Abstract
Background
Guidelines recommend exercise for cardiovascular health, although evidence from trials linking exercise to cardiovascular health through intermediate biomarkers remains inconsistent. We performed a meta-analysis of randomized controlled trials to quantify the impact of exercise on cardiorespiratory fitness and a variety of conventional and novel cardiometabolic biomarkers in adults without cardiovascular disease.
Methods and Results
Two researchers selected 160 randomized controlled trials (7487 participants) based on literature searches of Medline, Embase, and Cochrane Central (January 1965 to March 2014). Data were extracted using a standardized protocol. A random-effects meta-analysis and systematic review was conducted to evaluate the effects of exercise interventions on cardiorespiratory fitness and circulating biomarkers. Exercise significantly raised absolute and relative cardiorespiratory fitness. Lipid profiles were improved in exercise groups, with lower levels of triglycerides and higher levels of high-density lipoprotein cholesterol and apolipoprotein A1. Lower levels of fasting insulin, homeostatic model assessment–insulin resistance, and glycosylated hemoglobin A1c were found in exercise groups. Compared with controls, exercise groups had higher levels of interleukin-18 and lower levels of leptin, fibrinogen, and angiotensin II. In addition, we found that the exercise effects were modified by age, sex, and health status such that people aged <50 years, men, and people with type 2 diabetes, hypertension, dyslipidemia, or metabolic syndrome appeared to benefit more.
Conclusions
This meta-analysis showed that exercise significantly improved cardiorespiratory fitness and some cardiometabolic biomarkers. The effects of exercise were modified by age, sex, and health status. Findings from this study have significant implications for future design of targeted lifestyle interventions.
Keywords: biomarker, cardiometabolic health, cardiovascular disease prevention, exercise training
Cardiovascular disease (CVD) remains a leading cause of morbidity and mortality affecting ≈84 million people in the United States.1–3 Current guidelines recommend exercise for both primary and secondary prevention of CVD.4–6 Observational studies have associated exercise with lower CVD risk in populations free of preexisting CVD.7–9 Substantial evidence from secondary prevention studies also confirms better survival and reduced CVD recurrence after exercise interventions.10,11 Because of apparent ethical and feasibility issues, however, no long-term randomized controlled trials (RCTs) have directly investigated the benefits and risks of exercise training in relation to CVD incidence.12 Consequently, exercise interventions among healthy populations have focused on intermediate CVD biomarkers. Changes in circulating CVD biomarkers and cardiorespiratory fitness (CRF) are reasonable indicators for the favorable effects of exercise training on cardiovascular health.
An important component of health-related fitness, CRF refers to the capacity of respiratory and cardiovascular systems to provide muscles with oxygen during sustained and/or intense exercise. Available evidence has shown that CRF can significantly improve the predictive ability of both short- and long-term CVD risk when added to traditional risk factors.13 In addition to serving as a diagnostic and prognostic health indicator in clinical settings, CRF has been used as an indicator of habitual exercise.14,15
Traditional CVD biomarkers, such as non–high-density lipoprotein cholesterol and high-sensitivity C-reactive protein, may also have the potential to be used in CVD risk prediction.16–19 Although most previous studies examining the relationship between exercise and circulating biomarkers focus on commonly measured CVD biomarkers, an increasing number of studies are evaluating novel biomarkers.20,21 Evidence has implicated, for example, relevant biomarkers in insulin resistance and inflammation that contribute to CVD development.22–26
Nevertheless, much remains uncertain concerning the effects of exercise on both traditional and novel CVD biomarkers for targeted interventions and clinical evaluations.20,21,27 The primary objective of this meta-analysis was to assess the effects of exercise training on CRF and a variety of both traditional and novel circulating CVD biomarkers. Furthermore, we aimed to investigate the sources of heterogeneity, especially by potential effect modifiers such as age, sex, obesity, lifestyle, preexisting conditions (type 2 diabetes, hypertension, hyperlipidemia, or metabolic syndrome), and intervention duration and intensity.
Methods
Data Sources and Searches
We developed and followed a standardized protocol to do this meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.28 Two investigators (X.L., X.Z.) independently conducted literature searches of Medline, Embase, and the Cochrane Central Register of Controlled Trials published from January 1965 (index date) to March 2014, using keywords and Medical Subject Headings (Table1). All relevant studies and review articles (including meta-analysis) and the reference lists of the identified articles were checked manually. Any disagreements between 2 investigators were resolved by consensus. Institutional review board approval is not applicable because the current study is a systematic review and meta-analysis, which is not considered research involving human subjects.
Table 1.
Search Strategy for Medline
| 1. | exp Exercise/ |
| 2. | physical activity.ab. |
| 3. | aerobic*.ab. |
| 4. | or/1 to 3 |
| 5. | exp Biological Markers/ |
| 6. | Exercise Tolerance/ |
| 7. | Exercise Test/ |
| 8. | exp Oxygen Consumption/ |
| 9. | Physical Fitness/ |
| 10. | or/5 to 9 |
| 11. | randomized controlled trial.pt. |
| 12. | controlled clinical trial.pt. |
| 13. | Randomized Controlled Trials/ |
| 14. | Random Allocation/ |
| 15. | Intervention Studies/ |
| 16. | or/11 to 15 |
| 17. | 4 and 10 |
| 18. | 17 and 16 |
| 19. | limit 18 to English language |
| 20. | limit 19 to humans |
Study Selection
Articles were included (1) if the study was an RCT that assigned at least 1 group of participants to exercise training and 1 group to control and (2) if CRF (absolute and relative maximal oxygen uptake) or circulating CVD biomarkers of lipid and lipoprotein metabolism, glucose intolerance and insulin resistance, systemic inflammation, or hemostasis were measured at baseline and at the end of the trial.
All abstracts about RCTs reporting the effect of exercise training on CVD-related biomarkers or CRF were included for screening. We excluded studies (1) if the study design was not a RCT; (2) if the exercise intervention was acute (≤1 week), because we are interested in the effects of exercise interventions of moderate to long duration; (3) if interventions were based on education or counseling rather than a structured exercise training assignment; (4) if maximal oxygen consumption, or VO2max, was indirectly calculated through heart rate or fixed time testing and no other biomarkers of interest to this study were reported; (5) if levels of circulating biomarkers were not directly measured; (6) if values of outcome measures at the end of trials were not reported; (7) if participants had severe chronic diseases (preexisting CVD, liver or kidney diseases, or cancers), any other conditions that could potentially compromise participants’ capacity to exercise (disability, frailty, declined activities of daily living, or wheelchair dependency), or any mental conditions (depression, anxiety, schizophrenia, bipolar disorder, Parkinson’s disease, or Alzheimer’s disease); (8) if participants were identified as trained professionals, athletes, or soldiers; (9) if participants were infants, children, or adolescents; or (10) if participants were pregnant, postpartum, nursing, had recent surgery, or were undergoing rehabilitation exercise. If multiple articles were published based on the same trial, data were retrieved as 1 independent trial. If there were duplicate results from the same trial, the most updated and comprehensive ones were extracted.
Data Extraction and Quality Assessment
In total, 6135 articles were retrieved from the literature search. We excluded 5796 articles after abstract review and 170 after full-text examination. Data extraction was conducted independently by 2 investigators (X.L., X.Z.), and discrepancies were resolved through consensus. The following information was extracted from all eligible studies: general information (first author’s name, article title, and country of origin), study characteristics (study design, eligibility criteria, randomization, blinding, cointervention, dropout rate, and reason for dropping out), participant characteristics (age, sex, ethnicity, body mass index, life style, health status, and number of participants in each group), intervention and setting (exercise type, duration, intensity, and supervision), and outcome measures (definition of outcomes, statistical techniques, pre- and postintervention means, standard deviation, sample size of each arm, and adverse events). Maximal oxygen uptake VO2max was measured directly and determined based on the highest VO2 obtained prior to volitional fatigue. In this meta-analysis, we focused on biomarkers in blood samples, including plasma, serum, and whole blood. All samples for fasting glucose and insulin measurement in the studies were collected after >10 hours of fasting.
Data Synthesis and Analysis
Methodological quality was assessed by 2 investigators (X.L., X.Z.) using the Cochrane Collaboration’s tool for assessing risk of bias.29 This included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. For each trial, the risk of bias was reported as low risk, unclear risk, or high risk. Disagreement was resolved by discussion. All eligible comparisons from each trial were extracted separately according to exercise intensity. The criteria for classifying exercise interventions as moderate exercise or vigorous exercise are summarized in Table2. If the intensity measures were not reported in individual studies, maximum heart rate, maximum heart rate percentage, speed of running, metabolic equivalent, oxygen uptake, or relative metabolic rate were used to classify exercise intensity. To maintain independence, the most vigorous intervention and the control group in each trial were included in the primary analysis if multiple training groups of different intensities were compared with a single control group. Sensitivity analyses were performed by conducting separate analyses of all eligible comparisons for moderate and vigorous exercise interventions, respectively.
Table 2.
Criteria Used for Exercise Intensity Classification
| Moderate | Vigorous | |
|---|---|---|
| Maximum heart rate, beats/min | <140 | ≥140 |
| Maximum heart rate, % | <75 | ≥75 |
| Speed of running, m/s | <6.8 | ≥6.8 |
| Metabolic equivalents | Women: <6 | Women: ≥6 |
| Men: <8 | Men: ≥8 | |
| Oxygen uptake (% of VO2max) | <70 | ≥70 |
| Relative metabolic rate | <8 | ≥8 |
Mean levels and standard deviations of CRF and CVD biomarkers after the exercise interventions from individual trials were used to calculate weighted mean differences (WMDs) and 95% CIs using DerSimonian and Laird random-effects models.30 Between-study heterogeneity was examined using Q statistics and I2 statistics.31,32 I2 ≈25%, 50%, and 75% is suggestive, respectively, of low, medium, and high heterogeneity. Egger’s tests were used to formally test publication bias.33 If there was any evidence of publication bias, the trim and fill method was used to evaluate the impact of publication bias.34
All eligible trials were analyzed in subgroup analyses conducted within the strata of the predetermined potential modifiers, including age (mean or median <50 versus ≥50 years), sex (women versus men), body mass index (obese versus nonobese), lifestyle (active versus sedentary), health status (having at least 1 of the following comorbidities: type 2 diabetes, hypertension, hyperlipidemia, and metabolic syndrome versus none), and trial duration (≥16 versus <16 weeks). Obesity was defined as body mass index ≥30 kg/m2. Active lifestyle was defined according to the report of individual trials. Health status was confirmed by clinical diagnosis or reported medication use. Metaregressions were performed to evaluate the overall impact of potential modifiers.
Two-sided P≤0.05 was used as the significance level except for the Q statistic and the Egger’s tests (P=0.10).35 All statistical analyses were performed with Stata statistical software version 12 (Stata Corp).
Results
Figure1 shows the number of trials included in the analysis for each outcome. A total of 7487 participants aged between 18 and 90 years, from 169 articles based on 160 RCTs, were included in the meta-analysis. Characteristics of eligible studies are summarized in Table3. Among all participants, 4276 (57.1%) were women; 3211 (42.9%) were men; 5845 (78.1%) were free of type 2 diabetes, hypertension, hyperlipidemia, or metabolic syndrome; and 1640 (21.9%) had at least 1 of those conditions. The median duration of trials was 12 weeks (range: 2 weeks to 2 years).
Figure 1.

Summary of study selection process. In total, 6135 articles were retrieved from the literature search that evaluated the effect of exercise interventions on CRF or cardiometabolic biomarkers. We excluded 5796 articles after abstract review and 170 after full text examination. After exclusion, 160 RCTs reported in 169 articles were included in the meta-analysis. Apo AI indicates apolipoprotein A1; Apo AII, apolipoprotein A2; Apo B, apolipoprotein B; CRF, cardiorespiratory fitness; CRP, C-reactive protein; FFA, free fatty acid; HbA1c, glycosylated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOME-B, homeostatic model assessment-beta cell function; HOMA-IR, homeostatic model assessment–insulin resistance; HOMA-S, homeostatic model assessment-insulin sensitivity; ICAM-1, intercellular adhesion molecule 1; IGF-1, insulin-like growth factor 1; IGF-BP, insulin-like growth factor binding protein; IL, interleukin; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); PAI-1, plasminogen activator inhibitor-1; RCTs, randomized controlled trials; TC, total cholesterol; TG, triglycerides; TNF-α, tumor necrosis factor α; VCAM-1, vascular cell adhesion molecule 1; VLDL-C, very low-density lipoprotein cholesterol.
Table 3.
