Abstract
Background
Mild transverse aortic arch (TAA) hypoplasia is common after coarctation treatment, but is considered benign in the absence of an arm-leg systolic blood pressure (SBP) difference. Hypertension (HTN) is a common long-term morbidity after successful coarctation treatment. We examined whether mild TAA hypoplasia after coarctation treatment is associated with late systemic HTN.
Methods and Results
We retrospectively reviewed 92 patients (median age, 19.9 years; range, 4.9 to 57.8; 60% male) 14.1±10.3 years after successful coarctation treatment (surgery in 63, stent in 16, and balloon dilation in 13), excluding those with resting right arm-leg blood pressure gradient >20 mm Hg, atypical coarctation, and major associated heart defects. Minimum body-surface area (BSA)-adjusted TAA cross-sectional area (CSA) was calculated from cardiac magnetic resonance (CMR) images. On follow-up, 38 of 92 (41%) patients had systemic HTN using standard criteria. Systemic HTN was independently associated with smaller TAA CSA/BSA (P=0.006; odds ratio [OR], 6.41 per 0.5 cm2/m2 decrease), higher age at CMR (P=0.03; OR, 1.57 per 5-year increase), and in a subset (n=61), higher arm-leg SBP difference during exercise (P=0.05; OR, 1.03 per 1-mm-Hg increase). Lower ratio of TAA diameter/descending aorta diameter was associated with a larger increase in right arm SBP during peak exercise (P=0.006; r2=0.11).
Conclusions
Persistent mild aortic arch hypoplasia, even in the absence of an arm-leg SBP difference at rest, is associated with late systemic HTN. Further studies should be undertaken to determine whether more-aggressive arch reconstruction at initial repair can reduce the incidence of systemic HTN.
Keywords: cardiac MRI, coarctation, hypertension, transverse aortic arch
Systemic hypertension (HTN) is an important long-term morbidity after treatment of coarctation of the aorta, occurring in nearly 30% patients.1 HTN contributes to the relatively high cardiovascular (CV) morbidity (including a high incidence of coronary artery disease and stroke) and mortality in these patients.2,3 The high prevalence of HTN has been attributed to various factors, including residual arch obstruction, abnormal aortic arch shape, and abnormal vascular properties of the aorta and systemic vasculature.4–6 Initial treatment of coarctation is usually focused on relieving obstruction at the aortic isthmus. Mild hypoplasia of the transverse aortic arch (TAA) is common in coarctation of the aorta, but unless severe, it is usually not addressed at the initial repair. During follow-up, mild TAA hypoplasia is considered benign in the absence of a significant arm-leg systolic blood pressure (SBP) difference. We hypothesized that mild residual TAA hypoplasia after coarctation treatment is not benign and examined whether it is associated with HTN.
Methods
Subjects
A retrospective review of existing clinical databases at Boston Children’s Hospital (Boston, MA) was used to identify subjects for this study who met the following inclusion criteria: (1) undergone treatment of coarctation of the aorta; (2) residual arm-leg SBP difference <20 mm Hg; and (3) cardiac magnetic resonance (CMR) study between January 2005 and July 2014. Those subjects with significant associated congenital heart disease (aside from an atrial or ventricular septal defect), mid-thoracic or abdominal coarctation, >mild atrioventricular or semilunar valve regurgitation, or aberrant subclavian artery origin were excluded. Demographic, clinical, and surgical data were abstracted from medical records. The Department of Cardiology Scientific Review Committee and the Boston Children’s Hospital Committee on Clinical Investigation approved this study and waived the requirement for informed consent.
Blood Pressure Data
All available blood pressure (BP) recordings postrepair were abstracted from the patient’s medical record. Following standard practice at our institution, right arm BP was recorded while seated using commercial oscillometric BP devices and size-appropriate cuffs. Four-extremity BP was recorded in the supine position. Arm-leg BP difference was calculated as the difference between the SBP in the right arm and the leg with the higher SBP. The most recent recording of arm-leg BP difference was used for analysis. Patients satisfying standard pediatric (for patients <18 years of age: SBP and/or diastolic BP [DBP] ≥95th percentile for age, gender, and height) or adult (for patients ≥18 years of age: SBP ≥140 and/or DBP ≥90 mm Hg) criteria for resting right arm HTN on 2 separate outpatient visits were labeled as having systemic HTN.7,8 Patients currently on antihypertensive medication were classified into the “hypertensive group” if they satisfied standard criteria for HTN on 2 separate outpatient visits before initiation of treatment. Patients who had transient postprocedure HTN (with or without antihypertensive treatment) that resolved with normal blood pressure recordings on at least 2 subsequent outpatient visits (without antihypertensive treatment) were classified into the “normotensive” group.
