Abstract
Background
Cardiovascular disease is the most common cause of death for both genders. Debates are ongoing as to whether gender-specific differences in clinical course, diagnosis, and management of acute myocardial infarction (MI) exist.
Methods and Results
We compared all men and women who were treated for acute MI at cardiac care units in Västra Götaland, Sweden, between January 1995 and October 2014 by obtaining data from the prospective SWEDEHEART (Swedish Web-System for Enhancement of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies) registry. We performed unadjusted and adjusted Cox proportional hazards and logistic regression analyses on complete case data and on imputed data sets. Overall, 48 118 patients (35.4% women) were diagnosed with acute MI. Women as a group had better age-adjusted prognosis than men, but this survival benefit was absent for younger women (aged <60 years) and for women with ST-segment elevation MI. Compared with men, younger women and women with ST-segment elevation MI were more likely to develop prehospital cardiogenic shock (adjusted odds ratio 1.67, 95% CI 1.30 to 2.16, P<0.001 and adjusted odds ratio 1.31, 95% CI 1.16 to 1.48, P<0.001) and were less likely to be prescribed evidence-based treatment at discharge (P<0.001 for β-blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, statins, and P2Y12 antagonists). Differences in treatment between the genders did not decrease over the study period (P>0.1 for all treatments).
Conclusions
Women on average have better adjusted prognosis than men after acute MI; however, younger women and women with ST-segment elevation MI have disproportionately poor prognosis and are less likely to be prescribed evidence-based treatment.
Keywords: acute myocardial infarction, evidence-based treatment, gender, non–ST-segment elevation myocardial infarction, ST-segment elevation myocardial infarction
Cardiovascular disease is the most common cause of death for both genders in industrialized countries. Women have an apparent biological protection from coronary artery disease, which translates to a decades-long delay of the onset of clinical cardiovascular disease.1
Short- and long-term mortality after an acute myocardial infarction (AMI) has decreased over past decades worldwide, but some reports imply less effective reduction in women.2–6 Debates are ongoing as to whether sex-specific differences exist in clinical course, diagnosis, and management of ischemic heart disease and the extent to which these differences can be alleviated by changes in clinical praxis.7–11
Sweden has national medical and health care quality registries that contain individualized data concerning diagnoses, interventions, and outcomes. This resource allows data to be obtained about a variety of risk factors, including traditional cardiovascular risk factors, for each patient treated at a Swedish hospital.12,13
We compared mortality, risk of complications, and likelihood of receiving evidence-based treatment for men and women who suffered an AMI between 1995 and 2014 in Västra Götaland County in Sweden.
Methods
We compared men and women who were treated for AMI in Västra Götaland County in western Sweden between January 1995 and October 2014. Data were obtained from the SWEDEHEART (Swedish Web-System for Enhancement of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies) registry about ST-segment elevation myocardial infarction (STEMI) and non-STEMI (NSTEMI) in Västra Götaland County, Sweden. The switch from thrombolysis to primary percutaneous coronary intervention (PCI) as the preferred reperfusion strategy within the region took place during 2004. By 2005, PCI was established as the primary strategy.
The RIKS-HIA Registry
RIKS-HIA (Registry of Information and Knowledge About Swedish Heart Intensive Care Admissions) is a component of the nationwide SWEDEHEART registry. RIKS-HIA contains a list of variables for all patients who are treated for acute coronary syndromes at cardiac care units in Sweden. The patients are informed about the registration of data as part of the everyday clinical routine. According to Swedish law, no additional informed consent is needed. All data in RIKS-HIA are prospectively entered into the registry by the treating physicians and/or nurses. Patients were diagnosed with non–ST-segment elevation or ST-segment elevation AMI if they fulfilled the criteria for the respective diagnosis at the end of their hospitalization.14,15 RIKS-HIA gathers >100 variables with information about patient demographics, risk factors, past medical history, medical treatment before admission, in-hospital treatment and interventions, and treatment at discharge.16 Long-term survival data were obtained by merging the RIKS-HIA databases with the national Cause of Death Register based on each patient’s unique 10-digit personal identification number.16
End Point
The primary end point of the study was all-cause mortality. We also studied the likelihood of patients receiving the recommended treatment, the risk of suffering prehospital cardiac arrest or arriving at the hospital in cardiogenic shock, and the risk of developing in-hospital heart failure. Patients were considered to have suffered from diabetes, hypertension, hyperlipidemia, previous myocardial infarction (MI), previous PCI, previous stroke, cardiogenic shock, or heart failure if they had been diagnosed with the respective International Classification of Diseases codes (including subcategories).13
Statistics
Continuous variables were presented as mean±SD, and categorical variables were reported as frequencies. Normal distribution of variables was assessed by inspecting the distribution of values on histograms and by the Shapiro–Wilk test. Differences between the genders in continuous variables were tested with the Student t test. Differences in categorical variables were tested by the chi-square test.
Matching
For the purpose of removing the influence of age on unadjusted survival and hazard estimates, we randomly matched male and female patients 1:1 by age. The matched data set was used solely for comparisons of these estimates. In all other analyses, the full data set was used.
Imputation protocol
Missing data and observations in the database were imputed using multiple imputation with the chain-equation method17 for 20 data sets. This approach was applied to each of the variables listed in Table1, together with calendar year, hospital, indicator of missingness, and an event indicator.18 Continuous variables were imputed by ordinary least squares multiple regression, whereas binary variables were imputed using logistic regression, categorical variables were imputed by multinomial logistic regression, and ordered categorical variables were imputed by ordinal logistic regression. The imputation procedure and subsequent analyses were performed according to Rubin’s protocol19 under the assumption that missing data were missing at random.