Characteristics of the Trials Included in the Meta-Analysis
| Study | Age, y | Sex | BMI, kg/m2 | Duration, wk | Activity/Medical Condition | Exercise Type, Intensity and Cointerventions | NT/NC | Marker |
|---|---|---|---|---|---|---|---|---|
| Abderrahman, 201336 | Mean: 20.6 | Male only | Mean: 22.8 | 7 | NR/Health | Running/Vigorous/No | 9/6 | Absolute CRF, Relative CRF |
| Ahmaidi, 199837 | 53 to 74 | NR | NR | 12 | Sedentary/Health | Walking/Jogging/Vigorous/No | 11/11 | Absolute CRF, Relative CRF |
| Aldred, 199538 | 41 to 55 | Female only | T: 24.8±1.0 C: 26.8±0.8 | 12 | Sedentary/Health | Walking/Moderate/No | 11/11 | TC, HDL-C2 LDL-C, FFA |
| Ashutosh, 199739 | 20 to 60 | Female only | Overweight or obesity | 46 | NR/Health | Aerobic exercise/NR/Dietary intervention | 9/6 | Absolute CRF, Relative CRF |
| Asikainen, 200240 | 48 to 63 | Female only | Mean: 26.2 | 24 | Sedentary/Health | Walking/Vigorous/No | 20/38 | Relative CRF |
| Baker, 198641 | Mean: 58.2 | Male only | NR | 20 | Sedentary/Health | Aerobic training/Vigorous/No | 20/14 | Absolute CRF, Relative CRF, TC, HDL-C, LDL-C, VLDL-C |
| Balducci, 2010, 201242 | C: 58.8±8.6 T: 58.8±8.5 | NR | C: 31.9±4.6 T: 31.2±4.6 | 52 | Sedentary/Diabetes mellitus | Aerobic and resistance training/Moderate/No | 288/275 | Relative CRF, TC, TG, HDL-C, LDL-C, CRP, Fasting glucose, Insulin, HOMA-IR HbA1c |
| Beavers, 201043 | 60 to 79 | Female: 67% | >28.0 | 78 | Active/Health | Walking and interactive, health education in control | 97/93 | Leptin |
| Bell, 201044 | Male: 49±11 Female: 50±9 | NR | Mean: 30 | 24 | Sedentary/Health | Walking/Moderate/No | 43/45 | Absolute CRF, Relative CRF, TC, TG, HDL-C, LDL-C, Fasting glucose |
| Bermon, 199945 | 67 to 80 | Male: 16 | T: 24.9±0.5 C: 25.9±0.6 | 8 | Sedentary/Health | Strength training/Vigorous/No | 16/16 | IGF-1, IGF-BP |
| Biddle, 201146 | Mean: 34.8±12.6 | Female 13 | Mean: 36.3±6.7 | 4 | Sedentary/Health | Small-sided games-based exercise/NR/No | 9/7 | Absolute CRF, Relative CRF, TC, TG, HDL-C, LDL-C, CRP, Fasting glucose, HbA1c |
| Blumenthal, 199147 | 60 to 83 | Female: 50% | NR | 60 | Sedentary/Health | Aerobic exercise or yoga/Vigorous/No | 15/15 | Absolute CRF, Relative CRF |
| Blumenthal, 199148 | 29 to 59 | Male: 62% | Mean: 26.9 | 16 | NR/untreated mild hypertension | Aerobic exercise training/Joging | 39/22 | Absolute CRF, Relative CRF |
| Boardley, 200749 | ≥65 | Male: 27% | NR | 16 | Sedentary/Health | Resistance training and aerobic walking/Moderate/No | 33/35 | TC, TG, HDL-C, LDL-C |
| Bobeuf, 201150 | 59 to 73 | Female: 52.6% | Mean: 26.2±2.6 | 24 | Sedentary/Health | Resistance training/Vigorous/Vitamins C/E supplementation | 17/12 | TC, TG, HDL-C, LDL-C |
| Boreham, 200051 | 18 to 22 | Female only | NR | 7 | Sedentary/Health | Stair climbing/Moderate/No | 12/10 | TC, HDL-C |
| Boudou, 200352 | Mean: 45.4±7.2 | Male only | Mean: 29.6±4.6 | 8 | NR/Diabetes mellitus | Endurance exercise/Vigorous/No | 8/8 | Adiponectin, Leptin, Insulin |
| Bourque, 199753 | 23 to 43 | Female only | Mean: 23.1±4.9 | 12 | Sedentary/Health | Endurance exercise/Vigorous/No | 6/7 | Relative CRF |
| Braith, 199454 | 60 to 79 | Female: 54.5% | NR | 24 | Sedentary/Health | Walking/Vigorous/No | 14/11 | Relative CRF |
| Broeder, 199255 | 18 to 35 | Male only | Mean: 25.3 | 12 | NR/Health | Walk or jog/Vigorous/No | 15/19 | Relative CRF |
| Broman, 200656 | 69±4 | Female only | NR | 8 | NR/Health | In deep water running/Walking/Vigorous/No | 15/9 | Absolute CRF, Relative CRF |
| Burr, 201157 | Mean: 26 | NR | NR | 6 | Sedentary/Health | Vehicle riding/Vigorous/No | 34/12 | Relative CRF, Fasting glucose |
| Camargo, 200858 | Mean: 29 | Male only | Mean: 27.3 | 12 | Sedentary/Health | Aerobic training/Moderate/No | 7/7 | Relative CRF |
| Campbell, 200759 | 40 to 75 | Female only | 29.9 to 28.7 | 52 | Sedentary/Health | Aerobic Exercise/Moderate/No | 17/15 | Absolute CRF, Relative CRF, CRP |
| Canuto, 201260 | 18 to 64 | Female only | Mean: 34.8 | 12 | NR/Health | Resistance training/Moderate/Education | 29/30 | TC, TG, HDL-C, LDL-C, CRP, Fasting glucose, Insulin, HbA1c |
| Carroll, 201261 | T: 39.3±7.8 C: 41.0±7.7 | Female only | T: 39.9±7.4 C: 41.0±7.7 | 12 | Sedentary/Health | Treadmill walking/Moderate/Lifestyle intervention | 22/22 | Absolute CRF, Relative CRF |
| Chan, 201362 | Mean: 54±11 | Female only | Mean: 31±7 | 10 | Sedentary/Hypertension | Treadmill walking/Vigorous/Education | 10/13 | Relative CRF, |
| Chandler, 199663 | 60 to 79 | Female: 38.6% | NR | 24 | NR/Health | Endurance training/Moderate/No | 16/11 | Relative CRF, PAI-1 |
| Cho, 201164 | 34 to 60 | Female only | Mean: 25.6 | 12 | Sedentary/Health | Walking/Moderate/No | 13/10 | Relative CRF,TG, HDL-C FFA, Fasting glucose, Insulin, HOMA-IR |
| Christiansen, 201065 | 18 to 45 | Female: 38 | 30 to 40 | 12 | Sedentary/Health | Aerobic exercise/Vigorous/Dietary intervention | 21/19 | Absolute CRF,TC, TG, HDL-C, FFA, IL-6, IL-18, Adiponectin, Fasting glucose, Insulin, HOMA-IR |
| Church, 200766 | 45 to 75 | Female only | 25 to 43 | 24 | Sedentary/Health | Aerobic exercise/Moderate/No | 103/102 | Absolute CRF, relative CRF, TG, HDL-C, LDL-C, Fasting glucose |
| Ciolac, 201167 | 20 to 30 | Female only | Mean: 23.78 | 16 | Sedentary/Health | Endurance exercise/Vigorous/No | 11/12 | Relative CRF,TC, TG, HDL-C, LDL-C, Fasting glucose, Insulin, HOMA-IR |
| Coker, 200968 | 65 to 90 | Female: 50% | 26 to 37 | 12 | NR/Health | Cycle ergometer/Moderate/No | 6/6 | Absolute CRF |
| Cortez-Cooper, 200869 | 40 to 80 | Female: 73.8% | <30 | 13 | Sedentary/Health | Aerobic exercise strength training vs stretching/Moderate/No | 12/12 | Relative CRF,TC, TG, HDL-C, LDL-C, Endothelin-1, Fasting glucose |
| Cox, 199370 | 20 to 45 | Male only | Mean: 26.3 (25.7 to 26.9) | 4 | Sedentary/Health | Not report/Vigorous/Drink low-alcohol beer or continue their normal drinking habits | 19/16 | TC, TG, HDL-C, HDL-C2, HDL-C3, LDL-C, Apo AI, Apo AII, Apo B |
| Cox, 200371 | Mean: 42.4±5.0 | Male only | Overweight or obesity | 16 | Sedentary/Hypertension | NR/Moderate & vigorous/Dietary intervention and usual dietary | 13/17 | Absolute CRF |
| Dalleck, 200972 | 45 to 75 year | Female only | Normal | 12 | Sedentary/Health | NR/Moderate/No | 8/10 | Absolute CRF, Relative CRF,TC, TG, HDL-C, LDL-C, Fasting glucose |
| De Vito, 199973 | 60 to 70 | Female only | NR | 12 | Sedentary/Health | Walking/Moderate/No | 11/9 | Absolute CRF, Relative CRF |
| Dimeo, 201274 | 42 to 78 | Female: 58% | Mean: 29.4 | 12 | NR/Hyperlipidemia | Walking on a treadmill/NR/No | 22/25 | Relative CRF |
| Dipietro, 200675 | 62 to 84 | Female only | Mean: 27.3 | 36 | Sedentary/Health | Aerobic training/Moderate/No | 9/7 | Relative CRF,FFA, Fasting glucose, Insulin |
| Duncan, 199176 | 20 to 40 | Female only | NR | 24 | Sedentary/Health | Walk/Moderate/No | 12/13 | Relative CRF,TC, TG, HDL-C, LDL-C |
| Duscha, 200577 | 40 to 65 | NR | 25 to 35 | 36 | NR/Hyperlipidemia | Walking/Moderate/No | 25/37 | Absolute CRF, Relative CRF |
| Eguchi, 201278 | 20 to 65 | Female only | Mean: 25.1±3.9 | 12 | NR/Health | Endurance training using bicycle ergometers/Moderate/No | 8/10 | Absolute CRF, Relative CRF,TC, TG, HDL-C, LDL-C, Fasting glucose, HbA1c |
| Fatouros, 200579 | 65 to 78 | Male only | 28.7 to 30.2 | 24 | Sedentary/Health | Resistance exercises/Moderate/No | 12/10 | Relative CRF, Adiponectin, Leptin, Fasting glucose, HOMA-IR |
| Finucane, 201080 | 67.4 to 76.3 | Female: 44% | Mean: 27.2 | 12 | NR/Health | Cycle ergometer/Moderate/No | 48/48 | TC, TG, HDL-C, LDL-C, Fasting glucose, HbA1c |
| Friedenreich, 201181 | 50 to 74 | Female only | 22 to 40 | 52 | Sedentary/Health | Aerobic exercise/Vigorous/No | 154/154 | Adiponectin, Leptin, Fasting glucose, Insulin, HOMA-IR, IGF-1, IGF-BP |
| Garber, 199282 | 24 to 50 | Female: 75% | NR | 8 | Sedentary/Health | Walk-jog/Moderate/No | 13/9 | Relative CRF |
| Geogiades, 200083 | ≥29 | Female: 44% | 25 to 37 | 24 | Sedentary/Hypertension | Aerobic exercise/Vigorous/No | 36/19 | Relative CRF |
| Gormley, 200884 | 18 to 31 | Female: 65.5% | Mean: 24.3 | 6 | Sedentary/Health | Aerobic/Moderate/No | 14/13 | Relative CRF |
| Gram, 201085 | 25 to 80 | Female: 45.6% | NR | 52 | NR/Diabetes mellitus | Strength training and aerobic exercise/Moderate/No | 21/20 | Absolute CRF,TC, HDL-C, LDL-C, HbA1c |
| Grandjean, 199686 | NR | Female only | NR | 24 | Sedentary/Health | Walking and jog and cycling/Vigorous/No | 20/17 | Absolute CRF,TC, TG, HDL-C, LDL-C, VLDL-C |
| Gray, 200987 | 18 to 65 | Female: 77% | Mean: 28.6 | 12 | Sedentary/Health | Pedometer-based walking/Moderate/No | 24/24 | CRP, IL-6, TNF-α, Fasting glucose, Insulin, HOMA-IR |
| Guadalupe-Grau, 200988 | Mean: 23.9±2.4 | Female: 34.8% | C: 24.0±3.6 T: 22.8±2.0 | 9 | Active/Health | Strength combined with plyometric jumps training/Vigorous/No | 8/15 | Leptin |
| Hagan, 198689 | Mean: 36.6 | Female: 50% | Normal | 12 | Sedentary/Health | Aerobic training/Moderate/Dietary training | 12/12 | Absolute CRF, Relative CRF,TC, TG, HDL-C, LDL-C, VLDL-C |
| Hass, 200190 | 35 to 55 | Female: 50% | NR | 12 | Sedentary/Health | NR/Moderate/No | 17/9 | Absolute CRF, Relative CRF |
| Hendrickson, 201091 | 18 to 26 | Female only | NR | 12 | Active/Health | Aerobic endurance and strength training/Vigorous/No | 13/10 | Relative CRF |
| Heydari, 201392 | Mean: 24.9±4.3 | Male only | Mean: 28.7±3.1 | 12 | Sedentary/Health | High-intensity intermittent exercise/Vigorous/No | 20/18 | Absolute CRF, Relative CRF |
| Heydari, 201392 | Mean: 24.9±4.3 | Male only | Mean: 28.7 | 12 | Active/Health | High-intensity intermittent exercise/Vigorous/No | 25/21 | Absolute CRF, Relative CRF |
| Hilberg, 201393 | T: 49±6 C: 48±6 | Male only | NR | 12 | NR/Health | NR/Vigorous/No | 22/22 | Relative CRF |
| Hiruntrakul, 201094 | 18 to 25 | Male only | C: 21.35±3.54 T: 20.99±3.35 | 12 | Sedentary/Health | Aerobic exercise/Moderate/No | 19/18 | Relative CRF, HDL-C |
| Ho, 201295 | 40 to 66 | Female: 83.5% | 25 to 40 | 12 | Sedentary/Health | Aerobic resistance training/Moderate/No | 15/16 | Relative CRF, TC, TG, HDL-C, LDL-C, Fasting glucose, Insulin |
| Hu, 200996 | 20 to 45 | Male only | NR | 10 | Sedentary/Health | Progressive strength training/Moderate/No | 48/21 | Absolute CRF, Relative CRF, |
| Huttunen, 197997 | 40 to 45 | Male only | NR | 16 | Sedentary/Health | Walking, Jogging, Swimming, Skiing, or Cycling/Moderate/No | 44/46 | Relative CRF, HDL-C, Apo AI, Apo AII |
| Tsuji, 200098 | 60 to 81 | Female: 53% | NR | 25 | Active/Health | Endurance session with a bicycle ergometer, and a resistance exercise training session using rubber films/Moderate/Education | 31/33 | Relative CRF |
| Irwin, 201299 | 59 to 86 | Female: 61% | NR | 9 | Sedentary/Health | Tai Chi Chih vs health education/Moderate/No | 46/37 | CRP, IL-6, IL-18 |
| Larose, 2011100 | 39 to 70 | Female 36.2% | Mean: 34.9 | 24 | Sedentary/Diabetes mellitus | Aerobic or resistance training/Vigorous/No | 60/63 | Relative CRF, HbA1c |
| Jessup, 1998101 | 61 to 77 | Female: 52% | NR | 16 | Sedentary/Health | Treadmills and stair-climbers/Vigorous/No | 11/10 | Relative CRF |
| Kadoglou, 2012102 | Mean: 61.3±2.1 | Female: 67.6% | T: 32.74±4.05 C: 31.58±5.71 | 12 | NR/Diabetes mellitus | Resistance Exercise/Vigorous/No | 23/24 | Relative CRF, TC, TG, HDL-C, LDL-C, Fasting glucose, Insulin, HOMA-IR, HbA1c, Fibrinogen |
| Karstoft, 2013103 | C: 57.1±3.0 T: 60.8±2.2 | Female: 31% | NR | 16 | NR/Diabetes mellitus | Walking/Moderate/No | 12/8 | Absolute CRF, Relative CRF,TC, TG, HDL-C, LDL-C, Fasting glucose, Insulin, HbA1c |
| King, 1989104 | Male 49±6 Female 47±5 | Female: 50% | NR | 24 | Sedentary/Health | Aerobic/Exercise/Training/Moderate/No | 29/28 | Relative CRF |
| Kirk, 2003105 | 19 to 30 | Male only | 27 to 32 | 36 | Sedentary/Health | Aerobic exercise/Moderate/No | 16/15 | Absolute CRF, Relative CRF |
| Kirk, 2003105 | 19 to 30 | Female only | 27 to 32 | 36 | Sedentary/Health | Aerobic Exercise/Moderate/No | 25/18 | Absolute CRF, Relative CRF |
| Kiviniemi, 2007106 | T: 31±6 C 35±8 | Male only | T: 24±2 C: 25±1 | 4 | Active/Health | Running/Vigorous/No | 9/10 | Absolute CRF, Relative CRF |
| Kokkinos, 1998107 | 35 to 76 | Male only | T: 30±4 C: 31±5 | 16 | Sedentary/Hypertension | Aerobic/Exercise/Moderate/No | 15/19 | TC, TG, HDL-C, HDL-C2, HDL-C3, LDL-C, Apo AI, Apo B |
| Kraemer, 1997,108 1999109 | Mean: 35.4±8.5 | Female only | C: 28.2±4.0 T: 28.3±4.2 | 12 | NR/Health | Aerobic endurance exercise/Vigorous/Dietary intervention | 9/8 | Absolute CRF, Relative CRF, TG, Fasting glucose |
| Krogh, 2012110 | 18 to 60 | Female: 67% | NR | 12 | NR/Health | Aerobic exercise/Vigorous/No | 56/59 | Relative CRF, TC, TG, HDL-C, Fasting glucose, Insulin |
| Krustrup, 2009111 | 20 to 43 | Male only | Mean: 25.7 | 12 | Sedentary/Health | Recreational soccer/Vigorous/No | 12/10 | Relative CRF, TC, HDL-C, LDL-C,Absolute CRF, CRP, Fasting glucose, Insulin |
| Kukkonen-Harjula, 1998112 | 31 to 52 | Female: 53% | 18.5 to 32.7 | 15 | Sedentary/Health | Walking/Training/Moderate/No | 58/58 | Absolute CRF, Relative CRF, Fibrinogen |
| Kurban, 2011113 | T: 53.77±8.2 C: 53.57±6.6 | Female: 51.7% | T: 30.90±4.64 C: 30.23±4.74 | 12 | Sedentary/Diabetes Mellitus | Walking/Moderate/No | 30/30 | Fasting glucose, HbA1c |
| Laaksonen, 2000114 | 20 to 40 | Male only | Mean: 24.4 | 16 | Active/Diabetes mellitus | Sustained running/Moderate/No | 20/22 | Relative CRF, TC, TG, HDL-C, LDL-C, Apo AI, Apo B, HbA1c |
| Labrunee, 2012115 | Mean: 52.7±8.2 | Female: 82.6% | Mean: 38.5±7.6 | 12 | NR/Diabetes mellitus | Cyclergometer training/NR/No | 11/12 | Relative CRF, TC, TG, HDL-C, LDL-C, Fasting glucose, HOMA-IR, HbA1c |
| Lake, 1996116 | 18 to 28 | Male only | NR | 6 | Active/Health | Running training/Moderate/No | 8/7 | Relative CRF |
| LaPerriere, 1994117 | 18 to 40 | Male only | NR | 10 | Sedentary/Health | Aerobic exercise/Vigorous/No | 7/7 | Relative CRF |
