Abstract
Background
It is well recognized that right ventricular apical pacing can have deleterious effects on ventricular function. We performed a head-to-head comparison of the SafeR pacing algorithm versus DDD pacing with a long atrioventricular delay in a heterogeneous population of patients with dual-chamber pacemakers.
Methods and Results
In a multicenter prospective double-blinded randomized trial conducted at 10 centers in Canada, 373 patients, age 71±11 years, with indications for dual chamber DC pacemakers were randomized 1:1 to SafeR or DDD pacing with a long atrioventricular delay (250 ms). The primary objective was twofold: (1) reduction in the proportion of ventricular paced beats at 1 year; and (2) impact on atrial fibrillation burden at 3 years, defined as the ratio between cumulative duration of mode-switches divided by follow-up time. Statistical significance of both co-primary end points was required for the trial to be considered positive. At 1 year of follow-up, the median proportion of ventricular-paced beats was 4.0% with DDD versus 0% with SafeR (P<0.001). At 3 years of follow-up, the atrial fibrillation burden was not significantly reduced with SafeR versus DDD (median 0.00%, interquartile range [0.00% to 0.23%] versus median 0.01%, interquartile range [0.00% to 0.44%], respectively, P=0.178]), despite a persistent reduction in the median proportion of ventricular-paced beats (10% with DDD compared to 0% with SafeR).
Conclusions
A ventricular-paced rate <1% was safely achieved with SafeR in a population with a wide spectrum of indications for dual-chamber pacing. However, the lower percentage of ventricular pacing did not translate into a significant reduction in atrial fibrillation burden.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/ Unique identifier: NCT01219621.
Keywords: adverse arrhythmic events, atrial fibrillation, dual-chamber pacemaker, long atrioventricular delay, right ventricular pacing
The association between extent of right ventricular (RV) pacing and adverse cardiac outcomes is well established: RV pacing modifies left ventricular (LV) contraction by inducing a left bundle branch block–like activation sequence. The resulting dyssynchrony is associated with adverse LV remodeling,1 reduced LV ejection fraction,2,3 and increased risk for heart failure and death.4 In patients with intrinsic atrioventricular (AV) conduction and dual-chamber (DDD) pacemakers, RV pacing can potentially be avoided by programming long AV delays. However, this approach is not always effective5,6 and may be associated with tachycardias and cycle timing conflicts.7–10 To address these concerns, several pacemaker algorithms were developed to minimize potentially avoidable RV pacing. Clinical benefits from such algorithms have been confirmed, predominantly in patients with sinus node dysfunction.11,12 Specifically, a reduction in the percentage of RV pacing from 99% to 9.1% was associated with a significantly lower risk of developing persistent atrial fibrillation (AF).11
The SafeR algorithm (Sorin CRM, Clamart, France) was developed to provide a pacing mode that combines the benefits of AAI with the safety of DDD pacing.13–16 The recent randomized SaveR study confirmed the superiority of SafeR in reducing RV pacing when compared to standard DDD pacing in selected patients without high-degree AV block and in a more general pacemaker population. The prospective “CANadian multi-centre randomised study–Spontaneous AtrioVEntricular conduction pReservation” (CAN-SAVE R; https://www.ClinicalTrials.gov identifier NCT01219621) was designed to assess the impact of the SafeR mode compared to DDD programming with long AV delays in a broad population of patients with dual-chamber pacemakers. Programming long AV delays in patients randomized to DDD pacing permitted the comparison of 2 different methods of moderating RV pacing. The primary objectives were twofold: (1) to evaluate the effectiveness of SafeR in patients with spontaneous AV conduction; and (2) to determine whether a reduction in RV pacing is associated with a lower rate of atrial arrhythmias. The study design and preliminary results on 208 patients regarding short-term effectiveness and safety were previously published.17 Herein, we present the final results of the CAN SAVE R trial, including long-term clinical effects of SafeR.
Methods
Study Design and Eligibility Criteria
The CAN-SAVE R trial was a multicenter prospective double-blinded randomized trial conducted at 10 centers in Canada between April 2006 (first patient enrolled) and May 2012 (last 3-year follow-up visit). The study was conducted in accordance with the ethical principles expressed in the declaration of Helsinki18 and was approved by the institutional review boards at all participating centers. All patients provided written informed consent to participate in the study.
Patients were eligible for inclusion if they were ≥18 years of age and fulfilled the criteria for implantation of a dual-chamber pacemaker as defined by standard practice guidelines.19,20 Patients were excluded if they had permanent complete AV block, permanent atrial tachyarrhythmias, ventricular arrhythmias, implantable cardioverter-defibrillators, anticipated cardiac surgery within 6 months, or a life expectancy <1 year.
