<0.10. Analyses were conducted using SAS (9.3; SAS Institute Inc., Cary, NC) and STATA 12.
Results
Sample Characteristics
Table1 lists the baseline characteristics by menopausal status in blacks (n=9669) and whites (n=13 417). Among blacks, men were similar in age and education level to women, but men reported higher income. Black men were more likely to smoke, exercise, and had lower BMI than black women, but blood pressure and HDL were poorer. Among whites, similar patterns were observed, except that smoking prevalence and BMI were similar by sex groups.
Table 1.
Baseline Characteristics of REGARDS Cohort Members Without CHD at Baseline
| Black (n=9669) | White (n=13 417) | |||||||
|---|---|---|---|---|---|---|---|---|
| Men (n=3744) | Natural Menopause (n=3420) | Hysterectomy Only (n=1386) | Bilateral Oophorectomy With or Without Hysterectomy (n=1119) | Men (n=6423) | Natural Menopause (n=4130) | Hysterectomy Only (n=1361) | Bilateral Oophorectomy With or Without Hysterectomy (n=1503) | |
| Age, y | 64 (9) | 65 (9) | 64 (9) | 64 (8) | 65 (9) | 65 (9) | 66 (9) | 66 (9) |
| Age at last menstrual period, y | — | 48 (8) | 40 (8) | 43 (8) | — | 48 (7) | 39 (8) | 44 (8) |
| Age at last menstrual period <45 y, % | — | 1290 (38) | 904 (65) | 590 (53) | — | 1151 (28) | 957 (70) | 697 (47) |
| Region, % | ||||||||
| Stroke belt | 1247 (33) | 1106 (32) | 503 (36) | 384 (34) | 2221 (35) | 1338 (32) | 578 (43) | 609 (41) |
| Stroke buckle | 604 (16) | 637 (19) | 275 (20) | 215 (19) | 1261 (20) | 978 (24) | 429 (32) | 399 (27) |
| Nonstroke belt/buckle | 1893 (51) | 1677 (49) | 608 (44) | 520 (47) | 2941 (46) | 1814 (44) | 354 (26) | 495 (33) |
| Education less than high school, % | 685 (18) | 696 (20) | 263 (19) | 201 (18) | 380 (6) | 235 (6) | 124 (9) | 126 (8) |
| Annual income <$35 000, % | 1688 (45) | 1930 (56) | 797 (58) | 622 (56) | 1665 (26) | 1521 (37) | 616 (45) | 631 (42) |
| Diabetes, % | 1052 (29) | 950 (29) | 373 (28) | 311 (29) | 896 (14) | 432 (11) | 203 (15) | 175 (12) |
| Never exercises, % | 1079 (29) | 1396 (41) | 541 (39) | 443 (40) | 1566 (25) | 1445 (36) | 519 (39) | 592 (40) |
| Current smoker, % | 731 (20) | 536 (16) | 196 (14) | 160 (14) | 738 (12) | 539 (13) | 164 (12) | 183 (12) |
| Body mass index, kg/m2 | 28.9 (5.4) | 31.4 (7.1) | 32.2 (6.7) | 32.0 (7.0) | 28.2 (4.8) | 27.9 (6.3) | 28.5 (6.3) | 28.5 (6.3) |
| Waist circumference, cm | 99 (15) | 96 (16) | 97 (15) | 97 (15) | 100 (13) | 88 (15) | 90 (15) | 90 (16) |
| Total cholesterol, mg/dL | 187 (40) | 199 (41) | 200 (41) | 202 (41) | 187 (36) | 205 (39) | 202 (40) | 201 (38) |
| HDL, mg/dL | 48 (15) | 57 (16) | 57 (15) | 58 (17) | 45 (13) | 59 (16) | 56 (17) | 58 (17) |
| Systolic blood pressure, mm Hg | 132 (17) | 130 (18) | 130 (16) | 130 (17) | 127 (15) | 123 (16) | 124 (16) | 124 (15) |
| Antihypertensive med use, % | 2082 (56) | 2132 (64) | 942 (69) | 783 (71) | 2423 (38) | 1576 (39) | 631 (47) | 701 (47) |
| Statin use, % | 975 (26) | 984 (29) | 398 (29) | 341 (31) | 1898 (30) | 1123 (28) | 436 (32) | 469 (32) |