| Lee, 2003118 | 18 to 30 | Male only | NR | 2 | Sedentary/Health | Cycle ergometer/Vigorous/No | 12/12 | Relative CRF |
| Lee, 2012119 | 30 to 50 | Female only | ≥25 | 14 | NR/Health | NR/Moderate/No | 8/7 | Relative CRF, TC, TG, HDL-C, LDL-C, CRP, IL-6, TNF-α |
| LeMura, 2000120 | Mean: 20.4±1 | Female only | T: 20.8±2.1 C: 21.8±2.3 | 16 | Sedentary/Health | Resistance training and aerobic training/Vigorous/No | 10/12 | Relative CRF,TC, TG, HDL-C, LDL-C |
| Libardi, 2012121 | T 48.6+5.0 C 49.1+5.5 | Male only | T: 27.5+4.1 C: 24.7+3.3 | 24 | Sedentary/Health | Resistance training/Moderate/No | 12/13 | Relative CRF, TC, TG, HDL-C, LDL-C, CRP, IL-6, TNF-α, Fasting glucose |
| de Lima, 2012122 | 20 to 35 | Female only | C: 23.0±2.4 T: 22.8±3.6 | 12 | Sedentary/Health | Muscular endurance/Moderate/No | 10/8 | Relative CRF |
| Lovell, 2011123 | 70 to 80 | Male only | NR | 20 | Active/Health | Cycle ergometer and stretching/Vigorous/No | 12/12 | Absolute CRF, Relative CRF |
| Martin, 1990124 | T: 58.6±4.6 C 60.6±7.4 | Female only | NR | 12 | Sedentary/Health | Cycle ergometer training/Vigorous/No | 14/14 | Absolute CRF, Relative CRF |
| McAuley, 2002125 | 25 to 70 | Female: 67% | <27 | 16 | NR/Health | NR/Moderate/Dietary intervention | 29/23 | TC, TG, HDL-C, LDL-C, Fasting glucose, Insulin |
| Meckling, 2007126 | 20 to 62 | Female only | 25 to 30 | 12 | NR/Health | Resistance training and endurance training/Moderate and vigorous/Dietary intervention or high protein | 11/8 | TC, TG, HDL-C, Fasting glucose, Insulin |
| Meyer, 2006127 | 30 to 60 | Female: 47% | NR | 12 | Sedentary/Health | Walking or running/Vigorous/No | 12/13 | Relative CRF |
| Miyaki, 2012128 | Mean: 60±6 | Female only | NR | 8 | Sedentary/Health | Walking and cycling/Moderate/No | 11/11 | Relative CRF, TC, TG, HDL-C, LDL-C, Fasting glucose |
| Morey, 2012129 | 60 to 89 | Female: 3% | 25 to 45 kg/m2 | 52 | NR/Health | Enhanced fitness intervention/NR/No | 180/122 | TC, TG, HDL-C, LDL-C, Fasting glucose, Insulin, HOMA-IR HbA1c |
| Morgan, 2010130 | 50 to 70 | Females: 73.3% | NR | 15 | Sedentary/Health | Walk/Moderate/No | 14/15 | TC, HDL-C |
| Morton, 2010131 | T: 61+10 C: 63+9 | Females: 22.2% | T: 32+7 C: 30.9+7.0 | 7 | Sedentary/Diabetes mellitus | Walking/Vigorous/No | 15/12 | Absolute CRF, Relative CRF, Fasting glucose, HbA1c |
| Murphy, 2006132 | Mean: 41.5±9.3 | Female: 64.9% | T: 26.8±5.6 C: 24.4±3.6 | 8 | Sedentary/Health | Walking/Moderate/No | 21/12 | TC, TG, HDL-C, LDL-C, CRP |
| Murtagh, 2005133 | Mean: 45.7±9.4 | Female: 64.6% | <30 | 12 | Sedentary/Health | Walking/Vigorous/No | 18/11 | Relative CRF, TC, TG, HDL-C, LDL-C |
| Musa, 2009134 | 21 to 36 | Male only | Normal | 8 | Sedentary/Health | Interval running/Moderate/No | 20/16 | TC, HDL-C |
| Nemoto, 2007135 | Mean: 63±6 | Female: 75.6% | C: 22.8 T moderate: 22.8 vigorous: 22.9 | 20 | NR/Health | Walking/Moderate/No | 43/37 | Absolute CRF |
| Nicklas, 2009136 | 50 to 70 | Female only | 25 to 40 | 20 | Sedentary/Health | Calorie restriction and aerobic exercise/Moderate/dietary intervention | 36/29 | TG, HDL-C, LDL-C, Fasting glucose, Insulin |
| Niederseer, 2011137 | T: 66.6±2.1 C: 67.3±4.4 | Female: 47.6% | T: 27.1±3.3 C: 25.4±2.8 | 12 | Active/Health | Skiing/Moderate/No | 22/20 | Relative CRF, TC, TG, HDL-C, LDL-C, CRP VCAM-1, ICAM-1, Endothelin-1, e_selectin |
| Nieman, 1993138 | 67 to 85 | Female only | Mean: 23.7 | 12 | Sedentary/Health | Walk/Moderate/No | 14/16 | Relative CRF, TC, TG, HDL-C, LDL-C, |
| Nieman, 1998139 | Mean: 45.6±1.1 | Female only | Mean: 33.1±0.6 | 12 | Active/Health | Walking/Moderate and vigorous/dietary intervention | 22/26 | Absolute CRF, TC, Fasting glucose |
| Nordby, 2012140 | 20 to 40 | Male only | 25 to 30 | 12 | Sedentary/Health | Endurance training (cycling, running, cross-training, or rowing)/Moderate/Dietary intervention | 12/12 | Absolute CRF, Relative CRF, Fasting glucose, Insulin, HbA1c |
| O’donovan, 2005141 | 30 to 45 | Male only | NR | 24 | Sedentary/Health | NR/Moderate/No | 14/15 | Absolute CRF, Relative CRF, TC, TG, HDL-C, LDL-C, Fibrinogen |
| Panton, 1990142 Pollock, 1991143 | 70 to 79 | Female: 53.1% | NR | 24 | Sedentary/Health | Aerobic and resistance training/NR/No | 13/15 | Relative CRF |
| Phillips, 2012144 | 62 to 67 | Female only | Overweight or Obesity | 12 | Active/Health | Aerobic training/Vigorous/No | 11/12 | Leptin |
| Poehlman, 2000145 | 18 to 35 | Female only | C: 22±2 T: 22±2 | 24 | Sedentary/Health | Endurance training (N=14), resistance training/Vigorous/No | 14/20 | Absolute CRF |
| Posner, 1992146 | 60 to 86 | Female: 61.9% | NR | 16 | Sedentary/Health | Cycle ergometer/Moderate/No | 166/81 | Absolute CRF, Relative CRF |
| Probart, 1991147 | ≥70 | Female only | Mean: 24.6 | 26 | NR/Health | Walking on a treadmill/Vigorous/No | 10/6 | Absolute CRF, Relative CRF |
| Pyka, 1994148 | 64 to 78 | Female: 60% | NR | 104 | NR/Health | Resistance exercise (walking and stretching)/Moderate/No | 8/6 | IGF-1 |
| Chow, 1987149 | 50 to 62 | Female only | NR | 52 | NR/Health | Aerobic exercise or aerobic and strengthening exercises/Vigorous/No | 17/15 | Relative CRF |
| Raz, 1988150 | 24 to 26 | Male only | Mean: 22.8 | 9 | Sedentary/Health | Aerobic exercise/Vigorous/No | 28/27 | Relative CRF, TC, TG, HDL-C, HDL-C2, HDL-C3, LDL-C, HbA1c |
| Ready, 1996151 | ≥50 | Female only | NR | 24 | Sedentary/Health | Walk/Moderate/No | 17/18 | Absolute CRF, Relative CRF, TC, TG, HDL-C, LDL-C |
| Romero-Arenas, 2013152 | 55 to 75 | NR | Mean: 29.9 | 12 | Active/Health | Resistance training/Moderate/No | 16/10 | Relative CRF |
| Santa-Clara, 2003,153 2006154 | 45 to 70 | Female only | Caucasian-American T: 25±3 C: 27±5 African-American T: 29±7 C: 29±6 | 24 | Sedentary/Health | Treadmill walking/Jogging, stationary cycling, and rowing/Vigorous/No | 17/16 | Relative CRF, IGF I |
| Santiago, 1995155 | 22 to 40 | Female only | ≥31 | 40 | Sedentary/Hyperlipidemia | Walking/Vigorous/No | 16/11 | Relative CRF, TC, TG, HDL-C, LDL-C |
| Scanga, 1998156 | Mean: 38±7 | Female only | C: 35.2±3.9 T: 36.6±4.3 | 8 | NR/Health | Aerobic and resistance training/Moderate/Dietary intervention | 10/12 | Absolute CRF, Relative CRF |
| Seifert, 2009157 | C: 30±5 T: 32±6 | Male only | 25 to 30 | 12 | Sedentary/Health | Endurance training/Moderate/Endurance training | 10/7 | Fasting glucose |
| Lamina, 2011158 | 50 to 70 | Male only | 20 to 30 | 8 | Sedentary/Hypertension | Bicycle ergometer/Vigorous/No | 112/105 | Relative CRF |
| Sillanpaa, 2009,159 2010160 | 39 to 64 | Female only | Normal | 21 | NR/Health | NR/Vigorous/ | 15/12 | TC, TG, HDL-C, LDL-C, Fasting glucose, Insulin |
| Sloan, 2013161 | T: 54.1±5.8 C: 54.1±4.9 | Female only | T: 29.2±4.9 C: 27.1±5.9 | 16 | Sedentary/Health | Walking/Moderate/No | 16/16 | Relative CRF |
| Spence, 2013162 | NR | Male only | T: 73.0±16.9 C: 81.7±15.23 | 24 | Active/Health | Endurance vs resistance/Moderate/No | 10/13 | Absolute CRF, Relative CRF |
| Stachenfeld, 1998163 | > 65 | Female only | NR | 24 | Active/Health | Aerobic training/Vigorous/No | 9/8 | Relative CRF |
| Stein, 1992164 | T: 46.2±6.1 C: 45.0±6.1 | Male only | NR | 8 | Sedentary/Health | Aerobic exercise training/Moderate/No | 19/14 | Absolute CRF |
| Stensel, 1993165 | 42 to 59 | Male only | Normal | 52 | Sedentary/Health | Brisk/Walking/Moderate/No | 24/24 | TC, TG, HDL-C, LDL-C, VLDL-C, Apo AI, Apo B, Lp-A |
| Stensvold, 2010166 | Mean: 50.2±9.5 | Female: 39.5% | C: 31.9±4.1 T: 32.2±4.2 | 12 | Sedentary/Health | Strength training vs aerobic interval training/Vigorous/No | 11/10 | Relative CRF, TC, TG, HDL-C, Fasting glucose, C-peptide, HbA1c |
| Strasser, 2009167 | >70 | Females: 55.6% | Mean: 26.9 | 24 | Sedentary/Health | Endurance training or–and resistance training/Vigorous/No | 13/14 | Relative CRF |
| Sung, 2012168 | >70 | Female: 65% | NR | 24 | NR/Diabetes mellitus | Walking/Moderate/No | 22/18 | TC, TG, HDL-C, LDL-C, Fasting glucose, HbA1c |
| Takeshima, 2002169 | 60 to 75 | Female only | NR | 7 | Sedentary/Health | Stretching, endurance-type exercise (walking and dancing, 30 min), Resistance exercise/Vigorous/No | 15/15 | TC, TG, HDL-C, LDL-C |
| Takeshima, 2004170 | 60 to 83 | 8 Males and 10 Females | NR | 12 | Sedentary/Health | Progressive accommodating circuit exercise/Vigorous/No | 18/17 | Absolute CRF, TC, TG, HDL-C, LDL-C |
| Thomas, 1984171 | 18 to 32 | Female only | NR | 12 | Active/Health | Running/Vigorous/No | 9/6 | Absolute CRF, Relative CRF, TC, TG, HDL-C |
| Thompson, 2010172 | 45 to 64 | Male only | C: 28.0±2.7 T: 28.5±2.9 | 24 | Sedentary/Health | NR/Moderate/Dietary intervention | 20/21 | Relative CRF,TC, TG, HDL-C, CRP, IL-6, Fasting glucose, Insulin, HOMA-IR |
| Tjonna, 2008173 | Mean: 52.3±3.7 | Female: 53.6% | C: 32.1±3.3 T: 29.4±4.9 | 16 | NR/Health | Aerobic interval training/Vigorous/No | 8/9 | Relative CRF, TG, HDL-C, Adiponectin, Fasting glucose, Insulin, HOMA-B |
| Toledo, 2008174 | >30 | Female: 62.5% | T: 34.8±1.1 C: 33.4±1.2 | 16 | Sedentary/Health | Walking/Moderate/Dietary training | 9/7 | FFA, Fasting glucose, Insulin |
| Tseng, 2013175 | 18 to 29 | Male only | 12 | NR/Health | Aerobic, resistance or combined aerobic and resistance training/Moderate/ | 10/10 | TG, HDL-C, Fasting glucose | |
| Tulppo, 2003176 | 35±10 | Male only | Moderate: 25±3 Vigorous: 25±2 C: 25±3 | 8 | Sedentary/Health | Walking and Jogging/Vigorous/No | 16/11 | Absolute CRF, Relative CRF |
| Utter, 1998177 | 25 to 75 | Female only | 25 to 65 | 12 | Sedentary/Health | Walk/Moderate and vigorous/Dietary intervention | 21/22 | Absolute CRF, Relative CRF |
| Van Aggel-Leijssen, 2001,178 2001179 | C: 38.6±6.5 T: 39.3±7.7 | Male only | C: 32.6±2.5 T: 32.0±2.1 | 12 | Sedentary/Health | Cycling on an ergometer, walking, and aqua-jogging/Moderate/Energy restriction and dietary intervention | 20/17 | Absolute CRF, FFA, Fasting glucose, Insulin |
| Van Den Berg, 2010180 | 18 to 30 | Male only | NR | 7 | Sedentary/Health | Motor-driven treadmill/Moderate/No | 9/13 | Absolute CRF, Relative CRF |
| Vicente-Campos, 2012181 | 62 to 67 | Female: 60% | NR | 28 | Sedentary/Health | Aerobic training/Vigorous/No | 22/21 | TC, TG, HDL-C, LDL-C |
| Vincent, 2002182 | 60 to 83 | Female and Male | NR | 24 | Sedentary/Health | Resistance training/Moderate/No | 24/16 | Relative CRF |
| Vissers, 2010183 | C: 44.8±11.4 T: 44.7±13.0 | Female: 74.7% | C: 29.8±2.6 T: 33.1±3.4 | 52 | Active/Health | Bicycle ergometer/Vigorous/No | 20/20 | TG, HDL-C |
| Vitiello, 1997184 | Male: 66.9±1.0 Female: 67.1±1.7 | Female: 40.3% | NR | 24 | Sedentary/Health | Endurance or stretching/Flexibility/Moderate/No | 30/22 | Relative CRF, IGF-1 |
| Volpe, 2008185 | Mean: 44.2±7.2 | Female only | Mean: 30.5±2.7 | 52 | Sedentary/Health | Skiing/NR/Dietary intervention | 14/14 | TC, TG, HDL-C, LDL-C |
| Waib, 2011186 | 47 to 56 | Training: 60.8% | T: 30.0 (28.8 to 31.2) C: 29.6 (27.8 to 31.5) | 15 | Sedentary/Hypertension | Aerobic training jogging on an electronic treadmill/Moderate/No | 55/24 | Relative CRF, HOMA-IR, C-peptide |
| Wallman, 2009187 | 18 to 64 | Female: 75% | Mean: 30±2 | 8 | Sedentary/Health | Aerobic Exercise/Vigorous/Dietary education | 6/8 | TC, TG, HDL-C, LDL-C |
| Wang, 2005188 | C: 24.7±2.3 T: 23.5±1.6 | Male only | C: 22.7±1.7 T: 23.1±0.6 | 8 | Sedentary/Health | Bicycle ergometer/Moderate/No | 15/15 | Relative CRF |
| Wang, 2011189 | T: 21.5±0.7 C: 22.9±0.4 | Male only | T: 22.9±0.4 C: 23.3±0.7 | 4 | Sedentary/Health | Bicycle ergometer/Moderate/No | 10/10 | Relative CRF |
| Warner, 1989190 | 27 to 63 | Female: 35.3% | NR | 12 | Sedentary/Hyperlipidemia | Aerobic training/Vigorous/Fish oil intervention | 7/7 | Relative CRF, LDL-C, Apo B |
| Warren, 1993191 | Mean: 73.6±0.7 | Female only | Normal | 12 | Sedentary/Health | Walking or calisthenics control/Moderate/No | 14/16 | Relative CRF |
| Watkins, 2003192 | NR | NR | T: 33.4±4.5 C: 34.0±5.2 | 24 | Sedentary/Health | Aerobic training/Vigorous/Weight lost | 14/9 | Relative CRF,TC, TG, HDL-C, LDL-C, Fasting glucose, Insulin |
| Wong, 1990193 | Mean: 62.7±3.1 | Male only | Normal | 52 | NR/Health | Treadmill walking/Moderate/No | 69/69 | Absolute CRF, |
| Woods, 1999194 | Mean: 65±0.8 | NR | NR | 24 | Sedentary/Health | Aerobic exercise/Moderate/No | 14/15 | Absolute CRF, Relative CRF |
| Wu, 2011195 | 45 to 64 | Female: 71.9% | 16.0 to 33.3 | 36 | NR/Health | Aerobic exercise, stretching exercise/Vigorous/No | 68/67 | TG, Adiponectin, Fasting glucose, Insulin, HOMA-IR |
| Yoshizawa, 2009196 | 50 to 65 | Female only | Mean: 23.7 | 8 | Sedentary/Health | Resistance training/Moderate/No | 12/13 | Relative CRF, TC, TG, HDL-C, LDL-C |
| Yoshizawa, 2009197 | 32 to 59 | Female only | T: 24.6±1.1 C: 21.8±1.0 | 12 | Sedentary/Health | Aerobic exercise training/Moderate/No | 12/12 | Relative CRF, TC, HDL-C, LDL-C |
| You, 2006198 | 50 to 70 | Female only | 25 to 40 | 20 | Sedentary/Health | Treadmill/Moderate/Dietary intervention | 13/14 | Absolute CRF, Relative CRF |
| Ziemann, 2011199 | T: 21.6±1.1 C: 21.0±0.9 | Male only | T: 24.5±1.8 C: 23.0±1.9 | 6 | Active/Health | NR/Vigorous/Physical education | 10/11 | Absolute CRF, Relative CRF |
Apo AI indicates apolipoprotein A1; Apo AII, apolipoprotein A2; Apo B, apolipoprotein B; BMI, body mass index; C, control group; CRF, cardiorespiratory fitness; CRP, C-reactive protein; FFA, free fatty acid; HbA1c, glycosylated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment–insulin resistance; ICAM-1, intercellular adhesion molecule 1; IGF-1, insulin-like growth factor 1; IGF-BP, insulin-like growth factor binding protein; IL, interleukin; LDL-C, low-density lipoprotein cholesterol; NR, not reported; PAI-1, plasminogen activator inhibitor-1; T, training group; TC, total cholesterol; TNF, tumor necrosis factor; VCAM-1, vascular cell adhesion molecule 1; VLDL-C, very low-density lipoprotein cholesterol.