Table 1.
Patient Characteristics and Treatment
| Variable | Men n=31 050 | Women n=17 068 | Missing n (%) | P Value | P Value PS |
|---|---|---|---|---|---|
| Age, mean (SD) | 68±12 | 75±12 | — | <0.001 | 0.946 |
| STEMI, n (%) | 11 636 (39) | 5821 (35) | 1452 (3) | <0.001 | 0.923 |
| BMI, mean (±SD) | 269 (53) | 263 (68) | 25 313 (55) | <0.001 | 0.930 |
| Smoker, n (%) | 6978 (24) | 3162 (21) | 4200 (9) | <0.001 | 0.966 |
| Diabetes, n (%) | 5609 (18) | 3614 (21) | 400 (1) | <0.001 | 0.915 |
| Hypertension, n (%) | 11 488 (38) | 7982 (48) | 1221 (2) | <0.001 | 0.909 |
| Hyperlipidemia, n (%) | 3440 (14) | 1754 (13) | 551 (1) | 0.013 | 0.928 |
| Serum creatinine, mean (SD) | 104 (65) | 90 (52) | 20 471 (43) | <0.001 | 0.471 |
| LV function, n (%) | 17 088 | 8532 | 22 498 (47) | 0.155 | 0.911 |
| >50% | 9843 (58) | 4991 (59) | n/a | n/a | n/a |
| 40% to 49% | 3951 (23) | 1888 (22) | n/a | n/a | n/a |
| 30% to 39% | 2025 (12) | 1050 (12) | n/a | n/a | n/a |
| <30% | 1269 (7) | 603 (7) | n/a | n/a | n/a |
| Previous myocardial infarction, n (%) | 6196 (20) | 2890 (17) | 699 (1) | <0.001 | 0.968 |
| Previous PCI, n (%) | 1810 (6) | 629 (4) | 676 (1) | <0.001 | 0.939 |
| Previous cardiac surgery, n (%) | 2422 (8) | 705 (4) | 462 (1) | <0.001 | 0.988 |
| Reperfusion, n (%) | 10 878 (35) | 4734 (28) | 443 (1) | <0.001 | 0.856 |
| ASA after discharge, n (%) | 25 363 (84) | 13 397 (81) | 1426 (3) | <0.001 | 0.961 |
| Other antiplatelet at discharge, n (%) | 13 924 (46) | 6602 (40) | 1109 (2) | <0.001 | 0.985 |
| β-blocker at discharge, n (%) | 25 917 (85) | 13 591 (82) | 1117 (2) | <0.001 | 0.965 |
| ACEI or ARB at discharge, n (%) | 16 663 (55) | 8498 (51) | 1135 (2) | <0.001 | 0.988 |
| Oral anticoagulants at discharge, n (%) | 2897 (10) | 1262 (8) | 1163 (2) | <0.001 | 0.969 |
| Statins at discharge, n (%) | 18 907 (62) | 8698 (52) | 1158 (2) | <0.001 | 0.583 |
| IV diuretics during hospitalization, n (%) | 7784 (25) | 5761 (34) | 516 (1) | <0.001 | 0.161 |
| IV inotropes during hospitalization, n (%) | 1547 (5) | 865 (5) | 551 (1) | 0.674 | 0.139 |
| CPAP during hospitalization, n (%) | 1756 (6) | 1317 (8) | 572 (1) | <0.001 | 0.297 |
| Prehospital cardiogenic shock, n (%) | 1614 (5) | 1046 (6) | 1511 (3) | <0.001 | 0.941 |
| Prehospital cardiac arrest, n (%) | 586 (2) | 201 (1) | 2407 (5) | <0.001 | 0.861 |
| In-hospital heart failure, n (%) | 9388 (31) | 6816 (41) | 1833 (4) | <0.001 | 0.986 |
ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid; BMI, body mass-index; CPAP, continuous positive airway pressure; IV, intravenous; LV, left ventricle; PCI, percutaneous coronary intervention; PS, propensity score adjusted; STEMI, ST-segment elevation myocardial infarction.
Statistical models
Men and women were compared using Cox proportional hazards regression or logistic or multiple linear regression. The analyses were performed on patients with complete data and used the multiple imputation method. Differences in patient characteristics were accounted for by adjusting for covariates or by propensity scores.20 Multilevel models accounting for clustering of patients within different hospitals were fitted and compared to single-level models (Table2).
Table 2.
Outcomes and Statistical Models
| End point |
| Death within study period* |
| Prehospital cardiogenic shock |
| Prehospital cardiac arrest |
| In-hospital heart failure |
| Evidence-based therapies: reperfusion treatment, aspirin, β-blockers, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, statin, P2Y12 antagonist |
| Statistical models |
| Unadjusted models |
| Unadjusted Cox proportional hazards regression or unadjusted logistic regression models |
| Age-adjusted models |
| Cox proportional hazards regression or logistic regression adjusted for age |
| Risk factor–adjusted models |
| Cox proportional hazards regression model/logistic regression adjusted for risk factors†age, smoking habits, hypertension, diabetes, hyperlipidemia, previous myocardial infarction, previous cardiac surgery, previous PCI, STEMI, and calendar year |
| Risk factor– and treatment-adjusted models |
| Propensity score–adjusted Cox proportional hazards regression model adjusted for risk factors and treatment. Propensity scores were calculated from the variables age; smoking habits; hypertension; diabetes; hyperlipidemia; previous myocardial infarction; previous cardiac surgery; previous PCI; STEMI; calendar year; BMI; prehospital cardiac arrest; prehospital cardiogenic shock; in-hospital heart failure; revascularization treatment; and discharged with β-blocker, aspirin, ACEI/ARB, antiplatelet therapy, oral anticoagulant, and/or statin. |
All statistical models were multilevel models with patient as the first-level unit and treating hospital as the second-level unit. ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Primary end point.