| Current estrogen therapy, % | — | 252 (7) | 201 (15) | 286 (26) | — | 591 (14) | 392 (29) | 576 (28) |
| C-reactive protein, mg/L* | 2.1 [0.9 to 4.4] | 3.2 [1.3 to 7.1] | 3.6 [1.5 to 7.7] | 4.1 [1.7 to 8.1] | 1.5 [0.7 to 3.3] | 1.9 [0.9 to 4.3] | 2.7 [1.2 to 5.6] | 2.8 [1.2 to 6.0] |
| Albumin-creatinine ratio* | 7.2 [4.1 to 20.9) | 8.3 [5.1 to 18.3] | 7.4 [4.8 to 15.3] | 7.1 [4.7 to 15.9] | 6.0 [4.0 to 12.0] | 8.1 [5.3 to 14.3] | 7.1 [4.8 to 12.7] | 7.0 [4.8 to 11.8] |
Number (%) or means (SD) shown, unless otherwise indicated. CHD indicates coronary heart disease; HDL, high-density lipoprotein; REGARDS, Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort.
Median (interquartile ratio).
Among blacks, women who had undergone natural menopause were of similar age, education, and income, compared to women in surgical menopause, although the proportion of women with menopause at <45 years of age was lower among women in natural menopause. Surgical menopause was only slightly more common among women living in the stroke belt or buckle than those residing elsewhere. Behaviors such as smoking and exercise were similar across menopause categories, as were metabolic profiles. Black women in natural menopause were less likely to use blood pressure medications and estrogen therapy, compared to black women in surgical menopause. Among whites, similar patterns were observed, except that white women in natural menopause had higher income than white women in surgical menopause. Surgical menopause was more common among white women living in the stroke belt or buckle. Regional differences in menopause type were more pronounced among whites than among blacks.
Total CHD Events
Events by nonfatal versus fatal status, sex, menopause type, and race are shown in Table2. Table3 shows the risk of total CHD, fatal CHD, and nonfatal CHD by menopause type, stratified by race. Black women had a significantly lower hazard of any CHD event, compared to black men, regardless of menopause type, and white women also had a significantly lower hazard of any CHD event, compared to white men, regardless of menopause type. In the fully adjusted model combining blacks and whites with total CHD events as the outcome, the race by menopause category interaction term had a P=0.09. This interaction was owing to differences in association between menopause category and nonfatal CHD events by race.
Table 2.
Incident CHD Events Through December 2011
| Black (n=9669) | White (n=13 417) | |||||||
|---|---|---|---|---|---|---|---|---|
| Men (n=3744) | Natural Menopause (n=3420) | Hysterectomy Only (n=1386) | Bilateral Oophorectomy With or Without Hysterectomy (n=1119) | Men (n=6423) | Natural Menopause (n=4130) | Hysterectomy Only (n=1361) | Bilateral Oophorectomy With or Without Hysterectomy (n=1503) | |
| Person-years of follow-up | 21 001 | 18 684 | 7732 | 6326 | 38 489 | 23 620 | 7859 | 8541 |
| Total CHD events | 192 | 111 | 42 | 37 | 351 | 83 | 39 | 37 |
| Fatal CHD events | 91 | 47 | 13 | 12 | 99 | 27 | 11 | 8 |
| Nonfatal CHD events | 101 | 64 | 29 | 25 | 252 | 56 | 28 | 29 |
CHD indicates coronary heart disease.
Table 3.