CMR
CMR was performed using a commercially available whole-body 1.5 T scanner (Achieva; Philips Healthcare, Best, the Netherlands). In the case of multiple CMR studies, the most recent one was analyzed. In young patients who could not cooperate with the examination (generally <8 years age), imaging was performed under general anesthesia. Steady-state free precession cine imaging in the ventricular short-axis plane was performed using the following imaging parameters: echo time 1.5 to 2 ms; repetition time 2.8 to 4.0 ms; flip angle 45 degrees; turbo factor 10 to 20; and 30 reconstructed images per cardiac cycle. Ventricular short-axis images were analyzed using commercial software (QMass; Medis, Leiden, the Netherlands) to calculate left ventricular (LV) mass which was adjusted to body surface area (BSA), calculated using Haycock’s formula.9 After placement of a peripheral intravenous cannula, 0.15 to 0.2 mmol/kg of gadopentate dimeglumine (Magnevist; Berlex, Seattle, WA) was injected at a rate of 2 mL/s. Magnetic resonance angiography (MRA) image acquisition was initiated using bolus tracker to optimize visualization of the aorta. Contrast-enhanced 3-dimensional (3D) MRA of the chest without electrocardiographic gating was performed using a commercial T1-weighted fast gradient echo sequence, as previously described.10
CMR Image Analysis
Images were analyzed by a single observer (A.P.) using a commercial computer workstation (Extended Workstation; Philips Healthcare). Aortic arch measurements were performed in cross-sectional multiplanar reformatted images from the ungated gadolinium 3D MRA using the thinnest possible slice and adjusting the image contrast and brightness such that the chest wall was just visible and lung tissue remained dark (Figure1).11 Standard anatomic landmarks were used to define segments of the aortic arch. Cross-sectional area (CSA) of the TAA was measured by direct planimetry at the narrowest segment of the TAA (narrowest among proximal and distal TAA segments) and adjusted to BSA. Two orthogonal diameters each of the proximal and distal TAA, isthmus, and thoracic descending aorta (DAO) were also measured. The DAO was measured at the level of the left atrium, avoiding the dilated segment just distal to the isthmus. The smallest TAA and isthmus diameters measured at the narrowest segment were used for analysis. In addition to the TAA CSA/BSA, the ratio of the minimum TAA diameter and the DAO diameter (TAA/DAO) was calculated as another indicator of TAA hypoplasia. Similarly, the ratio of the minimum isthmus diameter and the DAO diameter (isthmus/DAO) was calculated. In patients treated with a stent, susceptibility artifact precluded isthmus measurement on CMR. In these subjects (n=16), the minimum stent diameter measured on most recent X-ray angiography was recorded in lieu of isthmus diameter (mean time difference, 1.8±1.4 years between CMR and X-ray angiography).
Figure 1.
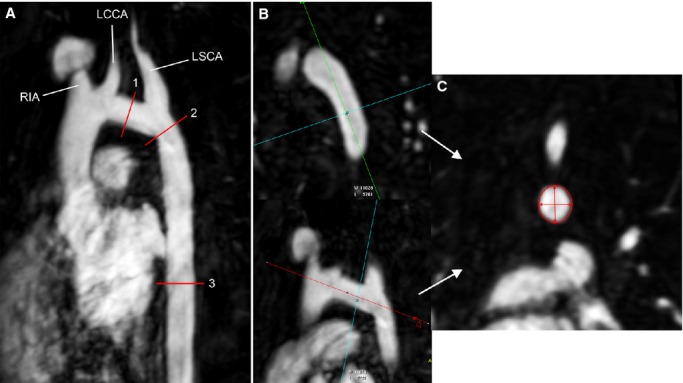
A, Maximum intensity projection from the gadolinium 3-dimensional (3D) MRA showing the sites of measurements. The transverse aortic arch (1) extending from the origin of the RIA to the origin of the LSCA was measured at its narrowest segment. The isthmus (2) was measured just distal to the origin of the LSCA. The thoracic descending aorta (3) was measured at the level of the left atrium. B and C, Example of multiplanar reformatting of the 3D MRA to measure 2 orthogonal diameters and cross-sectional area of the transverse aortic arch. LCCA indicates left common carotid artery; LSCA, left subclavian artery; MRA, magnetic resonance angiogram; RIA, right innominate artery.
Exercise Stress Test
Exercise test data were included if performed within 1 year of CMR imaging and if the patient achieved maximal aerobic capacity (respiratory exchange ratio, >1.09). Testing was performed using a standard Bruce treadmill or bicycle ergometer protocol, as previously described.12 Following institutional practice, antihypertensive medications were continued on the day of testing. BP in the right arm and a leg were measured using a commercial oscillometric BP recorder in the supine position at baseline and immediately after peak exercise in early recovery. Baseline and peak arm-leg SBP differences and the increase in right arm BP with peak exercise were recorded.