Primary model.
Primary model
A Cox proportional hazards model adjusted for age, smoking habits, hypertension, diabetes, hyperlipidemia, previous MI, previous cardiac surgery, previous PCI, STEMI, and calendar year fitted on imputed data was predefined as the primary model. These covariates were entered into the model as a main effect only. When we referred to adjusted risk in this paper, we referred to the above risk factor–adjusted risk ratio between women and men.
Propensity scores
In addition to the primary analysis with traditional multivariable modeling, we used propensity scores in secondary analyses to adjust for differences in patient characteristics and treatment.20 To generate the propensity scores for each patient, logistic regression was used to calculate the likelihood of belonging to the respective gender. The covariates used were the same as those in multivariable logistic regression with the addition of body mass index; prehospital cardiac arrest; prehospital cardiogenic shock; in-hospital heart failure; whether the patient received revascularization treatment; and whether the patient was discharged with β-blocker, aspirin, angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB), antiplatelet therapy, oral anticoagulant, and/or statin. The calculated propensity score was entered in the Cox regression model as a continuous variable. We also compared 30-day mortality between men and women by fitting logistic regression models.
Secondary models
We fitted unadjusted and adjusted multivariate logistic regression models, using both complete cases and imputed models, to detect the difference in risk between developing prehospital cardiac arrest or cardiogenic shock and the risk of developing in-hospital heart failure. We also compared the likelihood of developing major bleeding during the index hospitalization, a variable that is available from 2005, between women and men. The same covariates used in the other adjusted Cox models were included. Multivariate adjusted models fitted on imputed data were considered the primary analysis for predicting the risk of presenting with cardiogenic shock or of developing in-hospital heart failure. If model fitting was inadequate, interaction terms between the variables were included if they improved model fitting.
A multivariate logistic regression model containing the same covariates as the multivariate Cox regression was fitted on imputed data to detect differences between the genders in the likelihood of receiving reperfusion treatment and prescriptions for recommended long-term medications at discharge.
Subgroup analyses
We performed subgroup analyses by including interaction terms. We performed subgroup analyses in Cox proportional hazards models to detect associations between gender and calendar year, diabetes mellitus, smoking, age, or presence of ST-segment elevation, respectively. Subgroup analyses, with the same interaction terms, were also performed in the logistic models that predicted likelihood of receiving evidence-based treatment after AMI.
Trends over time were assessed by inclusion of a time variable as well as an interaction term between the time variable and gender in the statistical models. The time variable was included in addition to the traditional risk factors described above and was continuous (per calendar year), categorical (per 5-year period), or binary (before or after introduction of primary PCI as the preferred reperfusion strategy).
Postestimation diagnostics
Goodness of fit (calibration) for the models was assessed with the Hosmer–Lemeshow test. Multicollinearity among the variables in the model was assessed by calculation of the variance inflation factor. Cox proportional hazards models were tested for proportionality of hazards by visual inspection of the log–log plots. All statistical analyses were performed using Stata software (version 13.1; StataCorp). All tests were 2-tailed, and a P<0.05 was considered statistically significant. Because of multiple analyses, P<0.05 was expected to occur accidently in 1 of 20 analyses.
Results
Patient Characteristics and Treatments
During the study period, 130 667 patients were hospitalized in 11 cardiac care units in Västra Götaland County. Of these, 48 118 (36.8%) were diagnosed with AMI and were included in the analysis. Of all patients with AMI, 17 068 (35.4%) were women (Figure1). Women were less likely to present with STEMI, to smoke, and to have had a previous MI. Women were also less likely to have undergone previous PCI or cardiac surgery and to present with prehospital cardiac arrest. Women were more likely to have been diagnosed with hypertension or diabetes mellitus and to develop prehospital cardiogenic shock or in-hospital heart failure. Women were more likely to receive intravenous diuretics or continuous positive airway pressure treatment during hospitalization but were less likely to receive prescriptions for recommended therapies at discharge (ie, aspirin, β-blockers, ACE inhibitor or ARB, other antiplatelets, or statins) (Table1).
Figure 1.
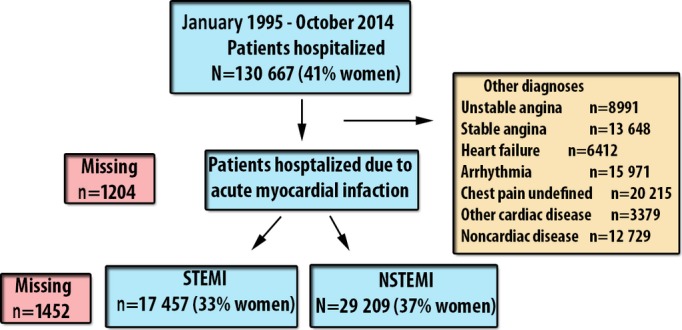
Flow chart. Patient selection and number of patients. NSTEMI indicates non–ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction.