Total, Fatal, and Nonfatal Acute CHD Events in Cox Regression Models, Hazard Ratios, and 95% Confidence Intervals
| Black (n=9669) | White (n=13 417) | |||||
|---|---|---|---|---|---|---|
| Natural Menopause (n=3420) | Surgical Menopause | Natural Menopause (n=4130) | Surgical Menopause | |||
| Hysterectomy Only (n=1386) | Bilateral Oophorectomy With or Without Hysterectomy (n=1119) | Hysterectomy Only (n=1361) | Bilateral Oophorectomy With or Without Hysterectomy (n=1503) | |||
| Total CHD | ||||||
| Adjusted for age and region | 0.63 (0.50 to 0.80) | 0.58 (0.41 to 0.80) | 0.64 (0.45 to 0.91) | 0.39 (0.30 to 0.49) | 0.51 (0.36 to 0.71) | 0.45 (0.32 to 0.63) |
| Fully adjusted* | 0.58 (0.43 to 0.78) | 0.55 (0.35 to 0.83) | 0.61 (0.38 to 0.94) | 0.40 (0.29 to 0.55) | 0.44 (0.28 to 0.70) | 0.46 (0.30 to 0.72) |
| 0.57 (0.40 to 0.82) | 0.45 (0.31 to 0.66) | |||||
| Fatal CHD | ||||||
| Adjusted for age and region | 0.57 (0.40 to 0.81) | 0.38 (0.21 to 0.68) | 0.45 (0.24 to 0.82) | 0.46 (0.30 to 0.70) | 0.50 (0.27 to 0.94) | 0.35 (0.17 to 0.72) |
| Fully adjusted* | 0.45 (0.28 to 0.71) | 0.31 (0.15 to 0.63) | 0.37 (0.18 to 0.76) | 0.28 (0.15 to 0.52) | 0.20 (0.08 to 0.49) | 0.17 (0.06 to 0.42) |
| 0.34 (0.18 to 0.61) | 0.18 (0.08 to 0.40) | |||||
| Nonfatal CHD | ||||||
| Adjusted for age and region | 0.69 (0.50 to 0.94) | 0.75 (0.50 to 1.14) | 0.81 (0.53 to 1.26) | 0.36 (0.27 to 0.48) | 0.51 (0.34 to 0.75) | 0.49 (0.33 to 0.72) |
| Fully adjusted* | 0.69 (0.47 to 1.03) | 0.79 (0.46 to 1.33) | 0.84 (0.49 to 1.45) | 0.45 (0.31 to 0.66) | 0.62 (0.36 to 1.04) | 0.67 (0.41 to 1.10) |
| 0.81 (0.51 to 1.29) | 0.65 (0.42 to 0.99) | |||||
Reference group is men. CHD indicates coronary heart disease.
Adjusted for age, age at menopause <45 years (yes/no), less than high school education, annual income ≤$35 000; body mass index, waist circumference, level of total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, albumin-creatinine ratio, and C-reactive protein; current smoking (yes/no), diabetes (yes/no), never exercise (yes/no), use of antihypertensive (yes/no), statins (yes/no), estrogen therapy (yes/no).
Fatal CHD Events
In minimally- and fully adjusted models, black and white women had a markedly lower hazard of fatal CHD events than men, regardless of menopause type (Table3).These associations did not differ by race (P interaction of menopause category × race = 0.89). Figure1 illustrates how the point estimates and 95% confidence intervals (CIs) for the hazard ratios differed with adjustment for each set of covariates. Whereas 95% CIs overlapped, adjustment for age at menopause <45 years decreased the point estimates, particularly in white women who had undergone hysterectomy (P=0.04 for interaction for menopause type and age at menopause <45 years).
Figure 1.