Description of Study Quality
The quality of studies included was heterogeneous (Figure2). Random sequence generation was reported in 50 trials, and allocation concealment was reported in 20 trials; only 1 of these trials showed a high probability of selection bias because the random allocation was not concealed. The risk of potential performance bias was high in all trials because it was not possible to blind participants and trainers in exercise interventions. Among 26 trials reporting the blinding of outcome assessment, the risk of detection bias was high in only 1 trial. The risk of other bias was high in 46 trials because of poor compliance, the use of intention-to-treat analysis, limited sample sizes, or limitations discussed in individual articles.
Figure 2.

Assessment of risk of bias: summary for items of bias.
Cardiorespiratory Fitness
A total of 67 and 123 independent comparisons were included in the primary analysis for absolute and relative CRF, respectively (Table4). Both measures were significantly raised by exercise interventions (both P<0.001). The WMDs comparing exercise groups and control groups were 0.28 L/min (95% CI 0.23 to 0.33; I2=93.7%; P<0.001 for heterogeneity) for absolute CRF and 3.90 mL/kg per minute (95% CI 3.45 to 4.35; I2=91.4%; P<0.001 for heterogeneity) for relative CRF. The Egger’s tests showed evidence of publication bias in both instances (P<0.05). When applying the trim and fill method, the conclusion regarding the associations between exercise training and CRF did not change (filled analysis for absolute CRF: WMD 0.14 L/min, 95% CI 0.20 to 5.28, P<0.001; filled analysis for relative CRF: WMD 2.56 mL/kg per minute, 95% CI 3.06 to 10.16, P<0.001).
Table 4.
WMDs in Cardiorespiratory Fitness and Circulating Concentrations of Biomarkers Between Exercise Groups and Control Groups
| Outcome | No.* | Number of Participants | WMD | 95% CI | P WMD | |
|---|---|---|---|---|---|---|
| Exercise | Control | |||||
| Cardiorespiratory fitness | ||||||
| Absolute, L/min | 67 | 1448 | 1272 | 0.28 | 0.23 to 0.33 | <0.001 |
| Relative, mL/kg per minute | 122 | 2543 | 2249 | 3.94 | 3.48 to 4.39 | <0.001 |
| Lipid and lipoprotein markers | ||||||
| TC, mg/dL | 68 | 1754 | 1604 | 1.16 | −9.28 to 11.99 | 0.82 |
| TG, mg/dL | 66 | 1851 | 1703 | −5.31 | −10.63 to −0.89 | 0.02 |
| HDL-C, mg/dL | 74 | 1967 | 1800 | 2.32 | 1.16 to 3.87 | <0.001 |
| HDL2-C, mg/dL | 5 | 91 | 92 | 0.39 | −1.93 to 2.32 | 0.8 |
| HDL3-C, mg/dL | 3 | 62 | 62 | −0.08 | −1.55 to 1.55 | 0.94 |
| LDL-C, mg/dL | 59 | 1681 | 1525 | 3.87 | −8.12 to 0.39 | 0.08 |
| VLDL-C, mg/dL | 7 | 130 | 102 | −3.09 | −8.51 to 2.32 | 0.29 |
| Apo AI, g/L | 5 | 63 | 62 | 0.03 | 0.02 to 0.04 | <0.001 |
| Apo AII, g/L | 2 | 140 | 126 | 0.01 | −0.01 to 0.03 | 0.2 |
| Apo B, g/L | 5 | 103 | 87 | 0.01 | −0.01 to 0.03 | 0.4 |
| FFA, mmol/L | 6 | 70 | 62 | −0.06 | −0.14 to 0.03 | 0.21 |
| Adipokine and inflammatory markers | ||||||
| CRP, mg/L | 13 | 598 | 554 | −0.22 | −0.78 to 0.34 | 0.44 |
| IL-6, pg/mL | 6 | 130 | 121 | −0.05 | −0.27 to 0.17 | 0.66 |
| IL-18, pg/mL | 2 | 67 | 56 | 18.3 | 0.10 to 36.6 | 0.05 |
| TNF-α, pg/mL | 3 | 43 | 44 | 0.21 | −0.37 to 0.79 | 0.48 |
| Adiponectin, μg/mL | 6 | 273 | 267 | 0.52 | −0.20 to 1.23 | 0.16 |
| Leptin, ng/mL | 7 | 312 | 315 | −2.72 | −4.03 to −1.42 | <0.001 |
| Glucose/insulin metabolism markers | ||||||
| Glucose, mmol/L | 49 | 1720 | 1569 | −0.07 | −0.13 to 0.004 | 0.06 |
| Insulin, μIU/mL | 29 | 1272 | 1149 | −1.03 | −1.69 to −0.37 | 0.002 |
| HOMA-IR | 14 | 1033 | 912 | −0.3 | −0.49 to −0.11 | 0.002 |
| HbA1c, % | 19 | 972 | 878 | −0.28 | −0.42 to −0.14 | <0.001 |
| C-peptide, nmol/L | 2 | 66 | 34 | −0.08 | −0.29 to 0.46 | 0.67 |
| IGF-1, ng/mL | 5 | 230 | 207 | 3.16 | −2.98 to 9.31 | 0.31 |
| IGF-BP3, μg/mL | 2 | 170 | 164 | −0.002 | −0.23 to 0.23 | 0.99 |
| Hemostatic factors | ||||||
| Fibrinogen, g/L | 2 | 36 | 39 | −0.39 | −0.75 to −0.03 | 0.04 |
| Endothelin-1, pg/mL | 2 | 34 | 32 | −0.22 | −0.62 to 0.19 | 0.29 |
| Angiotensin II, pg/mL | 2 | 24 | 25 | −1.32 | −2.11 to −0.54 | 0.001 |
Apo AI indicates apolipoprotein A1; Apo AII, apolipoprotein A2; Apo B, apolipoprotein B; CRP, C-reactive protein; FFA, free fatty acid; HbA1c, glycosylated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment–insulin resistance; IGF-1, insulin-like growth factor 1; IGF-BP3, insulin-like growth factor binding protein 3; IL, interleukin; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; TNF-α, tumor necrosis factor α; VLDL-C, very low-density lipoprotein cholesterol; WMDs, weighted mean differences.
Number of eligible independent comparisons.
Lipid and Lipoprotein Markers
The number of comparisons for each lipid and lipoprotein marker is shown in Table4. Exercise training significantly lowered the levels of triglycerides (P=0.02) and increased the levels of high-density lipoprotein cholesterol (HDL-C; P<0.001) and apolipoprotein A1 (P<0.001). The WMDs were −5.31 mg/dL (95% CI −10.63 to −0.89; I2=71.8%; P<0.001 for heterogeneity) for triglycerides, 2.32 mg/dL (95% CI 1.16 to 3.87; I2=87.5%; P<0.001 for heterogeneity) for HDL-C, and 0.03 g/L (95% CI 0.02 to 0.04; I2=0.0%; P=0.81 for heterogeneity) for apolipoprotein A1. The P value of the Egger’s test for HDL-C was 0.03, suggesting possible publication bias; however, the results from the trim and fill analysis did not show substantial impact of publication bias on the estimates or the statistics (filled analysis: WMD 2.32 mg/dL, 95% CI 1.16 to 3.87, P<0.001).
Adipokine and Inflammatory Markers
Significant associations were found for interleukin-18 (WMD 18.3 pg/mL; 95% CI 0.10 to 36.6; I2=0.0%; P=0.95 for heterogeneity) but not for C-reactive protein, interleukin-6, or tumor necrosis factor α in the primary analysis (Table4). Although there was no effect on adiponectin, exercise training was significantly associated with reduced levels of leptin (WMD −2.72 ng/mL; 95% CI −4.03 to −1.42; I2=82.10%; P<0.001 for heterogeneity) (Table4).
Markers of Glucose Intolerance and Insulin Resistance
Table4 also shows the effects of exercise training on markers of glucose intolerance and insulin resistance. Fasting insulin levels; homeostatic model assessment–insulin resistance, or HOMA-IR; and glycosylated hemoglobin A1c were significantly lowered in exercise groups compared with control groups (P=0.002, P=0.002, and P<0.001) (Table4). The WMDs between exercise groups and control groups were −1.03 μIU/mL (95% CI −1.69 to −0.37; I2=79.8%; P<0.001 for heterogeneity) for fasting insulin. The WMD for HOMA-IR was −0.30 (95% CI −0.49 to −0.11; I2=77.5%; P<0.001 for heterogeneity), whereas the WMD for hemoglobin A1c was −0.28% (95% CI −0.42 to −0.14; I2=80.1%; P<0.001 for heterogeneity). The Egger’s tests for fasting glucose and insulin were not suggestive of substantial publication bias (P=0.18 and P=0.24, respectively). The results from the trim and fill analysis suggested that there was no substantial impact of publication bias on the results for HOMA-IR or hemoglobin A1c (filled analysis for HOMA-IR: WMD −0.30, 95% CI −0.49 to −0.11, P=0.002; filled analysis for hemoglobin A1c: WMD −0.28%, 95% CI −0.42 to −0.14, P<0.001).
Hemostatic Factors
The primary analysis examined 3 hemostatic factors: fibrinogen, endothelin-1, and angiotensin II (Table4). On average, the levels of fibrinogen and angiotensin II were 0.39 g/L (95% CI 0.03 to 0.75; I2=45.00%; P=0.18 for heterogeneity) and 1.32 pg/mL (95% CI 0.54 to 2.11; I2=0.00%; P=0.71 for heterogeneity) lower in exercise groups than in control groups. No significant association was found for endothelin-1.
Subgroup Analyses
Our metaregression results suggest that the differences in CRF between exercise and control groups were modified by age and sex (absolute CRF: P=0.008 and P<0.001 for age and sex, respectively; relative CRF: P=0.003 and P=0.001 for age and sex, respectively) (Table5, Figure3). In addition, the effects of exercise on levels of total cholesterol (P=0.04), low-density lipoprotein cholesterol (LDL-C; P=0.06), and fasting insulin (P=0.05) were modified by the presence of at least 1 of the following comorbidities: type 2 diabetes, hypertension, hyperlipidemia, and metabolic syndrome (Tables6 and 7, Figure3). Sex differences in the effects of exercise were also found for fasting insulin (P=0.04).
Table 5.
WMDs in Absolute and Relative Cardiorespiratory Fitness Comparing Exercise Intervention Groups to Control Groups by Specific Modifiers
| Modifier | Absolute CRF (L/min) | Relative CRF (mL/kg per minute) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | WMD | 95% CI | I2, % | P interaction * | n | WMD | 95% CI | I2, % | P interaction | |
| Age, y | ||||||||||
| <50 | 16 | 0.47 | 0.34 to 0.60 | 93.4 | 0.008 | 28 | 5.60 | 4.56 to 6.65 | 85.1 | 0.003 |
| ≥50 | 12 | 0.21 | 0.11 to 0.32 | 84.0 | 30 | 3.31 | 2.46 to 4.15 | 91.0 | ||
| Sex | ||||||||||
| Women | 25 | 0.19 | 0.13 to 0.24 | 92.3 | <0.001 | 48 | 3.24 | 2.61 to 3.87 | 88.7 | 0.001 |
| Men | 27 | 0.42 | 0.32 to 0.53 | 90.4 | 37 | 5.43 | 4.32 to 6.53 | 90.2 | ||
| Lifestyle | ||||||||||
| Active | 9 | 0.33 | 0.15 to 0.51 | 97.0 | 0.89 | 14 | 3.62 | 1.39 to 5.85 | 96.5 | 0.83 |
| Sedentary | 43 | 0.31 | 0.25 to 0.37 | 88.4 | 88 | 3.85 | 3.36 to 4.33 | 90.5 | ||
| BMI† | ||||||||||
| Obese | 19 | 0.28 | 0.20 to 0.36 | 93.3 | 0.65 | 19 | 3.85 | 2.83 to 4.87 | 94.9 | 0.96 |
| Nonobese | 20 | 0.26 | 0.17 to 0.36 | 89.1 | 46 | 4.01 | 3.22 to 4.79 | 85.7 | ||
| Health status‡ | ||||||||||
| Yes | 8 | 0.33 | 0.07 to 0.60 | 88.2 | 0.84 | 16 | 3.34 | 2.63 to 4.04 | 74.8 | 0.46 |
| None | 53 | 0.27 | 0.22 to 0.33 | 94.6 | 94 | 4.10 | 3.51 to 4.71 | 92.7 | ||
| Duration, wk | ||||||||||
| <16 | 39 | 0.33 | 0.25 to 0.40 | 91.3 | 0.09 | 69 | 3.83 | 3.12 to 4.54 | 90.7 | 0.72 |
| ≥16 | 28 | 0.21 | 0.15 to 0.28 | 92.3 | 54 | 3.90 | 3.34 to 4.35 | 90.4 | ||
BMI indicates body mass index; CRF, cardiorespiratory fitness; WMDs, weighted mean differences,
P values for the impact of potential modifiers on the exercise effects.
BMI in kg/m2: obese ≥30; nonobese <30.
Health status: participants having at least 1 of type 2 diabetes, hypertension, hyperlipidemia, or metabolic syndrome (yes) vs those with none of them (none).
Figure 3.

Forest plot of effects of exercise interventions on cardiorespiratory fitness, TC, TG, HDL-C, LDL-C, Fasting glucose, and fasting insulin within subgroups. The WMDs (diamonds) and corresponding CIs (extended line) between exercise groups and control groups are shown for each subgroup. Abs. CRF indicates absolute cardiorespiratory fitness; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Rel. CRF, relative cardiorespiratory fitness; TC, total cholesterol; TG, triglycerides; WMDs, weighted mean differences.
Table 6.
WMDs in Lipid Biomarkers Comparing Exercise Intervention and Control Groups by Specific Modifiers
| Modifier | Total Cholesterol (mg/dL) | Total Triglycerides (mg/dL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | WMD | 95% CI | I2, % | P interaction * | n | WMD | 95% CI | I2, % | P interaction | |
| Age, y | ||||||||||
| <50 | 12 | −4.25 | −10.1 to 1.55 | 0.0 | 0.43 | 12 | −6.20 | −14.2 to 2.66 | 34.3 | 0.21 |
| ≥50 | 15 | 0.77 | −5.41 to 7.35 | 72.5 | 13 | 1.77 | −8.86 to 13.3 | 75.5 | ||
| Sex | ||||||||||
| Women | 28 | 1.16 | −5.41 to 7.73 | 91.6 | 0.61 | 27 | −1.77 | −9.74 to 5.31 | 76.1 | 0.25 |
| Men | 15 | −0.39 | −5.80 to 5.03 | 54.3 | 13 | −8.86 | −14.2 to −4.43 | 12.8 | ||
| Lifestyle | ||||||||||
| Active | 6 | 8.12 | −7.73 to 24.0 | 92.5 | 0.71 | 5 | −8.86 | −30.1 to 12.4 | 61.2 | 0.64 |
| Sedentary | 47 | 1.93 | −13.9 to 17.4 | 99.1 | 43 | −3.54 | −9.74 to 2.66 | 75.1 | ||
| BMI† | ||||||||||
| Obese | 16 | 12.8 | −22.4 to 47.6 | 99.7 | 0.20 | 19 | −7.97 | −14.2 to −1.77 | 53.0 | 0.70 |
| Nonobese | 29 | −1.55 | −7.73 to 4.25 | 83.6 | 28 | −5.31 | −14.2 to 4.43 | 80.7 | ||
| Health status‡ | ||||||||||
| Yes | 10 | −11.2 | −19.3 to −3.48 | 75.2 | 0.04 | 9 | −9.74 | −26.6 to 6.20 | 63.9 | 0.48 |
| None | 47 | −1.55 | −5.41 to 2.32 | 81.6 | 44 | −4.43 | −11.5 to 2.66 | 75.2 | ||
| Duration, wk | ||||||||||
| <16 | 39 | 3.87 | −15.5 to 22.8 | 82.9 | 0.34 | 35 | −6.20 | −13.3 to 0.89 | 71.1 | 0.76 |
| ≥16 | 29 | −3.09 | −7.73 to 1.55 | 99.2 | 31 | −5.31 | −11.5 to 1.77 | 72.7 | ||
| Modifier | HDL-C (mg/dL) | LDL-C (mg/dL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | WMD | 95% CI | I2, % | P interaction * | N | WMD | 95% CI | I2, % | P interaction | |
| Age, y | ||||||||||
| <50 | 16 | 4.25 | 2.32 to 6.19 | 73.9 | 0.94 | 9 | −3.87 | −10.8 to 3.09 | 49.3 | 0.38 |
| ≥50 | 15 | 3.87 | 0.77 to 6.96 | 84.5 | 14 | 0.39 | −5.03 to 6.19 | 73.0 | ||
| Sex | ||||||||||
| Women | 28 | 2.32 | 0.08 to 4.64 | 84.8 | 0.80 | 24 | −1.93 | −9.67 to 5.80 | 95.0 | 0.93 |
| Men | 19 | 2.71 | 0.39 to 5.03 | 92.5 | 13 | −2.32 | −8.89 to 4.25 | 79.7 | ||
| Lifestyle | ||||||||||
| Active | 5 | 4.25 | 0.39 to 8.51 | 86.9 | 0.52 | 2 | 8.12 | −10.4 to 27.1 | 54.9 | 0.21 |
| Sedentary | 52 | 2.32 | 0.77 to 3.87 | 18.6 | 45 | −3.87 | −8.12 to 0.39 | 88.3 | ||
| BMI‡ | ||||||||||
| Obese | 19 | 4.25 | 1.93 to 6.96 | 88.1 | 0.13 | 14 | −0.08 | −4.64 to 4.25 | 62.0 | 0.33 |
| Nonobese | 30 | 1.16 | −1.16 to 3.87 | 83.0 | 25 | −4.25 | −10.4 to 2.32 | 91.4 | ||
| Health status‡ | ||||||||||
| Yes | 11 | 2.71 | −2.32 to 7.73 | 91.2 | 0.89 | 12 | −11.6 | −19.7 to −3.09 | 80.8 | 0.06 |
| None | 50 | 2.32 | 0.77 to 3.87 | 87.3 | 39 | −3.09 | −7.73 to 1.55 | 89.3 | ||
| Duration, wk | ||||||||||
| <16 | 39 | 2.71 | 1.16 to 4.64 | 83.0 | 0.55 | 29 | −3.09 | −9.28 to 3.48 | 89.6 | 0.63 |
| ≥16 | 35 | 1.93 | 0.15 to 0.28 | 90.4 | 30 | −4.64 | −10.4 to 1.16 | 92.2 | ||
BMI indicates body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; WMDs, weighted mean differences.
