Abstract
Background
Cardiovascular disease (CVD), myocarditis and fibrosis are comorbidities of HIV+ individuals on durable antiretroviral therapy (ART). Although mechanisms for these vary, monocytes/macrophages are increasingly demonstrated to be key players.
Methods and Results
We directly blocked monocyte/macrophage traffic to the heart in an SIV model of AIDS using an anti-alpha-4 integrin antibody (natalizumab). Nineteen Rhesus macaques were SIVmac251 infected and CD8-lymphocyte depleted for the development of rapid AIDS. Ten animals received natalizumab once a week, for 3 weeks, and were sacrificed 1 week later. Six animals began treatment at the time of infection (early) and the remaining 4 began treatment 28 days post-infection (late), a time point we have previously established when significant cardiac inflammation occurs. Nine animals were untreated controls; of these, 3 were sacrificed early and 6 were sacrificed late. At necropsy, we found decreased SIV-associated cardiac pathology in late natalizumab-treated animals, compared to untreated controls. Early and late treatment resulted in significant reductions in numbers of CD163+ and CD68+ macrophages in cardiac tissues, compared to untreated controls, and a trend in decreasing numbers of newly recruited MAC387+ and BrdU+ (recruited) monocytes/macrophages. In late treated animals, decreased macrophage numbers in cardiac tissues correlated with decreased fibrosis. Early and late treatment resulted in decreased cardiomyocyte damage.
Conclusions
These data demonstrate a role for macrophages in the development of cardiac inflammation and fibrosis, and suggest that blocking monocyte/macrophage traffic to the heart can alleviate HIV- and SIV-associated myocarditis and fibrosis. They underscore the importance of targeting macrophage activation and traffic as an adjunctive therapy in HIV infection.
Keywords: animal model, cardiomyopathy, fibrosis, HIV, myocarditis
Combination antiretroviral therapy (cART) has increased the life expectancy of HIV+ individuals, but comorbidities, including neurological,1 renal,2 bone,3 and cardiovascular (CV) disease (CVD)4–9 exist.10–12 HIV-associated CVD is a leading cause of HIV-associated mortality, where there is a 2-fold increase in the relative risk compared to noninfected, age-matched individuals.13 HIV-associated CVD, which includes atherosclerosis, dilated cardiomyopathy, myocarditis, and myocardial infarction,7,14,15 likely has multiple etiologies, including toxic effects of cART,16 opportunistic infections,17 and chronic immune activation,18 but monocyte/macrophages are emerging as central players.
Dilated cardiomyopathy and myocarditis with HIV infection was evident in 40% to 50% of AIDS patients at necropsy in the pre-cART era.19 With effective cART in developed countries, incidence has decreased approximately 30%, suggesting that dilated cardiomyopathy and myocarditis with HIV and cART has declined.7,19,20 Despite this, magnetic resonance imaging and spectroscopy show that HIV+ individuals continue to have subclinical myocardial disease with myocardial fibrosis and alterations in cardiac function.21,22 Recent data comparing the rates of mortality in the pre- and post-cART era support these findings, where HIV+ individuals had a 6.3-fold increase in mortality resulting from cardiomyopathy and myocarditis in the post-cART era. Additionally, a recent study examining myocardial and microvascular inflammation showed that myocarditis is still present with HIV infection.23 This suggests that HIV infection with effective cART can still lead to increased cardiac fibrosis and myocarditis.
SIV-infected rhesus macaques are an excellent model to study the effects of SIV on cardiac inflammation and fibrosis. Previous work established that SIV-infected monkeys have dilated cardiomyopathy and myocarditis.24,25 Overall few SIV- or HIV-RNA or protein positive cells are found in cardiac tissues, similar to what is found in HIV-infected human cardiac tissues.26,27 Despite this, levels of SIV-RNA correlate with diastolic dysfunction, underscoring the role of lentiviral infection and cardiac dysfunction with AIDS.27 We have previously demonstrated that SIV-infected, CD8-lymphocyte-depleted animals develop rapid and consistent AIDS with macrophage accumulation in the heart, cardiomyocyte damage, and fibrosis28 and accumulation of CD163+ macrophages correlated with increased fibrosis.28,29 In these studies, bromodeoxyuridine (BrdU)-labeled monocyte/macrophages, which were labeled in the bone marrow and traffic to the heart, were increased late in infection. Overall, these observations support the hypothesis that macrophage activation and accumulation with SIV and HIV infection play a critical role in the development of cardiac pathology. To date, no studies have directly blocked monocyte/macrophage traffic to the heart in experimental infection with SIV.
Although no studies have directly blocked monocyte/macrophage traffic to the heart with SIV or HIV infection, data from experimental and clinical studies that diminished macrophage activation or accumulation in the heart support the notion that these cells are major players in cardiac pathogenesis. Thus, studies that blocked chemokine (C-C motif) ligand 5 (CCR5) using chemokine (C-C motif) ligand 5/regulated on activation normal T-cell expressed and secreted resulted in decreased monocyte/macrophage and T-lymphocyte accumulation,30,31 likely by indirect mechanisms. Similarly, blocking CCR5 with the anti-CCR5 antibody, maraviroc, resulted in reduced cardiac CD163 expression by macrophages and prevented diastolic dysfunction in SIV-infected monkeys.32 Similarly, studies blocking macrophage inhibitory factor showed decreased T-cell and macrophage migration and inhibition of onset of myocarditis in a rodent model of experimental autoimmune myocarditis.33 Treatment with the statin, pravastatin, decreased macrophage numbers in abdominal aortic plaques of uninfected monkeys.34 Another statin, atorvastatin, that did not reduce aortic inflammation in HIV+ infected individuals, did decrease the volume and high-risk features of noncalcified plaques.35 In humans with coronary artery disease, angiotensin receptor blocker treatment resulted in decreased numbers of atherosclerotic lesions and significantly decreasing levels of soluble markers of inflammation C-reactive protein, interleukin-6 (IL-6), and monocyte chemotactic protein 1.36,37
In this study, we examined whether directly blocking leukocyte and monocyte/macrophage traffic to cardiac tissues with an anti-α4 integrin antibody, natalizumab, decreased SIV-associated cardiac pathology (inflammation, fibrosis, and cardiomyocyte damage). Natalizumab is an anti-α4 antibody that binds to the α4 subunit of α4β1 and α4β7 integrins and blocks interactions between α4 and its ligands.38 Natalizumab has been used effectively to treat multiple sclerosis39 and Crohn’s disease,40 blocking accumulation of lymphocytes and monocytes/macrophages in the brain and gut, but not lymph nodes. In rodents, blocking α4 integrins reduced macrophage homing to atherosclerotic plaques.41 We have shown that natalizumab treatment in SIV-infected rhesus macaques with AIDS blocked monocyte/macrophage traffic to the central nervous system (CNS) and leukocytes to the gut, resulting in decreased numbers of SIV-RNA and SIV-p28-positive cells.42 Furthermore, natalizumab treatment of monkeys at the time of SIV infection resulted in undetectable SIV- RNA, -DNA and -p28 in the CNS and gut in the majority of animals, as well as the absence of leukocyte inflammation. In the current study, we examined whether natalizumab treatment decreased monocyte/macrophage accumulation in the heart and whether such treatment decreased cardiac fibrosis and myocyte damage.