Prognosis After Acute Myocardial Infarction
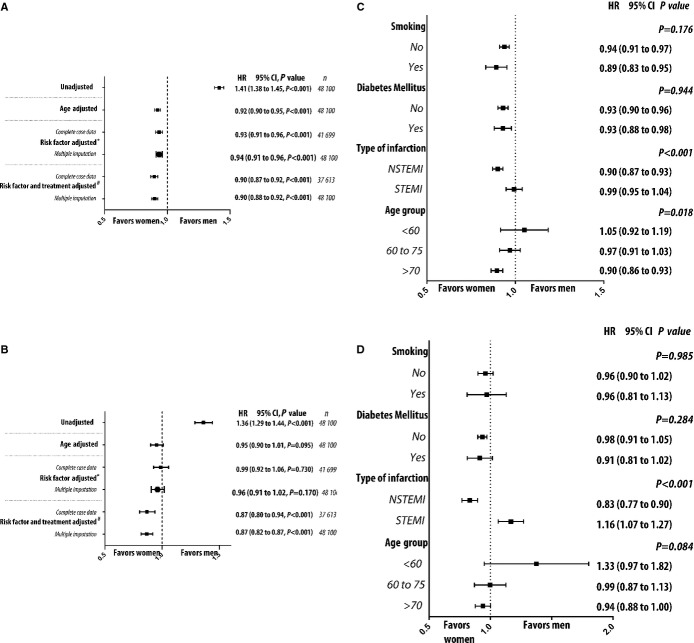
From 1995 to 2013, in-hospital mortality, 30-day mortality, and 1-year mortality decreased from 3.7%, 15.9%, and 24.7% to 1.2%, 4.9%, and 11.9%, respectively, for men and from 3.8%, 17.8%, and 26.4% to 2.3%, 9.8%, and 18.0%, respectively, for women. The adjusted reduction over time in mortality was not different between the genders (P=0.136). Unadjusted survival estimates were worse for women as a group than for men as a group (Figure2A). On average, however, women were older, and when we randomly selected age-matched cohorts of patients, the estimated survival and hazard was better for women than for men (Figure2B). Our prespecified primary analysis (ie, a risk factor–adjusted Cox proportional hazards regression model on imputed data) revealed that among patients diagnosed with AMI, women had a lower risk of dying than men (Figure3A). Because women were older on average, unadjusted analyses showed them to be at increased risk of dying. We observed a trend toward lower risk of death for women for 30-day mortality (Figure3B). Subgroup analyses revealed significant interactions between gender and age as well as between gender and type of MI (ie, STEMI or NSTEMI). Older women had better long- and short-term prognoses than older men, whereas prognosis was similar for women and men in younger age categories. Women with NSTEMI had better prognosis than men, but prognosis was similar between the genders for STEMI. The risk was particularly high for younger women with STEMI (3-way interaction term among gender, age, and type of MI; P=0.005). No interaction was found between gender and diabetes mellitus and between gender and smoking (Figure3C and 3D). Risk reduction after the introduction in 2005 of primary PCI as therapy of choice was similar for men and women with regard to death within 30 days, with a nonsignificant trend toward better risk reduction in men (P=0.055 for interaction term between gender and time).
Figure 2.

Survival and hazard estimates. Kaplan–Meier cumulative survival estimates and smoothed hazard estimates for men and women after acute myocardial infarction. A, All patients with acute myocardial infarction. B, Randomly selected age-matched set of patients with acute myocardial infarction.
Figure 3.
Hazard ratio, risk of death. Six different statistical models were fitted to compare estimated Cox proportional hazard ratios for death at any time during the study period (A) or death within 30 days (B) for men and women after acute myocardial infarction. We also performed subgroup analyses by Cox proportional hazards regression models for death at any time during the study period (C) or death within 30 days (D). Subgroup analyses were performed using models adjusted for risk factors and including interaction terms. P values refer to the interaction term between categories and gender. #Primary model. *Adjusted for age, smoking habits, hypertension, diabetes, hyperlipidemia, previous myocardial infarction, previous cardiac surgery, previous PCI, STEMI, and calendar year. #Variables included in propensity scores: age; smoking habits; hypertension; diabetes; hyperlipidemia; previous myocardial infarction; previous cardiac surgery; previous PCI; STEMI and calendar-year BMI; prehospital cardiac arrest; cardiogenic shock; in-hospital heart failure; whether the patient received revascularization treatment; and whether the patient was discharged with β-blocker, aspirin, ACEI/ARB, antiplatelet therapy, oral anticoagulant, and/or statin. ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; HR, hazard ratio; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Complications After Acute Myocardial Infarction
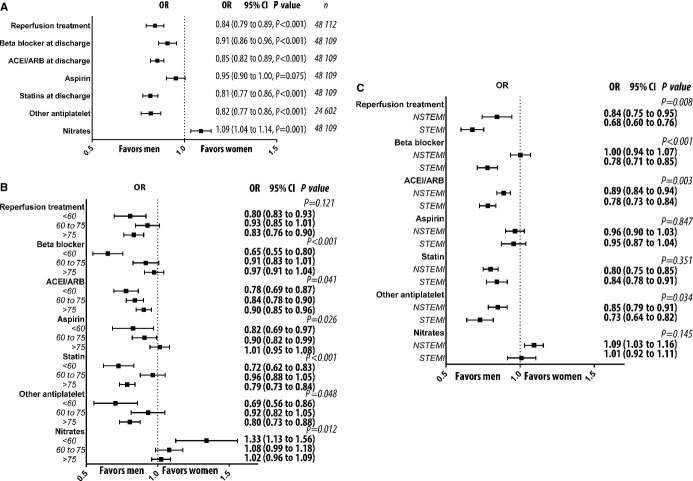