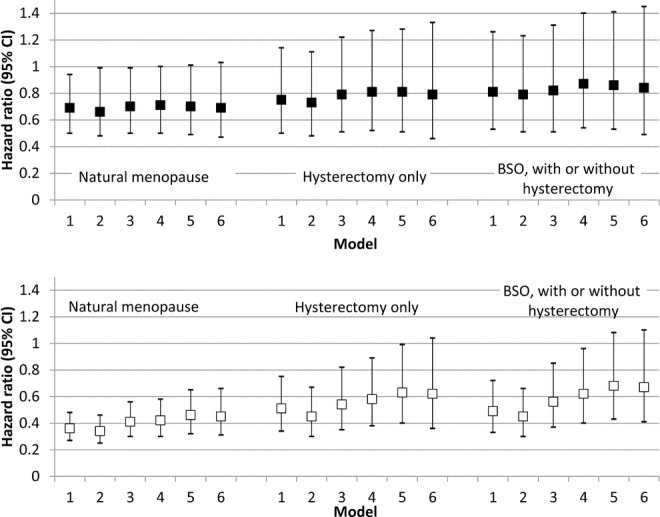
Risk of acute fatal CHD events by menopause category (hazard ratios and 95% confidence intervals), with men as referent group. Black participants are shown in the top panel and white participants shown in the bottom panel. Model 1 adjusts for age and region. Model 2 adjusts for less than high school education and annual income <$35 000. Model 3 adjusts for level of total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, albumin-creatinine ratio, and C-reactive protein; current smoking (yes/no), diabetes (yes/no), never exercise (yes/no), use of antihypertensives (yes/no), and statins (yes/no). Model 4 further adjusts for estrogen therapy. Model 5 further adjusts for waist circumference (cm) and body mass index (kg/m2). Model 6 further adjusts for age at last menstrual period <45 years (yes/no). BSO indicates bilateral oophorectomy; CHD, coronary heart disease; CI, confidence interval.
Nonfatal CHD Events
When we examined risk for nonfatal CHD events, a different pattern emerged. Among blacks, women in natural menopause had a slightly lower hazard for nonfatal events than men, although these results did not reach statistical significance (Table3). Women in any type of surgical menopause had a statistically similar hazard for nonfatal CHD events. Among whites, women in natural menopause had lower risk than men after adjustment for other covariates (Figure2). White women in surgical menopause also had a decreased hazard for nonfatal events, compared to men, although the CIs for subcategories of surgical menopause were wider (Table3). This difference in patterns between blacks and whites was statistically significant (P=0.03 for interaction term between race and menopause type in fully adjusted models). For white women, in models stratified by age at menopause <45 years, similar patterns as the nonstratified analysis were noted (results not shown).
Figure 2.
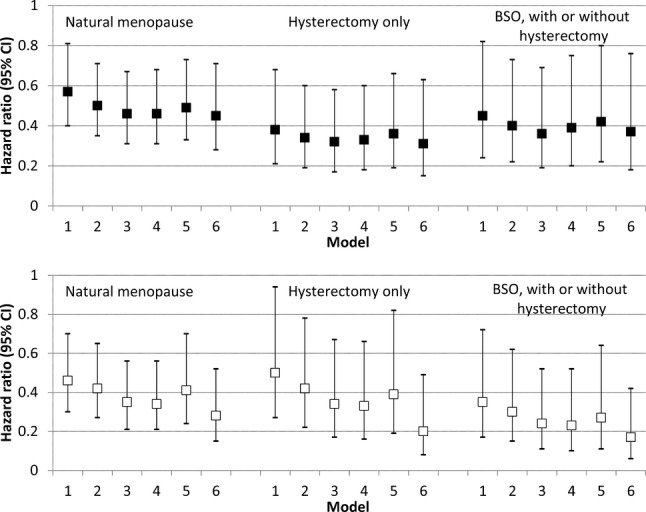
Risk of acute nonfatal CHD events by menopause category (hazard ratios and 95% confidence intervals), with men as referent group. Black participants are show in the top panel and white participants shown in the bottom panel. Model 1 adjusts for age and region. Model 2 adjusts for less than high school education and annual income <$35 000. Model 3 adjusts for level of total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, albumin-creatinine ratio, and C-reactive protein; current smoking (yes/no), diabetes (yes/no), never exercise (yes/no), use of antihypertensives (yes/no), and statins (yes/no). Model 4 further adjusts for estrogen therapy. Model 5 further adjusts for waist circumference (cm) and body mass index (kg/m2). Model 6 further adjusts for age at last menstrual period <45 years (yes/no). BSO indicates bilateral oophorectomy; CHD, coronary heart disease; CI, confidence interval.