P values for the impact of potential modifiers on the exercise effects.
BMI in kg/m2: obese ≥30; nonobese <30.
Health status: participants having at least 1 of type 2 diabetes, hypertension, hyperlipidemia, or metabolic syndrome (yes) vs those with none of them (none).
Table 7.
WMDs in Biomarkers of Glucose Intolerance and Insulin Resistance Comparing Exercise Intervention Groups to Control Groups by Specific Modifiers
| Modifier | Fasting Glucose (mmol/L) | Fasting Insulin (μIU/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | WMD | 95% CI | I2, % | P interaction * | n | WMD | 95% CI | I2, % | P interaction | |
| Age, y | ||||||||||
| <50 | 5 | 0.09 | −0.11 to 0.29 | 91.3 | 0.57 | 4 | −1.34 | −3.44 to 0.76 | 76.9 | 0.22 |
| ≥50 | 7 | 0.01 | −0.06 to 0.07 | 36.9 | 3 | 0.45 | −1.23 to 2.13 | 75.6 | ||
| Sex | ||||||||||
| Women | 16 | −0.06 | −0.19 to 0.08 | 91.4 | 0.93 | 9 | −0.27 | −1.12 to 0.57 | 68.7 | 0.04 |
| Men | 9 | −0.07 | −0.25 to 0.12 | 84.3 | 6 | −2.86 | −3.55 to −2.17 | 0.0 | ||
| Lifestyle | ||||||||||
| Active | 2 | −0.20 | −0.74 to 0.34 | 99.0 | 0.63 | 0 | NA | NA | NA | NA |
| Sedentary | 29 | −0.06 | −0.16 to 0.03 | 80.2 | 17 | −0.94 | −1.75 to −0.13 | 78.5 | ||
| BMI† | ||||||||||
| Obese | 20 | −0.06 | −0.20 to 0.07 | 90.7 | 0.90 | 13 | −0.93 | −2.18 to 0.32 | 82.0 | 0.88 |
| Nonobese | 18 | −0.05 | −0.17 to 0.07 | 80.7 | 10 | −0.86 | −1.52 to −0.19 | 32.8 | ||
| Health status‡ | ||||||||||
| Yes | 9 | −0.18 | −0.40 to 0.05 | 0.0 | 0.40 | 6 | −2.68 | −4.67 to −0.70 | 75.2 | 0.05 |
| None | 27 | −0.03 | −0.11 to 0.06 | 87.2 | 14 | −0.70 | −1.60 to 0.21 | 77.5 | ||
| Duration, wk | ||||||||||
| <16 | 30 | −0.10 | −0.22 to 0.03 | 90.0 | 0.70 | 13 | −1.35 | −2.50 to −0.20 | 79.3 | 0.58 |
| ≥16 | 19 | −0.02 | −0.09 to 0.06 | 47.5 | 16 | −0.83 | −1.83 to 0.17 | 78.7 | ||
BMI indicates body mass index; NA, not available due to the lack of comparisons reported for active participants; WMDs, weighted mean differences.
P-values for the impact of potential modifiers on the exercise effects.
BMI in kg/m2: obese ≥30; nonobese <30.
Health status: participants having at least 1 of type 2 diabetes, hypertension, hyperlipidemia, or metabolic syndrome (yes) vs those with none of them (none).
After conducting metaregressions, analyses within subgroups were performed. Compared with older people, those aged <50 years appeared to have larger changes in CRF. Consistent with the metaregression results, men seemed to have greater exercise-related improvement in CRF, LDL-C, and fasting insulin than women did (Figure3). Exercise interventions appreciably improved the levels of total cholesterol, LDL-C, and fasting insulin (P=0.004, P=0.01, and P=0.01, respectively) in people having at least 1 of type 2 diabetes, hypertension, hyperlipidemia, and metabolic syndrome (Tables6 and 7, Figure3); no such improvements were observed among people without any of those health conditions (P=0.44, P=0.19, and P=0.13, respectively) (Tables6 and 7, Figure3).
Sensitivity Analyses
In light of the potential impact of exercise intensity, we conducted separate analyses of all eligible comparisons for moderate and vigorous exercise interventions, respectively. The 95% CIs for moderate and vigorous interventions overlapped for both CRF measures and for all biomarkers (Table8).
Table 8.
WMDs in Cardiorespiratory Fitness and Circulating Concentrations of Biomarkers Comparing Moderate and Vigorous Exercise Intervention Groups to Control Groups
| Outcome | Moderate | Vigorous | ||||
|---|---|---|---|---|---|---|
| No.* | WMD | 95% CI | No.* | WMD | 95% CI | |
| Cardiorespiratory fitness | ||||||
| Absolute, L/min | 39 | 0.22 | 0.16 to 0.29 | 33 | 0.31 | 0.22 to 0.40 |
| Relative, mL/kg per minute | 64 | 3.22 | 2.61 to 4.18 | 68 | 3.26 | 2.63 to 3.89 |
| Lipids markers | ||||||
| TC, mg/dL | 41 | 4.25 | −7.73 to 16.6 | 28 | 3.87 | −31.7 to 39.8 |
| TG, mg/dL | 37 | −5.31 | −12.4 to 1.77 | 32 | −5.31 | −11.5 to 0.09 |
| HDL-C, mg/dL | 44 | 1.16 | −0.39 to 2.71 | 33 | 2.71 | 0.39 to 5.03 |
| HDL2-C, mg/dL | 2 | 1.16 | −0.77 to 3.48 | 2 | 1.55 | −1.16 to 4.25 |
| HDL3-C, mg/dL | 1 | −1.16 | −5.80 to 3.87 | 2 | 0.04 | −1.55 to 1.55 |
| LDL-C, mg/dL | 35 | −3.09 | −8.12 to 2.32 | 26 | −4.64 | −12.0 to 2.32 |
| VLDL-C, mg/dL | 5 | −1.93 | −5.41 to 1.93 | 2 | −7.35 | −22.9 to 6.19 |
| Apo AI, g/L | 4 | 0.03 | 0.02 to 0.04 | 1 | 0.00 | −0.12 to 0.12 |
| Apo AII, g/L | 1 | −0.001 | −0.24 to 0.24 | 1 | 0.01 | −0.01 to 0.03 |
| Apo B, g/L | 3 | 0.01 | −0.01 to 0.03 | 2 | −0.02 | −0.21 to 0.18 |
| FFA, mmol/L | 5 | −0.06 | −0.16 to 0.03 | 3 | −0.04 | −0.17 to 0.10 |
| Inflammatory markers | ||||||
| CRP, mg/L | 9 | −0.23 | −1.01 to 0.55 | 4 | 0.04 | −0.24 to 0.31 |
| IL-6, pg/mL | 5 | 0.02 | −0.22 to 0.25 | 2 | −0.39 | −0.83 to 0.06 |
| IL-18, pg/mL | 1 | 14.0 | −128 to 156 | 1 | 18.4 | 0.02 to 36.8 |
| TNF-α, pg/mL | 3 | 0.06 | −0.48 to 0.60 | 1 | −0.01 | −0.93 to 0.91 |
| Adiponectin, μg/mL | 1 | 3.52 | 1.17 to 5.87 | 6 | 0.52 | −0.20 to 1.23 |
| Leptin, ng/mL | 1 | −0.70 | −1.19 to −0.21 | 6 | −2.56 | −4.04 to −1.08 |
| Insulin resistance markers | ||||||
| Glucose, mmol/L | 31 | −0.04 | −0.24 to 0.17 | 22 | 0.03 | −0.08 to 0.12 |
| Insulin, μIU/mL | 17 | −0.91 | −2.08 to 0.26 | 17 | −1.32 | −2.15 to −0.50 |
| HOMA-IR | 7 | −0.30 | −0.66 to 0.06 | 7 | −0.47 | −0.82 to −0.12 |
| HbA1c, % | 11 | −0.28 | −0.46 to −0.11 | 7 | −2.71 | −0.54 to −0.002 |
| C-peptide, nmol/L | 1 | 0.22 | 0.19 to 0.25 | 1 | −0.18 | −0.62 to 0.26 |
| IGF-1, ng/mL | 2 | −4.64 | −29.58 to 20.30 | 3 | 3.91 | −2.87 to 10.69 |
| IGF-BP3, μg/mL | 0 | NA | NA | 2 | −0.002 | −0.23 to 0.23 |
| Hemostatic factors | ||||||
| Fibrinogen, g/L | 0 | NA | NA | 2 | −0.39 | −0.75 to −0.03 |
| Endothelin-1, pg/mL | 2 | −0.22 | −0.62 to 0.19 | 0 | NA | NA |
| Angiotensin II, pg/mL | 2 | −1.32 | −2.11 to −0.54 | 0 | NA | NA |
Apo AI indicates apolipoprotein A1; Apo AII, apolipoprotein A2; Apo B, apolipoprotein B; CRP, C-reactive protein; FFA, free fatty acid; HbA1c, glycosylated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment–insulin resistance; IGF-1, insulin-like growth factor 1; IGF-BP3, insulin-like growth factor binding protein 3; IL, interleukin; LDL-C, low-density lipoprotein cholesterol; NA, not available due to the lack of comparisons reported; TC, total cholesterol; TG, triglycerides; TNF-α, tumor necrosis factor α; VLDL-C, very low-density lipoprotein cholesterol; WMDs, weighted mean differences.
Number of eligible independent comparisons.
Discussion
This systematic review and meta-analysis of 160 RCTs involving 7487 participants indicates that exercise training may significantly improve CRF and CVD biomarkers of lipid and lipoprotein metabolism, glucose intolerance and insulin resistance, systemic inflammation, and hemostasis (Figure4). In addition, we identified several important modifiers, including age, sex, and health status, that may partially modify the exercise effects on cardiovascular health.
Figure 4.
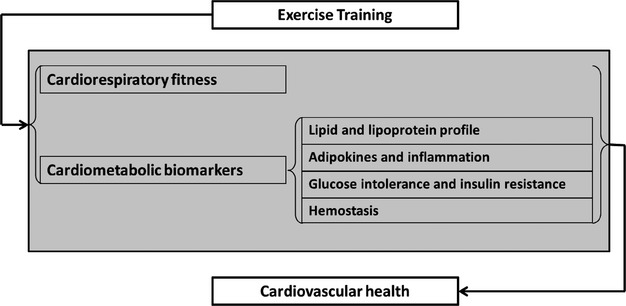
Mechanisms by which exercise training may improve cardiovascular health.
The current meta-analysis shows that exercise, with relatively low risk of side effects compared with medications, may be an effective way to prevent CVD through impact on various biomarkers. Our results from the meta-analysis showed that exercise training significantly raised CRF, which has been demonstrated to be an independent predictor of CVD risk, CVD mortality, and total mortality.200,201 Lower levels of triglycerides and higher levels of HDL-C were observed in exercise groups. Aside from conventional CVD biomarkers, our meta-analysis also examined the effects on biomarkers that have not been well studied in previous studies, including biomarkers of insulin resistance and hemostasis, adipokines, and novel lipid and inflammatory biomarkers. We found evidence supporting the favorable effects of exercise on apolipoprotein A1, interleukin-18, fasting insulin, HOMA-IR, and hemoglobin A1c. Although the exact biological mechanisms are not clear, our findings indicate that exercise may exert cardioprotective effects by altering dyslipidemia, inflammation, insulin resistance, and hemostasis.19
As a major component of HDL, apolipoprotein A1 plays an important role in the cardioprotective effects of HDL-C.202–204 Our findings on apolipoprotein A1 strengthen the hypothesis that exercise may accelerate reverse cholesterol transport. Another plausible mechanism by which exercise improves the lipid profile is by regulation of lipoprotein lipase. Various studies have suggested that exercise may decrease the levels of triglycerides and increase the levels of HDL-C through its impact on lipoprotein lipase expression and activity, which were consistent with the results from our meta-analysis.205–207 In addition, our analysis also confirmed that the proportion of CVD risk that could have been reduced by exercise via effects on total cholesterol and LDL-C is much lower than what has been observed previously.208,209 Consequently, the results from our meta-analysis provide additional evidence in support of the notion that, in addition to modifying total cholesterol and LDL-C, exercise training may also affect cardiovascular health through other pathways. We found that people in exercise groups also had significantly lower levels of IL-18 and several biomarkers of insulin resistance and hemostatic factors, indicating that exercise may exert its effects via pathways of inflammation-characterized atherothrombosis and insulin resistance. A recent review suggested that exercise training may regulate white adipose tissue mass and the expression of adipokines.210 Obesity has become widely regarded as a chronic proinflammatory state, and substantial evidence indicates that chronic inflammation in adipose tissues, especially in white adipose tissue, could lead to insulin resistance.211,212 Consequently, it is biologically plausible that by reducing the white adipose tissue mass and regulating the expression of adipokines, exercise could mitigate the chronic inflammation in adipose tissues, resulting in improved insulin sensitivity. Nevertheless, the exact mechanism remains to be elucidated.
The results from the subgroup analyses also may have important clinical implications. Consistent with previous evidence,213 both moderate and vigorous exercise training appeared to have favorable effects on cardiorespiratory fitness and cardiometabolic health. We found that the differences in CVD risk between exercise groups and control groups were not significantly modified by lifestyle, body mass index, or intervention duration. These findings suggest that exercise interventions may have similar effects on cardiovascular health in populations regardless of these factors. Alternatively, the effectiveness of exercise training appeared to be different across strata of age, sex, and health status. The effects of exercise interventions on CRF measures were modified by age, sex, and health status such that people aged <50 years, men, and people with type 2 diabetes, hypertension, hyperlipidemia, or metabolic syndrome appeared to benefit more from exercise interventions. We also observed significant modification of the effects on total cholesterol and LDL-C by preexisting medical conditions (type 2 diabetes, hypertension, hyperlipidemia, or metabolic syndromes), and that may explain why we did not find significant effects of exercise on total cholesterol and LDL-C. This finding also suggests that exercise interventions may provide significant benefits for people with those preexisting conditions by lowering total cholesterol and LDL-C.
Strengths of this meta-analysis include the comprehensive and systematic review of both conventional and novel CVD biomarkers, detailed subgroup analyses for potential effect modifiers that have not been conducted previously, assessment of robustness with regard to exercise intensity, and evaluation of the risk of different bias. The 2008 Physical Activity Guidelines Advisory Committee Report included a number of comprehensively systematic reviews and meta-analyses based mostly on observational studies.214 The evidence from RCTs has been relatively scarce, especially for novel cardiometabolic biomarkers. Our study is the first that synthesized evidence from the RCT setting and covered a comprehensive set of both traditional and novel biomarkers. Our findings are corroborated by several previous meta-analyses of RCTs,20,215 but the inclusion of both sexes, more studies, subgroup analyses, and sensitivity analyses allowed us to achieve higher precision in the estimates and to determine the effect modification in subgroups.
This meta-analysis had some limitations. First, the evidence for hemostatic factors is based on a limited number of available trials, and we were not able to synthesize evidence for some novel biomarkers, such as plasminogen activator inhibitor 1, lipoprotein(a), and homocysteine due to sparse available data. Second, subgroup analyses were restricted to outcomes with >20 studies included, and cutoff points used for categorizing modifiers were arbitrarily selected. Third, due to the heterogeneity of exercise training programs and the limited number of RCTs that provided separate data, this meta-analysis can neither perform a dose-response analysis nor distinguish exercise types. We maximized the utility of data regarding exercise duration and intensity available from original RCTs and found that exercise effects were not significantly different across subgroups defined by duration and intensity. Our findings are consistent with previous evidence showing that both moderate and vigorous exercise training has similarly favorable effects on cardiometabolic health.213 The duration threshold at which exercise exerts its effects needs further investigation. Fourth, to maintain independence, we selected 1 comparison from each trial with exercise groups of different intensities compared with 1 single control group. The results may potentially be subject to bias by excluding several eligible intervention groups with moderate intensity; however, we found that the direction and magnitude of the effects on most of the outcome measures were quite similar between moderate and vigorous interventions (Table8). Finally, like any meta-analysis, our results may be prone to publication bias and inherent weaknesses of individual studies.
In conclusion, this large meta-analysis of RCTs clearly shows that exercise training significantly improved CRF and some traditional and novel CVD biomarkers in adults without CVD, indicating the causal role of exercise in the primary prevention of CVD morbidity and mortality.
