Abstract
Background
Early clopidogrel administration to patients with acute myocardial infarction (AMI) has been demonstrated to improve outcomes in a large Chinese trial. However, patterns of use of clopidogrel for patients with AMI in China are unknown.
Methods and Results
From a nationally representative sample of AMI patients from 2006 and 2011, we identified 11 944 eligible patients for clopidogrel therapy and measured early clopidogrel use, defined as initiation within 24 hours of hospital admission. Among the patients eligible for clopidogrel, the weighted rate of early clopidogrel therapy increased from 45.7% in 2006 to 79.8% in 2011 (P<0.001). In 2006 and 2011, there was significant variation in early clopidogrel use by region, ranging from 1.5% to 58.0% in 2006 (P<0.001) and 48.7% to 87.7% in 2011 (P<0.001). While early use of clopidogrel was uniformly high in urban hospitals in 2011 (median 89.3%; interquartile range: 80.1% to 94.5%), there was marked heterogeneity among rural hospitals (median 50.0%; interquartile range: 11.5% to 84.4%). Patients without reperfusion therapy and those admitted to rural hospitals were less likely to be treated with clopidogrel.
Conclusions
Although the use of early clopidogrel therapy in patients with AMI has increased substantially in China, there is notable wide variation across hospitals, with much less adoption in rural hospitals. Quality improvement initiatives are needed to increase consistency of early clopidogrel use for patients with AMI.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT01624883.
Keywords: acute myocardial infarction, clopidogrel, quality of care
China, a country of more than 1.3 billion people, faces a marked increase in the incidence of acute myocardial infarction (AMI). The World Bank estimates that by 2030, 23 million Chinese patients will experience AMI annually.1 To manage this rising burden of heart disease, it is important that the Chinese healthcare system consistently deliver highly effective interventions, especially those treatments that can be delivered within existing infrastructure. The early use of clopidogrel, defined as use of the drug within the first 24 hours of presentation for AMI, is an important example of such intervention. The benefit of clopidogrel has been established in numerous clinical trials.2–4 Notably, the ClOpidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT), which was published in 2005 and conducted exclusively in China, demonstrated that in-hospital clopidogrel treatment within 24 hours of presentation of AMI resulted in a significant reduction in major cardiovascular events.2 Based on this evidence, and the COMMIT trial in particular, the Chinese guidelines released in 2007 and 2010 have strongly endorsed the early use of clopidogrel for patients with AMI. The European Society of Cardiology and American College of Cardiology/American Heart Association guidelines also make similar recommendations.5–10
Antiplatelet therapies such as clopidogrel are generally safe and have proven efficacy in the management of AMI and the benefit of antiplatelet could extend beyond the acute setting. In addition, because they are easy to administer, they are particularly useful in a broad range of practice settings, especially in rural hospitals with limited resources, which are responsible for the care of more than 700 million Chinese people.11
A registry analysis from the China Patient-centered evaluative assessment of cardiac events (PEACE)–Retrospective AMI Study demonstrated that early clopidogrel use for patients with ST-segment elevation myocardial infarction (STEMI) increased substantially in the years following the publication of COMMIT from 47.4% in 2006 to 82.1% in 2011.12 However, use in the broader Chinese AMI population eligible for treatment, and the extent of variation in use according to patient or hospital characteristics, such as region and rural/urban location, have not been described. A detailed assessment of changes in and correlates of early clopidogrel use could facilitate future quality-improvement efforts that aim to ensure that all eligible patients with AMI receive clopidogrel appropriately.
Accordingly, we sought to evaluate the use of early clopidogrel among patients with AMI in China from 2006 to 2011 using a nationally representative sample from the China PEACE Retrospective AMI Study. Our objectives were the following: (1) to examine regional and hospital-level variation in the use of early clopidogrel therapy; and (2) to identify characteristics of patients who did not receive early clopidogrel therapy and the hospitals that seldom employed this intervention. This analysis is part of the China PEACE study, which was funded by the Chinese government and designed to generate the knowledge to support future national quality-improvement initiatives to enhance clinical care for patients with AMI in China.