Intervention
All patients enrolled in the trial received a Symphony DR 2550 cardiac pacemaker (SORIN CRM SAS, Clamart, France) equipped with the SafeR algorithm. Details of this algorithm have been previously described.14 In summary, SafeR provides AAI(R) pacing while continuously monitoring AV conduction and reverts to DDD(R) pacing when required for AV block of any degree. The mode-switching occurs after detection of one of the following: 2 consecutive nonconducted atrial events (third-degree atrioventricular block criteria); missing AV conduction following 3 of 12 atrial events (second-degree atrioventricular block criteria); 7 consecutive AV intervals ≥350 (with atrial sensing) or 450 ms (with atrial pacing) (first-degree atrioventricular block criteria); or a ventricular pause ≥2 to 4 s (programmable value). Reassessment of the AAI mode occurs after 100 ventricular paced beats, 12 consecutive cycles with spontaneous R waves, or at 8 am each morning.
Run-in Phase, Randomization, and Follow-up
Following pacemaker implantation, patients entered a 2-month run-in phase with the SafeR algorithm enabled in order to ensure proper device functioning, including atrial sensing and appropriateness of mode switches. At the end of this phase, patients were considered eligible for randomization if they remained without any exclusion criteria, had <40% ventricular pacing during this period (low likelihood of becoming pacemaker dependent over 3 years), and spent <30% of their time in AF (low likelihood of imminently developing permanent AF). Eligible patients were then randomized 1:1 to SafeR or DDD pacing. In the SafeR group, the algorithm was enabled. The ventricular pause was set at 3 s and the first-degree AV block criterion was enabled during exercise only. The SafeR mode was deactivated in the DDD group. Resting and exercise AV delays were set at 250 ms, with a 65-ms extension after an atrial pace. All other parameters were identically programmed in both groups (Table1).
Table 1.
Programming Instructions for the SafeR and DDD Groups, Respectively
| Parameter | SafeR Group | DDD Group |
|---|---|---|
| Mode | SafeR | DDD |
| Sensed AV delay at rest | N/A | 250 ms |
| Sensed AV delay at exercise | N/A | 250 ms |
| Sensed AV delay extension | N/A | 65 ms |
| Ventricular pause | 3 s | N/A |
| AVB 1 commutation | Exercise only | N/A |
| Basic rate | 30 to 70 bpm | |
| Max rate | Nominal settings | |
| Hysteresis | 0% | |
| Mode switch | On | |
| Postventricular atrial blanking period | 155 ms | |
| Smoothing | Off | |
| Atrial sensing threshold | 0.6 mV if possible | |
| Atrial sensing polarity | Bipolar | |
| Ventricular sensing polarity | Bipolar if possible | |
| AIDA* follow-up duration | Automatic 6-month (day/day) | |
AV indicates atrioventricular; AVB, atrioventricular block; DDD, intrinsic atrioventricular conduction and dual-chamber pacemaker; N/A, not applicable.
AIDA (Automatic Interpretation for Data Analysis) provides device-based diagnosis of recorded statistics, including heart rate, paced/sensed events, automatic mode switches on AF episodes and SafeR statistics such as atrioventricular block episodes of first, second, and third degree.
Patients were followed for 3 years with visits scheduled at 1, 2, and 3 years (±3 months) after the initial enrollment visit.
Study Objectives and End Points
The main prespecified primary objective was twofold: (1) to assess the effectiveness of the SafeR mode at preserving intrinsic AV conduction compared to DDD pacing with a long AV delay (250 ms) at 1 year of follow-up; and (2) to determine the impact of the SafeR algorithm on incident AF over the 3 years of follow-up.
Preserved AV conduction was assessed by the percentage of ventricular-paced beats derived from pacemaker interrogation. AF burden was defined as the ratio between the cumulative duration of documented mode-switches divided by the total follow-up time.
Secondary end points consisted of the percentage of ventricular-paced beats at 3 years, AF-related clinical events (ie, cardioversion or AV node ablation) at 3 years, and adverse events. Ancillary analyses included the identification of factors independently associated with AF burden and a prespecified subgroup analysis of AF burden according to whether or not subjects had a prior history of AF.
Data Collection and Measurements
Ventricular pacing and AF burden data were ascertained from device recordings by investigators blinded to treatment allocation. Predefined adverse events were documented throughout the study and were adjudicated by an independent committee (Appendix S1). Adverse events included device reprogramming, device-related complications, changes in pharmacological therapy, chemical or electrical cardioversion, catheter ablation of the AV node, and death.
Statistical Analysis
The trial was powered to assess both components of the primary end point. The sample size calculation was based on assumed rates of ventricular pacing at 1 year (ie, 17% control group; 10% SafeR group)2,16 and AF burden at 3 years (ie, 17.5% control group; 7.4% SafeR group).2 In order to achieve a statistical power of 80% with a 2-sided alpha of 0.05, a total of 160 and 270 patients were required to assess the first and second components of the primary outcome, respectively. Factoring in a loss to follow-up rate of 10% per year, we sought to randomize a total of 370 patients.
For normally distributed data, mean values and standard deviations are shown for continuous data. For non-normally distributed data, median values and interquartile ranges are shown. For categorical data, frequencies and percentages are presented.