Women had higher adjusted risk to develop cardiogenic shock and in-hospital heart failure than men (Figure4A and 4B), but the risk difference between the genders with regard to developing cardiogenic shock decreased after the introduction of primary PCI as the reperfusion strategy of choice (P=0.021 for interaction term between gender and time). In contrast, women had lower adjusted risk of suffering prehospital cardiac arrest (Figure4C). Comparison of subgroups revealed that estimated risk to develop prehospital cardiogenic shock was even larger for younger women (aged <60 years) compared with younger men (adjusted odds ratio [OR] 1.67, 95% CI 1.30 to 2.16, P<0.001 for the interaction term between gender and age group). We found higher risk for cardiogenic shock (OR 1.31, 95% CI 1.16 to 1.48) in women with STEMI but not in women with NSTEMI (OR 0.92, 95% CI 0.82 to 1.04, P<0.001 for the interaction term). The information about time from medical contact to reperfusion was available from 2005. Data about reperfusion times (ie, time from ECG to arterial puncture) was missing in one-third of the patients with STEMI. We did not find any significant difference in time to reperfusion between men and women in unadjusted and adjusted analyses in STEMI. The adjusted risk of being treated for in-hospital heart failure was higher for women than for men (Figure4C). Women also had higher adjusted risk of major bleeding (Figure4D). The difference in risk of developing major bleeding during the index hospitalization between women and men was similar regardless of age category and did not change significantly over time (P>0.05 for interaction terms).
Figure 4.
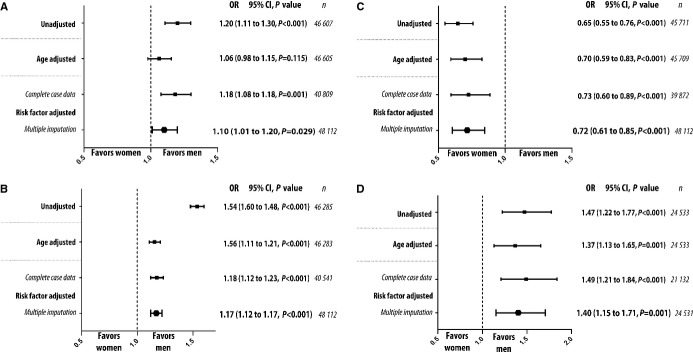
Risk of complications. Risk of developing complications, as assessed by logistic regression models adjusted for risk factors. P values refer to test for trend. A, Risk of developing cardiogenic shock. B, Risk of developing in hospital heart failure. C, Risk of developing prehospital cardiac arrest. D, Risk of major bleeding. OR indicates odds ratio.
Treatment After Acute Myocardial Infarction
After 2005, 75% of women and 88% of men with STEMI underwent coronary angiography (P<0.001). Women with STEMI were less likely to undergo coronary angiography than men with STEMI in adjusted analysis that included an interaction term between gender and whether or not the patient was diagnosed with STEMI or NSTEMI (OR 0.72, 95% CI 0.57 to 0.90, P<0.001). The difference between the genders in the likelihood of undergoing coronary angiography if presenting with STEMI did not change over time (P=0.62). We did not find a significant interaction between age group and gender for chance of undergoing coronary angiography (P=0.15).
Overall, 85% of the men and 82% of the women (P<0.001) were prescribed β-blockers at discharge. For aspirin, ACE inhibitor or ARB, and statins, these numbers were 84%, 55%, and 64%, respectively, for men and 81%, 51%, and 52%, respectively, for women (each P<0.001). Fewer men than women were prescribed nitrates (22% versus 25%, P<0.001). After 2005, 78% of the men and 69% of women received a P2Y12 antagonist (P<0.001). When we adjusted for traditional cardiovascular risk factors, we found that women were less likely to receive β-blockers, ACE inhibitors or ARBs, statins, and P2Y12 receptor inhibitors. The likelihood of being prescribed P2Y12 antagonists was also lower for women if we included the variable major bleeding during hospitalization in the statistical model (OR 0.84, 95% CI 0.79 to 0.90, P=0.001). We also found that women were more likely to be prescribed long-acting nitrates (Figure5A). The increased likelihood of not being prescribed evidence-based pharmacological treatment in women did not change over time (Table3). Major bleeding during the hospitalization was associated with a lower likelihood of receiving antiplatelet drugs (adjusted OR 0.34, 95% CI 0.32 to 0.35 P<0.001).
Figure 5.
Likelihood of receiving evidence-based treatment. A, Likelihood of receiving evidence-based treatment, as assessed by logistic regression models adjusted for risk factors. Women were compared with men. B, Subgroup analyses by inclusion of interaction terms between gender and age category. Women were compared with men within each age category. All models were fitted on imputed data sets. C, Subgroup analysis by inclusion terms between gender and type of myocardial infarction (ie, STEMI or NSTEMI). P values refer to interaction between age category and gender. ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; NSTEMI, non–ST-segment elevation myocardial infarction; OR, odds ratio; STEMI, ST-segment elevation myocardial infarction.
Table 3.