Methods
Design Overview of China PEACE–Retrospective AMI Study
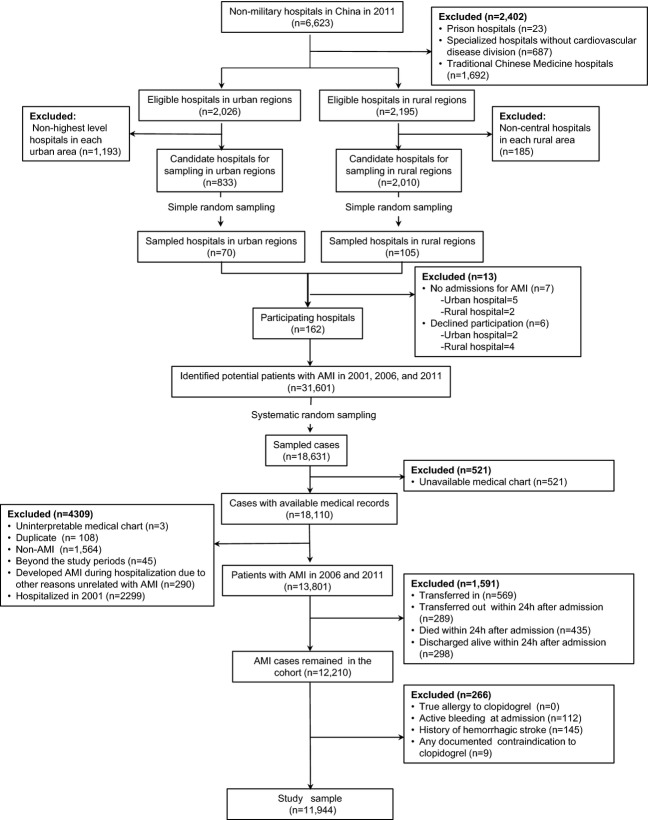
The design and methods of the China PEACE–Retrospective AMI study have been published previously.12,13 In brief, we developed a nationally representative sample of hospitalizations for AMI during 2001, 2006, and 2011 using a 2-stage random sampling design. In the first stage, we identified hospitals using a simple random sampling procedure within each of the 5 study strata: Eastern-rural, Central-rural, Western-rural, Eastern-urban, and Central/Western-urban regions, since hospital volumes and clinical capacities differ between urban and rural areas as well among the 3 official economic–geographic regions (Eastern, Central, and Western) of Mainland China. We considered Central and Western urban regions together, given their similar per-capita income and health services capacity. According to government documents, there were 6623 nonmilitary hospitals in 2011 in China. We excluded prison hospitals, specialized hospitals without a cardiovascular disease division, and traditional Chinese medicine hospitals. In the 3 rural strata, the sampling framework consisted of the central hospital (the largest general hospital in the rural region with the greatest clinical capacity for treating acute illness, including patients with AMI) in each of the predefined rural regions (2010 central hospitals in 2010 rural regions). In each of the 2 urban strata, the sampling framework consisted of the highest-level hospitals (ie, tertiary referral hospitals with advanced facilities) in each of the predefined urban regions (833 hospitals in 287 urban regions) (Figure1). Since the majority of hospitals in China are publicly owned and administered, hospital closure is rare. We selected representative hospitals from 2011 to reflect current practices and trace this cohort backward to 2006 and 2001 to describe temporal trends. In the second stage, we drew cases based on the local hospital database for patients with AMI in each year at each sampled hospital using systematic random sampling procedures. Patients with AMI were identified using International Classification of Diseases–Clinical Modification codes, including versions 9 (410.xx) and 10 (I21.xx), when available or through principal discharge diagnosis if available. Hospitals in China are mandated by the Ministry of Health of China to list this information. If such a database was not available, site coordinators manually searched the written hospitalization log or electronic medical record system for hospitalizations for AMI. Site coordinators reviewed the original medical records in cases where the diagnosis of AMI was uncertain.
Figure 1.
Flow diagram of study sample. AMI indicates acute myocardial infarction.
We sampled 175 hospitals; 7 did not have admissions for AMI and 6 declined participation. Examination of patient databases from participating 162 hospitals yielded 31 601 hospitalizations for AMI (3859 in 2001, 8863 in 2006, and 18 879 in 2011). We sampled 18 631 cases and finally acquired medical records for 18 110 (97.2%) cases (Figure1).
The Chinese government, which provided financial support for the study, had no role in the design or conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation or approval of the manuscript. The central ethics committee of the China National Center for Cardiovascular Diseases approved this study. All collaborating hospitals accepted the central ethics approval except for 5 hospitals, which obtained local approval by internal ethics committees. The study is registered at www.clinicaltrials.gov (NCT01624883).
Study Sample
Patients with AMI eligible for early clopidogrel therapy were identified as follows: only those patients with a definite discharge diagnosis of AMI were included in the study sample. In this study, we first excluded patients hospitalized in 2001 because clopidogrel was not approved for use in China until August 2001.14 Among patients with AMI admitted in 2006 and 2011, we excluded patients who had been transferred from another hospital because they might have received acute treatments at another facility that were not documented in the medical charts included in our study. In addition, we excluded patients who transferred to another hospital, were discharged, or died during the first 24 hours of admission because these patients might not have had the opportunity to receive clopidogrel therapy. Finally, we excluded patients who had documented contraindications to clopidogrel therapy: true allergy to clopidogrel, history of hemorrhagic stroke, active or major bleeding (eg, active gastrointestinal bleeding, acute hemorrhagic stroke, urogenital system bleeding, retroperitoneal bleeding) at admission, or any other contraindication documented by a physician in the medical record (eg, thrombocytopenia). The resulting cohorts are those who were eligible for treatment with clopidogrel.