The co-primary end points were assessed on the basis of intention-to-treat (ITT) principle. Missing outcome data were considered in the analysis applying the Last Observation Carried Forward method for continuous variables. Between-group comparisons were performed by Wilcoxon test. Both co-primary end points were required to be significant at the 0.05 level in order to declare the trial “positive.” Secondary end points were carried out on the intention-to-treat population without adjusting alpha (ie, considered statistically significant at a P-value of 0.05). Safety categorical data were compared with χ2 test or Fisher’s exact test when appropriate. As per protocol, patients who were enrolled in the trial but did not undergo randomization were excluded from safety analyses.
Univariate and multivariate linear regression analyses were performed to explore potential predictors of AF burden (in patients with positive values) at 3 years follow-up. Variables with <20% missing data associated with P<0.100 in univariate analyses were considered in a multivariate automated stepwise model with forced inclusion of randomized pacemaker mode. Candidate variables were as follows: sex, age, implant indication, AF history, known cardiomyopathy, previous cardiac surgery, mitral valve regurgitation, class II anti-arrhythmic drug use, baseline LV end-systolic and end-diastolic volume, left atrial volume, AF at randomization, and percent ventricular pacing and percent atrial pacing at end of follow-up. To satisfy the normality assumption of the linear model, AF burden at 3 years follow-up was log-transformed. Candidate variables with P<0.05 in the multivariate analysis were retained in the final model.
All analyses were performed using Medcalc™ and SAS™ software at the Clinical Affairs Department, Sorin CRM SAS, or at the Montreal Health Innovations Coordinating Centre. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Study Population
A total of 450 patients were enrolled, of whom 373 were randomized: 191 to the SafeR algorithm and 182 to DDD pacing. Reasons for nonrandomizing enrolled patients and subsequent withdrawals are listed in Figure1. Baseline characteristics are presented in Table2. The groups were evenly balanced at baseline. Average age of the randomized population was 71±11 years (median 73.5 years). Sinus node dysfunction was present in 73% (81% of whom had no concomitant AV block) and AV block in 41% (66% of whom had no concomitant sinus node dysfunction). Nonpermanent AF was detected prior to randomization (ie, at screening or in the run-in phase) in one third of randomized patients. In the overall randomized population, 15% received drug therapy for AF.
Figure 1.
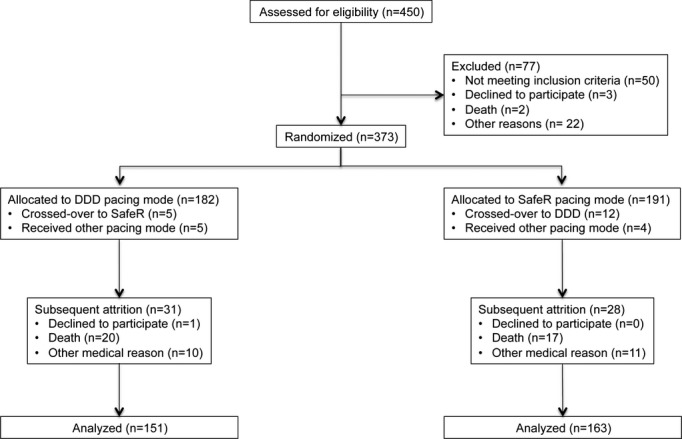
Flow chart of patients’ disposition. Inclusion, randomization, and follow-up of CAN-SAVE R patients. CAN-SAVE R indicates CANadian multi-centre randomised study–Spontaneous AtrioVEntricular conduction pReservation; DDD, intrinsic atrioventricular conduction and dual-chamber pacemaker.
Table 2.
Demographic Characteristics
| Enrolled Patients* | Randomized in DDD | Randomized in SafeR | P Value | |
|---|---|---|---|---|
| Number of patients | 450 | 182 | 191 | — |
| Age at baseline, y | 71±11 | 72±11 | 70±10 | 0.137 |
| Males, % | 65 | 60 | 65 | 0.373 |
| Documented SND at baseline, % | 56 | 75 | 71 | 0.452 |
| Documented AVB at baseline, % | 44 | 41 | 41 | 0.916 |
| Other pacing indications, % | 14 | 14 | 14 | 0.881 |
| Documented AF at baseline, % | 34.0 | 35.2 | 33.0 | 0.735 |
| AF at randomization (%) as documented by mode-switch statistics | 34.4 | 34.1 | 36.6 | 0.692 |
| Amiodarone at baseline, % | 6.0 | 9.3 | 4.2 | 0.078 |
| LVEF at randomization, % | — | 60±10 | 61±9 | 0.103 |
| LVESV at randomization, mL | — | 37±18 | 36±18 | 0.666 |
| LA Vol index at randomization, mL/m2 | — | 24±10 | 23±9 | 0.400 |
AF indicates atrial fibrillation; AVB, atrioventricular block; DDD, intrinsic atrioventricular conduction and dual-chamber pacemaker; LA Vol, left atrium volume index; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; SND, sinus node dysfunction.
Patients enrolled but not randomized did not have any echocardiographic exam reviewed by the core-lab.