Statistical Analysis
Systemic HTN, as previously defined, was evaluated as the primary outcome variable. TAA CSA/BSA and isthmus/DAO diameter ratios were the primary independent variables. Increase in right arm SBP with exercise and LV mass/BSA on CMR were evaluated as secondary outcomes. Associations between outcome and predictor variables were examined using multivariable logistic or linear regression with forward selection. Variables demonstrating univariate associations with P value <0.2 qualified for inclusion into the final multivariable models. For multivariable analyses, statistical significance was evaluated with respect to a type 1 error probability threshold of 0.05. Statistical analysis was performed using commercially available software (Stata version 13.0; StataCorp LP, College Station, TX).
Results
Subjects
A database search yielded 92 patients who met inclusion and exclusion criteria. Characteristics of these subjects are summarized in Table1. The cohort consisted of predominantly children and young adults who were evaluated at a median time interval of 13.5 years after their initial coarctation procedure. A second procedure for recurrent or residual coarctation had been performed in 37% patients. During follow-up, 41% of the patients satisfied standard adult or pediatric criteria for HTN on at least 2 outpatient visits (median, 3 visits; range, 2 to 6) and were classified as the “hypertensive” group. Of the 38 patients in the hypertensive group, 87% were receiving antihypertensive medication for a median duration of 4 years (range, 1 to 33). Antihypertensive treatment consisted of a single medication in 22 patients (58%), 2 medications in 9 (24%), 3 medications in 2 (5%), and lifestyle modification without medication in 5 (13%). No patient was receiving medication for prevention or treatment of aortopathy alone in the absence of HTN. Among the patients receiving antihypertensive medication (n=33), 4 (12%) satisfied criteria for HTN on most recent evaluation and 2 of these 4 were not fully compliant with their medication. Among the 54 “normotensive” patients, 9 (17%) had received antihypertensive medication temporarily in the past (all during the first year after initial repair for treatment of postprocedure HTN). Patients in the hypertensive and normotensive groups were similar with respect to age at CMR, age at repair, resting arm-leg BP difference before repair, type of initial repair, need for a second arch procedure, and the presence of a ventricular septal defect or bicommissural aortic valve. Both TAA/DAO and isthmus/DAO diameter ratios were <1 in 75% of subjects.
Table 1.
Patient Characteristics
| Parameter | All Patients (n=92) | Hypertensive (n=38) | Normotensive (n=54) | P Value |
|---|---|---|---|---|
| Age at CMR, years | 19.9 (4.9 to 57.8) | 21.4 (10.2 to 57.8) | 19.4 (4.9 to 49.5) | 0.13 |
| Males (%) | 56 (61) | 21 (58) | 35 (63) | 0.83 |
| Age at initial repair, years | 4.6 (0 to 57.1) | 4.3 (0 to 57.1) | 5.0 (0 to 38.9) | 0.95 |
| Time since repair, years | 13.5 (0 to 48.2) | 13.9 (0 to 48.2) | 12.5 (1.1 to 33.5) | 0.48 |
| Initial arm-leg SBP gradient, mm Hg | 39 (13 to 100) | 40 (20 to 100) | 35 (13 to 75) | 0.29 |
| Type of initial repair (%) | 0.47 | |||
| Surgery | 63 (68) | 29 (76) | 34 (63) | |
| Stent | 16 (17) | 5 (13) | 11 (20) | |
| Balloon dilation | 13 (15) | 4 (11) | 9 (17) | |
| Second arch procedure (%) | 31 (37) | 14 (37) | 17 (31) | 0.66 |
| Ventricular septal defect (%) | 15 (16) | 7 (18) | 8 (15) | 0.39 |
| Bicommissural aortic valve (%) | 49 (53) | 20 (53) | 29 (54) | 0.99 |
| TAA CSA/BSA, cm2/m2 | 1.06 (0.47 to 2.2) | 0.92 (0.50 to 2.20) | 1.18 (0.47 to 2.0) | 0.004 |
| TAA/DAO diameter ratio | 0.92 (0.43 to 1.27) | 0.87 (0.43 to 1.27) | 0.94 (0.67 to 1.27) | 0.02 |
| Arm-leg SBP gradient, mm Hg | −8 (−60 to 18) | −5 (−45 to 18) | −9 (−60 to 14) | 0.14 |
| Isthmus/DAO diameter ratio | 0.85 (0.43 to 1.75) | 0.77 (0.43 to 1.31) | 0.88 (0.56 to 1.75) | 0.02 |
| Right arm SBP, mm Hg | 123 (86 to 174) | 129 (101 to 174) | 120 (86 to 152) | 0.003 |
| Right arm DBP, mm Hg | 63 (35 to 87) | 63 (48 to 83) | 63 (35 to 87) | 0.84 |
| Antihypertensive medication (%) | 33 (36) | 33 (87) | 0 | <0.0001 |
| Arm-leg SBP gradient during exercise, mm Hg | 33 (−39 to 120) | 47 (−15 to 120) | 29 (−39 to 73) | 0.04 |
| Increase in right arm SBP during exercise, mm Hg | 51 (1 to 108) | 64 (1 to 103) | 42 (3 to 108) | 0.28 |
| Right arm hypertension during exercise, >220 mm Hg (%) | 10/57 (18) | 7/22 (32) | 3/35 (9) | 0.03 |
| LV mass/BSA, g/m2 | 54.7 (33.8 to 86.3) | 53.5 (38.3 to 83.0) | 56.1 (33.8 to 86.3) | 0.67 |
| LV mass/volume ratio | 0.62 (0.43 to 1.09) | 0.62 (0.49 to 1.09) | 0.62 (0.43 to 1.00) | 0.67 |
All data presented as median (range) or frequency (%). Patients were classified into “hypertensive” group if they satisfied standard pediatric or adult criteria for hypertension on at least 2 separate occasions. BSA indicates body-surface area; CMR, cardiac magnetic resonance study; CSA, cross-sectional area; DAO, descending aorta; DBP, diastolic blood pressure; LV, left ventricle; SBP, systolic blood pressure; TAA, transverse aortic arch.