Data Collection
Data were collected via central medical chart abstraction using standardized data definitions. We adopted a rigorous approach to monitoring data abstraction quality, randomly auditing 5% of medical charts throughout the study; overall variable abstraction accuracy exceeded 98%. Data elements included demographic information, clinical characteristics at admission, laboratory parameters, in-hospital treatments, timing of care delivery, and in-hospital clinical outcomes. Hospitals were defined as percutaneous coronary intervention capable if at least 1 patient received percutaneous coronary intervention at that hospital in the given study year. Hospital teaching status was derived from an independent survey of hospital characteristics.
Outcome Variable
The primary outcome of this analysis was early use of clopidogrel therapy, defined as the initiation of clopidogrel within 24 hours of hospital admission.
Statistical Analysis
Continuous variables were described as median values with interquartile ranges (IQR), and categorical variables were described as frequencies with percentages. For age, which was missing at a low rate (0.1%), we imputed the missing values as the overall median. All the remaining variates had no missing values. We employed Wilcoxon rank sum tests for continuous variables and χ2 tests for categorical variables to compare differences between patients who did and did not receive early clopidogrel therapy. We described the distribution of early clopidogrel use among eligible patients within the 5 defined regions, as well as in urban and rural hospitals. We calculated rates of use for hospitals with at least 5 patients with AMI in the given study year. To estimate the use of early clopidogrel therapy among the entire Chinese population with AMI, we applied weights proportional to the inverse sampling fraction of hospitals within each stratum, as well as to patients within each hospital, to account for differences in the sampling fraction for each time period. Changes in the early use of clopidogrel between the 2 time periods were evaluated by the χ2 test.
Factors independently associated with early clopidogrel therapy were identified using a logistic regression model using a generalized estimating equation accounting for clustering of patients within hospitals. Predictor variables of interest were selected on the basis of prior literature and clinical context. These variables included demographics, cardiovascular risk factors, medical history, clinical characteristics at admission, use of reperfusion therapy, year of hospitalization, and hospital characteristics. We transformed continuous variables, such as age and blood pressure, into categorical variables using a fractional polynomial approach according to clinically meaningful cut-off values, and then created dummy variables. Backward selection (with a cutoff significance level of 0.05) was used to identify factors predictive of early clopidogrel use. Odds ratios (OR) and 95% CI were reported for the fully adjusted models. All tests of statistical significance were 2-sided, with a P<0.05 considered statistically significant. Statistical analysis was performed using SAS software (version 9.2, SAS Institute, Cary, NC) and R software (version 3.0.2, R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Sample
There were 4468 patients with AMI in 2006 and 9333 in 2011. We excluded patients who were transferred in from another hospital (n=569), those who died (n=435), were discharged (n=298), or were transferred out (n=289) within 24 hours of hospital admission, and patients with documented contraindications to clopidogrel (n=266), to identify a sample of 11 944 patients with AMI who were eligible for early clopidogrel therapy (Figure1). The 3977 patients in 2006 represented 103 950 patients in 2006 across China, and the 7967 patients with AMI in our 2011 sample represented 216 558 patients across China, respectively.
Overall, the median age of the cohort was 66 years (IQR: 56 to 75), 69.6% were men, and 35.6% current smokers (Table 1). Comorbidities were common: 51.0% of patients had hypertension, 11.0% had a prior myocardial infarction, 10.4% had a prior ischemic stroke, and 18.4% had diabetes.
Table 1.