Trends Over Time for Odds Ratio With 95% CI and P Values for Women to Receive Specific Treatment at Discharge
| β-Blocker | ACEI/ARB | ASA | P2Y12* | Statin | Nitrates | |
|---|---|---|---|---|---|---|
| Per calendar year | 1.01 (1.00 to 1.01) | 0.98 (0.97 to 0.99) | 0.99 (0.98 to 1.00) | 1.03 (1.01 to 1.05) | 0.94 (0.78 to 0.86) | 1.09 (1.04 to 1.15) |
| P=0.089 | P<0.001 | P=0.205 | P=0.014 | P<0.001 | P=0.001 | |
| Over 5 years | 1995–2000 | 1995–2000 | 1995–2000 | N/A | 1995–2000 | 1995–2000 |
| 0.83 (0.74 to 0.92) | 0.94 (0.86 to 1.09) | 0.91 (0.82 to 1.02) | 1.17 (1.03 to 1.33) | 0.96 (0.86 to 1.06) | ||
| 2000–2005 | 2000–2005 | 2000–2005 | 2000–2005 | 2000–2005 | ||
| 0.96 (0.87 to 1.05) | 0.91 (0.84 to 0.98) | 1.08 (0.99 to 1.18) | 1.03 (0.95 to 1.12) | 0.95 (0.87 to 1.04) | ||
| 2005–2010 | 2005–2010 | 2005–2010 | 2005–2010 | 2005–2010 | ||
| 0.93 (0.84 to 1.03) | 0.80 (0.75 to 0.87) | 0.95 (0.86 to 1.05) | 0.65 (0.60 to 0.72) | 1.22 (1.11 to 1.35) | ||
| 2010–2014 | 2010–2014 | 2010–2014 | 2010–2014 | 2010–2013 | ||
| 0.94 (0.84 to 1.05) | 0.75 (0.69 to 0.82) | 0.83 (0.73 to 0.94) | 0.58 (0.51 to 0.64) | 1.27 (1.14 to 1.42) | ||
| P=0.191 | P=0.001 | P=0.005 | P<0.001 | P<0.001 | ||
| Before and after 2005 | Before 2005 | Before 2005 | Before 2005 | N/A | Before 2005 | Before 2005 |
| 0.89 (0.83 to 0.96) | 0.92 (0.87 to 0.98) | 1.00 (0.94 to 1.08) | 1.07 (1.00 to 1.15) | 0.95 (0.89 to 1.02) | ||
| After 2005 | After 2005 | After 2005 | After 2005 | After 2005 | ||
| 0.93 (0.87 to 1.00) | 0.78 (0.74 to 0.83) | 0.90 (0.83 to 0.98) | 0.62 (0.58 to 0.67) | 1.24 (1.15 to 1.34) | ||
| P=0.388 | P<0.001 | P=0.039 | P<0.001 | P<0.001 |
ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid; N/A, not available.
From 2005 to 2014.
Risk factor–adjusted subgroup analysis revealed an interaction between gender and age in the likelihood of receiving evidence-based treatment. Younger women were particularly unlikely to receive β-blockers, ACE inhibitors or ARBs, aspirin, or statins and were more likely to receive a long-acting nitrate (Figure5B). When we analyzed interaction among gender, NSTEMI, and STEMI, we saw that the adjusted likelihood of receiving reperfusion treatment, P2Y12 antagonists, β-blockers, and ACE inhibitors and/or ARBs was lower in women with STEMI (Figure5C).
Data Analysis and Postestimation Diagnostics
Most variables had missing information, but information was missing for >5% of patients in only 7 variables, namely, body mass index (55%), smoking status (9%), serum creatinine levels (43%), left ventricle function (47%), and whether or not the patient suffered from prehospital cardiac arrest (5%).
We verified the assumption of proportionality of hazards by inspecting Schoenfeld residuals on −ln(−ln(survival)) plots for each Cox proportional hazards model. Postestimation analysis for the logistic regression models, including propensity score estimation, by the Hosmer–Lemeshow test showed adequate goodness of fit for the models, and all P values were >0.05. We evaluated balancing properties of the calculated propensity scores with multivariate linear and logistic regressions. When we included propensity scores as a covariate in the regression models, we found no statistical differences in baseline characteristics between the groups (Table1). The average variance inflation factor was <4.58 for the adjusted models and 4.47 for the propensity score models, demonstrating that there was no significant multicollinearity among the variables in the models. Multilevel modeling that attempted to account for potential clustering of patients between different calendar years or between different hospitals did not improve the predictive power of the models and thus was abandoned.
Discussion
We investigated the effect of gender on mortality and health care during the last 2 decades in 41 118 patients with AMI, using data from the prospective RIKS-HIA registry. We found that, in general, women have better prognosis than men, but this benefit was not present for younger women or for women with STEMI. We also found that cardiogenic shock and major bleeding were disproportionately more common in younger women and that women were less likely to receive evidence-based treatment after discharge from the hospital. Perhaps the most important and novel finding in our study was that these gender disparities did not decrease over the study period.
The large number of prospectively followed patients with AMI in RIKS-HIA allowed us to address associations between gender and mortality in several clinically important subgroups. Although women as a group had better prognosis than men, we did not find this survival advantage for younger women. These findings support a previous report from Sweden21 and extend those observations to the present time. On the one hand, younger women in our study were more likely to develop cardiogenic shock, which is associated with higher mortality. On the other hand, they were less likely to receive evidence-based pharmacological treatment with statins, aspirin, and ACE inhibitors, which improve survival after MI. Both of these realities could have had a negative impact on the survival of younger women.