Methods
Ethical Treatment of Animals
Treatment of animals in this study was in accord with the Guide for the Care and Use of Laboratory Animals, 8th edition. Animals were housed at the New England Regional Primate Center (NERPC; Southborough, MA), Tulane National Primate Research Center (TNPRC; Covington, LA), or BIOQUAL (Baltimore, MD). The NEPRC Protocol Number for this study is 04420 and the Animal Welfare Assurance Number is A3431-01. The TNPRC Number for this study is 3497 and the Animal Welfare Assurance Number is A4499-01. Animals were monitored daily for evidence of disease progression and changes in appetite or behavior, with clinical support administered under the direction of an attending veterinarian.
Animals, SIV Infection, and CD8 Lymphocyte Depletion
Nineteen rhesus macaques (Macaca mulatta) were infected with SIV mac251 (2 ng of SIV-p27) intravenously (kindly provided by Ronald Desrosiers, University of Miami; Table1). All animals were CD8-lymphocyte depleted using cM-T807, a human anti-CD8 antibody, administered subcutaneously (10 mg/kg) on day 6 post-infection (pi) and intravenously (5 mg/kg) on days 8 and 12 pi, as previously described.1 Ten animals (n=4 late natalizumab treated, n=6 untreated) were sacrificed at similar time points with progression to AIDS (49 to 65 days postinfection [dpi]). All CD8-lymphocyte-depleted animals had high viral load at peak viremia that remained elevated and was not different between early and late treated animals and controls. Six early natalizumab-treated animals were sacrificed at 21 dpi and 3 untreated controls were sacrificed at 22 dpi. Sections of left ventricular myocardium (hereafter referred to as cardiac tissue) were analyzed by a board-certified veterinary pathologist (A.D.M.), and scored based on degree of inflammation, fibrosis, and cardiomyocyte degeneration. Sections were scored as either no significant findings (NSF), mild, moderate, or severe, based on degree of change in cardiac tissues, as previously described.28
Table 1.
Animals Used in This Study and Cardiac Pathology
| Animal Groups | ID | Primate Center | Start of Natalizumab (dpi) | BrdU Administration (dpi) | Survival (dpi) | Cardiac Pathology | ||
|---|---|---|---|---|---|---|---|---|
| Inflammation | Fibrosis | Cardiomyocyte Degeneration | ||||||
| Early untreated n=3 | A1 | TNPRC | — | 6, 20 | 22 | NSF | NSF | NSF |
| A2 | TNPRC | — | 6, 20 | 22 | NSF | NSF | NSF | |
| A3 | TNRPC | — | 22 | 22 | Mild | Mild | NSF | |
| Early natalizumab n=6 | A4 | NERPC | 0 | 6, 20 | 21 | NSF | NSF | NSF |
| A5 | NERPC | 0 | 6, 20 | 21 | NSF | NSF | NSF | |
| A6 | BIOQUAL | 0 | 6, 20 | 21 | NSF | NSF | NSF | |
| A7 | BIOQUAL | 0 | 6, 20 | 21 | NSF | NSF | NSF | |
| A8 | BIOQUAL | 0 | 6, 20 | 21 | Mild | Mild | Mild | |
| A9 | BIOQUAL | 0 | 6, 20 | 21 | Mild | Mild | NSF | |
| Late untreated without cardiac pathology n=3 | A10 | NERPC | — | 49 | 56 | NSF | NSF | NSF |
| A11 | NERPC | — | pre, 7, 20, 41, 54 | 56 | NSF | NSF | NSF | |
| A12 | NERPC | — | pre, 7, 20, 41, 54 | 55 | Mild | Mild | NSF | |
| Late untreated with cardiac pathology n=3 | A13 | TNPRC | — | pre, 7, 26, 55 | 56 | Moderate | Moderate | Mild |
| A14 | TNPRC | — | pre, 7, 26, 55 | 65 | Moderate | Moderate | Mild | |
| A15 | NERPC | — | 6, 20 | 60 | Severe | Moderate | Moderate | |
| Late natalizumab n=4 | A16 | NERPC | 28 | pre, 26, 47 | 49 | Mild | NSF | NSF |
| A17 | NERPC | 28 | pre, 26, 47 | 49 | Mild | NSF | NSF | |
| A18 | NERPC | 28 | 33, 47 | 49 | Mild | Mild | NSF | |
| A19 | NERPC | 28 | 33, 47 | 49 | Moderate | Mild | Mild | |
Nineteen animals were used in this study, housed at either the New England Regional Primate Center (NERPC), Tulane National Primate Research Center (TNPRC), or BIOQUAL, as indicated. Six animals began natalizumab treatment at the time of infection at 0 days postinfection (dpi) and were sacrificed at 21 dpi. Three early untreated controls were sacrificed at 22 dpi. Four late natalizumab-treated animals began treatment at 28 dpi and were sacrificed at 49 dpi. Three animals each for late untreated controls without cardiac pathology and with cardiac pathology were sacrificed at 56 to 65 dpi. Pathology was assessed based on the degree of inflammation, fibrosis, and cardiomyocyte degeneration. To investigate whether blocking monocyte/macrophage traffic to the heart decreased SIV-associated cardiac pathology, 10 randomly chosen, ×200 fields of view were chosen and analyzed blindly by a veterinary pathologist. Sections of cardiac tissue were scored based on the degree of change as having no significant findings (NSF), mild, moderate, or severe inflammation, fibrosis, and cardiomyocyte degeneration. BrdU indicates bromodeoxyuridine.
Anti-α4 Integrin (Natalizumab) and BrdU Administration
The anti-α4 integrin monoclonal antibody (natalizumab) was provided by Biogen Idec (Cambridge, MA) in a sterile concentrated solution. Natalizumab has specificity for the α4 subunit of α4β1 and α4β7 integrins expressed on surfaces of all leukocytes, except neutrophils.43 Natalizumab was administered weekly for 3 weeks beginning on the day of infection (0 dpi; n=6) or on 28 dpi (n=4), as previously described.42 This treatment regimen maintains high levels of natalizumab in serum of rhesus macaques during treatment.44 To study monocyte/macrophage traffic to the heart, animals were administered BrdU (60 mg/kg) at indicated time points (Table1), as previously described.42
Immunohistochemistry
Numbers of macrophages and T lymphocytes present in formalin-fixed, paraffin-embedded tissues were determined by immunohistochemistry and cell counting. Cardiac tissues were stained with antibodies against CD163 (1:250; AbD Serotec, Kidlington, UK), CD68 (1:200; Dako, Carpinteria, CA), MAC387 (1:100; Dako) macrophages, and CD3+ T lymphocytes (1:300; Dako). The number of macrophages that traffic to cardiac tissues was determined using a mouse monoclonal BrdU antibody (1:50), as previously described.28 Twenty random, nonoverlapping, ×200 microscopic fields of view were taken for each animal and the number of positive cells/mm2 calculated for each. Data are represented as the average number of positive cells/mm2 from the 20 random fields.
Masson’s Trichrome Stain
Percent of collagen per tissue area used as a marker of fibrosis29 was measured using a modified Massons Trichrome Stain kit (Newcomer Supply, Middleton, WI), according to the manufacturer’s recommendation. Tissue sections were imaged using a Zeiss Axio Imager M1 microscope (Zeiss, Oberkochen, Germany) using Plan-Apochromat ×20/0.8 Korr objectives, as previously described.28,45 The area of red and blue dyes corresponding to cytoplasm and collagen, respectively, were measured to determine the percentage of total tissue area.