Baseline Characteristics for Patients Hospitalized With and Without Early Clopidogrel Therapy
| Characteristics | Total N (%) | Early Clopidogrel Use N (%) | Not Early Clopidogrel Use N (%) | P Value |
|---|---|---|---|---|
| All eligible patients | 11 944 | 7795 (65.3) | 4149 (34.7) | |
| Demographics | ||||
| Age, y | 0.086 | |||
| <55 | 2616 (21.9) | 1849 (23.7) | 767 (18.5) | |
| 55 to 64 | 2791 (23.4) | 1873 (24.0) | 918 (22.1) | |
| 65 to 74 | 3487 (29.2) | 2177 (27.9) | 1310 (31.6) | |
| ≥75 | 3050 (25.5) | 1896 (24.3) | 1154 (27.8) | |
| Gender | <0.001 | |||
| Female | 3626 (30.4) | 2242 (28.8) | 1384 (33.4) | |
| CVD risk factors | ||||
| Prior hypertension | 6086 (51.0) | 4179 (53.6) | 1907 (46.0) | <0.001 |
| Prior diabetes | 2195 (18.4) | 1539 (19.7) | 656 (15.8) | <0.001 |
| Currently smoking | 4253 (35.6) | 3092 (39.7) | 1161 (28.0) | <0.001 |
| Medical histories | ||||
| Ischemic stroke | 1244 (10.4) | 787 (10.1) | 457 (11.0) | 0.118 |
| Myocardial infarction | 1311 (11.0) | 859 (11.0) | 452 (10.9) | 0.834 |
| PCI | 258 (2.2) | 216 (2.8) | 42 (1.0) | <0.001 |
| Clinical characteristics at admission | ||||
| Chest discomfort | 10 968 (91.8) | 7333 (94.1) | 3635 (87.6) | <0.001 |
| Cardiogenic shock | 517 (4.3) | 314 (4.0) | 203 (4.9) | 0.027 |
| BP, mm Hg | 0.049 | |||
| SBP ≥180 or DBP ≥110 | 998 (8.4) | 623 (8.0) | 375 (9.0) | |
| AMI type | 0.969 | |||
| STEMI | 10 049 (84.1) | 6559 (84.1) | 3490 (84.1) | |
| Reperfusion therapies | <0.001 | |||
| No reperfusion | 8334 (69.8) | 5065 (65.0) | 3269 (78.8) | |
| Fibrinolytic therapy | 2251 (18.8) | 1446 (18.6) | 805 (19.4) | |
| Primary PCI | 1359 (11.4) | 1284 (16.5) | 75 (1.8) | |
| Hospital characteristics | ||||
| Teaching hospital | 9552 (80.0) | 6836 (87.7) | 2716 (65.5) | <0.001 |
| PCI-capable hospital | 7863 (65.8) | 6333 (81.2) | 1530 (36.9) | <0.001 |
| Economic–geographic region | <0.001 | |||
| Eastern | 6883 (57.6) | 4789 (61.4) | 2094 (50.5) | |
| Central | Central | 1435 (18.4) | 1161 (28.0) | |
| Western | 2465 (20.6) | 1571 (20.2) | 894 (21.5) | |
| Urban/rural | <0.001 | |||
| Urban | 7271 (60.9) | 5613 (72.0) | 1658 (40.0) | |
| Rural | 4673 (39.1) | 2182 (28.0) | 2491 (60.0) | |
| Year | <0.001 | |||
| 2006 | 3977 (33.3) | 1651 (21.2) | 2326 (56.1) | |
| 2011 | 7967 (66.7) | 6144 (78.8) | 1823 (43.9) | |
BP indicates blood pressure; CVD, cardiovascular disease; DBP, diastolic blood pressure; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; STEMI, ST-segment elevation myocardial infarction.
Use of Early Clopidogrel Therapy
There was a significant increase in the use of early clopidogrel from 41.5% in 2006 to 77.1% in 2011 (weighted proportions of use: 45.7% in 2006, 79.8% in 2011; P<0.001). However, there were significant geographic variations in the early use of clopidogrel therapy (Figure2). In 2006, the rate of early clopidogrel therapy ranged from a low of 1.5% in the Western-rural region to a high of 58.0% in the Eastern-urban region (P<0.001), while in 2011 it varied from a low of 48.7% in the Central-rural region to a high of 87.7% in the Central/Western-urban region (P<0.001). In addition, there were marked differences between the rate of early clopidogrel use between rural and urban hospitals (P<0.001; Figure3). From 2006 to 2011, the rate of early clopidogrel use in rural hospitals increased significantly, but there was wide variation in 2011 (median rate of early clopidogrel therapy in 2006: 0% [IQR: 0% to 13.6%]; median rate of early clopidogrel therapy in 2011: 50.0% [IQR: 11.5% to 84.4%]; P<0.001). In contrast, there was substantial heterogeneity in early clopidogrel therapy between urban hospitals in 2006 (median rate of use in 2006: 52.6% [IQR 6.1% to 82.1%]), but by 2011 the rate of use had increased markedly and there was much less variation (median rate of early clopidogrel therapy in 2011: 89.3% [IQR 80.1% to 94.5%]; P<0.001).
Figure 2.