Another clinically important subgroup is women with STEMI. Whereas women with NSTEMI had a lower risk of dying than men, women with STEMI had similar long-term mortality and higher short-term mortality than men. Higher mortality in women early after STEMI was reported previously.6,22–24 Some studies have attributed this finding to lower rates of revascularization in women.25 In our cohort, women with STEMI were less likely to undergo coronary angiography. This finding was unexpected because coronary angiography should be performed in all eligible patients with STEMI regardless of gender.15,26 The reason for this difference between the genders is not clear. On average, women were older than men, and more women at extreme age with STEMI were hospitalized and did not have angiography performed. Subgroup analyses, however, showed that women with STEMI were less likely than men to undergo coronary angiography and PCI regardless of age category, and that suggests the presence of systematic undertreatment.
Women with STEMI were more likely to develop acute heart failure and cardiogenic shock. The risk of these complications increases if reperfusion treatment is delayed; however, we have not found a difference between the genders in time from first ECG to reperfusion. It is known that women, on average, seek medical attention later in the course of the disease and that the time course of myocardial injury differs between the genders.27,28 These facts could partially explain the higher risk of acute heart failure and cardiogenic shock in women. Similar to previous reports, we found that women had a higher risk of major bleeding.28
Gender disparities in mortality and treatment did not decrease over the past 2 decades. This finding is novel and somewhat surprising, given Sweden’s egalitarian social and political traditions, particularly with regard to equality between the genders. Despite several nationwide campaigns and administrative regulations aimed at reducing inequalities in health care over past decades, inequalities between the genders are still present in substantial measure.29 The decreased risk of developing cardiogenic shock in women, however, could be a positive result of these campaigns.
Younger women and women with STEMI were less likely than their male counterparts to receive evidence-based treatment at discharge. Instead, they were more likely to be treated with long-acting nitrates. Some women may have suffered from atypical MI in which no overt coronary artery disease was diagnosed on the coronary angiogram. Angiographic absence of coronary artery disease may have led the treating physician to omit evidence-based medication. The differences between the genders in prescription of antiplatelet medication could be related to the higher risk of major bleeding among women, which has been reported in many studies.28 In our study, major bleeding during hospitalization was more common among female patients, and major bleeding was associated with lower prescription of antiplatelet agents. Consequently, the underprescription of antiplatelet drugs for women may be explained partially by physicians’ fear of risk of future bleeding after discharge from the hospital; however, the decision to withhold any evidence-based pharmacological treatment from patients diagnosed with AMI, regardless of angiographic findings, has no foundation in the scientific literature and is not supported by the current guidelines. It is true that the large multicenter clinical trials in which specific pharmacological treatment after MI was evaluated have been dominated by male patients, but female patients were also substantially represented in these studies. Current guidelines for secondary prevention after MI are based on the information from many studies in which coronary angiography was not performed. Evidence-based treatment should thus be prescribed to all eligible patients after MI, independent of gender.15,26
This study has several limitations. First, it is an observational retrospective study and, as such, provides only associative, not causative, evidence. In contrast, the observational nature of our study provides real-world data on a large cohort of patients. Second, we do not have data on cause-specific mortality, including cardiovascular mortality; however, current Academic Research Consortium criteria favor reporting of all-cause mortality rather than cardiac mortality in cardiovascular research.30 Third, a proportion of patients had missing data. The exclusion of these patients from the analysis might have produced biased results; however, results from the multiple imputation model were congruent with the data from the complete case analysis. Last, we performed many statistical analyses; therefore, statistical significance could occur by chance in 1 of 20 comparisons.
Conclusion
On average, women have better adjusted prognosis than men after AMI; however, younger women and women with STEMI have disproportionately poor short- and long-term prognosis and are less likely than men to be prescribed evidence-based treatment. Women are less likely than men to be prescribed evidence-based treatment after AMI. We cannot rule out that this reflects a sexist bias. Effective measures within the health care system are needed to neutralize undertreatment of women after MI.
Sources of Funding
This work was supported by AFA Insurance [AFA 110115]; Swedish Heart and Lung Foundation [HLF 20120670]; Swedish Scientific Research Council [VR 2008-2487]; ALF Västra Götaland; and University of Gothenburg, Sweden [ALFGBG 141131].
Disclosures
None.