Discussion
In the REGARDS study, in both blacks and whites, women had a lower risk of total CHD events than men, regardless of menopause category. This reduced risk persisted after adjustment for an extensive list of potential confounders. Compared to black men, black women had approximately half the hazard of any CHD event, compared to men. Compared to white men, white women also had a significantly reduced hazard of CHD. These sex differences were even more pronounced for risk of acute fatal CHD events in both blacks and whites. However, sex differences were less pronounced for nonfatal CHD events. Patterns varied by race, with no significant sex differences observed in nonfatal CHD risk among blacks. White women in natural menopause and surgical menopause continued to have lower nonfatal CHD risk than men. Thus, frequency of surgical menopause was not responsible for the lack of sex differences in nonfatal CHD in blacks as opposed to whites.
Our results confirm that the risk of having any incident CHD event in women is lower than in men.2 We also note that this low risk persists despite the postmenopausal state, regardless of menopause type, and despite comprehensive adjustment for traditional and unconventional CHD risk factors, including renal function, CRP, and medication use. This suggests that other factors besides menopause status and CHD risk factors are driving sex differences in mid-life. Previous investigators noted that although the risk of incident CHD increases rapidly in women after 50 years of age, the approximate age of menopause, evaluation of risk for CHD changes on a log-scale reveal a gradual increase in risk rather than a sharp change in risk (as is observed for breast cancer).2,5 Therefore, men may have a slower rate of increase in CHD events in mid-life, whereas women’s rate of increase remains constant.2,5 In other words, the greater sex differences observed at younger ages, as opposed to older ages, may be owing to changing risk in men rather than women, as well as the difficulty in demonstrating greater relative differences in events as the number of events increases.
To our knowledge, other reports have not examined the impact of menopause type upon sex differences in CHD risk. Previous literature has conflicted regarding the degree of CHD risk conferred by different types of menopause. In the Women’s Health Initiative Observational Study, the risk of any CVE associated with menopause type was examined, using natural menopause as a referent group; CV disease was defined as MI, stroke, coronary revascularization, or CHD death. Women who had undergone hysterectomy had a poorer CHD risk profile than women in natural menopause, regardless of oophorectomy status, and the increased risk associated with hysterectomy was diminished after adjustment for CHD risk factors.28 In the Nurses’ Health Study, BSO was associated with increased CHD risk, compared to natural menopause, after adjustment for age and smoking.7 A more recent analysis suggested that BSO was associated with greater CHD risk, compared to hysterectomy alone, after adjustment for a more extensive list of covariates than age and smoking.8 Our results are similar to these findings, in that we found that, of all the menopause categories, black and white women in natural menopause had the lowest risk of nonfatal CHD events, compared to men.
Our report differs from previous studies that suggested women in surgical menopause might lose their advantage, compared to men, even as men were not examined along with subtype of menopause. Analyses in other cohorts have reported that women who undergo surgical menopause tend to have poorer CHD risk factor profiles29 than other women, particularly among blacks.30 One exception reported that annual changes in CHD risk factors did not vary by menopausal type.18 We found that surgical menopause was not associated with a greater risk of fatal CHD events. Though the sudden cardiac death that comprises a subset of fatal CHD is more common in men than women, myocardial ischemia is believed to be the primary etiology.31 However, other reports have noted that testosterone levels are inversely associated with QT intervals in men, but not women,32 and it is possible that the higher androgen levels observed in men confer a relative disadvantage regarding arrhythmias. The risk of acute CHD events has also declined with time, a finding attributed to more-intensive CHD risk factor management with lifestyle modification and pharmacotherapy.33 It is possible that these secular trends may have contributed to the decrease in CHD risk associated with surgical menopause.
We also found that the black women who underwent surgical menopause and natural menopause in REGARDS had a similar risk of nonfatal CHD events, compared to black men, regardless of menopause type. White women, particularly white women who underwent natural menopause, had a lower risk of nonfatal CHD events, compared to white men. It is possible that the androgen production that distinguishes menopause types22 is influential in the pathophysiology of the diffuse atherosclerosis that characterizes nonfatal events, and racial/ethnic differences by androgen level exist. Previous studies have conflicted as to whether black and white women have different levels of androgens,34–37 so it is unknown whether differences in androgen profile might explain risk in whites, but not blacks. Persistence of these patterns after adjustment for an extensive list of CHD risk factors suggests that a higher risk-factor burden in blacks does not contribute.