Statistical Analysis
Statistical analyses were conducted using Prism software (version 6.0; GraphPad Software Inc., La Jolla, CA). P values were calculated using the nonparametric Mann–Whitney t test with significance accepted at P<0.05 when comparing early and late natalizumab-treated animals to early and late untreated controls. ANOVA was used to compare late natalizumab-treated animals to late untreated animals with and without cardiac pathology. If the ANOVA was significant (P<0.05), then a post-hoc nonparametric Mann–Whitney t test was performed. To determine whether changes in numbers of macrophages in cardiac tissues correlates with changes in fibrosis, a nonparametric Spearman rank correlation was used where P<0.05 was significant.
Results
Natalizumab Treatment Decreases the Frequency and Severity of Pathology in SIV-Infected, CD8-Lymphocyte-Depleted Rhesus Macaque Cardiac Tissues
The relative degree of pathology in cardiac tissues was assessed based on levels of inflammation, fibrosis, and cardiomyocyte degeneration. Normal sections were scored as having no significant findings (NSF). When present, inflammation, fibrosis, and cardiomycocyte degeneration were scored as mild, moderate, or severe. We found no significant changes in the pathology of cardiac tissues in early natalizumab-treated animals, compared to untreated controls. Of 3 SIV-infected untreated animals sacrificed early, 21 dpi (n=3), 2 had no significant findings with regard to inflammation or fibrosis. The remaining SIV-infected untreated controls, sacrificed at 21 dpi, had mild inflammation and mild fibrosis. In the early natalizumab-treated group (n=6), sacrificed at 21 dpi, 4 animals had no significant findings in regard to inflammation and fibrosis and 2 had mild inflammation and fibrosis (Table1).
Overall, natalizumab treatment decreased cardiac pathology in late treated animals (n=4), compared to late untreated controls (n=6; Table1). Three of 4 late treated animals had no significant findings with respect to cardiomyocyte degeneration, and 2 of 4 had no significant findings with regard to fibrosis. SIV-infected, untreated late control animals with cardiac pathology (n=3) had an increased severity, compared to late treated SIV-infected animals (Table1). Two of the late controls with pathology had moderate inflammation and 1 had mild inflammation. All 3 late controls with pathology had moderate fibrosis, whereas 2 had mild and 1 had moderate cardiomycocyte degeneration. Compared to the late controls with pathology, the severity of pathology in SIV-infected, late natalizumab-treated animals (n=4) was diminished (Table1). Three late treated animals had mild inflammation, whereas the remaining had moderate inflammation. Two late treated animals had no inflammation and 2 had only mild inflammation. Three late treated animals had no significant findings with regard to cardiomyocyte degeneration and 1 had mild cardiomyocyte degeneration (Table1).
Natalizumab Treatment Decreases the Number of Macrophages Present in Cardiac Tissue in Early and Late Treated Animals
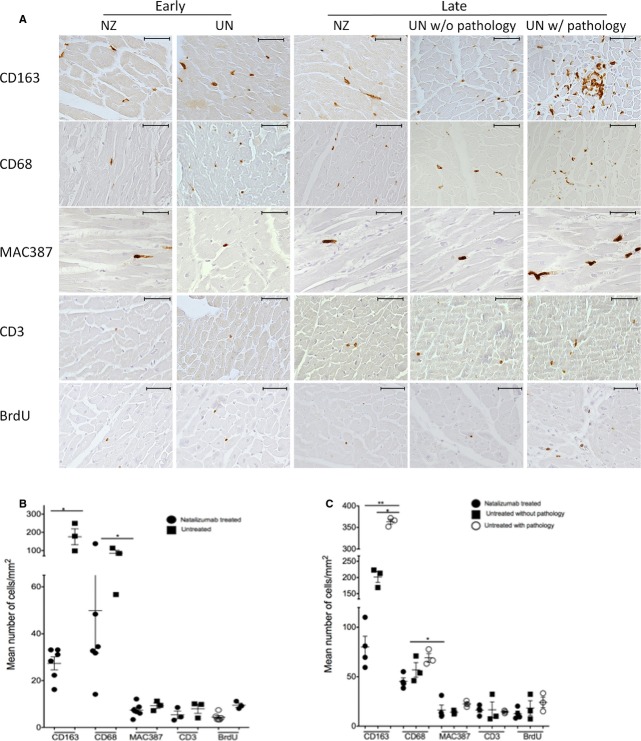
In animals that began natalizumab treatment early at 0 dpi, there was a significant decrease in the number of CD163+ and CD68+ macrophages in heart tissues, compared to untreated controls sacrificed at the same time point (Figure1). There was a significant 3.35- and 3.74-fold decrease in the numbers of CD163+ and CD68+ macrophages, respectively, in cardiac tissue in early natalizumab-treated animals, compared to controls (Figure1B; Table2; nonparametric Mann–Whitney t test, P<0.05). Though not significant, there were decreased numbers of newly infiltrating MAC387+ macrophages and CD3 T-lymphocytes in early treated animals (Figure1B; Table2).
Figure 1.
Natalizumab treatment decreases the number of macrophages in cardiac tissues in SIV-infected, CD8-lymphocyte-depleted rhesus macaques. A, Sections of left ventricular tissues from early and late natalizumab-treated animals and matched controls were immunohistochemically stained with antibodies recognizing CD163+, CD68+, and MAC387+ macrophages and CD3+ T lymphocytes. B and C, Twenty random, nonoverlapping, ×200 fields of view were taken for each animal and the average number of cells/mm2 calculated. In both early and late natalizumab-treated animals, there was a decrease in the numbers of CD163+ and CD68+ macrophages when compared to controls, with no differences in T lymphocytes detected. Statistical analysis between early natalizumab-treated animals and controls was done using a nonparametric Mann–Whitney t test. For late natalizumab-treated animals and untreated controls with and without cardiac pathology, an ANOVA was performed first, and, if significant, a post-hoc nonparametric Mann–Whitney t test was performed (*P<0.05; **P<0.01). Scale bar=50 μm, ×400 magnification. Error bars represent the average number of positive cells/mm2±SEM. BrdU indicates bromodeoxyuridine; NZ, natalizumab treated; UN, untreated.
Table 2.
Numbers of Macrophages and T Lymphocytes in Natalizumab-Treated Animals and Controls
| Immune Markers | Early | Late | ||||||
|---|---|---|---|---|---|---|---|---|
| Untreated (n=3) | NZ (n=6) | P Value | Fold Decrease | Untreated (n=6) | NZ (n=4) | P Value | Fold Decrease | |
| CD163 | 158.84 (±55.78) | 47.36 (±18.77) | * | 3.35 | 282.45 (±36.97) | 80.06 (±10.95) | ** | 3.53 |
| CD68 | 84.34 (±16.67) | 22.54 (±5.09) | * | 3.74 | 63.01 (±4.71) | 52.87 (±10.83) | * | 1.19 |
| MAC387 | 9.33 (±1.17) | 7.43 (±3.19) | ns | — | 18.25 (±2.11) | 15.91 (±7.46) | ns | — |
| CD3 | 8.05 (±2.13) | 5.13 (±3.02) | ns | — | 15.55 (±3.60) | 12.27 (±5.42) | ns | — |
| BrdU | 9.31 (±1.57) | 4.39 (±0.64) | ns | — | 21.19 (±5.85) | 16.42 (±3.19) | ns | — |
Numbers represent the mean number of positive cells (cells/mm2)±SEM, in parentheses. All animals were SIV-infected and CD8-lymphocyte depleted, with 10 of the animals receiving natalizumab. Twenty random, nonoverlapping, ×200 fields of view were counted for each animal and the average number of positive cells/mm2 calculated. P values were calculated by comparing the mean number of positive cells for the indicated groups using the nonparametric Mann–Whitney t test
P<0.05
P<0.01). Fold change was calculated for the numbers of cells where there was a significant difference between the indicated groups. Early natalizumab-treated animals began treatment at the time of infection, 0 days postinfection (dpi). Late natalizumab-treated animals began treatment 28 dpi. All treated animals were treated weekly for 3 weeks with a dose of 30 mg/kg of α-VLA-4. BrdU indicates bromodeoxyuridine; ns, no significance; NZ, natalizumab treated.