One and 3-year follow-up visits were at 324±53 days (median=322 days) and 1059±66 days (median=1050 days), respectively. Complete data were available at 1 and 3 years of follow-up in 348 (93.3%) and 315 (84.5%) patients, respectively. At the end of follow-up, 86.9% and 88.5% of patients randomized to SafeR and DDD modes, respectively, remained in the same pacing mode (P=0.754).
Primary Outcome
At 1 year of follow-up, the median proportion of ventricular-paced beats was significantly reduced by the SafeR algorithm compared to DDD pacing (0% versus 4.0%, P<0.001; Table3). However, the overall AF burden at 3 years of follow-up was not significantly reduced by the SafeR algorithm compared to DDD pacing (median 0.00%, interquartile range [0.00% to 0.23%] versus median 0.01%, interquartile range [0.00% to 0.44%], respectively, P=0.178; Table4).
Table 3.
Percentage of Ventricular Pacing Per Subgroups of Patients
| Randomized in DDD | Randomized in SafeR | P Value | |
|---|---|---|---|
| All pts, Year 1, median % (Q1 to Q3) 95% CI | 4.0 (0 to 55) 1 to 12 | 0.0 (0 to 1) 0 to 0 | <0.001 |
| All pts, Year 3, median % (Q1 to Q3) 95% CI | 10.0 (0 to 59) 4 to 19 | 0.0 (0 to 4) 0 to 0 | <0.001 |
| No-AVB pts, Year 1, median % (Q1 to Q3) 95% CI | 1.0 (0 to 20) 0 to 2 | 0.0 (0 to 0) 0 to 0 | <0.001 |
| No-AVB pts, Year 3, median % (Q1 to Q3) 95% CI | 2.3 (0 to 24) 0 to 7 | 0.0 (0 to 1) 0 to 0 | <0.001 |
| AVB pts, Year 1, median % (Q1 to Q3) 95% CI | 37.0 (3 to 78) 17 to 60 | 0.0 (0 to 5) 0 to 1 | <0.001 |
| AVB pts, Year 3, median % (Q1 to Q3) 95% CI | 43.7 (6 to 80) 18 to 60 | 0.7 (0 to 21) 0 to 8 | <0.001 |
AVB indicates atrioventricular block; DDD, intrinsic atrioventricular conduction and dual-chamber pacemaker.
Table 4.
AF Burden in the Randomized Population and in the Subpopulation of Patients Free From AF, as Documented by Mode-Switch Statistics at Randomization
| AF Burden, % | |||
|---|---|---|---|
| SafeR | DDD | P Value* | |
| All randomized patients (N=353) | |||
| Q1 | 0.00 | 0.00 | 0.178 |
| Median | 0.00 | 0.01 | |
| Q3 | 0.23 | 0.44 | |
| Patients free from AF at randomization (N=239) | |||
| Q1 | 0.00 | 0.00 | 0.015 |
| Median | 0.00 | 0.00 | |
| Q3 | 0.04 | 0.32 | |
AF indicates atrial fibrillation; DDD, intrinsic atrioventricular conduction and dual- chamber pacemaker.
Wilcoxon test.
Effectiveness of the SafeR Mode on Preserving Intrinsic AV Conduction
Ventricular pacing rates remained significantly reduced by the SafeR algorithm at 3 years of follow-up, from a median of 10.0% with DDD pacing to 0.0% with SafeR, P<0.001. In the 41% of patients with some degree of AV block at baseline, median RV pacing rates at 1 and 3 years of follow-up remained ≤1% with SafeR compared to 37.3% and 41.8% in the DDD group (both P<0.001). At 3 years follow-up, 64% of patients in the SafeR group had <1% RV pacing and 91% had <40% RV pacing. In comparison, corresponding RV pacing rates in the DDD group were 34% (P<0.001) and 67% (P<0.001).
Impact of SafeR on AF-Related Adverse Events
AF-related clinical events were significantly less frequent in patients randomized to SafeR versus DDD pacing (7 [3.7%] versus 16 [8.8%], P=0.040): 3 patients (1.6%) with SafeR and 10 (5.5%) with DDD required electrical or chemical cardioversion for AF, while 4 patients (2.1%) with SafeR and 6 (3.3%) with DDD underwent AV node ablation for rate control.
Safety
A total of 300 adverse events were documented in 179 patients (Table5). Overall, 37 patients died, 10 from cardiovascular causes. No death was related to the procedure or pacemaker. In patients randomized to SafeR versus DDD pacing, no significant differences were observed with regard to stroke (5 versus 2), syncope (3 versus 11), and episodes of heart failure (6 versus 4). During the 3-year follow-up period, 3 SafeR-related adverse events were reported in 3 patients (1.6% of SafeR population) linked to suspected or documented ventricular pauses and 2 DDD-related adverse events were reported in 2 patients (1.1% of DDD population) related to pacemaker-mediated tachycardia (Table5). All pacing mode-related adverse events were resolved by reprogramming the device to the alternate mode under study.
Table 5.