TAA Size and HTN
Results of uni- and multivariable analysis are shown in Table2. Several measures of TAA and isthmus size were associated with systemic HTN. Among these, the TAA CSA/BSA had the lowest P value and was carried forward into the multivariable model. On multivariable analysis, patients with lower TAA CSA/BSA, older age at evaluation, and higher arm-leg SBP gradient at peak exercise were more likely to have systemic HTN. The model fit was good (C-statistic=0.802) and a TAA CSA/BSA <100 cm2/m2 predicted systemic HTN with 60% sensitivity and 68% specificity. The following factors were not associated with systemic HTN in the final model: age or type of repair; gender; second coarctation procedure; and arm-leg SBP gradient at rest.
Table 2.
Factors Associated With Systemic Hypertension
| Parameter | OR | 95% CI | P Value |
|---|---|---|---|
| Univariate analysis | |||
| Age at CMR (per 1 year ↑) | 1.03 | 0.99 to 1.07 | 0.11 |
| Male gender | 1.02 | 0.44 to 2.39 | 0.95 |
| Age at repair (per 1 year ↑) | 1.01 | 0.97 to 1.05 | 0.68 |
| Second arch procedure | 1.27 | 0.59 to 3.04 | 0.59 |
| Ventricular septal defect | 1.79 | 0.58 to 5.45 | 0.31 |
| Bicommissural aortic valve | 0.96 | 0.42 to 2.20 | 0.91 |
| Arm-leg SBP gradient (per 1 mm Hg ↑) | 1.02 | 0.99 to 1.05 | 0.19 |
| Arm-leg SBP gradient at peak exercise (per 1 mm Hg ↑) | 1.02 | 1.00 to 1.05 | 0.02 |
| TAA/DAO diameter ratio (per 0.1 point ↓) | 1.36 | 1.03 to 1.81 | 0.02 |
| TAA CSA/BSA (per 0.5 cm2/m2 ↓) | 2.30 | 1.19 to 4.44 | 0.009 |
| Ratio of isthmus/DAO diameter ratio (per 0.1 point ↓) | 1.26 | 1.04 to 1.54 | 0.01 |
| Multivariable analysis (C-statistic=0.802) | |||
| TAA CSA/BSA (per 0.5 cm2/m2 ↓) | 6.41 | 1.72 to 23.8 | 0.006 |
| Age at CMR (per 5-year ↑) | 1.57 | 1.04 to 2.36 | 0.03 |
| Arm-leg SBP gradient at peak exercise (per 1 mm Hg ↑) | 1.03 | 1.01 to 1.05 | 0.05 |
BSA indicates body-surface area; CI, confidence interval; CMR, cardiac magnetic resonance study; CSA, cross-sectional area; DAO, descending aorta; OR, odds ratio; SBP, systolic blood pressure; TAA, transverse aortic arch.
In a separate analysis, we divided all patients into 3 equal-sized groups (tertiles) based on their TAA CSA/BSA and found that prevalence of systemic HTN was inversely related to TAA CSA/BSA (P=0.01; Figure2).
Figure 2.