Natalizumab treatment beginning on 28 dpi (late) resulted in a significant decrease in numbers of CD163+ and CD68+ macrophages, when compared to all SIV-infected untreated late control animals (Figure1). We found a 3.53- and 1.19-fold decrease in numbers of CD163+ and CD68+ macrophages, respectively, in cardiac tissues of late natalizumab-treated tissues, compared to controls (Table2; nonparametric Mann–Whitney t test, P<0.05; P<0.01). We next examined whether the numbers of macrophages in late natalizumab-treated animals differed between late untreated control animals with and without cardiac pathology.
Late treated animals had decreased numbers of CD163+ macrophages, compared to untreated late controls without cardiac pathology (Figure1A). There was a 2.51-fold decrease in the number of CD163+ macrophages in cardiac tissue in late treated animals, compared to controls without cardiac pathology (Figure1B; Table3; nonparametric Mann–Whitney t test, P<0.05). There were no differences in numbers of CD68+, MAC387+ macrophages and CD3 T lymphocytes in cardiac tissues of late natalizumab-treated animals and untreated controls without pathology (Table3).
Table 3.
Numbers of Macrophages and T Lymphocytes in Late Natalizumab-Treated Animals and Controls Without and With Cardiac Pathology
| Immune Markers | Late | ||||||
|---|---|---|---|---|---|---|---|
| Untreated w/o Pathology n=3 | Untreated w/Pathology n=3 | NZ n=4 | P Value NZ vs w/o | P Value NZ vs w/ | Fold Decrease NZ vs w/o | Fold Decrease NZ vs w/ | |
| CD163 | 195.33 (±16.37) | 363.23 (±15.87) | 80.06 (±10.95) | * | ** | 2.51 | 4.53 |
| CD68 | 56.96 (±7.37) | 84.13 (±4.38) | 52.87 (±10.83) | ns | * | — | 1.59 |
| MAC387 | 14.01 (±1.09) | 22.51 (±1.77) | 15.91 (±7.46) | ns | ns | — | — |
| CD3 | 13.17 (±3.76) | 16.63 (±7.92) | 12.27 (±5.42) | ns | ns | — | — |
| BrdU | 19.86 (±10.08) | 22.51 (±5.23) | 16.17 (±3.19) | ns | ns | — | — |
Numbers represent the mean number of positive cells (cells/mm2)±SEM, in parentheses. All animals were SIV-infected and CD8-lymphocyte depleted. Twenty random, nonoverlapping, ×200 fields of view were counted for each animal and the average number of positive cells/mm2 calculated. ANOVA was used to compare late natalizumab-treated animals to late untreated animals with and without cardiac pathology. If the ANOVA was significant (P<0.05), then post-hoc Mann–Whitney t tests were performed. BrdU indicates bromodeoxyuridine; ns, no significance; NZ, natalizumab treated; w/, untreated with cardiac pathology; w/o, untreated without cardiac pathology.
P<0.05
P<0.01.
Significant reductions in the number of CD163+ and CD68+ macrophages were found in late treated animals, compared to late untreated controls with cardiac pathology (Figure1A). There was a 4.53- and 1.59-fold decrease in numbers of CD163+ and CD68+ macrophages present in cardiac tissues, compared to late natalizumab treated animals (Figure1C; Table3; nonparametric Mann–Whitney t test, P<0.05, P<0.01). Similar to early treated animals, late treated animals had a trend of decreased numbers of newly infiltrating macrophages expressing MAC387 and CD3 T lymphocytes, compared to late untreated animals with cardiac pathology (Table3).
Natalizumab Treatment Blocks Traffic of Macrophages to the Cardiac Tissues
BrdU experiments were used to further determine whether natalizumab treatment blocks traffic of monocyte/macrophages to the heart. Previously, we have shown that the majority of BrdU+ macrophages in the heart are MAC387+ macrophages.28 In early natalizumab-treated animals, there were few BrdU+ cells (4.38±0.64 cells/mm2) and a trend of decreasing numbers of BrdU+ cells, compared to untreated controls (9.31±1.57 cells/mm2; Figure1A and 1B; Table2). Animals that began natalizumab treatment at 28 dpi had decreased numbers of BrdU+ cells (16.42±3.19 cells/mm2), compared to untreated animals with cardiac pathology (22.51±5.23 cells/mm2; Figure1A and 1C; Table3). The number of BrdU+ cells in late natalizumab-treated animals did not differ, when compared to untreated animals without cardiac pathology (Figure1A and 1C; Table3).
Decreased Fibrosis in Cardiac Tissues of Natalizumab-Treated Animals Correlates With Significant Decreases in Macrophage Numbers
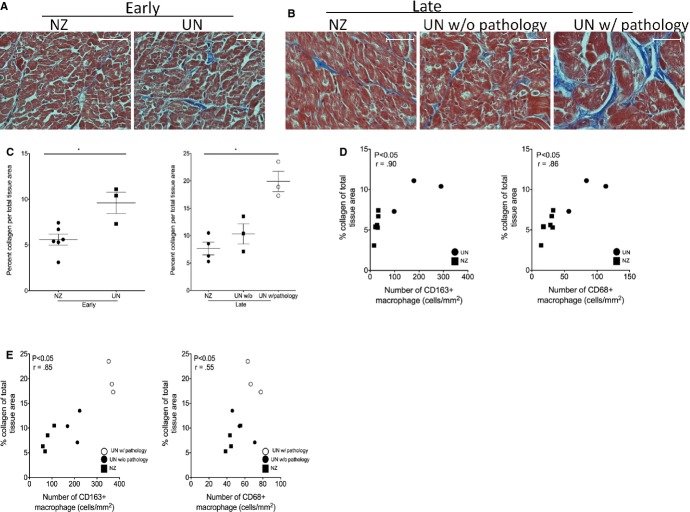
Using a modified Masson’s trichrome stain, the percent collagen per total tissue area in cardiac tissues of natalizumab-treated animals and untreated controls was quantified (Figure2A and 2B). Compared to controls, both early and late natalizumab-treated animals had decreased amounts of collagen (Figure2C). In early natalizumab-treated animals, the average percent collagen per total tissue area was 5.58±2.56%, compared to 9.6±2.06% for untreated controls, a significant 1.72-fold decrease in percent collagen (Figure2C, left; P<0.05, nonparametric Mann–Whitney t test).
Figure 2.