Adverse Events in Randomized Patients
| N (%) | DDD (N=182) | SafeR (N=191) |
|---|---|---|
| All-cause death | 20 (11) | 17 (9) |
| Cardiac* death | 4 (2) | 4 (2) |
| Pneumonia/respiratory failure | 10 (5) | 6 (3) |
| Cancer | 5 (3) | 4 (2) |
| Septic shock | 1 (1) | 1 (1) |
| Unwitnessed | 0 (N) | 2 (1) |
| Clinical cause excluding death | 94 (57) | 99 (56) |
| Cardiac* | 62 (39) | 65 (36) |
| AF-related events† | 16 (16) | 7 (7) |
| Pneumonia/respiratory failure | 5 (5) | 6 (5) |
| Cancer | 2 (2) | 5 (5) |
| Other | 9 (9) | 16 (16) |
| Procedure-related event | 18 (15) | 15 (13) |
| A lead‡ | 6 (6) | 2 (2) |
| V lead‡ | 4 (4) | 3 (3) |
| Pocket§ | 3 (3) | 5 (5) |
| Pneumothorax | 4 (3) | 1 (1) |
| Other‖ | 1 (1) | 4 (4) |
| Device-related event | 14 (12) | 23 (17) |
| Pacing mode related | 2 (2) | 3 (3) |
| Pacemaker related | 2 (2) | 4 (4) |
| Atrial lead | 2 (2) | 6 (5) |
| Ventricular lead | 6 (5) | 10 (6) |
| Pacemaker programming system | 2 (2) | 0 |
| Total | 146 (92) | 154 (87) |
AF indicates atrial fibrillation; DDD, intrinsic atrioventricular conduction and dual-chamber pacemaker.
Includes heart failure, myocardial infarction, and sudden cardiac death.
Chemical or electrical cardioversion and ablation for AF.
Includes dislodgment, perforation, and fracture.
Includes infection, hematoma/bleeding, and pain at pocket.
Includes thrombosis, epi/pericardial hemorrhage, and tamponade.
Ancillary Analyses
In multivariate analyses, factors associated with a higher AF burden at 3 years of follow-up were as follows: proportion of ventricular-paced beats >1%, prior history of AF, male sex, and sinus node dysfunction as the pacing indication (Table6). Moreover, we observed a small but significant reduction of AF burden at 3 years in the SafeR arm as compared to DDD in the subgroup of patients free from AF at randomization (P=0.015, Table4).
Table 6.
Multivariate Analysis of AF Burden at 3 Years of Follow-Up
| Variable* | % Increase of AF Burden† | B Coefficient | Standard Error | P Value |
|---|---|---|---|---|
| AF burden (n=236) | ||||
| Randomized mode (SafeR vs DDD) | −11 | −0.113 | 0.506 | 0.824 |
| Vp (≥1% vs <1%) | 936 | 2.338 | 0.518 | <0.001 |
| History of AF | 1450 | 2.741 | 0.522 | <0.001 |
| Sex (female vs male) | −74 | −1.342 | 0.505 | 0.008 |
| SND indication | 565 | 1.895 | 0.597 | 0.002 |
History of AF=yes if AF burden at randomization is greater than 0 or if AF/flutter was documented at enrollment. AF indicates atrial fibrillation; DDD, intrinsic atrioventricular conduction and dual-chamber pacemaker; SND, sinus node dysfunction; Vp, ventricular pacing at Y3 follow-up.
Variables retained in the multivariate model after stepwise selection.
% increase of AF burden is obtained by using the formula: (eβ−1)×100, where β is the parameter estimate from the multivariate model.
Discussion
In this randomized multicenter clinical trial, the SafeR algorithm was highly effective in further reducing the percentage of RV pacing compared to DDD pacing with a long AV delay in a broad spectrum of patients with indications for dual-chamber pacing. However, the primary end point was not reached since the lower proportion of ventricular-paced beats did not result in a significant reduction in AF burden in this patient population at low risk of developing AF. Nevertheless, the risk of developing AF-related events was significantly decreased by SafeR compared to DDD with long AV delay.
Preserving Intrinsic AV Conduction
In this mixed dual-chamber pacemaker population, the proportion of RV pacing was reduced from ≈10% to near zero by the SafeR algorithm. Importantly, the study is unique in its inclusion of patients with abnormal but existing AV conduction, which represented 41% of the study population. Within this subgroup of patients, the impact of SafeR remained substantial, with the proportion of RV pacing reduced from 42% to 1%. Moreover, this marked reduction in ventricular pacing was achieved without compromising patient safety.
Benefits From Avoiding Preventable Ventricular Pacing
Assessing the impact of a pacemaker intervention on AF remains complex. As such, our study included several predefined analyses to address this objective, including overall AF burden and proportion of patients with adverse events related to AF. The decrease in RV pacing resulted in measurable but modest and not significant reductions in AF burden. As an end point, AF burden has important limitations. It is a pacemaker metric as opposed to a hard clinical outcome. Adjudicating all mode-switch events is not feasible considering that device storage of EGMs is limited to a maximum of 7 episodes. Nevertheless, a run-in phase was primarily designed to validate appropriate device functioning and exclude issues such as atrial oversensing, which may impact accuracy of the computed AF burden. During this run-in phase, all available mode-switch events were reviewed and pacing and sensitivity parameters were modified, when relevant, to minimize inappropriate mode-switches.