Younger age at menopause was associated with increased hazard of fatal CHD in white women, given that adjustment for this factor increased women’s advantage, compared to men. Previous studies have noted consistent relationships with younger age at menopause in white populations and increased risk of any CHD event,13,14 particularly fatal CHD. Smoking increases risk of earlier menopause,14 although the association persisted after adjustment for smoking. Autoimmune disease may also increase risk of early menopause,38 although such conditions are uncommon. It is possible that the changes in sex steroid profile that characterize the menopause may be cumulative over time, with younger ages at menopause leading to increased risk. Adjustment for age at menopause led to minimal change among blacks, perhaps related to the reduced sex differences among blacks.
Despite the large numbers of black and white participants, the number of fatal CHD events was modest, and estimates of fatal CHD risk should be interpreted with caution. Age at menopause was self-reported. Women who had undergone menopause as a result of hysterectomy alone have been reported to have slight reductions in androgens, but with smaller reductions compared to women in natural menopause or with BSO (presumably owing to unintended ovarian infarction during oophorectomy) and thus were defined as such.22 Women tend to under-report BSO in the context of hysterectomy,39 and thus the category of women with hysterectomy only may have included women with bilateral oophorectomy as well. Studies have reported high correlation between ultrasound scans and self-report of hysterectomy,40 whereas the positive predictive value for self-report of oophorectomy is lower.39 Precise measures of diet and physical activity were not available. Strengths of this study include its large sample size and national sampling frame, allowing adjustment for regional variation in surgeries and CHD risk, as well as stratified analyses by race. Additional strengths included adjudication of CHD endpoints as well as examination of CHD alone instead of a composite endpoint. In addition, REGARDS measured a comprehensive list of both classic and nontraditional CHD risk factors that were included in adjustment. Finally, the examination of race and sex differences has not previously been examined.
In a national population-based sample of blacks and whites, we found that menopausal women had a lower risk of CHD events than men after consideration of an extensive list of potential confounders. This advantage was particularly pronounced for fatal CHD events, regardless of menopause type, in both whites and blacks. Regarding nonfatal CHD events, white women retained their advantage regardless of menopause type, whereas black women had a similar risk to black men for nonfatal CHD events regardless of menopause type. For black women, our findings are reassuring, in that CHD risk does not seem to vary significantly by menopause type, compared to men, and, moreover, that women have lower risk for fatal CHD, even in postmenopause, compared to men. For white women, women are still at lower risk than men for any type of CHD event. Exploration of factors that contribute nonfatal MI, and, particularly, to the sex advantage in white women, compared to white men, and the absence of this advantage in black women and black men, is needed.
Sources of Funding
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Additional funding is provided by grants R01 HL080477 and K24 HL111154 from the National Heart, Lung, and Blood Institute. Funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgments
A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Disclosures
None.