Natalizumab treatment decreases fibrosis in the left ventricle of SIV-infected, CD8-lymphocyte-depleted rhesus macaques. A and B, Modified Masson’s trichrome stain was used to compare the percent collage per total tissue area, a marker of fibrosis, in the left ventricle of early and late natalizumab-treated animals compared to untreated controls. C, Natalizumab treatment resulted in decreased fibrosis in cardiac tissues, compared to controls, regardless of when treatment began. In animals that began natalizumab treatment at 0 days postinfection (dpi), there was a significant decrease in the amount of collagen per tissue area (5.58±2.56%), compared to untreated controls (9.6±2.06%; nonparametric Mann–Whitney t test, P<0.05). Animals that began natalizumab treated at 28 dpi also showed a significant decrease in the amount of collagen per total tissue area (8.66±2.31%), when compared to untreated controls with cardiac pathology (19.91±1.85%). There was no difference in the percent collagen per total tissue area in late natalizumab-treated animals, compared to untreated controls without cardiac pathology. Spearman rank test was used to determine whether there was a correlation between decreases in fibrosis in natalizumab-treated animals and numbers of macrophages. D, In early natalizumab-treated animals (closed square), there was a correlation between the decrease in numbers of CD163+ and CD68+ macrophages and decreases in fibrosis, when compared to untreated controls (closed circle). E, In late natalizumab-treated animals (closed square), there was a correlation between decreases in CD163+ and CD68+ macrophages, compared to untreated controls with (open circle) and untreated controls without pathology (closed circle). r=spearman coefficient, P<0.05. Statistical analysis between early natalizumab-treated animals and controls was done using a nonparametric Mann–Whitney t test. For late natalizumab-treated animals and untreated controls with and without cardiac pathology, an ANOVA was performed first, and, if significant, a post-hoc nonparametric Mann–Whitney t test was performed (*P<0.05). Scale bar=50 μm, ×400 magnification. Error bars represent the average number of positive cells/mm2±SEM. NZ indicates natalizumab treated; UN, untreated.
Animals that began natalizumab treatment at 28 dpi (late) had a significantly higher average percentage of collagen per tissue area in the left ventricle (8.66±2.31%), compared to animals that began treatment early (5.58±1.47; Figure2C, right; P<0.05, nonparametric Mann–Whitney t test). Late natalizumab-treated animals had no significant differences in the percent of collagen per total tissue area, compared to untreated animals without cardiac pathology (8.66±2.31% versus 10.33±1.84%). However, when compared to untreated animals with cardiac pathology, there was a significant decrease in the percentage of collagen per tissue area. Whereas late natalizumab-treated animals had an average percentage of collagen of 8.66±2.31%, untreated animals with cardiac pathology had an average of 19.91±1.85%, a significant 2.29-fold decrease in the average percent collagen per total tissue area (Figure2C, right; P<0.05, nonparametric Mann–Whitney t test).
We next examined whether there was a correlation between decreased fibrosis in natalizumab-treated animals and changes in macrophage numbers in cardiac tissues if significant differences in macrophage numbers were found between groups. There was a correlation between increased fibrosis and increased numbers of CD163+ (r=0.9; P<0.05) and CD68+ (r=0.86; P<0.05) macrophages in untreated controls sacrificed at 21 dpi, compared to early natalizumab-treated animals (Figure2D). A correlation also existed in late natalizumab-treated animals, compared to all late untreated controls for CD163+ (r=0.85; P<0.05), CD68+ (r=0.55; P<0.05) macrophages and fibrosis (Figure2E).
Discussion
Chronic inflammation persists within HIV-infected individuals despite effective cART and decreased plasma viral load to undetectable levels.46–48 With chronic inflammation, there are increased comorbidities, compared to the general non-HIV-infected population.49–51 In particular, there is an increased incidence of CVD4 where monocytes/macrophages are increasingly considered to play a role.52,53 Previously, we have shown that SIV-infected, CD8-lymphocyte-depleted monkeys have increased numbers of macrophages (CD163+, CD68+, and MAC387+) in cardiac tissues that positively correlate increased fibrosis.28 In this study, we examined whether an anti-α4 integrin antibody, natalizumab, diminishes leukocyte and monocyte/macrophage traffic to the heart resulting in decreased fibrosis.
Natalizumab blocks the interaction between α4 integrin and its ligand, vascular cell adhesion molecule 1 (VCAM-1).38 VCAM-1 is expressed on endothelial cells of the arterial lumen with atherosclerosis.54 Studies in mice showed that inhibiting the interaction between α4 and VCAM-1 decreased macrophage recruitment to atherosclerotic plaques.41 Previously, we have shown that there is a higher level of macrophage traffic to the heart later in SIV infection (>21 dpi) and that there are few macrophages present in untreated animals sacrificed at 21 dpi.28 In the current study, we found that natalizumab treatment beginning at 0 dpi resulted in decreased numbers of CD163+ and CD68+ macrophages, compared to uninfected controls, but cardiac pathology in early infection is minimal and most of the pathology occurred in the later stages of infection.
When compared to untreated controls with cardiac pathology, late treated animals had significant decreases in the number of CD163+ and CD68+ macrophages. In fact, the numbers of macrophages in late natalizumab-treated animals were similar to untreated animals without cardiac pathology. Additionally, we found a correlation between decreased macrophage numbers in natalizumab-treated animals with decreased cardiac fibrosis. Overall, these data show that blocking monocyte/macrophage traffic to the heart alleviates HIV- and SIV-associated cardiac pathology that resulted in reduced inflammation, fibrosis, and cardiomyocyte degeneration.
Though not significant, we found a trend of decreasing numbers of MAC387+ macrophages in the left ventricle of late treated animals, compared to untreated animals with cardiac pathology. The finding of a decrease in newly recruited macrophages is supported by our observation of fewer BrdU-labeled macrophages (that traffic from the bone marrow), suggesting that macrophage traffic to the heart results in increased fibrosis. Whereas previous research showed that natalizumab decreased traffic of CD3 T lymphocytes and MAC387+ to the brain and gut,42 in the current study, we did not find significant differences in the number of CD3 T lymphocytes or MAC387+ macrophages in cardiac tissues of SIV-infected animals with or without natalizumab treatment. This possibly suggests that CD3+ T lymphocytes and MAC387+ macrophages use different integrins to traffic to the heart than to the brain or gut; however, we have previously shown that MAC387+ macrophages and not CD3+ T lymphocytes correlate with increased fibrosis in cardiac tissues.28 Our lack of finding a statistically significant reduction in the number MAC387+ macrophages may be owing to the relatively few numbers of those cells in cardiac tissues.
Previously, we have shown that the rate of monocyte/macrophage traffic to the heart is increased later in infection (after 48 dpi), as opposed to early infection.28 Using BrdU labeling, we found a trend of decreased traffic of monocytes/macrophages to the heart in late natalizumab-treated animals, compared to untreated animals that developed cardiac pathology. Late natalizumab-treated animals had a similar rate of traffic of newly released monocytes/macrophages to the heart as untreated animals without cardiac pathology. This provides evidence that potentially blocking traffic of monocyte/macrophages later during SIV infection can alleviate SIV-associated cardiac pathology.
Although cART can decrease HIV to nondetectable levels in plasma, it does not necessarily target monocyte/macrophages that play a role in the development of cardiac pathology and CVD.32 Chronic immune activation with HIV infection is posited to play a role in HIV-associated cardiovascular pathology. Previous studies show that HIV-infected individuals have increased inflammation in the ascending aorta that correlates with levels of sCD163 in plasma.55 Increased inflammation in the aorta is also been linked to high-risk noncalcified plaques that are prone to rupture.8 18-Fluorodeoxyglucose positron emission tomography imaging studies have demonstrated that such plaque areas are comprised of areas with accumulation of macrophages.8,56,57 Whereas macrophage accumulation in the aorta and cardiac plaques are critical in HIV-associated cardiac disease, it is not surprising that also there is increased macrophage inflammation in cardiac tissues at the same time. Unpublished data from our laboratory, using matched cardiac tissues (left ventricle) and aorta from HIV− and HIV+ individuals, shows that, with HIV infection, there is an increased macrophage accumulation inflammation in ventricular tissues and the aorta. Moreover, increased macrophage inflammation in cardiac tissues correlates with increased fibrosis, macrophage accumulation in the aorta, and increased aortic intima-media thickness. To date, there are few therapies that target macrophages specifically or indirectly to diminish HIV-associated CV pathology. Emergent data underscore the importance of such therapy strategies.