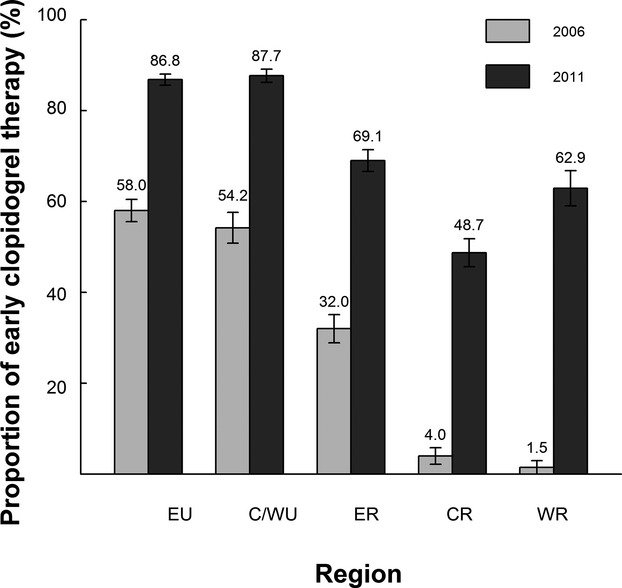
The proportion of early clopidogrel therapy stratified by region among patients with acute myocardial infarction. P<0.001 for changes between 2006 and 2011, P<0.001 for changes in all regions. Error bar indicates 95% CI. C/WU indicates Central/Western-urban; CR, Central-rural; ER, Eastern-rural; EU, Eastern-urban; WR, Western-rural.
Figure 3.
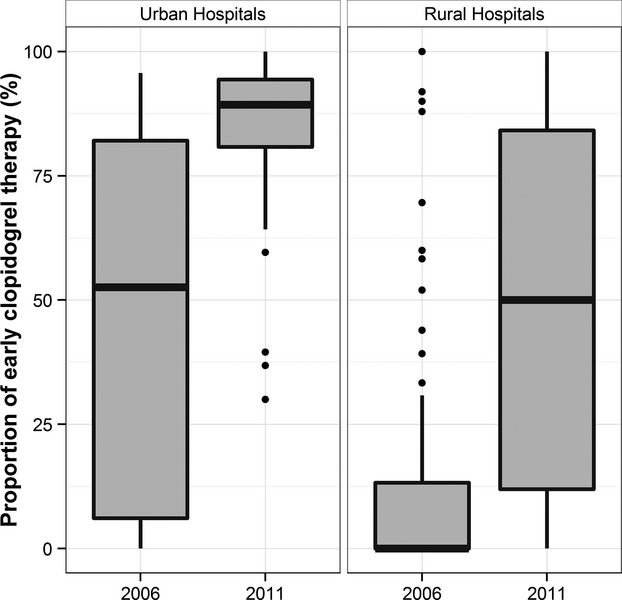
The variation in early clopidogrel therapy between hospitals in rural areas and urban hospitals in 2006 and 2011. P<0.001 for the difference between rural and urban.
Patient and Hospital Characteristics Associated With Early Clopidogrel Therapy
In the multivariable model, several patient characteristics were associated with early clopidogrel therapy (Figure4). For example, patients with a prior ischemic stroke (OR 0.77; 95% CI 0.64 to 0.93, P<0.001) and history of myocardial infarction (OR 0.80; 95% CI 0.68 to 0.95, P<0.001) were significantly less likely to receive early clopidogrel therapy. In contrast, patients with STEMI (OR 1.32; 95% CI 1.11 to 1.56, P<0.001 compared to non-ST elevation myocardial infarction) or those presenting with chest discomfort (OR 2.53; 95% CI 2.09 to 3.04, P<0.001) were more likely to receive early clopidogrel therapy. Compared with patients not receiving reperfusion therapy, those who received primary percutaneous coronary intervention or fibrinolytic therapy were more likely to receive early clopidogrel therapy (OR 4.16; 95% CI 2.22 to 7.80, P<0.001 and OR 1.49; 95% CI 1.20 to 1.84, P<0.001, respectively).
Figure 4.
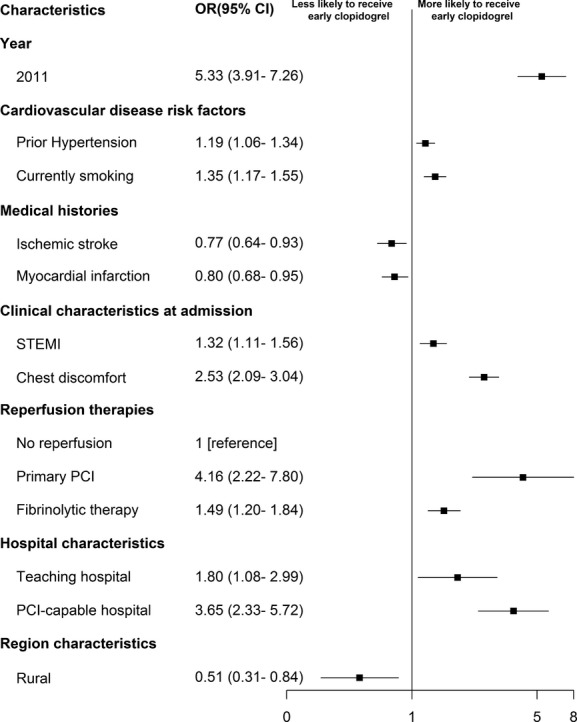
Factors associated with early use of clopidogrel in multivariable model. Variables with a significant association with early use of clopidogrel are shown along the vertical axis. The strength of effect is shown along the horizontal axis with the vertical solid line demarking an odds ratio (OR) of 1 (that is, no association); estimates to the right (that is, >1) are associated with greater likelihood of early clopidogrel use, while those to the left (that is, <1) indicate association with reduced likelihood of early clopidogrel use. Each square and line represents the point estimate of the effect of that variable in the model, while the line shows the 95% CI. PCI indicates percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Hospital characteristics were also associated with use of early clopidogrel therapy. Patients admitted to rural hospitals (OR 0.51; 95% CI 0.31 to 0.84, P<0.001) were less likely to receive early clopidogrel than patients admitted to urban hospitals. In contrast, patients admitted to percutaneous coronary intervention–capable hospitals and teaching hospitals were more likely to receive early clopidogrel therapy (OR 3.65; 95% CI 2.33 to 5.72, P<0.001 and OR 1.80; 95% CI 1.08 to 2.99, P<0.001, respectively).