The proportion of patients who developed AF-related clinical events (cardioversion or AV node ablation) was modestly but significantly reduced by the SafeR algorithm. Taken together, the impact of minimizing RV pacing on AF observed in this CAN-SAVE R trial is less pronounced than in SAVE-PACe11 and Long-MinVPace21 trials and is consistent with findings from the DANPACE22 trial and more recent PreFER MVP23 and ANSWER24 trials. However, AF burden was not reported in 4 of these 5 trials. In the Long-MinVPace study,12 AF burden was significantly reduced from 47.6±42.2% to 12.8±15.3% with an algorithm designed to minimize ventricular pacing. This discrepancy may be explained, in part, by differences in study populations. The markedly lower AF burden observed in our study (median <1% in both groups) reflects our study design, with the deliberate exclusion of patients who spent ≥30% of their time in AF during the run-in phase. While the intention was to capture patients with little AF at baseline, it is possible that a more significant impact on AF burden may have been observed in a higher-risk population.
In 2 previous studies that demonstrated a beneficial impact of algorithms designed to minimize unnecessary ventricular pacing on AF, the proportion of paced ventricular beats varied between 74% and 99% in studies with DDD pacing, reduced to 5.8% to 9.1% in the treatment groups.11,12 In contrast, the DANPACE study22 failed to demonstrate a benefit from AAIR pacing for AF when the proportion of paced ventricular beats was 65±33% in the DDDR group.22 In the SAVE PACe study, 7.9% of patients in the group assigned to minimal ventricular pacing developed persistent AF compared to 12.7% of patients in the group assigned to conventional dual-chamber pacing. In the Long-MinVPACE study,12 9% of patients in the group assigned to minimal ventricular pacing developed persistent AF compared to 42% of patients with conventional dual-chamber pacing. This contrasts with the ANSWER study24 where hospitalization rates for AF or cardioversion were similar between the SafeR group and DDD group (HR: 1.09). Finally, in the DANPACE study,22 28% of patients assigned to AAIR pacing presented with paroxysmal AF compared to 23% of patients with conventional dual-chamber pacing.
Benefits from reduced ventricular pacing accrue over time. Brief follow-up periods, such as the 6-month primary analysis of the MinVPace trial, failed to show a significant impact on rates of AF despite marked reductions in rates of ventricular pacing (eg, from 86% to 2%).21 Significant differences emerged over a longer follow-up of 1.4 years.12 Other trials, such as CTOPP,25 required even longer follow-up periods to demonstrate that different pacing modes may influence propensity for AF. In the recent PreFER MVP trial, no significant impact of the pacing mode could be observed at a follow-up of 2 years.24
Risk–Benefit Assessment
A risk–benefit assessment should be individualized when considering algorithms to minimize RV pacing. The current study excluded patients with >40% RV pacing during the 2-month run-in phase. In qualifying patients, both treatment alternatives were well tolerated and associated with similar overall rates of adverse events. There are, however, reservations around programming DDD pacemakers with long AV intervals, since deleterious consequences such as endless loop (or pacemaker-mediated) tachycardias7,10 and timing cycle conflicts that limit the detection of atrial tachyarrhythmias8,9 have been reported. In light of recently expressed concerns about possible proarrhythmic effects of algorithms designed to minimize ventricular pacing,26 it is reassuring to note that no ventricular tachyarrhythmic events were associated with the SafeR mode in the CAN-SAVE R trial.
Conclusions
The SafeR algorithm is highly effective in preventing RV pacing when compared to a DDD mode with a long AV delay in a patient population with a wide spectrum of indications for dual-chamber pacing including paroxysmal high-degree AV block. Ventricular pacing rates <1% were achieved without compromising patient safety, a finding that has clinical implications with regard to improving battery longevity. However, in this patient population at low risk of developing AF, AF burden remained low and was not significantly reduced by SafeR.
Acknowledgments
All investigators and co-ordinators are listed in the Appendix S1. The authors are grateful to Céline Pitre for directing the echocardiography core laboratory, Michele Buys-Topart for data monitoring, Malorie Chabot-Blanchet and Pierre-Henri Siot for statistical support, and Anne Rousseau-Plasse and Pelle Stolt for editorial assistance.
Sources of Funding
Sorin CRM SAS sponsored the study: It provided resources for study development on sites, data monitoring, data entry, and data analysis.