References
- Tillmanns H, Waas W, Voss R, Grempels E, Holschermann H, Haberbosch W, Waldecker B. Gender differences in the outcome of cardiac interventions. Herz. 2005;30:375–389. doi: 10.1007/s00059-005-2716-3. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Jacobsen JB, Lash TL, Botker HE, Sorensen HT. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ. 2012;344:e356. doi: 10.1136/bmj.e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman C, Bots ML, van Oeffelen AA, van Dis I, Verschuren WM, Engelfriet PM, Capewell S, Vaartjes I. Population trends and inequalities in incidence and short-term outcome of acute myocardial infarction between 1998 and 2007. Int J Cardiol. 2013;168:993–998. doi: 10.1016/j.ijcard.2012.10.036. [DOI] [PubMed] [Google Scholar]
- Dudas K, Lappas G, Rosengren A. Long-term prognosis after hospital admission for acute myocardial infarction from 1987 to 2006. Int J Cardiol. 2012;155:400–405. doi: 10.1016/j.ijcard.2010.10.047. [DOI] [PubMed] [Google Scholar]
- Smolina K, Wright FL, Rayner M, Goldacre MJ. Determinants of the decline in mortality from acute myocardial infarction in England between 2002 and 2010: linked national database study. BMJ. 2012;344:d8059. doi: 10.1136/bmj.d8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- Hemingway H, Langenberg C, Damant J, Frost C, Pyorala K, Barrett-Connor E. Prevalence of angina in women versus men: a systematic review and meta-analysis of international variations across 31 countries. Circulation. 2008;117:1526–1536. doi: 10.1161/CIRCULATIONAHA.107.720953. [DOI] [PubMed] [Google Scholar]
- Shin JY, Martin R, Suls J. Meta-analytic evaluation of gender differences and symptom measurement strategies in acute coronary syndromes. Heart Lung. 2010;39:283–295. doi: 10.1016/j.hrtlng.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Lagerqvist B, Safstrom K, Stahle E, Wallentin L, Swahn E. Is early invasive treatment of unstable coronary artery disease equally effective for both women and men? FRISC II Study Group Investigators. J Am Coll Cardiol. 2001;38:41–48. doi: 10.1016/s0735-1097(01)01308-0. [DOI] [PubMed] [Google Scholar]
- Mueller C, Neumann FJ, Roskamm H, Buser P, Hodgson JM, Perruchoud AP, Buettner HJ. Women do have an improved long-term outcome after non-ST-elevation acute coronary syndromes treated very early and predominantly with percutaneous coronary intervention: a prospective study in 1,450 consecutive patients. J Am Coll Cardiol. 2002;40:245–250. doi: 10.1016/s0735-1097(02)01949-6. [DOI] [PubMed] [Google Scholar]
- Glaser R, Herrmann HC, Murphy SA, Demopoulos LA, DiBattiste PM, Cannon CP, Braunwald E. Benefit of an early invasive management strategy in women with acute coronary syndromes. JAMA. 2002;288:3124–3129. doi: 10.1001/jama.288.24.3124. [DOI] [PubMed] [Google Scholar]
- Chung SC, Gedeborg R, Nicholas O, James S, Jeppsson A, Wolfe C, Heuschmann P, Wallentin L, Deanfield J, Timmis A, Jernberg T, Hemingway H. Acute myocardial infarction: a comparison of short-term survival in national outcome registries in Sweden and the UK. Lancet. 2014;383:1305–1312. doi: 10.1016/S0140-6736(13)62070-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frobert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angeras O, Calais F, Danielewicz M, Erlinge D, Hellsten L, Jensen U, Johansson AC, Karegren A, Nilsson J, Robertson L, Sandhall L, Sjogren I, Ostlund O, Harnek J, James SK, Trial T. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587–1597. doi: 10.1056/NEJMoa1308789. [DOI] [PubMed] [Google Scholar]
- Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van ‘t Hof A, Widimsky P, Zahger D. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART) Heart. 2010;96:1617–1621. doi: 10.1136/hrt.2010.198804. [DOI] [PubMed] [Google Scholar]
- van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- White IR, Royston P. Imputing missing covariate values for the cox model. Stat Med. 2009;28:1982–1998. doi: 10.1002/sim.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB. Inference and missing data. Biometrika. 1976;63:581–590. [Google Scholar]
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- Nielsen S, Bjorck L, Berg J, Giang KW, Zverkova Sandstrom T, Falk K, Maatta S, Rosengren A. Sex-specific trends in 4-year survival in 37 276 men and women with acute myocardial infarction before the age of 55 years in Sweden, 1987–2006: a register-based cohort study. BMJ Open. 2014;4:e004598. doi: 10.1136/bmjopen-2013-004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra NC, Ziegelstein RC, Rogers WJ, Tiefenbrunn AJ, Gore JM, French WJ, Rubison M. Observations of the treatment of women in the United States with myocardial infarction: a report from the National Registry of Myocardial Infarction-I. Arch Intern Med. 1998;158:981–988. doi: 10.1001/archinte.158.9.981. [DOI] [PubMed] [Google Scholar]
- Gan SC, Beaver SK, Houck PM, MacLehose RF, Lawson HW, Chan L. Treatment of acute myocardial infarction and 30-day mortality among women and men. N Engl J Med. 2000;343:8–15. doi: 10.1056/NEJM200007063430102. [DOI] [PubMed] [Google Scholar]
- Greenland P, Reicher-Reiss H, Goldbourt U, Behar S. In-hospital and 1-year mortality in 1,524 women after myocardial infarction. Comparison with 4,315 men. Circulation. 1991;83:484–491. doi: 10.1161/01.cir.83.2.484. [DOI] [PubMed] [Google Scholar]
- Kudenchuk PJ, Maynard C, Martin JS, Wirkus M, Weaver WD. Comparison of presentation, treatment, and outcome of acute myocardial infarction in men versus women (the Myocardial Infarction Triage and Intervention Registry) Am J Cardiol. 1996;78:9–14. doi: 10.1016/s0002-9149(96)00218-4. [DOI] [PubMed] [Google Scholar]
- Kushner FG, Hand M, Smith SC, Jr, King SB, III, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE, Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Reimer KA, Jennings RB. The “wavefront phenomenon” of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest. 1979;40:633–644. [PubMed] [Google Scholar]
- Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, Van de Werf F, Aylward P, Topol EJ, Califf RM. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. N Engl J Med. 1999;341:226–232. doi: 10.1056/NEJM199907223410402. [DOI] [PubMed] [Google Scholar]
- Smirthwaite G, Tengelin E, Borrman T, Bjornstrom A. (In)equalites in health and healthcare. Stockholm: Sveriges kommuner och landsting; 2014. [Google Scholar]
- Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW Academic Research C. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]