Therapeutic agents that have been successful in the treatment noncalcified cardiac plaque in HIV+ individuals include 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins), which, fortuitously, have anti-inflammatory effects on macrophages.58,59 Statin therapy in conjunction with cART reduced serum levels of inflammatory markers, including IL-6, IL-8, and tumor necrosis factor alpha, more so than cART alone.60 Statins have similarly been used in monkeys, where they decreased the macrophage content in plaques in the abdominal aorta.34 A recent study in HIV-infected individuals showed that statins significantly decreased the volume on noncalcified plaques, but whether statin use in this study directly affected monocyte and/or macrophage activation and traffic was not studied.35 In rodent models of experimental autoimmune myocarditis, rosuvastatin-reduced numbers in macrophages, T lymphocytes, and multinucleated giant cells in the heart resulted in decreased numbers of apoptotic cardiomyocytes. The effects of statins on myocarditis with HIV infection have not been examined.61
Other studies found that maraviroc treatment decreased chemotaxis of monocyte/macrophages, in vitro,62 but in clinical studies with advanced HIV, it did not affect the development of immune reconstitution inflammatory syndrome,63 Maraviroc is used primarily to inhibit viral replication of R5-tropic HIV by blocking interactions between the virus and CCR5 on host cells.64,65 Studies using maraviroc in SIV-infected monkeys demonstrated fewer CD163+ macrophages in the heart, but this could have been owing to a decreased CD163 expression (activation) on macrophages already present in the heart and not a decrease in inflammatory cells. All together, these experiments add further evidence to the role that monocyte/macrophages play in cardiac pathology with HIV and SIV infection, and suggest that therapies blocking monocyte/macrophage traffic to the heart could diminish HIV-associated cardiac pathology.
In this study, we showed that directly blocking monocyte/macrophage traffic to cardiac tissues with natalizumab successfully decreased the numbers of macrophages present in tissues. Studies examining whether blocking traffic to vessels result in decreased high-risk vascular plaques with HIV infection are warranted. Our data suggest that studies examining the efficacy of blocking monocyte/macrophage traffic, or directly targeting monocyte/macrophage activation as an adjunctive therapy with cART, should be examined with an aim to decrease HIV-associated cardiac pathology.
Acknowledgments
The authors thank Biogen Idec (Cambridge, MA) for providing natalizumab for use in this study. In vivo CD8 depletion antibodies were kindly provided by the NIH Nonhuman Primate Reagent Resource. Author Contributions: Conceived and designed experiments: Walker, Campbell, Burdo, Williams. Performed the experiments: Walker, Beck. Analyzed the data: Walker, Beck. Scoring of cardiac tissue sections: Miller. Wrote the article: Walker, Burdo, Williams.
Sources of Funding
This work was supported by the following National Institutes of Health (NIH) grants: R01 NS040237 (Williams) and R01 NS082116 (Burdo).
Disclosures
None.
References
- Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocroft A, Kirk O, Gatell J, Reiss P, Gargalianos P, Zilmer K, Beniowski M, Viard JP, Staszewski S, Lundgren JD. Chronic renal failure among HIV-1-infected patients. AIDS. 2007;21:1119–1127. doi: 10.1097/QAD.0b013e3280f774ee. [DOI] [PubMed] [Google Scholar]
- Cazanave C, Dupon M, Lavignolle-Aurillac V, Barthe N, Lawson-Ayayi S, Mehsen N, Mercie P, Morlat P, Thiebaut R, Dabis F Groupe d’Epidemiologie Clinique du SeA. Reduced bone mineral density in HIV-infected patients: prevalence and associated factors. AIDS. 2008;22:395–402. doi: 10.1097/QAD.0b013e3282f423dd. [DOI] [PubMed] [Google Scholar]
- Ho JE, Hsue PY. Cardiovascular manifestations of HIV infection. Heart. 2009;95:1193–1202. doi: 10.1136/hrt.2008.161463. [DOI] [PubMed] [Google Scholar]
- Zaaqoq AM, Khasawneh FA, Smalligan RD. Cardiovascular complications of HIV-associated immune dysfunction. Cardiol Res Pract. 2015;2015:1–8. doi: 10.1155/2015/302638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, Rimland D, Rodriguez Barradas M, Brown S, Gibert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser S, Gelbrich G, Brockmeyer N, Goehler A, Schadendorf D, Erbel R, Neumann T, Reinsch N. Prevalence of cardiovascular diseases in HIV-infected outpatients: results from a prospective, multicenter cohort study. Clin Res Cardiol. 2013;102:203–213. doi: 10.1007/s00392-012-0519-0. [DOI] [PubMed] [Google Scholar]
- Tawakol A, Lo J, Zanni MV, Marmarelis E, Ihenachor EJ, MacNabb M, Wai B, Hoffmann U, Abbara S, Grinspoon S. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J Acquir Immune Defic Syndr. 2014;66:164–171. doi: 10.1097/QAI.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanni MV, Schouten J, Grinspoon SK, Reiss P. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol. 2014;11:728–741. doi: 10.1038/nrcardio.2014.167. [DOI] [PubMed] [Google Scholar]
- Ray M, Logan R, Sterne JA, Hernandez-Diaz S, Robins JM, Sabin C, Bansi L, van Sighem A, de Wolf F, Costagliola D, Lanoy E, Bucher HC, von Wyl V, Esteve A, Casbona J, del Amo J, Moreno S, Justice A, Goulet J, Lodi S, Phillips A, Seng R, Meyer L, Perez-Hoyos S, Garcia de Olalla P, Hernan MA. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24:123–137. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquet I, Pavie J, Palmer P, Barbier F, Legriel S, Mayaux J, Molina JM, Schlemmer B, Azoulay E. Survival trends in critically ill HIV-infected patients in the highly active antiretroviral therapy era. Crit Care. 2010;14:R107. doi: 10.1186/cc9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, Berti A, Rossi E, Roverato A, Palella F. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- Hemkens LG, Bucher HC. HIV infection and cardiovascular disease. Eur Heart J. 2014;35:1373–1381. doi: 10.1093/eurheartj/eht528. [DOI] [PubMed] [Google Scholar]
- Barbaro G, Fisher SD, Lipshultz SE. Pathogenesis of HIV-associated cardiovascular complications. Lancet Infect Dis. 2001;1:115–124. doi: 10.1016/S1473-3099(01)00067-6. [DOI] [PubMed] [Google Scholar]
- Patanè S, Marte F, Sturiale M, Dattilo G, Albanese A. Myocarditis and cardiomyopathy HIV associated. Int J Cardiol. 2011;146:e56–e57. doi: 10.1016/j.ijcard.2008.12.207. [DOI] [PubMed] [Google Scholar]