Disclosures
Thibault, Dubuc, Dyrda, Guerra, Macle, Mondésert, Rivard, Roy, Talajic, Andrade, and Khairy have received honoraria for lectures from, and performed clinical studies supported by: Bayer’s, Biotronik, Boston Scientific, Bristol-Myers-Squibb, Johnson & Johnson, Medtronic, Novartis, Pfizer, Servier, St. Jude Medical, and Sorin Group. Ducharme has received honoraria for lectures from, and performed clinical studies supported by: Abbot Vascular, Novartis, Otsuka, Pfizer, St. Jude Medical, and Thoratec. Baranchuk has received honoraria for lectures from, and performed clinical studies supported by: Boehringer Ingelheim, Boston Scientific, Medtronic, St. Jude Medical, and Sorin Group. Nitzsché is an employee of Sorin Group.
Supporting Information
Appendix S1. Members of the CAN-SAVE R Study Investigators.
References
- Prinzen FW, Cheriex EC, Delhaas T, van Oosterhout MF, Arts T, Wellens HJ, Reneman RS. Asymmetric thickness of the left ventricular wall resulting from asynchronous electric activation: a study in dogs with ventricular pacing and in patients with left bundle branch block. Am Heart J. 1995;130:1045–1053. doi: 10.1016/0002-8703(95)90207-4. [DOI] [PubMed] [Google Scholar]
- Nielsen JC, Kristensen L, Andersen HR, Mortensen PT, Pedersen OL, Pedersen AK. A randomized comparison of atrial and dual-chamber pacing in 177 consecutive patients with sick sinus syndrome: echocardiographic and clinical outcome. J Am Coll Cardiol. 2003;42:614–623. doi: 10.1016/s0735-1097(03)00757-5. [DOI] [PubMed] [Google Scholar]
- Nahlawi M, Waligora M, Spies SM, Bonow RO, Kadish AH, Goldberger JJ. Left ventricular function during and after right ventricular pacing. J Am Coll Cardiol. 2004;44:1883–1888. doi: 10.1016/j.jacc.2004.06.074. [DOI] [PubMed] [Google Scholar]
- Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, Sharma A. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288:3115–3123. doi: 10.1001/jama.288.24.3115. [DOI] [PubMed] [Google Scholar]
- Nielsen JC, Pedersen AK, Mortensen PT, Andersen HR. Programming a fixed long atrioventricular delay is not effective in preventing ventricular pacing in patients with sick sinus syndrome. Europace. 1999;1:113–120. doi: 10.1053/eupc.1998.0026. [DOI] [PubMed] [Google Scholar]
- Nitardy A, Langreck H, Dietz R, Stockburger M. Reduction of right ventricular pacing in patients with sinus node dysfunction through programming a long atrioventricular delay along with the DDIR mode. Clin Res Cardiol. 2008;98:25–32. doi: 10.1007/s00392-008-0716-z. [DOI] [PubMed] [Google Scholar]
- Stierle U, Krüger D, Vincent AM, Mitusch R, Giannitsis E, Wiegand U, Potratz J. An optimized AV delay algorithm for patients with intermittent atrioventricular conduction. Pacing Clin Electrophysiol. 1998;21:1035–1043. doi: 10.1111/j.1540-8159.1998.tb00149.x. [DOI] [PubMed] [Google Scholar]
- Sutton R, Kenny RA. The natural history of sick sinus syndrome. Pacing Clin Electrophysiol. 1986;9:1110–1114. doi: 10.1111/j.1540-8159.1986.tb06678.x. [DOI] [PubMed] [Google Scholar]
- Shivkumar K, Feliciano Z, Boyle NG, Wiener I. Intradevice interaction in a dual chamber implantable cardioverter defibrillator preventing ventricular tachyarrhythmia detection. J Cardiovasc Electrophysiol. 2000;11:1285–1288. doi: 10.1046/j.1540-8167.2000.01285.x. [DOI] [PubMed] [Google Scholar]
- Dennis MJ, Sparks PB. Pacemaker mediated tachycardia as a complication of the autointrinsic conduction search function. Pacing Clin Electrophysiol. 2004;27:824–826. doi: 10.1111/j.1540-8159.2004.00538.x. [DOI] [PubMed] [Google Scholar]
- Sweeney MO, Bank AJ, Nsah E, Koullick M, Zeng QC, Hettrick D, Sheldon T, Lamas GA. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007;357:1000–1008. doi: 10.1056/NEJMoa071880. [DOI] [PubMed] [Google Scholar]
- Veasey RA, Arya A, Silberbauer J, Sharma V, Lloyd GW, Patel NR, Sulke AN. The relationship between right ventricular pacing and atrial fibrillation burden and disease progression in patients with paroxysmal atrial fibrillation: the long-MinVPACE study. Europace. 2011;13:815–820. doi: 10.1093/europace/euq463. [DOI] [PubMed] [Google Scholar]
- Savouré A, Fröhlig G, Galley D, Defaye P, Reuter S, Mabo P, Sadoul N, Amblard A, Limousin M, Anselme F. A new dual-chamber pacing mode to minimize ventricular pacing. Pacing Clin Electrophysiol. 2005;28(suppl 1):S43–S46. doi: 10.1111/j.1540-8159.2005.00095.x. [DOI] [PubMed] [Google Scholar]