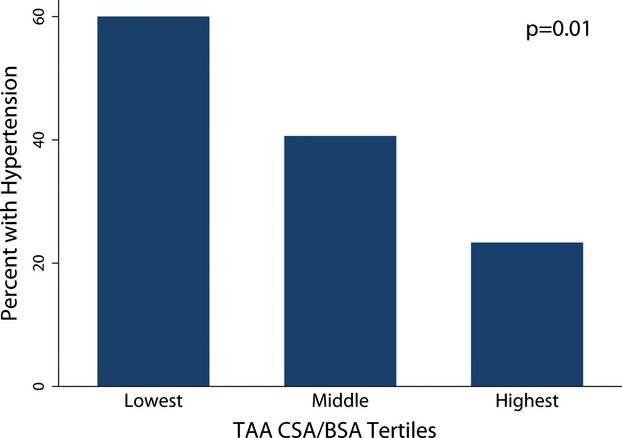
Prevalence of systemic hypertension in tertiles of TAA CSA indexed to BSA. Systemic hypertension was more prevalent in patients who were in the lower tertiles of TAA CSA/BSA. BSA indicates body-surface area; CSA, cross-sectional area; TAA, transverse aortic arch.
TAA Hypoplasia and SBP Response to Exercise
Exercise testing results were available and satisfactory for analysis in 61 of the 92 patients. At peak exercise, the mean increase in right arm SBP was 51±27 mm Hg. Uni- and multivariable predictors of SBP response to exercise are shown in Table3. In a multivariable linear model (r2=0.36), larger increase in right arm SBP was associated with lower TAA/DAO diameter ratio (Figure3) and with higher arm-leg SBP difference at peak exercise. SBP increase with exercise was not associated with current age, gender, age or type of repair, second arch procedure, TAA CSA/BSA, isthmus/DAO diameter ratio, and resting arm-leg SBP gradient. Right arm HTN during exercise (>220 mm Hg) was noted in 10 of 57 patients (18%; Table1) and was more common in the hypertensive group (32% vs. 9%; P=0.02). In multivariable analysis, factors independently associated with right arm HTN during exercise included lower TAA/DAO diameter ratio (P=0.02) and higher LV mass/BSA (P=0.02). The arm-leg SBP difference during peak exercise was larger in the hypertensive group (median, 47 mm Hg), compared to the normotensive group (median, 29 mm Hg; P=0.04).
Table 3.
Predictors of SBP Response to Exercise
| Parameter | Coefficient | r 2 | P Value |
|---|---|---|---|
| Univariate analysis | |||
| Age at CMR, y | −0.22 | 0.01 | 0.54 |
| Male gender | 11.36 | 0.05 | 0.12 |
| Age at repair, y | −0.22 | 0.01 | 0.55 |
| Second arch procedure | −11.00 | 0.04 | 0.14 |
| Ventricular septal defect | −2.46 | 0.01 | 0.80 |
| Bicommissural aortic valve | −0.53 | 0.01 | 0.94 |
| Arm-leg SBP difference, mm Hg | 0.04 | 0.09 | 0.02 |
| Exercise Arm-leg SBP difference, mm Hg | 0.51 | 0.29 | <0.0001 |
| TAA/DAO diameter ratio | −56.04 | 0.11 | 0.006 |
| TAA CSA/BSA, cm2/m2 | −0.04 | 0.03 | 0.66 |
| Isthmus/DAO diameter ratio | 0.16 | 0.01 | 0.6 |
| Multivariable analysis (model r2=0.36) | |||
| Arm-leg SBP difference at peak exercise, mm Hg | 0.48 | N/A | <0.0001 |
| TAA/DAO diameter ratio | −43.61 | N/A | 0.03 |
BSA indicates body-surface area; CMR, cardiac magnetic resonance study; CSA, cross-sectional area; DAO, descending aorta; SBP, systolic blood pressure; TAA, transverse aortic arch.
Figure 3.
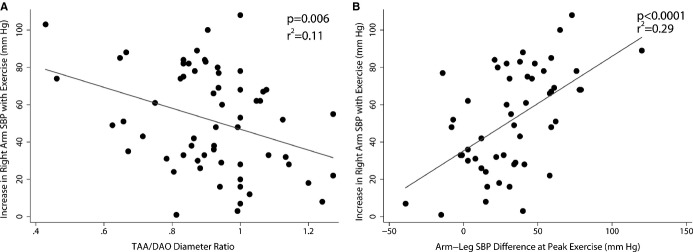
Factors associated with blood pressure response to exercise. Greater increase in right arm SBP was associated with lower TAA/DAO diameter ratio (A) and greater arm-leg SBP difference at peak exercise (B). DAO indicates descending aorta; SBP, systolic blood pressure; TAA, transverse aortic arch.
TAA Hypoplasia and Arm-Leg SBP Difference
No patient had an arm-leg SBP gradient >20 mm Hg at rest owing to our inclusion criteria. TAA/DAO and isthmus/DAO diameter ratios as well as TAA CSA/BSA were not significantly associated with arm-leg SBP difference at rest (P>0.1 for all comparisons). During exercise, the arm-leg SBP difference was higher, compared to the measurement at rest, in both hypertensive and normotensive groups (P<0.001 in both groups). There was a weak trend toward a higher arm-leg SBP difference during exercise with lower TAA CSA/BSA (r=−0.21; P=0.14).