Acknowledgments
Author contributions: Lin, Liu, and Song designed research; Lin and Zhang were involved in data collection; Lin analyzed data; Guo, Roberts, McKenzie, Wu, and Liu participated in interpretation of findings; Lin and Song wrote the first draft. All authors read, edited, and approved the final manuscript.
Sources of Funding
The study was supported by the Indiana University Health–Indiana University School of Medicine Strategic Research Initiative Grant (Zhang and Song), R01DK09406 (Roberts) and P50HL105188 (Roberts) from the National Institutes of Health (NIH), and Brown University. The NIH, Brown University, or Indiana University had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Disclosures
None.
References
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DS, Greene JA. The decline and rise of coronary heart disease: understanding public health catastrophism. Am J Public Health. 2013;103:1207–1218. doi: 10.2105/AJPH.2013.301226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, Kissela BM, Kittner SJ, Lichtman JH, Lisabeth LD, Schwamm LH, Smith EE, Towfighi A. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45:315–353. doi: 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire S. U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010. 7th edition, Washington, DC: U.S. Government Printing Office, January 2011. Adv Nutr. 2011;2:293–294. doi: 10.3945/an.111.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM, Hong Y, Miller NH, Lauer RM, Ockene IS, Sacco RL, Sallis JF, Jr, Smith SC, Jr, Stone NJ, Taubert KA. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd-Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- Berlin JA, Colditz GA. A meta-analysis of physical activity in the prevention of coronary heart disease. Am J Epidemiol. 1990;132:612–628. doi: 10.1093/oxfordjournals.aje.a115704. [DOI] [PubMed] [Google Scholar]
- Berry JD, Pandey A, Gao A, Leonard D, Farzaneh-Far R, Ayers C, DeFina L, Willis B. Physical fitness and risk for heart failure and coronary artery disease. Circ Heart Fail. 2013;6:627–634. doi: 10.1161/CIRCHEARTFAILURE.112.000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner J, Lambrick D, Woolley B, Stoner L, Wong L-K, McGonigal G. Health-enhancing physical activity programme (HEPAP) for transient ischaemic attack and non-disabling stroke: recruitment and compliance. N Z Med J. 2012;125:68–76. [PubMed] [Google Scholar]
- Alter DA, Oh PI, Chong A. Relationship between cardiac rehabilitation and survival after acute cardiac hospitalization within a universal health care system. Eur J Cardiovasc Prev Rehabil. 2009;16:102–113. doi: 10.1097/HJR.0b013e328325d662. [DOI] [PubMed] [Google Scholar]
- Niebauer J, Clark AL, Webb-Peploe KM, Coats AJ. Exercise training in chronic heart failure: effects on pro-inflammatory markers. Eur J Heart Fail. 2005;7:189–193. doi: 10.1016/j.ejheart.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Williams MA, Ades PA, Hamm LF, Keteyian SJ, LaFontaine TP, Roitman JL, Squires RW. Clinical evidence for a health benefit from cardiac rehabilitation: an update. Am Heart J. 2006;152:835–841. doi: 10.1016/j.ahj.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Gupta S, Rohatgi A, Ayers CR, Willis BL, Haskell WL, Khera A, Drazner MH, de Lemos JA, Berry JD. Cardiorespiratory fitness and classification of risk of cardiovascular disease mortality. Circulation. 2011;123:1377–1383. doi: 10.1161/CIRCULATIONAHA.110.003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukkanen JA, Laaksonen D, Lakka TA, Savonen K, Rauramaa R, Makikallio T, Kurl S. Determinants of cardiorespiratory fitness in men aged 42 to 60 years with and without cardiovascular disease. Am J Cardiol. 2009;103:1598–1604. doi: 10.1016/j.amjcard.2009.01.371. [DOI] [PubMed] [Google Scholar]
- Lee DC, Artero EG, Sui X, Blair SN. Mortality trends in the general population: the importance of cardiorespiratory fitness. J Psychopharmacol. 2010;24:27–35. doi: 10.1177/1359786810382057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation. 2005;112:3375–3383. doi: 10.1161/CIRCULATIONAHA.104.532499. [DOI] [PubMed] [Google Scholar]
- Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- Smith SC, Jr, Anderson JL, Cannon RO, III, Fadl YY, Koenig W, Libby P, Lipshultz SE, Mensah GA, Ridker PM, Rosenson R. CDC/AHA workshop on markers of inflammation and cardiovascular disease: application to clinical and public health practice: report from the clinical practice discussion group. Circulation. 2004;110:e550–e553. doi: 10.1161/01.CIR.0000148981.71644.C7. [DOI] [PubMed] [Google Scholar]
- Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- Swardfager W, Herrmann N, Cornish S, Mazereeuw G, Marzolini S, Sham L, Lanctot KL. Exercise intervention and inflammatory markers in coronary artery disease: a meta-analysis. Am Heart J. 2012;163:666–676. doi: 10.1016/j.ahj.2011.12.017. .e661–663. [DOI] [PubMed] [Google Scholar]
- Lavie CJ, Church TS, Milani RV, Earnest CP. Impact of physical activity, cardiorespiratory fitness, and exercise training on markers of inflammation. J Cardiopulm Rehabil Prev. 2011;31:137–145. doi: 10.1097/HCR.0b013e3182122827. [DOI] [PubMed] [Google Scholar]
- Patel N, Taveira TH, Choudhary G, Whitlatch H, Wu WC. Fasting serum C-peptide levels predict cardiovascular and overall death in nondiabetic adults. J Am Heart Assoc. 2012;1:e003152. doi: 10.1161/JAHA.112.003152. doi: 10.1161/JAHA.112.003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holten TC, Waanders LF, de Groot PG, Vissers J, Hoefer IE, Pasterkamp G, Prins MW, Roest M. Circulating biomarkers for predicting cardiovascular disease risk; a systematic review and comprehensive overview of meta-analyses. PLoS One. 2013;8:e62080. doi: 10.1371/journal.pone.0062080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- Robins SJ, Lyass A, Zachariah JP, Massaro JM, Vasan RS. Insulin resistance and the relationship of a dyslipidemia to coronary heart disease: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2011;31:1208–1214. doi: 10.1161/ATVBAHA.110.219055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzeri C, Sori A, Chiostri M, Gensini GF, Valente S. Prognostic role of insulin resistance as assessed by homeostatic model assessment index in the acute phase of myocardial infarction in nondiabetic patients submitted to percutaneous coronary intervention. Eur J Anaesthesiol. 2009;26:856–862. doi: 10.1097/EJA.0b013e32832a235c. [DOI] [PubMed] [Google Scholar]
- Kelley GA, Kelley KS. Effects of aerobic exercise on C-reactive protein, body composition, and maximum oxygen consumption in adults: a meta-analysis of randomized controlled trials. Metabolism. 2006;55:1500–1507. doi: 10.1016/j.metabol.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–1129. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- Ben Abderrahman A, Zouhal H, Chamari K, Thevenet D, de Mullenheim P-Y, Gastinger S, Tabka Z, Prioux J. Effects of recovery mode (active vs. passive) on performance during a short high-intensity interval training program: a longitudinal study. Eur J Appl Physiol. 2013;113:1373–1383. doi: 10.1007/s00421-012-2556-9. [DOI] [PubMed] [Google Scholar]
- Ahmaidi S, Masse-Biron J, Adam B, Choquet D, Freville M, Libert JP, Prefaut C. Effects of interval training at the ventilatory threshold on clinical and cardiorespiratory responses in elderly humans. Eur J Appl Physiol. 1998;78:170–176. doi: 10.1007/s004210050403. [DOI] [PubMed] [Google Scholar]
- Aldred HE, Hardman AE, Taylor S. Influence of 12 weeks of training by brisk walking on postprandial lipemia and insulinemia in sedentary middle-aged women. Metabolism. 1995;44:390–397. doi: 10.1016/0026-0495(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Ashutosh K, Methrotra K, Fragale-Jackson J. Effects of sustained weight loss and exercise on aerobic fitness in obese women. J Sports Med Phys Fitness. 1997;37:252–257. [PubMed] [Google Scholar]
- Asikainen TM, Miilunpalo S, Oja P, Rinne M, Pasanen M, Uusi-Rasi K, Vuori I. Randomised, controlled walking trials in postmenopausal women: the minimum dose to improve aerobic fitness? Br J Sports Med. 2002;36:189–194. doi: 10.1136/bjsm.36.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TT, Allen D, Lei KY, Willcox KK. Alterations in lipid and protein profiles of plasma lipoproteins in middle-aged men consequent to an aerobic exercise program. Metabolism. 1986;35:1037–1043. doi: 10.1016/0026-0495(86)90040-5. [DOI] [PubMed] [Google Scholar]
- Balducci S, Zanuso S, Nicolucci A, De Feo P, Cavallo S, Cardelli P, Fallucca S, Alessi E, Fallucca F, Pugliese G Italian Diabetes Exercise Study I. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES) Arch Intern Med. 2010;170:1794–1803. doi: 10.1001/archinternmed.2010.380. [DOI] [PubMed] [Google Scholar]
- Beavers KM, Hsu F-C, Isom S, Kritchevsky SB, Church T, Goodpaster B, Pahor M, Nicklas BJ. Long-term physical activity and inflammatory biomarkers in older adults. Med Sci Sports Exerc. 2010;42:2189–2196. doi: 10.1249/MSS.0b013e3181e3ac80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell GJ, Harber V, Murray T, Courneya KS, Rodgers W. A comparison of fitness training to a pedometer-based walking program matched for total energy cost. J Phys Act Health. 2010;7:203–213. doi: 10.1123/jpah.7.2.203. [DOI] [PubMed] [Google Scholar]
- Bermon S, Ferrari P, Bernard P, Altare S, Dolisi C. Responses of total and free insulin-like growth factor-I and insulin-like growth factor binding protein-3 after resistance exercise and training in elderly subjects. Acta Physiol Scand. 1999;165:51–56. doi: 10.1046/j.1365-201x.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- Biddle MG, Vincent G, McCambridge A, Britton G, Dewes O, Elley CR, Moyes SA, Edge J. Randomised controlled trial of informal team sports for cardiorespiratory fitness and health benefit in pacific adults. J Prim Health Care. 2011;3:269–277. [PubMed] [Google Scholar]
- Blumenthal JA, Emery CF, Madden DJ, Schniebolk S, Riddle MW, Cobb FR, Higginbotham M, Coleman RE. Effects of exercise training on bone density in older men and women. J Am Geriatr Soc. 1991;39:1065–1070. doi: 10.1111/j.1532-5415.1991.tb02870.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Emery CF, Madden DJ, Schniebolk S, Walsh-Riddle M, George LK, McKee DC, Higginbotham MB, Cobb FR, Coleman RE. Long-term effects of exercise on psychological functioning in older men and women. J Gerontol. 1991;46:P352–P361. doi: 10.1093/geronj/46.6.p352. [DOI] [PubMed] [Google Scholar]
- Boardley D, Fahlman M, Topp R, Morgan AL, McNevin N. The impact of exercise training on blood lipids in older adults. Am J Geriatr Cardiol. 2007;16:30–35. doi: 10.1111/j.1076-7460.2007.05353.x. [DOI] [PubMed] [Google Scholar]
- Bobeuf F, Labonte M, Dionne IJ, Khalil A. Combined effect of antioxidant supplementation and resistance training on oxidative stress markers, muscle and body composition in an elderly population. J Nutr Health Aging. 2011;15:883–889. doi: 10.1007/s12603-011-0097-2. [DOI] [PubMed] [Google Scholar]
- Boreham CA, Wallace WF, Nevill A. Training effects of accumulated daily stair-climbing exercise in previously sedentary young women. Prev Med. 2000;30:277–281. doi: 10.1006/pmed.2000.0634. [DOI] [PubMed] [Google Scholar]
- Boudou P, Sobngwi E, Mauvais-Jarvis F, Vexiau P, Gautier JF. Absence of exercise-induced variations in adiponectin levels despite decreased abdominal adiposity and improved insulin sensitivity in type 2 diabetic men. Eur J Endocrinol. 2003;149:421–424. doi: 10.1530/eje.0.1490421. [DOI] [PubMed] [Google Scholar]
- Bourque SP, Pate RR, Branch JD. Twelve weeks of endurance exercise training does not affect iron status measures in women. J Am Diet Assoc. 1997;97:1116–1121. doi: 10.1016/S0002-8223(97)00272-1. [DOI] [PubMed] [Google Scholar]
- Braith RW, Pollock ML, Lowenthal DT, Graves JE, Limacher MC. Moderate- and high-intensity exercise lowers blood pressure in normotensive subjects 60 to 79 years of age. Am J Cardiol. 1994;73:1124–1128. doi: 10.1016/0002-9149(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Broeder CE, Burrhus KA, Svanevik LS, Wilmore JH. The effects of either high-intensity resistance or endurance training on resting metabolic rate. Am J Clin Nutr. 1992;55:802–810. doi: 10.1093/ajcn/55.4.802. [DOI] [PubMed] [Google Scholar]
- Broman G, Quintana M, Lindberg T, Jansson E, Kaijser L. High intensity deep water training can improve aerobic power in elderly women. Eur J Appl Physiol. 2006;98:117–123. doi: 10.1007/s00421-006-0237-2. [DOI] [PubMed] [Google Scholar]
- Burr JF, Jamnik VK, Gledhill N. Physiological fitness and health adaptations from purposeful training using off-road vehicles. Eur J Appl Physiol. 2011;111:1841–1850. doi: 10.1007/s00421-010-1817-8. [DOI] [PubMed] [Google Scholar]
- Camargo MD, Stein R, Ribeiro JP, Schvartzman PR, Rizzatti MO, Schaan BD. Circuit weight training and cardiac morphology: a trial with magnetic resonance imaging. Br J Sports Med. 2008;42:141–145. doi: 10.1136/bjsm.2007.038281. discussion 145. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Westerlind KC, Harber VJ, Bell GJ, Mackey JR, Courneya KS. Effects of aerobic exercise training on estrogen metabolism in premenopausal women: a randomized controlled trial. Cancer Epidemiol Biomark Prev. 2007;16:731–739. doi: 10.1158/1055-9965.EPI-06-0784. [DOI] [PubMed] [Google Scholar]
- Canuto K, Cargo M, Li M, D’Onise K, Esterman A, McDermott R. Pragmatic randomised trial of a 12-week exercise and nutrition program for Aboriginal and Torres Strait Islander women: clinical results immediate post and 3 months follow-up. BMC Public Health. 2012;12:933. doi: 10.1186/1471-2458-12-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S, Marshall P, Ingle L, Borkoles E. Cardiorespiratory fitness and heart rate recovery in obese premenopausal women. Scand J Med Sci Sports. 2012;22:e133–e139. doi: 10.1111/j.1600-0838.2012.01522.x. [DOI] [PubMed] [Google Scholar]
- Chan L, Chin LMK, Kennedy M, Woolstenhulme JG, Nathan SD, Weinstein AA, Connors G, Weir NA, Drinkard B, Lamberti J, Keyser RE. Benefits of intensive treadmill exercise training on cardiorespiratory function and quality of life in patients with pulmonary hypertension. Chest. 2013;143:333–343. doi: 10.1378/chest.12-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler WL, Schwartz RS, Stratton JR, Vitiello MV. Effects of endurance training on the circadian rhythm of fibrinolysis in men and women. Med Sci Sports Exerc. 1996;28:647–655. doi: 10.1097/00005768-199606000-00001. [DOI] [PubMed] [Google Scholar]
- Cho JK, Lee SH, Lee JY, Kang HS. Randomized controlled trial of training intensity in adiposity. Int J Sports Med. 2011;32:468–475. doi: 10.1055/s-0031-1271789. [DOI] [PubMed] [Google Scholar]
- Christiansen T, Paulsen SK, Bruun JM, Ploug T, Pedersen SB, Richelsen B. Diet-induced weight loss and exercise alone and in combination enhance the expression of adiponectin receptors in adipose tissue and skeletal muscle, but only diet-induced weight loss enhanced circulating adiponectin. J Clin Endocrinol Metab. 2010;95:911–919. doi: 10.1210/jc.2008-2505. [DOI] [PubMed] [Google Scholar]
- Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007;297:2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- Ciolac EG, Bocchi EA, Greve JMD, Guimaraes GV. Heart rate response to exercise and cardiorespiratory fitness of young women at high familial risk for hypertension: effects of interval vs continuous training. Eur J Cardiovasc Prev Rehabil. 2011;18:824–830. doi: 10.1177/1741826711398426. [DOI] [PubMed] [Google Scholar]