Discussion
In this large nationally representative study of patients with AMI in China, we found a nearly 2-fold increase in the early use of clopidogrel between 2006 and 2011. However, significant gaps in the use of early clopidogrel therapy were noted, especially in rural hospitals. In 2011, 6 years after publication of the COMMIT trial, only 1 in 2 patients with AMI received this efficacious therapy in rural hospitals, with 1 quarter of such hospitals providing early clopidogrel therapy to only 1 in 9 eligible patients. The low rate of use in rural hospitals contrasts sharply with that in urban hospitals, where the median rate of use was almost 90%.
Although this study was descriptive and could not identify the reasons for the increase in the use of clopidogrel over this 5-year period, it is likely that the accumulation of evidence demonstrating the benefit of early clopidogrel therapy has contributed to the adoption of this strategy in recent years. Specifically, in 2005, the COMMIT trial, which enrolled more than 45 000 Chinese patients, established that early clopidogrel therapy reduced mortality and major vascular events in patients with AMI. Dissemination of the results of COMMIT, which are directly applicable to Chinese patients with AMI, was probably an important driver of the marked increase in the use of early clopidogrel therapy that we observed in this analysis. In addition, clopidogrel became a national basic drug in 2004, meaning that medical insurance covered part of its cost.15 Meanwhile, healthcare reform in China, which expanded medical insurance coverage to 90% of the population by 2011, may have also contributed to the improvement in the use of early clopidogrel therapy.16
A number of factors may explain the disparities between rural and urban hospitals. Differences in per capita income, which are marked between urban and rural China, may influence medical resource utilization.17 Despite the progress of healthcare reform, medical insurance still does not cover the full cost of clopidogrel therapy. A single dose of clopidogrel (75 mg) costs about 20 Yuan (≈3.25 US dollars or 3 Euros),18 which is approximately equal to the average daily income in rural regions in 2011, meaning that the cost of clopidogrel therapy may be limiting, especially in rural regions.11 In addition, most urban hospitals are tertiary hospitals, which are more commonly staffed with cardiovascular specialists; the availability of practitioners with greater condition-specific expertise in urban areas may explain the greater adoption of AMI-specific evidence-based care.
In addition to the lower rate of use of early clopidogrel therapy in rural hospitals, there was also notable variation among hospitals, suggesting heterogeneity in the structure and function of rural health systems. Differential investment in healthcare capacity may explain this variation. Our study noted that the Western-rural region had the largest increase in the use of early clopidogrel therapy. This may reflect governmental investments in this region, including a special program for physicians’ education with the objective of improving clinical practice.19 For example, urban hospitals in the Western region sent experts with more than 13 600 visits to support physicians in rural hospitals from 2005 to 2010,19 which might also improve physicians’ diagnosis and treatment level. There may be other reasons for this variation, and the sheer size of the population of rural China (700 million) underscores the importance of further analysis.11
Our study found that certain patient characteristics were associated with failure to provide early clopidogrel therapy. For example, patients with a history of ischemic stroke or myocardial infarction were less likely to receive early clopidogrel therapy, although these patients may even benefit more than other patients without these conditions.20 This observation may reflect a risk–treatment paradox that has been described in other settings. Patients who did not present with overt symptoms of AMI (such as chest discomfort) were less likely to be treated, which may reflect uncertainty about the diagnosis of AMI. Patients with non–ST-segment acute myocardial infarction were less likely to receive early clopidogrel therapy, similar to findings in the United States.21 This pattern might occur because patients with STEMI are usually identified rapidly after hospital presentation based on initial electronic cardiogram findings. Conversely, identification of patients with non–ST-segment acute myocardial infarction can be clinically challenging due to the frequent initial lack of definitive electrocardiographic changes and to uncertainty about the definition of AMI with elevated cardiac troponin levels for patients who do not have persistent ST elevation when first seen. In addition, the early treatment of patients with clopidogrel was strongly associated with the provision of reperfusion therapy, so this effect may be driven by greater cardiologist involvement in early hospitalization treatment.