LV Mass
Uni- and multivariable predictors of BSA-adjusted LV mass are shown in Table4. In a multivariable linear model (r2=0.37), higher BSA-adjusted LV mass was associated with male gender (P<0.0001) and greater increase in right arm SBP at peak exercise (P=0.02; Figure4). LV mass was not associated with baseline SBP z-score, classification into the “hypertensive group,” TAA CSA/BSA, TAA/DAO and isthmus/DAO diameter ratios, and resting arm-leg SBP gradient.
Table 4.
Predictors of BSA-Adjusted LV Mass
| Parameter | Coefficient | r 2 | P Value |
|---|---|---|---|
| Univariate analysis | |||
| Age at CMR, years | 0.09 | 0.005 | 0.49 |
| Male gender | 15.41 | 0.34 | <0.0001 |
| Age at repair, years | 0.14 | 0.01 | 0.28 |
| Second arch procedure | −6.57 | 0.06 | 0.02 |
| Ventricular septal defect | −1.75 | 0.003 | 0.64 |
| Bicommissural aortic valve | −0.39 | 0.002 | 0.89 |
| Arm-leg SBP gradient, mm Hg | 0.01 | 0.002 | 0.89 |
| Arm-leg SBP gradient at peak exercise, mm Hg | 0.05 | 0.01 | 0.48 |
| Increase in right arm SBP with exercise, mm Hg | 0.13 | 0.3 | 0.004 |
| Right arm hypertension (>220 mm Hg) during exercise | 10.62 | 0.08 | 0.02 |
| TAA/DAO diameter ratio | −9.52 | 0.01 | 0.26 |
| TAA CSA/BSA, cm2/m2 | 0.07 | 0.04 | 0.06 |
| Isthmus/DAO diameter ratio | 4.85 | 0.009 | 0.37 |
| Multivariable analysis (model r2=0.37) | |||
| Male gender | 13.5 | N/A | <0.0001 |
| Increase in right arm SBP during exercise, mm Hg | 0.14 | N/A | 0.02 |
BSA indicates body-surface area; CMR, cardiac magnetic resonance study; CSA, cross-sectional area; DAO, descending aorta; LV, left ventricle; N/A, not applicable; SBP, systolic blood pressure; TAA, transverse aortic arch.
Figure 4.
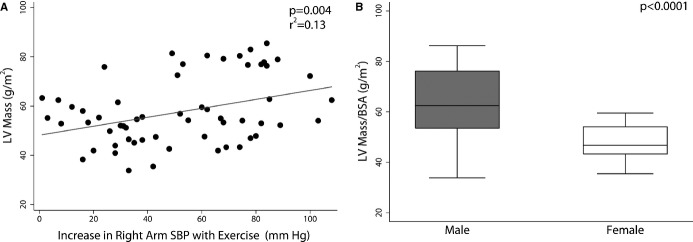
Factors associated with LV mass. Higher BSA-adjusted LV mass was associated with larger increase in right arm SBP with stress (A) and with male gender (B). BSA indicates body-surface area; LV, left ventricular; SBP, systolic blood pressure.
We also evaluated for predictors of LV mass-to-volume ratio and found that it was independently associated with older age at initial repair (coefficient, 0.004; P=0.001). LV mass-to-volume ratio was not associated with classification into the hypertensive group, TAA CSA/BSA, TAA/DAO and isthmus/DAO diameter ratios, or with resting arm-leg SBP gradient.
Discussion
In this study of children and young adults late after coarctation repair without a significant resting arm-leg SBP difference, systemic HTN was common. Patients with smaller TAA were more likely to have systemic HTN, despite the lack of a significant resting arm-leg SBP difference. Patients with a smaller TAA also demonstrated a greater increase in right arm SBP during exercise. Mild hypoplasia of the TAA late after coarctation repair in the absence of a significant arm-leg SBP gradient is generally considered a benign finding. Our results suggest that persistent TAA hypoplasia, even in the absence of a significant arm-leg SBP difference, may not be benign and may be a factor contributing to the high prevalence of late systemic HTN.
TAA Hypoplasia and HTN
The high prevalence of systemic HTN late after successful coarctation repair is well documented.1–3 This has been attributed to multiple factors, including residual arch obstruction, abnormal aortic arch shape, and abnormal vascular properties.4–6 However, the role of residual abnormalities of the TAA remains controversial. Ou et al. suggested that abnormal arch shape and increased aortic stiffness, but not arch size, were associated with late HTN.4 In contrast, in a more recent study, Ntsinjana et al. found that BP response to exercise had no relationship to arch shape, but was instead related to TAA hypoplasia.13 However, another recent study by Pedersen et al. found only a weak relationship between HTN and TAA size measured by CMR.14 Understanding the relationship between mild TAA hypoplasia and late systemic HTN is important because it may inform the initial surgical approach. There is some evidence that TAA augmentation at initial operation is associated with a lower risk of late HTN,15 but the relation between TAA size and HTN at long-term followup has not been directly studied previously.