- Friis-Møller N, Reiss P, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr W, Thiébaut R, De Wit S, Kirk O, Fontas E, Law MG, Phillips A, Lundgren JD. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- Liu R, Moroi M, Yamamoto M, Kubota T, Ono T, Funatsu A, Komatsu H, Tsuji T, Hara H, Hara H, Nakamura M, Hirai H, Yamaguchi T. Presence and severity of Chlamydia pneumoniae and Cytomegalovirus infection in coronary plaques are asssociated with acute coronary syndromes. Int Heart J. 2006;47:511–519. doi: 10.1536/ihj.47.511. [DOI] [PubMed] [Google Scholar]
- Stein JH, Hsue PY. Inflammation, immune activation, and CVD risk in individuals with HIV infection. JAMA. 2012;308:405–406. doi: 10.1001/jama.2012.8488. [DOI] [PubMed] [Google Scholar]
- Khunnawat C, Mukerji S, Havlichek D, Jr, Touma R, Abela GS. Cardiovascular manifestations in human immunodeficiency virus-infected patients. Am J Cardiol. 2008;102:635–642. doi: 10.1016/j.amjcard.2008.04.035. [DOI] [PubMed] [Google Scholar]
- Silwa K, Carrington MJ, Becker A, Thienemann F, Ntsekhe M, Stewart S. Contribution of the human immunodeficiency virus acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur Heart J. 2012;33:866–874. doi: 10.1093/eurheartj/ehr398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway CJ, Ntusi N, Suttie J, Mahmod M, Wainwright E, Clutton G, Hancock G, Beak P, Tajar A, Piechnik SK, Schneider JE, Angus B, Clarke K, Dorrell L, Neubauer S. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128:814–822. doi: 10.1161/CIRCULATIONAHA.113.001719. [DOI] [PubMed] [Google Scholar]
- Cheruvu S, Holloway CJ. Cardiovascular disease in human immunodeficiency virus. Intern Med J. 2014;44:315–324. doi: 10.1111/imj.12381. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Petrosillo N, Vizza D, Francone M, Badagliacca R, Verardo R, Fedele F, Ippolito G, Chimenti C. Myocardial and microvascular inflammation/infection in patients with HIV-associated pulmonary artery hypertension. AIDS. 2014;28:2541–2549. doi: 10.1097/QAD.0000000000000426. [DOI] [PubMed] [Google Scholar]
- Shannon RP. SIV cardiomyopathy in non-human primates. Trends Cardiovasc Med. 2011;11:242–246. doi: 10.1016/s1050-1738(01)00118-9. [DOI] [PubMed] [Google Scholar]
- Shannon RP, Simon MA, Mathier MA, Geng YJ, Mankad S, Lackner AA. Dilated cardiomyopathy associated with simian AIDS in nonhuman primates. Circulation. 2000;101:185–193. doi: 10.1161/01.cir.101.2.185. [DOI] [PubMed] [Google Scholar]
- Yearley JH, Pearson C, Carville A, Shannon RP, Mansfield K. Phenotypic variation in myocardial macrophage populations suggests a role for macrophage activation in SIV-associated cardiac disease. AIDS Res Hum Retroviruses. 2007;23:512–524. doi: 10.1089/aid.2006.0211. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Tarwater PM, Karper JM, Bedja D, Queen SE, Tunin RS, Adams RJ, Kass DA, Mankowski JL. Diastolic dysfunction is associated with myocardial viral load in simian immunodeficiency virus-infected macaques. AIDS. 2012;26:815–823. doi: 10.1097/QAD.0b013e3283518f01. [DOI] [PubMed] [Google Scholar]
- Walker JA, Sulciner ML, Nowicki KD, Miller AD, Burdo TH, Williams KC. Elevated numbers of CD163+ macrophages in hearts of simian immunodeficiency virus-infected monkeys correlate with cardiac pathology and fibrosis. AIDS Res Hum Retroviruses. 2014;30:685–694. doi: 10.1089/aid.2013.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower GL, Gardner JD, Forman MF, Murray DB, Voloshenyuk T, Levick SP, Janicki JS. The relationship between myocardial extracellular matrix remodeling and ventricular function. Eur J Cardiothorac Surg. 2006;30:604–610. doi: 10.1016/j.ejcts.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- Montecucco F, Braunersreuther V, Lenglet S, Delattre BM, Pelli G, Buatois V, Guilhot F, Galan K, Vuilleumier N, Ferlin W, Fischer N, Vallee JP, Kosco-Vilbois M, Mach F. CC chemokine CCL5 plays a central role impacting infarct size and post-infarction heart failure in mice. Eur Heart J. 2012;33:1964–1974. doi: 10.1093/eurheartj/ehr127. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Tocchetti CG, Lyashkov A, Tarwater PM, Bedja D, Graham DR, Beck SE, Metcalf Pate KA, Queen SE, Adams RJ, Paolocci N, Mankowski JL. CCR5 inhibition prevents cardiac dysfunction in the SIV/macaque model of HIV. J Am Heart Assoc. 2014;3:e000874. doi: 10.1161/JAHA.114.000874. doi: 10.1161/JAHA.114.000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y, Okamoto H, Jia N, Akino M, Uede T, Kitabatake A, Nishihira J. Blockade of macrophage migration inhibitory factor ameliorates experimental autoimmune myocarditis. J Mol Cell Cardiol. 2004;37:557–566. doi: 10.1016/j.yjmcc.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Sukhova GK. Statins reduce inflammation in atheroma of nonhuman primates independent of effects on serum cholesterol. Arterioscler Thromb Vasc Biol. 2002;22:1452–1458. doi: 10.1161/01.atv.0000030360.72503.56. [DOI] [PubMed] [Google Scholar]
- Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, Oh J, Zimmerman CO, Hwang J, Abbara S, Plutzky J, Robbins G, Tawakol A, Hoffmann U, Grinspoon SK. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2015;2:e52–e63. doi: 10.1016/S2352-3018(14)00032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliser D, Buchholz K, Haller H. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation. 2004;110:1103–1107. doi: 10.1161/01.CIR.0000140265.21608.8E. [DOI] [PubMed] [Google Scholar]
- Navalkar S, Parthasarathy S, Santanam N, Khan BV. Irbesartan, an angiotensin type 1 receptor inhibitor, regulates markers of inflammation in patients with premature atherosclerosis. J Am Coll Cardiol. 2001;37:440–444. doi: 10.1016/s0735-1097(00)01138-4. [DOI] [PubMed] [Google Scholar]
- Yu Y, Schurpf T, Springer TA. How natalizumab binds and antagonizes alpha4 integrins. J Biol Chem. 2013;288:32314–32325. doi: 10.1074/jbc.M113.501668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polma CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- Kane SV, Horst S, Sandborn WJ, Becker B, Neis B, Moscandrew M, Hanson KA, Tremaine WJ, Bruining DH, Faubion WA, Pardi DS, Harmsen WS, Zinsmeister AR, Loftus EV. Natalizumab for moderate to severe Crohn’s disease in clinical practice: the Mayo Clinic Rochester experience. Inflamm Bowel Dis. 2012;18:2203–2208. doi: 10.1002/ibd.22943. [DOI] [PubMed] [Google Scholar]
- Patel SS, Thiagarajan R, Willerson JT, Yeh ETH. Inhibition of 4 integrin and ICAM-1 markedly attenuate macrophage homing to atherosclerotic plaques in ApoE-deficient mice. Circulation. 1998;97:75–81. doi: 10.1161/01.cir.97.1.75. [DOI] [PubMed] [Google Scholar]