Although international guidelines strongly recommend early clopidogrel therapy for patients with AMI, this is the first nationally representative study to evaluate the use of this strategy in any country. The few available studies conducted in developed countries have shown that early clopidogrel therapy was widely used. In Europe, a prospective survey involving hospitals in 47 countries noted that 91% of patients with AMI received early clopidogrel therapy,22 while in the Unites States, a registry-based study found that the rate of this intervention in 2009 was 87.1% in patients with STEMI.21 While urban hospitals in China are now approaching the rates achieved in these developed healthcare systems, use in rural hospitals is lagging.
The findings of our study also have important implications for efforts to improve the quality of AMI care in China. Some 6 years after the results of the COMMIT study demonstrated direct evidence of benefit of this intervention in the Chinese population, early clopidogrel therapy is not a routine intervention in all Chinese hospitals, particularly those in rural areas. By quantifying the magnitude of underutilization, characterizing the disparity between urban and rural hospitals, identifying wide variations among rural hospitals, and understanding the factors associated with early clopidogrel therapy, our study provides important information to inform future quality-improvement initiatives. In addition, the factors associated with use of early clopidogrel therapy are likely to apply to new antiplatelet agents, such as ticagrelor. Future national quality-improvement efforts could include early clopidogrel therapy as a national quality measure, strengthening education to improve clinical knowledge, and improving the structural inadequacies of the systems of care.
Several limitations of our study should be considered. First, we cannot exclude the possibility that some patients had undocumented contraindications to early clopidogrel therapy and were classified as eligible; however, this is unlikely to explain the magnitude of underutilization of the therapy among patients cared for in rural hospitals. Second, some factors (eg, physicians’ opinion on clopidogrel therapy), which might affect the use of early clopidogrel therapy, were not measured and could have provided more insight into the observed variations. Finally, we were unable to investigate system-level factors that may have influenced prescribing practices, such as the availability of clopidogrel.
In conclusion, there has been marked improvement in the use of early clopidogrel therapy for patients with AMI in China. However, there are disparities in the use of this intervention between rural and urban regions, with lower rates of use and greater variation in use among hospitals in rural regions. National policies and initiatives, with a particular focus on rural hospitals, are needed to improve early clopidogrel therapy and patient outcomes.
Acknowledgments
We appreciate the multiple contributions made by study teams at the China Oxford Centre for International Health Research and the Yale-New Haven Hospital Center for Outcomes Research and Evaluation in the realms of study design and operations, particularly the data collection by Yi Pi, Jiamin Liu, Wuhanbilige Hundei, Haibo Zhang, Xue Du, Wenchi Guan, Xin Zheng, and Yuanlin Guo. We appreciate the advice of analysis by Zhenqiu Lin, Shuxia Li, Yongfei Wang, and Haiqun Lin. We are grateful for the funding support provided by the Chinese government. We are grateful to all the collaborators listed in Appendix S1.
Sources of Funding
This project was partly supported by the Research Special Fund for Public Welfare Industry of Health (201202025) from National Health and Family Planning Commission of China, and the International Science & Technology Cooperation Program (2010DFB33140) from the Ministry of Science and Technology of China. Dr Krumholz is supported by grant U01 HL105270-04 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. The sponsors had no role in the design or conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation or approval of the manuscript.
Disclosures
Dr Masoudi has received funding support from the American College of Cardiology for his role as the Senior Medical Officer of the National Cardiovascular Data Registries. Dr Spertus has received a grant from Lilly for different projects unrelated to the content of this article. The authors declare no other relevant conflicts of interest.
Supporting Information
Appendix S1. Members of the China PEACE-Retrospective AMI Study Site Investigators by Hospital and China PEACE Study Consultants.
References
- The World Bank. 2011. Toward a Healthy and Harmonious Life in China: Stemming the Rising Tide of Non-Communicable Diseases. Available at: http://www.worldbank.org/content/dam/Worldbank/document/NCD_report_en.pdf. Accessed January 16, 2014.