- Coker RH, Williams RH, Kortebein PM, Sullivan DH, Evans WJ. Influence of exercise intensity on abdominal fat and adiponectin in elderly adults. Metab Syndr Relat Disord. 2009;7:363–368. doi: 10.1089/met.2008.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez-Cooper MY, Anton MM, Devan AE, Neidre DB, Cook JN, Tanaka H. The effects of strength training on central arterial compliance in middle-aged and older adults. Eur J Cardiovasc Prev Rehabil. 2008;15:149–155. doi: 10.1097/HJR.0b013e3282f02fe2. [DOI] [PubMed] [Google Scholar]
- Cox KL, Puddey IB, Morton AR, Beilin LJ, Vandongen R, Masarei JR. The combined effects of aerobic exercise and alcohol restriction on blood pressure and serum lipids: a two-way factorial study in sedentary men. J Hypertens. 1993;11:191–201. doi: 10.1097/00004872-199302000-00012. [DOI] [PubMed] [Google Scholar]
- Cox KL, Burke V, Morton AR, Beilin LJ, Puddey IB. The independent and combined effects of 16 weeks of vigorous exercise and energy restriction on body mass and composition in free-living overweight men—a randomized controlled trial. Metabolism. 2003;52:107–115. doi: 10.1053/meta.2003.50017. [DOI] [PubMed] [Google Scholar]
- Dalleck LC, Allen BA, Hanson BA, Borresen EC, Erickson ME, De Lap SL. Dose-response relationship between moderate-intensity exercise duration and coronary heart disease risk factors in postmenopausal women. J Womens Health (Larchmt) 2009;18:105–113. doi: 10.1089/jwh.2008.0790. [DOI] [PubMed] [Google Scholar]
- De Vito G, Bernardi M, Forte R, Pulejo C, Figura F. Effects of a low-intensity conditioning programme on VO2max and maximal instantaneous peak power in elderly women. Eur J Appl Physiol. 1999;80:227–232. doi: 10.1007/s004210050586. [DOI] [PubMed] [Google Scholar]
- Dimeo F, Pagonas N, Seibert F, Arndt R, Zidek W, Westhoff TH. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension. 2012;60:653–658. doi: 10.1161/HYPERTENSIONAHA.112.197780. [DOI] [PubMed] [Google Scholar]
- DiPietro L, Dziura J, Yeckel CW, Neufer PD. Exercise and improved insulin sensitivity in older women: evidence of the enduring benefits of higher intensity training. J Appl Physiol. 2006;100:142–149. doi: 10.1152/japplphysiol.00474.2005. [DOI] [PubMed] [Google Scholar]
- Duncan JJ, Gordon NF, Scott CB. Women walking for health and fitness. How much is enough? JAMA. 1991;266:3295–3299. [PubMed] [Google Scholar]
- Duscha BD, Slentz CA, Johnson JL, Houmard JA, Bensimhon DR, Knetzger KJ, Kraus WE. Effects of exercise training amount and intensity on peak oxygen consumption in middle-age men and women at risk for cardiovascular disease. Chest. 2005;128:2788–2793. doi: 10.1378/chest.128.4.2788. [DOI] [PubMed] [Google Scholar]
- Eguchi Y, Ohta M, Inoue T, Honda T, Morita Y, Konno Y, Yamato H. Effects of transitory stimulation interval exercise on physical function: a randomized controlled pilot study among Japanese subjects. J UOEH. 2012;34:297–308. doi: 10.7888/juoeh.34.297. [DOI] [PubMed] [Google Scholar]
- Fatouros IG, Tournis S, Leontsini D, Jamurtas AZ, Sxina M, Thomakos P, Manousaki M, Douroudos I, Taxildaris K, Mitrakou A. Leptin and adiponectin responses in overweight inactive elderly following resistance training and detraining are intensity related. J Clin Endocrinol Metab. 2005;90:5970–5977. doi: 10.1210/jc.2005-0261. [DOI] [PubMed] [Google Scholar]
- Finucane FM, Sharp SJ, Purslow LR, Horton K, Horton J, Savage DB, Brage S, Besson H, De Lucia Rolfe E, Sleigh A, Martin HJ, Aihie SayerA, Cooper C, Ekelund U, Griffin SJ, Wareham NJ. The effects of aerobic exercise on metabolic risk, insulin sensitivity and intrahepatic lipid in healthy older people from the hertfordshire cohort study: a randomised controlled trial. Diabetologia. 2010;53:624–631. doi: 10.1007/s00125-009-1641-z. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM, Neilson HK, Woolcott CG, McTiernan A, Wang Q, Ballard-Barbash R, Jones CA, Stanczyk FZ, Brant RF, Yasui Y, Irwin ML, Campbell KL, McNeely ML, Karvinen KH, Courneya KS. Changes in insulin resistance indicators, IGFs, and adipokines in a year-long trial of aerobic exercise in postmenopausal women. Endocr Relat Cancer. 2011;18:357–369. doi: 10.1530/ERC-10-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber CE, McKinney JS, Carleton RA. Is aerobic dance an effective alternative to walk-jog exercise training? J Sports Med Phys Fitness. 1992;32:136–141. [PubMed] [Google Scholar]
- Georgiades A, Sherwood A, Gullette EC, Babyak MA, Hinderliter A, Waugh R, Tweedy D, Craighead L, Bloomer R, Blumenthal JA. Effects of exercise and weight loss on mental stress-induced cardiovascular responses in individuals with high blood pressure. Hypertension. 2000;36:171–176. doi: 10.1161/01.hyp.36.2.171. [DOI] [PubMed] [Google Scholar]
- Gormley SE, Swain DP, High R, Spina RJ, Dowling EA, Kotipalli US, Gandrakota R. Effect of intensity of aerobic training on VO2max. Med Sci Sports Exerc. 2008;40:1336–1343. doi: 10.1249/MSS.0b013e31816c4839. [DOI] [PubMed] [Google Scholar]
- Gram B, Christensen R, Christiansen C, Gram J. Effects of nordic walking and exercise in type 2 diabetes mellitus: a randomized controlled trial. Clin J Sport Med. 2010;20:355–361. doi: 10.1227/NEU.0b013e3181e56e0a. [DOI] [PubMed] [Google Scholar]
- Grandjean PW, Oden GL, Crouse SF, Brown JA, Green JS. Lipid and lipoprotein changes in women following 6 months of exercise training in a worksite fitness program. J Sports Med Phys Fitness. 1996;36:54–59. [PubMed] [Google Scholar]
- Gray SR, Baker G, Wright A, Fitzsimons CF, Mutrie N, Nimmo MA Scottish Physical Activity Research C. The effect of a 12 week walking intervention on markers of insulin resistance and systemic inflammation. Prev Med. 2009;48:39–44. doi: 10.1016/j.ypmed.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Guadalupe-Grau A, Perez-Gomez J, Olmedillas H, Chavarren J, Dorado C, Santana A, Serrano-Sanchez JA, Calbet JAL. Strength training combined with plyometric jumps in adults: sex differences in fat-bone axis adaptations. J Appl Physiol. 2009;106:1100–1111. doi: 10.1152/japplphysiol.91469.2008. [DOI] [PubMed] [Google Scholar]
- Hagan RD, Upton SJ, Wong L, Whittam J. The effects of aerobic conditioning and/or caloric restriction in overweight men and women. Med Sci Sports Exerc. 1986;18:87–94. [PubMed] [Google Scholar]
- Hass CJ, Garzarella L, de Hoyos DV, Connaughton DP, Pollock ML. Concurrent improvements in cardiorespiratory and muscle fitness in response to total body recumbent stepping in humans. Eur J Appl Physiol. 2001;85:157–163. doi: 10.1007/s004210100435. [DOI] [PubMed] [Google Scholar]
- Hendrickson NR, Sharp MA, Alemany JA, Walker LA, Harman EA, Spiering BA, Hatfield DL, Yamamoto LM, Maresh CM, Kraemer WJ, Nindl BC. Combined resistance and endurance training improves physical capacity and performance on tactical occupational tasks. Eur J Appl Physiol. 2010;109:1197–1208. doi: 10.1007/s00421-010-1462-2. [DOI] [PubMed] [Google Scholar]
- Heydari M, Boutcher YN, Boutcher SH. High-intensity intermittent exercise and cardiovascular and autonomic function. Clin Auton Res. 2013;23:57–65. doi: 10.1007/s10286-012-0179-1. [DOI] [PubMed] [Google Scholar]
- Hilberg T, Menzel K, Wehmeier UF. Endurance training modifies exercise-induced activation of blood coagulation: RCT. Eur J Appl Physiol. 2013;113:1423–1430. doi: 10.1007/s00421-012-2564-9. [DOI] [PubMed] [Google Scholar]
- Hiruntrakul A, Nanagara R, Emasithi A, Borer KT. Effect of once a week endurance exercise on fitness status in sedentary subjects. J Med Assoc Thai. 2010;93:1070–1074. [PubMed] [Google Scholar]
- Ho SS, Dhaliwal SS, Hills AP, Pal S. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health. 2012;12:704. doi: 10.1186/1471-2458-12-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Finni T, Zou L, Perhonen M, Sedliak M, Alen M, Cheng S. Effects of strength training on work capacity and parasympathetic heart rate modulation during exercise in physically inactive men. Int J Sports Med. 2009;30:719–724. doi: 10.1055/s-0029-1225329. [DOI] [PubMed] [Google Scholar]
- Huttunen JK, Lansimies E, Voutilainen E, Ehnholm C, Hietanen E, Penttila I, Siitonen O, Rauramaa R. Effect of moderate physical exercise on serum lipoproteins. A controlled clinical trial with special reference to serum high-density lipoproteins. Circulation. 1979;60:1220–1229. doi: 10.1161/01.cir.60.6.1220. [DOI] [PubMed] [Google Scholar]
- Tsuji I, Tamagawa A, Nagatomi R, Irie N, Ohkubo T, Saito M, Fujita K, Ogawa K, Sauvaget C, Anzai Y, Hozawa A, Watanabe Y, Sato A, Ohmori H, Hisamichi S. Randomized controlled trial of exercise training for older people (Sendai Silver Center Trial; SSCT): study design and primary outcome. J Epidemiol. 2000;10:55–64. doi: 10.2188/jea.10.55. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R. Mitigating cellular inflammation in older adults: a randomized controlled trial of Tai Chi Chih. Am J Geriatr Psychiatry. 2012;20:764–772. doi: 10.1097/JGP.0b013e3182330fd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larose J, Sigal RJ, Khandwala F, Prud’homme D, Boule NG, Kenny GP Diabetes A, Resistance Exercise trial I. Associations between physical fitness and HbA1(c) in type 2 diabetes mellitus. Diabetologia. 2011;54:93–102. doi: 10.1007/s00125-010-1941-3. [DOI] [PubMed] [Google Scholar]
- Jessup JV, Lowenthal DT, Pollock ML, Turner T. The effects of endurance exercise training on ambulatory blood pressure in normotensive older adults. Geriatr Nephrol Urol. 1998;8:103–109. doi: 10.1023/a:1008287320868. [DOI] [PubMed] [Google Scholar]
- Kadoglou NPE, Vrabas IS, Kapelouzou A, Angelopoulou N. The association of physical activity with novel adipokines in patients with type 2 diabetes. Eur J Intern Med. 2012;23:137–142. doi: 10.1016/j.ejim.2011.10.020. [DOI] [PubMed] [Google Scholar]
- Karstoft K, Winding K, Knudsen SH, Nielsen JS, Thomsen C, Pedersen BK, Solomon TPJ. The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2013;36:228–236. doi: 10.2337/dc12-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Taylor CB, Haskell WL, DeBusk RF. Influence of regular aerobic exercise on psychological health: a randomized, controlled trial of healthy middle-aged adults. Health Psychol. 1989;8:305–324. doi: 10.1037//0278-6133.8.3.305. [DOI] [PubMed] [Google Scholar]
- Kirk EP, Jacobsen DJ, Gibson C, Hill JO, Donnelly JE. Time course for changes in aerobic capacity and body composition in overweight men and women in response to long-term exercise: the Midwest Exercise Trial (MET) Int J Obes Relat Metab Disord. 2003;27:912–919. doi: 10.1038/sj.ijo.0802317. [DOI] [PubMed] [Google Scholar]
- Kiviniemi AM, Hautala AJ, Kinnunen H, Tulppo MP. Endurance training guided individually by daily heart rate variability measurements. Eur J Appl Physiol. 2007;101:743–751. doi: 10.1007/s00421-007-0552-2. [DOI] [PubMed] [Google Scholar]
- Kokkinos PF, Narayan P, Colleran J, Fletcher RD, Lakshman R, Papademetriou V. Effects of moderate intensity exercise on serum lipids in African-American men with severe systemic hypertension. Am J Cardiol. 1998;81:732–735. doi: 10.1016/s0002-9149(97)01020-5. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Volek JS, Clark KL, Gordon SE, Incledon T, Puhl SM, Triplett-McBride NT, McBride JM, Putukian M, Sebastianelli WJ. Physiological adaptations to a weight-loss dietary regimen and exercise programs in women. J Appl Physiol. 1997;83:270–279. doi: 10.1152/jappl.1997.83.1.270. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Volek JS, Clark KL, Gordon SE, Puhl SM, Koziris LP, McBride JM, Triplett-McBride NT, Putukian M, Newton RU, Hakkinen K, Bush JA, Sebastianelli WJ. Influence of exercise training on physiological and performance changes with weight loss in men. Med Sci Sports Exerc. 1999;31:1320–1329. doi: 10.1097/00005768-199909000-00014. [DOI] [PubMed] [Google Scholar]
- Krogh J, Videbech P, Thomsen C, Gluud C, Nordentoft M. DEMO-II trial. Aerobic exercise versus stretching exercise in patients with major depression-a randomised clinical trial. PLoS One. 2012;7:e48316. doi: 10.1371/journal.pone.0048316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Nielsen JJ, Krustrup BR, Christensen JF, Pedersen H, Randers MB, Aagaard P, Petersen AM, Nybo L, Bangsbo J. Recreational soccer is an effective health-promoting activity for untrained men. Br J Sports Med. 2009;43:825–831. doi: 10.1136/bjsm.2008.053124. [DOI] [PubMed] [Google Scholar]
- Kukkonen-Harjula K, Laukkanen R, Vuori I, Oja P, Pasanen M, Nenonen A, Uusi-Rasi K. Effects of walking training on health-related fitness in healthy middle-aged adults—a randomized controlled study. Scand J Med Sci Sports. 1998;8:236–242. doi: 10.1111/j.1600-0838.1998.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Kurban S, Mehmetoglu I, Yerlikaya HF, Gonen S, Erdem S. Effect of chronic regular exercise on serum ischemia-modified albumin levels and oxidative stress in type 2 diabetes mellitus. Endocr Res. 2011;36:116–123. doi: 10.3109/07435800.2011.566236. [DOI] [PubMed] [Google Scholar]
- Laaksonen DE, Atalay M, Niskanen LK, Mustonen J, Sen CK, Lakka TA, Uusitupa MI. Aerobic exercise and the lipid profile in type 1 diabetic men: a randomized controlled trial. Med Sci Sports Exerc. 2000;32:1541–1548. doi: 10.1097/00005768-200009000-00003. [DOI] [PubMed] [Google Scholar]
- Labrunee M, Antoine D, Verges B, Robin I, Casillas JM, Gremeaux V. Effects of a home-based rehabilitation program in obese type 2 diabetics. Ann Phys Rehabil Med. 2012;55:415–429. doi: 10.1016/j.rehab.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Lake MJ, Cavanagh PR. Six weeks of training does not change running mechanics or improve running economy. Med Sci Sports Exerc. 1996;28:860–869. doi: 10.1097/00005768-199607000-00013. [DOI] [PubMed] [Google Scholar]
- LaPerriere A, Antoni MH, Ironson G, Perry A, McCabe P, Klimas N, Helder L, Schneiderman N, Fletcher MA. Effects of aerobic exercise training on lymphocyte subpopulations. Int J Sports Med. 1994;15(suppl 3):S127–S130. doi: 10.1055/s-2007-1021127. [DOI] [PubMed] [Google Scholar]
- Lee CM, Wood RH, Welsch MA. Influence of short-term endurance exercise training on heart rate variability. Med Sci Sports Exerc. 2003;35:961–969. doi: 10.1249/01.MSS.0000069410.56710.DA. [DOI] [PubMed] [Google Scholar]
- Lee M-G, Park K-S, Kim D-U, Choi S-M, Kim H-J. Effects of high-intensity exercise training on body composition, abdominal fat loss, and cardiorespiratory fitness in middle-aged Korean females. Appl Physiol Nutr Metab. 2012;37:1019–1027. doi: 10.1139/h2012-084. [DOI] [PubMed] [Google Scholar]
- LeMura LM, von Duvillard SP, Andreacci J, Klebez JM, Chelland SA, Russo J. Lipid and lipoprotein profiles, cardiovascular fitness, body composition, and diet during and after resistance, aerobic and combination training in young women. Eur J Appl Physiol. 2000;82:451–458. doi: 10.1007/s004210000234. [DOI] [PubMed] [Google Scholar]
- Libardi CA, De Souza GV, Cavaglieri CR, Madruga VA, Chacon-Mikahil MPT. Effect of resistance, endurance, and concurrent training on TNF-alpha, IL-6, and CRP. Med Sci Sports Exerc. 2012;44:50–56. doi: 10.1249/MSS.0b013e318229d2e9. [DOI] [PubMed] [Google Scholar]
- de Lima C, Boullosa DA, Frollini AB, Donatto FF, Leite RD, Gonelli PRG, Montebello MIL, Prestes J, Cesar MC. Linear and daily undulating resistance training periodizations have differential beneficial effects in young sedentary women. Int J Sports Med. 2012;33:723–727. doi: 10.1055/s-0032-1306324. [DOI] [PubMed] [Google Scholar]