References
- Lerner D, Kannel W. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111:383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- Tunstall-Ped oe H. Myth and paradox of coronary risk and the menopause. Lancet. 1998;351:1425–1427. doi: 10.1016/S0140-6736(97)11321-6. [DOI] [PubMed] [Google Scholar]
- Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III): final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- Barrett-Connor E. Why women have less heart disease than men and how diabetes modifies women’s usual cardiac protection. Glob Heart. 2013;8:95–104. doi: 10.1016/j.gheart.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy R. Sex difference in coronary disease: two opposing views. J Chronic Dis. 1966;19:1245–1251. doi: 10.1016/0021-9681(66)90022-1. [DOI] [PubMed] [Google Scholar]
- Atsma F, Bartelink M, Grobbee D, van der Schouw Y. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13:265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- Colditz G, Willett W, Stampfer M, Rosner B, Speizer F, Hennekens C. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- Parker W, Broder M, Chang E, Feskanich D, Farquar C, Liu Z, Shoupe D, Berek J, Hankinson S, Manson J. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol. 2009;113:1027–1037. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard V, Cushman M, Pulley L, Gomez C, Go R, Prineas R, Graham A, Moy C, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- Gordon T, Kannel W, Kjortland M, McNamara P. Menopause and coronary heart disease. The Framingham study. Ann Intern Med. 1978;89:157–161. doi: 10.7326/0003-4819-89-2-157. [DOI] [PubMed] [Google Scholar]
- Verhoeven M, van der Mooren M, Teerlink T, Verheijen R, Scheffer P, Kenemans P. The influence of physiological and surgical menopause on coronary heart disease risk markers. Menopause. 2009;16:37–49. doi: 10.1097/gme.0b013e31817c42d6. [DOI] [PubMed] [Google Scholar]
- Jacoby V, Grady D, Sawaya G. Oophorectomy as a risk factor for coronary heart disease. Am J Obstet Gynecol. 2009;200:140.e141–140.e149. doi: 10.1016/j.ajog.2008.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellons M, Ouyang P, Schreiner P, Herrington D, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the multi-ethnic study of atherosclerosis (MESA) Menopause. 2012;19:1081–1087. doi: 10.1097/gme.0b013e3182517bd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Grodstein F, Hennekens C, Colditz G, Johson M, Manson J, Rosner B, Stampfer M. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159:1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Hillis S, Jamieson D, Morrow B, Podgornik M, Brett K, Marchbanks P. Inpatient hysterectomy surveillance in the United States, 2000–2004. Am J Obstet Gynecol. 2008;198:34.e31–34.e37. doi: 10.1016/j.ajog.2007.05.039. [DOI] [PubMed] [Google Scholar]
- McKnight K, Wellons M, Sites C, Roth D, Szychowski J, Halanych J, Cushman M, Safford M. Racial and regional differences in age at menopause in the United States: findings from the reasons for geographic and racial differences in stroke (regards) study. Am J Obstet Gynecol. 2011;205:353e351–353e358. doi: 10.1016/j.ajog.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Diez-Roux A, Nettleton J, Polak J, Post W, Siscovick D, Watson K, Vahratian A. Sex differences in subclinical atherosclerosis by race/ethnicity in the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2011;174:166–172. doi: 10.1093/aje/kwr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Gibson C, El Khoudary S, Thurston R. Changes in cardiovascular risk factors by hysterectomy status with and without oophorectomy: Study of Women’s Health Across the Nation. J Am Coll Cardiol. 2013;62:191–200. doi: 10.1016/j.jacc.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Society for Cardiovascular Angiography and Interventions. Risk factor modification and lifestyle changes for women. Available at: http://secondscount.org/treatments/treatments-detail-2/risk-factor-modification-lifestyle-changes-women-c#.VYKz8kaVlRo. Accessed October 31, 2013.
- Luepker R, Apple F, Chrstenson R, Crow R, Fortmann S, Goff D, Goldberg R, Hand M, Jaffe A. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- Prineas R, Crow R, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. Boston, MA: Wright-OSG; 1982. [Google Scholar]