- Chen ZM, Jiang LX, Chen YP, Xie JX, Pan HC, Peto R, Collins R, Liu LS COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45 852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1607–1621. doi: 10.1016/S0140-6736(05)67660-X. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Zhao F, Mehta S, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without st-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- Sabatine MS, Cannon CP, Gibson CM, López-Sendón JL, Montalescot G, Theroux P, Claeys MJ, Cools F, Hill KA, Skene AM, McCabe CH, Braunwald E CLARITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352:1179–1189. doi: 10.1056/NEJMoa050522. [DOI] [PubMed] [Google Scholar]
- China Society of Cardiology of Chinese Medical Association; Editorial Board of Chinese Journal of Cardiology. Guideline for diagnosis and treatment of patients with ST-elevation myocardial infarction. Zhonghua Xin Xue Guan Bing Za Zhi. 2010;38:675–690. [PubMed] [Google Scholar]
- O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- China Society of Cardiology of Chinese Medical Association; Editorial Board of Chinese Journal of Cardiology. Guideline for diagnosis and treatment of patients with unstable angina and non-ST-segment elevation myocardial infarction. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:295–304. [PubMed] [Google Scholar]
- Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg PG, Tubaro M, Verheugt F, Weidinger F, Weis M ESC Committee for Practice Guidelines (CPG) Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–2945. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernández-Avilés F, Fox KA, Hasdai D, Ohman EM, Wallentin L, Wijns W. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes: the Task Force for the Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of the European Society of Cardiology. Eur Heart J. 2007;28:1598–1660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- National Bureau of Statistics of China. 2011. China Statistical Yearbook. Available at: http://www.stats.gov.cn/tjsj/nds/2011/indexch.htm. Accessed January 8, 2014.
- Li J, Li X, Wang Q, Hu S, Wang Y, Masoudi FA, Spertus JA, Krumholz HM, Jiang L for the China PEACE Collaborative Group. ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet. 2015;385:441–451. doi: 10.1016/S0140-6736(14)60921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmarajan K, Li J, Li X, Lin Z, Krumholz HM, Jiang L. The China patient-centered evaluative assessment of cardiac events (China PEACE) retrospective study of acute myocardial infarction: study design. Circ Cardiovasc Qual Outcomes. 2013;6:732–740. doi: 10.1161/CIRCOUTCOMES.113.000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H. Plavix came to market in China. Chin J Cardiovasc Dis. 2001;29:679. [Google Scholar]
- Ministry of Labour and Social Security. Essential drug catalogue of the national basic medical insurance and industrial injury insurance. Beijing: China Labor Social Security Press; 2004. [Google Scholar]
- Cao Q, Shi L, Wang H, Dong K. Report from China: health insurance in China–evolution, current status, and challenges. Int J Health Serv. 2012;42:177–195. doi: 10.2190/HS.42.2.b. [DOI] [PubMed] [Google Scholar]
- Ministry of Health of the People’s Republic of China. China Public Health Statistical Yearbook 2011. Beijing: Peking Union Medical College Publishing House; 2011. [Google Scholar]
- Pan Y, Wang A, Liu G, Zhao X, Meng X, Zhao K, Meng X, Zhao K, Liu L, Wang C, Johnston SC, Wang Y, Wang Y CHANCE Investigators. Cost-effectiveness of clopidogrel-aspirin versus aspirin alone for acute transient ischemic attack and minor stroke. J Am Heart Assoc. 2014;3:e000912. doi: 10.1161/JAHA.114.000912. doi: 10.1161/JAHA.114.000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health of the People’s Republic of China. 2010. The development of the Western region health care in recent 10 years. Available at: http://www.gov.cn/xwfb/2010-08/10/content_1675599.htm. Accessed January 8, 2014.
- Budaj A, Yusuf S, Mehta SR, Fox KA, Tognoni G, Zhao F, Chrolavicius S, Hunt D, Keltai M, Franzosi MG Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) Trial Investigators. Benefit of clopidogrel in patients with acute coronary syndromes without ST-segment elevation in various risk groups. Circulation. 2002;106:1622–1626. doi: 10.1161/01.cir.0000029926.71825.e2. [DOI] [PubMed] [Google Scholar]
- Roe MT, Messenger JC, Weintraub WS, Cannon CP, Fonarow GC, Dai D, Chen AY, Klein LW, Masoudi FA, McKay C, Hewitt K, Brindis RG, Peterson ED, Rumsfeld JS. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol. 2010;56:254–263. doi: 10.1016/j.jacc.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Puymirat E, Battler A, Birkhead J, Bueno H, Clemmensen P, Cottin Y, Fox KA, Gorenek B, Hamm C, Huber K, Lettino M, Lindahl B, Müller C, Parkhomenko A, Price S, Quinn T, Schiele F, Simoons M, Tatu-Chitoiu G, Tubaro M, Vrints C, Zahger D, Zeymer U, Danchin N EHS 2009 Snapshot Participants. Euro Heart Survey 2009 Snapshot: regional variations in presentation and management of patients with AMI in 47 countries. Eur Heart J Acute Cardiovasc Care. 2013;2:359–370. doi: 10.1177/2048872613497341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Members of the China PEACE-Retrospective AMI Study Site Investigators by Hospital and China PEACE Study Consultants.