- Campbell JH, Ratai E-M, Autissier P, Nolan DJ, Tse S, Miller AD, González RG, Salemi M, Burdo TH, Williams KC. Anti-α4 antibody treatment blocks virus traffic to the brain and gut early, and stabilizes CNS injury late in infection. PLoS Pathog. 2014;10:e1004533. doi: 10.1371/journal.ppat.1004533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuve O, Marra CM, Jerome KR, Cook L, Cravens PD, Cepok S, Frohman EM, Phillips JT, Arendt G, Hemmer B, Monson NL, Racke MK. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol. 2006;59:743–747. doi: 10.1002/ana.20858. [DOI] [PubMed] [Google Scholar]
- Sasseville VG, Newmna W, Brodie SJ, Hesterberg P, Pauley D, Ringler DJ. Monocyte adhesion to endothelium in simian immunodeficiency virus-induced aids encephalitis is mediated by vascular cell adhesion molecule-1/alpha 4 beta 1 integrin interactions. Am J Pathol. 1994;144:27–40. [PMC free article] [PubMed] [Google Scholar]
- Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol. 2001;23:291–299. [PubMed] [Google Scholar]
- Lambotte O, Taoufik Y, de Goer MG, Wallon C, Goujard C, Delfraissy JF. Detection of infectious HIV in circulating monocytes from patients on prolonged highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;23:114–119. doi: 10.1097/00126334-200002010-00002. [DOI] [PubMed] [Google Scholar]
- Dornadula G, Zhang H, VanUitert B, Stern J, Livornese L, Jr, Ingerman MJ, Witek J, Kedanis RJ, Natkin J, DeSimone J, Pomerantz RJ. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA. 1999;282:1627–1632. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- Lichtfuss GF, Cheng WJ, Farsakoglu Y, Paukovics G, Rajasuriar R, Velayudham P, Kramski M, Hearps AC, Cameron PU, Lewin SR, Crowe SM, Jaworowski A. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J Immunol. 2012;189:1491–1499. doi: 10.4049/jimmunol.1200458. [DOI] [PubMed] [Google Scholar]
- Neuhaus J, Angus B, Kowalska JD, La Rosa A, Sampson J, Wentworth D, Mocroft A, Insight S Groups Es. Risk of all-cause mortality associated with nonfatal aids and serious non-AIDS events among adults infected with HIV. AIDS. 2010;24:697–706. doi: 10.1097/QAD.0b013e3283365356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Penney AT, Iudicello JE, Riggs PK, Doyle K, Ellis RJ, Letendre SL, Grant I, Woods SP Group HIVNRPH. Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDS. 2013;27:5–16. doi: 10.1089/apc.2012.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzinger JJ, Butterfield TR, Angelovich TA, Crowe SM, Palmer CS. Monocytes as regulators of inflammation and HIV-related comorbidities during cart. J Immunol Res. 2014;2014:569819. doi: 10.1155/2014/569819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medbury HJ, James V, Ngo J, Hitos K, Wang Y, Harris D, Fletcher JP. Differing association of macrophage subsets with atherosclerotic plaque stability. Int Angiol. 2013;32:74–84. [PubMed] [Google Scholar]
- Pamukcu B, Lip GYH, Devitt A, Griffiths H, Shantsila E. The role of monocytes in atherosclerotic coronary artery disease. Ann Med. 2010;42:394–403. doi: 10.3109/07853890.2010.497767. [DOI] [PubMed] [Google Scholar]
- OBrien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, McCarty J, Ferguson M, Hudkins K, Benjamin CD, Lobb R, Alpers CE. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92:945–951. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Tawakol A, Abbara S, Wei J, Vijayakumar J, Corsini E, Abdelbaky A, Zanni MV, Hoffman U, Williams KC, Lo J, Grinspoon SK. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelbaky A, Corsini E, Figueroa AL, Subramanian S, Fontanez S, Emami H, Hoffmann U, Narula J, Tawakol A. Early aortic valve inflammation precedes calcification: a longitudinal FDG-PET/CT study. Atherosclerosis. 2015;238:165–172. doi: 10.1016/j.atherosclerosis.2014.11.026. [DOI] [PubMed] [Google Scholar]
- Tahara N, Mukherjee J, de Haas HJ, Petrov AD, Tawakol A, Haider N, Tahara A, Constantinescu CC, Zhou J, Boersma HH, Imaizumi T, Nakano M, Finn A, Fayad Z, Virmani R, Fuster V, Bosca L, Narula J. 2-deoxy-2-[18F]fluoro-D-mannose positron emission tomography imaging in atherosclerosis. Nat Med. 2014;20:215–219. doi: 10.1038/nm.3437. [DOI] [PubMed] [Google Scholar]
- Giguere JF, Tremblay MJ. Statin compounds reduce human immunodeficiency virus type 1 replication by preventing the interaction between virion-associated host intercellular adhesion molecule 1 and its natural cell surface ligand LFA-1. J Virol. 2004;78:12062–12065. doi: 10.1128/JVI.78.21.12062-12065.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza L, Trapani F, Bartoletti M, Manfredi R, Colangeli V, Borderi M, Grossi G, Motta R, Viale P. Statin therapy decreases serum levels of high-sensitivity C-reactive protein and tumor necrosis factor-alpha in HIV-infected patients treated with ritonavir-boosted protease inhibitors. HIV Clin Trials. 2012;13:153–161. doi: 10.1310/hct1303-153. [DOI] [PubMed] [Google Scholar]
- Calza L, Vanino E, Salvadori C, Manfredi R, Colangeli V, Cascavilla A, Di Bari MA, Motta R, Viale P. Tenofovir/emtricitabine/efavirenz plus rosuvastatin decrease serum levels of inflammatory markers more than antiretroviral drugs alone in antiretroviral therapy-naive HIV-infected patients. HIV Clin Trials. 2014;15:1–13. doi: 10.1310/hct1501-1. [DOI] [PubMed] [Google Scholar]
- Liu X, Li B, Wang W, Zhang C, Zhang M, Zhang Y, Xia Y, Dong Z, Guo Y, An F. Effects of HMG-CoA reductase inhibitor on experimental autoimmune myocarditis. Cardiovasc Drugs Ther. 2012;26:121–130. doi: 10.1007/s10557-012-6372-6. [DOI] [PubMed] [Google Scholar]
- Rossi R, Lichtner M, De Rosa A, Sauzullo I, Mengoni F, Massetti AP, Mastroianni CM, Vullo V. In vitro effect of anti-human immunodeficiency virus CCR5 antagonist maraviroc on chemotactic activity of monocytes, macrophages and dendritic cells. Clin Exp Immunol. 2011;166:184–190. doi: 10.1111/j.1365-2249.2011.04409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Madero JG, Ellenberg SS, Rassool MS, Tierney A, Belaunzarán-Zamudio PF, López-Martínez A, Piñeirúa-Menéndez A, Montaner LJ, Azzoni L, Benítez CR, Sereti I, Andrade-Villanueva J, Mosqueda- Gómez JL, Rodriguez B, Sanne I, Lederman MM. Effect of the CCR5 antagonist maraviroc on the occurrence of immune reconstitution inflammatory syndrome in HIV (CADIRIS): a double-blind, randomised, placebo-controlled trial. Lancet HIV. 2014;1:e60–e67. doi: 10.1016/S2352-3018(14)70027-X. [DOI] [PubMed] [Google Scholar]
- MacArthur RD, Novak RM. Maraviroc: the first of a new class of antiretroviral agents. Clin Infect Dis. 2008;47:236–241. doi: 10.1086/589289. [DOI] [PubMed] [Google Scholar]
- Soriano V, Geretti AM, Perno CF, Fatkenheuer G, Pillay D, Reynes J, Tambussi G, Calvez V, Alcami J, Rockstroh J. Optimal use of maraviroc in clinical practice. AIDS. 2008;22:2231–2240. doi: 10.1097/QAD.0b013e3283136d95. [DOI] [PubMed] [Google Scholar]