- Laughlin G, Barrett-Connor E, Kritz-Silverstein D, von Muhlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85:645–651. doi: 10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- Laughlin G, Goodell V, Barrett-Connor E. Extremes of endogenous testosterone are associated with increased risk of incident coronary events in older women. J Clin Endocrinol Metab. 2010;95:740–747. doi: 10.1210/jc.2009-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond N, Richman J, Gamboa C, Albert M, Sims M, Durant R, Glasser S, Safford M. Perceived stress is associated with incident coronary heart disease and all-cause mortality in low- but not high-income participants in the REGARDS study. J Am Heart Assoc. 2013;2:e000447. doi: 10.1161/JAHA.113.000447. doi: 10.1161/JAHA.113.000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer H, Shoham D, McClure L, Durazo-Arvizu R, Howard G, Judd S, Muntner P, Safford M, Warnock D, McClellan W. Association of waist circumference and body mass index with all-cause mortality in CKD: the REGARDS study. Am J Kidney Dis. 2011;58:177–185. doi: 10.1053/j.ajkd.2011.02.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillett S, Boyle R, Zakai N, McClure L, Jenny N, Cushman M. Validating laboratory results in a national observational cohort study without field centers: the Reasons for Geographic and Racial Differences in Stroke cohort. Clin Biochem. 2014;47:243–246. doi: 10.1016/j.clinbiochem.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10:585–598. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- Howard B, Kuller L, Langer R, Manson J, Allen C, Assaf A, Cochrane B, Larson J, Lasser N, Rainford M, Van Horn L, Stefanick M, Trevisan M. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the women’s health initiative observational study. Circulation. 2005;111:1462–1470. doi: 10.1161/01.CIR.0000159344.21672.FD. [DOI] [PubMed] [Google Scholar]
- Appiah D, Winters S, Hornung CA. Bilateral oophorectomy and the risk of incident diabetes in postmenopausal women. Diabetes Care. 2014;37:725–733. doi: 10.2337/dc13-1986. [DOI] [PubMed] [Google Scholar]
- Bower J, Schreiner P, Lewis C. Black-white differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300–307. doi: 10.2105/AJPH.2008.133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel W, Cupples L, D’Agostino R. Sudden death risk in overt coronary heart disease: the Framingham Study. Am Heart J. 1987;113:799–804. doi: 10.1016/0002-8703(87)90722-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ouyang P, Post W, Dalal D, Vaidya D, Blasco-Colmenares E, Soliman E, Tomaselli G, Guallar E. Sex-steroid hormones and electrocardiographic QT-interval duaration: findings from the third national health and nutrition examination survey and the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2011;174:403–411. doi: 10.1093/aje/kwr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford E, Capewell S. Proportion of the decline in cardiovascular mortality disease due to prevention versus treatment: public health versus clinical care. Annu Rev Public Health. 2011;32:5–22. doi: 10.1146/annurev-publhealth-031210-101211. [DOI] [PubMed] [Google Scholar]
- Randolph J, Jr, Sowers M, Bondarenko I, Harlow S, Luborsky J, Little R. Change in estradiol and FSH across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab. 2004;89:1555–1561. doi: 10.1210/jc.2003-031183. [DOI] [PubMed] [Google Scholar]
- Setiawan V, Haiman C, Stanczyk F, Le Marchand L, Henderson B. Racial/ethnic differences in postmenopausal endogenous hormones: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15:1849–1855. doi: 10.1158/1055-9965.EPI-06-0307. [DOI] [PubMed] [Google Scholar]
- Golden S, Dobs A, Vaidya D, Szklo M, Gapstur S, Kopp P, Liu K, Ouyang P. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007;92:1289–1295. doi: 10.1210/jc.2006-1895. [DOI] [PubMed] [Google Scholar]
- Kim C, Golden S, Mather K, Laughlin G, Kong S, Nan B, Barrett-Connor E, Randolph J, Jr Diabetes Prevention Program Research Group. Racial/ethnic differences in sex hormone levels among postmenopausal women in the diabetes prevention program. J Clin Endocrinol Metab. 2012;97:4051–4060. doi: 10.1210/jc.2012-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakalov V, Anasti J, Calis K, Vanderhoof V, Premkumar A, Chen S, Furmaniak J, Smith B, Merino M, Nelson L. Autoimmune oophorotis as a mechanism of follicular dysfunction in women with 46, xx spontaneous premature ovarian failure. Fertil Steril. 2005;84:958–965. doi: 10.1016/j.fertnstert.2005.04.060. [DOI] [PubMed] [Google Scholar]
- Phipps A, Buist D. Validation of self-reported history of hysterectomy and oophorectomy among women in an integrated group practice setting. Menopause. 2009;16:576–581. doi: 10.1097/gme.0b013e31818ffe28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry-Maharaj A, Sharma A, Burnell M, Ryan A, Amso N, Seif M, Turner G, Brunell C, Fletcher G, Rangar R, Fallowfield L, Campbell S, Jacobs I, Menon U. Acceptance of transvaginal sonography by postmenopausal women participating in the United Kingdom collaborative trial of ovarian cancer screening. Ultrasound Obstet Gynecol. 2013;41:73–79. doi: 10.1002/uog.12262. [DOI] [PubMed] [Google Scholar]