- Fröhlig G, Gras D, Victor J, Mabo P, Galley D, Savouré A, Jauvert G, Defaye P, Ducloux P, Amblard A. Use of a new cardiac pacing mode designed to eliminate unnecessary ventricular pacing. Europace. 2006;8:96–101. doi: 10.1093/europace/euj024. [DOI] [PubMed] [Google Scholar]
- Pioger G, Leny G, Nitzsché R, Ripart A. AAIsafeR limits ventricular pacing in unselected patients. Pacing Clin Electrophysiol. 2007;30(suppl 1):S66–S70. doi: 10.1111/j.1540-8159.2007.00608.x. [DOI] [PubMed] [Google Scholar]
- Davy J-M, Hoffmann E, Frey A, Jocham K, Rossi S, Dupuis J-M, Frabetti L, Ducloux P, Prades E, Jauvert G. Near elimination of ventricular pacing in SafeR mode compared to DDD modes: a randomized study of 422 patients. Pacing Clin Electrophysiol. 2012;35:392–402. doi: 10.1111/j.1540-8159.2011.03314.x. [DOI] [PubMed] [Google Scholar]
- Thibault B, Simpson C, Gagné C-É, Blier L, Senaratne M, McNicoll S, Stuglin C, Williams R, Pinter A, Khaykin Y. Impact of AV conduction disorders on SafeR mode performance. Pacing Clin Electrophysiol. 2009;32:S231–S235. doi: 10.1111/j.1540-8159.2008.02293.x. [DOI] [PubMed] [Google Scholar]
- World Medical Association. 2008. WMA declaration of Helsinki—ethical principles for medical research involving human subjects. Amended by the 59th WMA General Assembly, Seoul, October 2008.. Available at: www.wma.net/en/30publications/10policies/b3/index.html.pdf. Accessed June 8, 2013.
- Vardas PE, Auricchio A, Blanc J-J, Daubert J-C, Drexler H, Ector H, Gasparini M, Linde C, Morgado FB, Oto A, Sutton R, Trusz-Gluza M ESC Committee for Practice Guidelines (CPG); Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Funck-Brentano C, Filippatos G, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL Document Reviewers. Priori SG, Blomstrom-Lundqvist C, Brignole M, Terradellas JB, Camm J, Castellano P, Cleland J, Farre J, Fromer M, Le Heuzey J-Y, Lip GY, Merino JL, Montenero AS, Ritter P, Schalij MJ, Stellbrink C. Guidelines for cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2007;28:2256–2295. doi: 10.1093/eurheartj/ehm305. [DOI] [PubMed] [Google Scholar]
- Epstein AE, DiMarco JP, Ellenbogen KA, Estes NAM, III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. J Am Coll Cardiol. 2008;51:e1–e62. doi: 10.1016/j.hrthm.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Veasey RA, Arya A, Freemantle N, Silberbauer J, Patel NR, Lloyd GW, Sulke AN. The usefulness of minimal ventricular pacing and preventive AF algorithms in the treatment of PAF: the “MinVPace” study. J Interv Card Electrophysiol. 2010;28:51–57. doi: 10.1007/s10840-009-9461-0. [DOI] [PubMed] [Google Scholar]
- Nielsen JC, Thomsen PEB, Hojberg S, Moller M, Vesterlund T, Dalsgaard D, Mortensen LS, Nielsen T, Asklund M, Friis EV, Christensen PD, Simonsen EH, Eriksen UH, Jensen GVH, Svendsen JH, Toff WD, Healey JS, Andersen HR on behalf of the DANPACE Investigators. A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur Heart J. 2011;32:686–696. doi: 10.1093/eurheartj/ehr022. [DOI] [PubMed] [Google Scholar]
- Botto GL, Ricci RP, Bénézet JM, Nielsen JC, De Roy L, Piot O, Quesada A, Quaglione R, Vaccari D, Garutti C, Vainer L, Kozák M on behalf of the PreFERMVP Investigators. Managed ventricular pacing compared to conventional dual-chamber pacing for elective replacement in chronically paced patients: results of the Prefer for Elective Replacement MVP (PreFER MVP) randomized study. Heart Rhythm. 2014;11:992–1000. doi: 10.1016/j.hrthm.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Stockburger M, Boveda S, Moreno J, Da Costa A, Hatala R, Brachmann J, Butter C, Garcia Serra J, Rolando M, Defaye P. Long-term clinical effects of ventricular pacing reduction with a changeover mode to minimize ventricular pacing in a general pacemaker population. Eur Heart J. 2015;36:151–157. doi: 10.1093/eurheartj/ehu336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr CR. Canadian Trial of Physiological Pacing: effects of physiological pacing during long-term follow-up. Circulation. 2004;109:357–362. doi: 10.1161/01.CIR.0000109490.72104.EE. [DOI] [PubMed] [Google Scholar]
- Lim HS. The prescription of minimal ventricular pacing. Pacing Clin Electrophysiol. 2012;35:1528–1536. doi: 10.1111/j.1540-8159.2012.03490.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Members of the CAN-SAVE R Study Investigators.