Our results provide evidence that mild persistent hypoplasia of the TAA is associated with higher prevalence of HTN, despite the absence of a significant arm-leg SBP difference at rest. A persistently small TAA in our study cohort was associated with systemic HTN and with a more-exaggerated SBP response to exercise. A more-exaggerated SBP response to exercise has been associated with an increased risk of developing clinical HTN and LV hypertrophy (LVH).12,16 TAA hypoplasia can be addressed at the initial repair by performing a more-extensive surgical reconstruction. However, the risks and benefits of this more-extensive repair and its impact on the growth rate of the TAA in the long-term remain unknown and warrant further investigation.
Arm-Leg SBP Difference
Arm-leg SBP difference is commonly used to assess the clinical severity of residual arch obstruction after coarctation repair. In our study, arm-leg SBP different at rest was not associated with the degree of TAA or isthmus hypoplasia, systemic HTN, SBP response to exercise, or BSA-adjusted LV mass. This suggests that contrary to conventional clinical practice, in patients with mild residual arch hypoplasia, arm-leg SBP difference at rest is not a good indicator of the risk for developing HTN or LVH. It is possible that the additive variance of multiple BP measurements makes this an insensitive test for evaluating mild degrees of arch obstruction. On the other hand, higher arm-leg SBP difference at exercise was associated with systemic HTN and with more-exaggerated SBP response to exercise and this measurement may provide additional prognostic information.
LV Hypertrophy
LVH is a known late complication of systemic HTN and is associated with increased CV morbidity and mortality.17,18 Coarctation patients are at risk for increased LV mass and mass-to-volume ratio, which have been correlated with sustained HTN, exaggerated BP response to exercise and increased central aortic stiffness.12,19,20 In our cohort, the only factors independently associated with higher LV mass and/or mass-to-volume ratio were older age at repair, male gender, and an exaggerated SBP response to exercise. Although TAA hypoplasia was associated with higher SBP response to exercise, it was not independently associated with either parameter of LVH. This is expected because a majority of patients in the “hypertensive group” had good BP control with medication and this mitigated the development of LVH. Furthermore, as previously shown in patients with idiopathic HTN, LVH occurs only in a subset of patients with HTN.21–23
Several limitations of this single-center retrospective analysis are worth considering. Because of the retrospective nature of this study, ambulatory BP monitoring (ABPM) was not performed in a majority of patients and it is likely that some reclassification in the HTN/no HTN groups would occur if ABPM, which is considered more reliable in diagnosing HTN, was performed. Nevertheless, the prevalence of systemic HTN in our cohort was similar to that reported in studies that utilized ABPM monitoring.5 Referral bias is another known limitation of retrospective study design. Finally, although we show an association between TAA hypoplasia and late HTN, our retrospective study design does not allow assessment of causality.
In conclusion, persistent mild TAA hypoplasia after coarctation treatment, even without a resting arm-leg BP difference, is associated with systemic HTN. Further studies are warranted to determine whether TAA hypoplasia has a causative role in the development of HTN and whether more-extensive reconstruction at initial repair will decrease the incidence of late HTN.
Disclosures
None.
References
- O’Sullivan J. Late hypertension in patients with repaired aortic coarctation. Curr Hypertens Rep. 2014;16:421. doi: 10.1007/s11906-014-0421-4. [DOI] [PubMed] [Google Scholar]
- Cohen M, Fuster V, Steele PM, Driscoll D, McGoon DC. Coarctation of the aorta. Long-term follow-up and prediction of outcome after surgical correction. Circulation. 1989;80:840–845. doi: 10.1161/01.cir.80.4.840. [DOI] [PubMed] [Google Scholar]
- Toro-Salazar OH, Steinberger J, Thomas W, Rocchini AP, Carpenter B, Moller JH. Long-term follow-up of patients after coarctation of the aorta repair. Am J Cardiol. 2002;89:541–547. doi: 10.1016/s0002-9149(01)02293-7. [DOI] [PubMed] [Google Scholar]
- Ou P, Bonnet D, Auriacombe L, Pedroni E, Balleux F, Sidi D, Mousseaux E. Late systemic hypertension and aortic arch geometry after successful repair of coarctation of the aorta. Eur Heart J. 2004;25:1853–1859. doi: 10.1016/j.ehj.2004.07.021. [DOI] [PubMed] [Google Scholar]
- O’Sullivan JJ, Derrick G, Darnell R. Prevalence of hypertension in children after early repair of coarctation of the aorta: a cohort study using casual and 24 hour blood pressure measurement. Heart. 2002;88:163–166. doi: 10.1136/heart.88.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehested J, Baandrup U, Mikkelsen E. Different reactivity and structure of the prestenotic and poststenotic aorta in human coarctation. Implications for baroreceptor function. Circulation. 1982;65:1060–1065. doi: 10.1161/01.cir.65.6.1060. [DOI] [PubMed] [Google Scholar]
- The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National Heart L, Blood Institute Joint National Committee on Prevention DE, Treatment of High Blood P, National High Blood Pressure Education Program Coordinating C. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–66. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- Prakash A, Torres AJ, Printz BF, Prince MR, Nielsen JC. Usefulness of magnetic resonance angiography in the evaluation of complex congenital heart disease in newborns and infants. Am J Cardiol. 2007;100:715–721. doi: 10.1016/j.amjcard.2007.03.090. [DOI] [PubMed] [Google Scholar]
- Nielsen JC, Powell AJ, Gauvreau K, Marcus EN, Prakash A, Geva T. Magnetic resonance imaging predictors of coarctation severity. Circulation. 2005;111:622–628. doi: 10.1161/01.CIR.0000154549.53684.64. [DOI] [PubMed] [Google Scholar]
- Krieger EV, Clair M, Opotowsky AR, Landzberg MJ, Rhodes J, Powell AJ, Colan SD, Valente AM. Correlation of exercise response in repaired coarctation of the aorta to left ventricular mass and geometry. Am J Cardiol. 2013;111:406–411. doi: 10.1016/j.amjcard.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Ntsinjana HN, Biglino G, Capelli C, Tann O, Giardini A, Derrick G, Schievano S, Taylor AM. Aortic arch shape is not associated with hypertensive response to exercise in patients with repaired congenital heart diseases. J Cardiovasc Magn Reson. 2013;15:101. doi: 10.1186/1532-429X-15-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TAL, Munk K, Andersen NH, Lundorf E, Pedersen EB, Hjortdal VE, Emmertsen K. High long-term morbidity in repaired aortic coarctation. Congenit Heart Dis. 2011;6:573–582. doi: 10.1111/j.1747-0803.2011.00575.x. [DOI] [PubMed] [Google Scholar]
- Lee MGY, Kowalski R, Galati JC, Cheung MMH, Jones B, Koleff J, d’Udekem Y. Twenty-four-hour ambulatory blood pressure monitoring detects a high prevalence of hypertension late after coarctation repair in patients with hypoplastic arches. J Thorac Cardiovasc Surg. 2012;144:1110–1118. doi: 10.1016/j.jtcvs.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Buys R, Van De Bruaene A, Muller J, Hager A, Khambadkone S, Giardini A, Cornelissen V, Budts W, Vanhees L. Usefulness of cardiopulmonary exercise testing to predict the development of arterial hypertension in adult patients with repaired isolated coarctation of the aorta. Int J Cardiol. 2013;168:2037–2041. doi: 10.1016/j.ijcard.2013.01.171. [DOI] [PubMed] [Google Scholar]
- Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson CJ, Thomson AD. High blood-pressure and stroke. Necropsy study of heart-weight and left ventricular hypertrophy. Lancet. 1960;2:342–345. doi: 10.1016/s0140-6736(60)91483-5. [DOI] [PubMed] [Google Scholar]
- de Divitiis M, Pilla C, Kattenhorn M, Donald A, Zadinello M, Wallace S, Redington A, Deanfield J. Ambulatory blood pressure, left ventricular mass, and conduit artery function late after successful repair of coarctation of the aorta. J Am Coll Cardiol. 2003;41:2259–2265. doi: 10.1016/s0735-1097(03)00480-7. [DOI] [PubMed] [Google Scholar]
- Ou P, Celermajer DS, Jolivet O, Buyens F, Herment A, Sidi D, Bonnet D, Mousseaux E. Increased central aortic stiffness and left ventricular mass in normotensive young subjects after successful coarctation repair. Am Heart J. 2008;155:187–193. doi: 10.1016/j.ahj.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Devereux RB, Roman MJ. Left ventricular hypertrophy in hypertension: stimuli, patterns, and consequences. Hypertens Res. 1999;22:1–9. doi: 10.1291/hypres.22.1. [DOI] [PubMed] [Google Scholar]
- Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, Vargiu P, Simongini I, Laragh JH. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–1558. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- Bella JN, Wachtell K, Palmieri V, Liebson PR, Gerdts E, Ylitalo A, Koren MJ, Pedersen OL, Rokkedal J, Dahlof B, Roman MJ, Devereux RB. Relation of left ventricular geometry and function to systemic hemodynamics in hypertension: the LIFE Study. Losartan Intervention for Endpoint Reduction in Hypertension Study. J Hypertens. 2001;19:127–134. doi: 10.1097/00004872-200101000-00017. [DOI] [PubMed] [Google Scholar]