- Lovell D, Cuneo R, Delphinus E, Gass G. Leg strength and the VO2 max of older men. Int J Sports Med. 2011;32:271–276. doi: 10.1055/s-0030-1269844. [DOI] [PubMed] [Google Scholar]
- Martin D, Kauwell GP. Continuous assistive-passive exercise and cycle ergometer training in sedentary women. Med Sci Sports Exerc. 1990;22:523–527. [PubMed] [Google Scholar]
- McAuley KA, Williams SM, Mann JI, Goulding A, Chisholm A, Wilson N, Story G, McLay RT, Harper MJ, Jones IE. Intensive lifestyle changes are necessary to improve insulin sensitivity: a randomized controlled trial. Diabetes Care. 2002;25:445–452. doi: 10.2337/diacare.25.3.445. [DOI] [PubMed] [Google Scholar]
- Meckling KA, Sherfey R. A randomized trial of a hypocaloric high-protein diet, with and without exercise, on weight loss, fitness, and markers of the metabolic syndrome in overweight and obese women. Appl Physiol Nutr Metab. 2007;32:743–752. doi: 10.1139/H07-059. [DOI] [PubMed] [Google Scholar]
- Meyer T, Auracher M, Heeg K, Urhausen A, Kindermann W. Does cumulating endurance training at the weekends impair training effectiveness? Eur J Cardiovasc Prev Rehabil. 2006;13:578–584. doi: 10.1097/01.hjr.0000198921.34814.4d. [DOI] [PubMed] [Google Scholar]
- Miyaki A, Maeda S, Choi Y, Akazawa N, Tanabe Y, Ajisaka R. Habitual aerobic exercise increases plasma pentraxin 3 levels in middle-aged and elderly women. Appl Physiol Nutr Metab. 2012;37:907–911. doi: 10.1139/h2012-069. [DOI] [PubMed] [Google Scholar]
- Morey MC, Pieper CF, Edelman DE, Yancy WS, Jr, Green JB, Lum H, Peterson MJ, Sloane R, Cowper PA, Bosworth HB, Huffman KM, Cavanaugh JT, Hall KS, Pearson MP, Taylor GA. Enhanced fitness: a randomized controlled trial of the effects of home-based physical activity counseling on glycemic control in older adults with prediabetes mellitus. J Am Geriatr Soc. 2012;60:1655–1662. doi: 10.1111/j.1532-5415.2012.04119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AL, Tobar DA, Snyder L. Walking toward a new me: the impact of prescribed walking 10,000 steps/day on physical and psychological well-being. J Phys Act Health. 2010;7:299–307. doi: 10.1123/jpah.7.3.299. [DOI] [PubMed] [Google Scholar]
- Morton RD, West DJ, Stephens JW, Bain SC, Bracken RM. Heart rate prescribed walking training improves cardiorespiratory fitness but not glycaemic control in people with type 2 diabetes. J Sports Sci. 2010;28:93–99. doi: 10.1080/02640410903365685. [DOI] [PubMed] [Google Scholar]
- Murphy MH, Murtagh EM, Boreham CA, Hare LG, Nevill AM. The effect of a worksite based walking programme on cardiovascular risk in previously sedentary civil servants [ NCT00284479] BMC Public Health. 2006;6:136. doi: 10.1186/1471-2458-6-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtagh EM, Boreham CAG, Nevill A, Hare LG, Murphy MH. The effects of 60 minutes of brisk walking per week, accumulated in two different patterns, on cardiovascular risk. Prev Med. 2005;41:92–97. doi: 10.1016/j.ypmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Musa DI, Adeniran SA, Dikko AU, Sayers SP. The effect of a high-intensity interval training program on high-density lipoprotein cholesterol in young men. J Strength Cond Res. 2009;23:587–592. doi: 10.1519/JSC.0b013e318198fd28. [DOI] [PubMed] [Google Scholar]
- Nemoto K-I, Gen-no H, Masuki S, Okazaki K, Nose H. Effects of high-intensity interval walking training on physical fitness and blood pressure in middle-aged and older people. Mayo Clin Proc. 2007;82:803–811. doi: 10.4065/82.7.803. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, Wang X, You T, Lyles MF, Demons J, Easter L, Berry MJ, Lenchik L, Carr JJ. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: a randomized, controlled trial. Am J Clin Nutr. 2009;89:1043–1052. doi: 10.3945/ajcn.2008.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederseer D, Ledl-Kurkowski E, Kvita K, Patsch W, Dela F, Mueller E, Niebauer J. Salzburg skiing for the elderly study: changes in cardiovascular risk factors through skiing in the elderly. Scand J Med Sci Sports. 2011;21(suppl 1):47–55. doi: 10.1111/j.1600-0838.2011.01341.x. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Warren BJ, O’Donnell KA, Dotson RG, Butterworth DE, Henson DA. Physical activity and serum lipids and lipoproteins in elderly women. J Am Geriatr Soc. 1993;41:1339–1344. doi: 10.1111/j.1532-5415.1993.tb06485.x. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Nehlsen-Cannarella SL, Henson DA, Koch AJ, Butterworth DE, Fagoaga OR, Utter A. Immune response to exercise training and/or energy restriction in obese women. Med Sci Sports Exerc. 1998;30:679–686. doi: 10.1097/00005768-199805000-00006. [DOI] [PubMed] [Google Scholar]
- Nordby P, Auerbach PL, Rosenkilde M, Kristiansen L, Thomasen JR, Rygaard L, Groth R, Brandt N, Helge JW, Richter EA, Ploug T, Stallknecht B. Endurance training per se increases metabolic health in young, moderately overweight men. Obesity. 2012;20:2202–2212. doi: 10.1038/oby.2012.70. [DOI] [PubMed] [Google Scholar]
- O’Donovan G, Owen A, Bird SR, Kearney EM, Nevill AM, Jones DW, Woolf-May K. Changes in cardiorespiratory fitness and coronary heart disease risk factors following 24 wk of moderate- or high-intensity exercise of equal energy cost. J Appl Physiol. 2005;98:1619–1625. doi: 10.1152/japplphysiol.01310.2004. [DOI] [PubMed] [Google Scholar]
- Panton LB, Graves JE, Pollock ML, Hagberg JM, Chen W. Effect of aerobic and resistance training on fractionated reaction time and speed of movement. J Gerontol. 1990;45:M26–M31. doi: 10.1093/geronj/45.1.m26. [DOI] [PubMed] [Google Scholar]
- Pollock ML, Carroll JF, Graves JE, Leggett SH, Braith RW, Limacher M, Hagberg JM. Injuries and adherence to walk/jog and resistance training programs in the elderly. Med Sci Sports Exerc. 1991;23:1194–1200. [PubMed] [Google Scholar]
- Phillips MD, Patrizi RM, Cheek DJ, Wooten JS, Barbee JJ, Mitchell JB. Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med Sci Sports Exerc. 2012;44:2099–2110. doi: 10.1249/MSS.0b013e3182644984. [DOI] [PubMed] [Google Scholar]
- Poehlman ET, Dvorak RV, DeNino WF, Brochu M, Ades PA. Effects of resistance training and endurance training on insulin sensitivity in nonobese, young women: a controlled randomized trial. J Clin Endocrinol Metab. 2000;85:2463–2468. doi: 10.1210/jcem.85.7.6692. [DOI] [PubMed] [Google Scholar]
- Posner JD, Gorman KM, Windsor-Landsberg L, Larsen J, Bleiman M, Shaw C, Rosenberg B, Knebl J. Low to moderate intensity endurance training in healthy older adults: physiological responses after four months. J Am Geriatr Soc. 1992;40:1–7. doi: 10.1111/j.1532-5415.1992.tb01820.x. [DOI] [PubMed] [Google Scholar]
- Probart CK, Notelovitz M, Martin D, Khan FY, Fields C. The effect of moderate aerobic exercise on physical fitness among women 70 years and older. Maturitas. 1991;14:49–56. doi: 10.1016/0378-5122(91)90147-i. [DOI] [PubMed] [Google Scholar]
- Pyka G, Lindenberger E, Charette S, Marcus R. Muscle strength and fiber adaptations to a year-long resistance training program in elderly men and women. J Gerontol. 1994;49:M22–M27. doi: 10.1093/geronj/49.1.m22. [DOI] [PubMed] [Google Scholar]
- Chow R, Harrison JE, Notarius C. Effect of two randomised exercise programmes on bone mass of healthy postmenopausal women. Br Med J (Clin Res Ed) 1987;295:1441–1444. doi: 10.1136/bmj.295.6611.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz I, Rosenblit H, Kark JD. Effect of moderate exercise on serum lipids in young men with low high density lipoprotein cholesterol. Arteriosclerosis. 1988;8:245–251. doi: 10.1161/01.atv.8.3.245. [DOI] [PubMed] [Google Scholar]
- Ready AE, Naimark B, Ducas J, Sawatzky JV, Boreskie SL, Drinkwater DT, Oosterveen S. Influence of walking volume on health benefits in women post-menopause. Med Sci Sports Exerc. 1996;28:1097–1105. doi: 10.1097/00005768-199609000-00004. [DOI] [PubMed] [Google Scholar]
- Romero-Arenas S, Blazevich AJ, Martinez-Pascual M, Perez-Gomez J, Luque AJ, Lopez-Roman FJ, Alcaraz PE. Effects of high-resistance circuit training in an elderly population. Exp Gerontol. 2013;48:334–340. doi: 10.1016/j.exger.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Santa-Clara H, Szymanski L, Fernhall B. Effect of exercise training on blood pressure in postmenopausal Caucasian and African-American women. Am J Cardiol. 2003;91:1009–1011. doi: 10.1016/s0002-9149(03)00128-0. [DOI] [PubMed] [Google Scholar]
- Santa-Clara H, Szymanski L, Ordille T, Fernhall B. Effects of exercise training on resting metabolic rate in postmenopausal African American and Caucasian women. Metabolism. 2006;55:1358–1364. doi: 10.1016/j.metabol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Santiago MC, Leon AS, Serfass RC. Failure of 40 weeks of brisk walking to alter blood lipids in normolipemic women. Can J Appl Physiol. 1995;20:417–428. doi: 10.1139/h95-033. [DOI] [PubMed] [Google Scholar]
- Scanga CB, Verde TJ, Paolone AM, Andersen RE, Wadden TA. Effects of weight loss and exercise training on natural killer cell activity in obese women. Med Sci Sports Exerc. 1998;30:1666–1671. doi: 10.1097/00005768-199812000-00002. [DOI] [PubMed] [Google Scholar]
- Seifert T, Rasmussen P, Brassard P, Homann PH, Wissenberg M, Nordby P, Stallknecht B, Secher NH, Nielsen HB. Cerebral oxygenation and metabolism during exercise following three months of endurance training in healthy overweight males. Am J Physiol Regul Integr Comp Physiol. 2009;297:R867–R876. doi: 10.1152/ajpregu.00277.2009. [DOI] [PubMed] [Google Scholar]
- Lamina S. Comparative effect of interval and continuous training programs on serum uric acid in management of hypertension: a randomized controlled trial. J Strength Cond Res. 2011;25:719–726. doi: 10.1519/JSC.0b013e3181d09edf. [DOI] [PubMed] [Google Scholar]
- Sillanpaa E, Laaksonen DE, Hakkinen A, Karavirta L, Jensen B, Kraemer WJ, Nyman K, Hakkinen K. Body composition, fitness, and metabolic health during strength and endurance training and their combination in middle-aged and older women. Eur J Appl Physiol. 2009;106:285–296. doi: 10.1007/s00421-009-1013-x. [DOI] [PubMed] [Google Scholar]
- Sillanpaa E, Hakkinen A, Laaksonen DE, Karavirta L, Kraemer WJ, Hakkinen K. Serum basal hormone concentrations, nutrition and physical fitness during strength and/or endurance training in 39-64-year-old women. Int J Sports Med. 2010;31:110–117. doi: 10.1055/s-0029-1242811. [DOI] [PubMed] [Google Scholar]
- Sloan CA, Engels HJ, Fahlman MM, Yarandi HE, Davis JE. Effects of exercise on S-IGA and URS in postmenopausal women. Int J Sports Med. 2013;34:81–86. doi: 10.1055/s-0032-1314817. [DOI] [PubMed] [Google Scholar]
- Spence AL, Carter HH, Naylor LH, Green DJ. A prospective randomized longitudinal study involving 6 months of endurance or resistance exercise. Conduit artery adaptation in humans. J Physiol. 2013;591:1265–1275. doi: 10.1113/jphysiol.2012.247387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachenfeld NS, Mack GW, DiPietro L, Morocco TS, Jozsi AC, Nadel ER. Regulation of blood volume during training in post-menopausal women. Med Sci Sports Exerc. 1998;30:92–98. doi: 10.1097/00005768-199801000-00013. [DOI] [PubMed] [Google Scholar]
- Stein PK, Boutcher SH. The effect of participation in an exercise training program on cardiovascular reactivity in sedentary middle-aged males. Int J Psychophysiol. 1992;13:215–223. doi: 10.1016/0167-8760(92)90071-i. [DOI] [PubMed] [Google Scholar]
- Stensel DJ, Hardman AE, Brooke-Wavell K, Vallance D, Jones PR, Norgan NG, Winder AF. Brisk walking and serum lipoprotein variables in formerly sedentary men aged 42-59 years. Clin Sci. 1993;85:701–708. doi: 10.1042/cs0850701. [DOI] [PubMed] [Google Scholar]
- Stensvold D, Tjonna AE, Skaug E-A, Aspenes S, Stolen T, Wisloff U, Slordahl SA. Strength training versus aerobic interval training to modify risk factors of metabolic syndrome. J Appl Physiol. 2010;108:804–810. doi: 10.1152/japplphysiol.00996.2009. [DOI] [PubMed] [Google Scholar]
- Strasser B, Keinrad M, Haber P, Schobersberger W. Efficacy of systematic endurance and resistance training on muscle strength and endurance performance in elderly adults—a randomized controlled trial. Wien Klin Wochenschr. 2009;121:757–764. doi: 10.1007/s00508-009-1273-9. [DOI] [PubMed] [Google Scholar]
- Sung K, Bae S. Effects of a regular walking exercise program on behavioral and biochemical aspects in elderly people with type II diabetes. Nurs Health Sci. 2012;14:438–445. doi: 10.1111/j.1442-2018.2012.00690.x. [DOI] [PubMed] [Google Scholar]
- Takeshima N, Rogers ME, Watanabe E, Brechue WF, Okada A, Yamada T, Islam MM, Hayano J. Water-based exercise improves health-related aspects of fitness in older women. Med Sci Sports Exerc. 2002;34:544–551. doi: 10.1097/00005768-200203000-00024. [DOI] [PubMed] [Google Scholar]
- Takeshima N, Rogers ME, Islam MM, Yamauchi T, Watanabe E, Okada A. Effect of concurrent aerobic and resistance circuit exercise training on fitness in older adults. Eur J Appl Physiol. 2004;93:173–182. doi: 10.1007/s00421-004-1193-3. [DOI] [PubMed] [Google Scholar]
- Thomas TR, Adeniran SB, Etheridge GL. Effects of different running programs on VO2 max, percent fat, and plasma lipids. Can J Appl Sport Sci. 1984;9:55–62. [PubMed] [Google Scholar]
- Thompson D, Markovitch D, Betts JA, Mazzatti D, Turner J, Tyrrell RM. Time course of changes in inflammatory markers during a 6-mo exercise intervention in sedentary middle-aged men: a randomized-controlled trial. J Appl Physiol. 2010;108:769–779. doi: 10.1152/japplphysiol.00822.2009. [DOI] [PubMed] [Google Scholar]
- Tjonna AE, Lee SJ, Rognmo O, Stolen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slordahl SA, Kemi OJ, Najjar SM, Wisloff U. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118:346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo FGS, Menshikova EV, Azuma K, Radikova Z, Kelley CA, Ritov VB, Kelley DE. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes. 2008;57:987–994. doi: 10.2337/db07-1429. [DOI] [PubMed] [Google Scholar]
- Tseng M-L, Ho C-C, Chen S-C, Huang Y-C, Lai C-H, Liaw Y-P. A simple method for increasing levels of high-density lipoprotein cholesterol: a pilot study of combination aerobic- and resistance-exercise training. Int J Sport Nutr Exerc Metab. 2013;23:271–281. doi: 10.1123/ijsnem.23.3.271. [DOI] [PubMed] [Google Scholar]
- Tulppo MP, Hautala AJ, Makikallio TH, Laukkanen RT, Nissila S, Hughson RL, Huikuri HV. Effects of aerobic training on heart rate dynamics in sedentary subjects. J Appl Physiol. 2003;95:364–372. doi: 10.1152/japplphysiol.00751.2002. [DOI] [PubMed] [Google Scholar]
- Utter AC, Nieman DC, Shannonhouse EM, Butterworth DE, Nieman CN. Influence of diet and/or exercise on body composition and cardiorespiratory fitness in obese women. Int J Sport Nutr. 1998;8:213–222. doi: 10.1123/ijsn.8.3.213. [DOI] [PubMed] [Google Scholar]
- van Aggel-Leijssen DP, Saris WH, Homan M, van Baak MA. The effect of exercise training on beta-adrenergic stimulation of fat metabolism in obese men. Int J Obes Relat Metab Disord. 2001;25:16–23. doi: 10.1038/sj.ijo.0801470. [DOI] [PubMed] [Google Scholar]
- van Aggel-Leijssen DP, Saris WH, Hul GB, van Baak MA. Short-term effects of weight loss with or without low-intensity exercise training on fat metabolism in obese men. Am J Clin Nutr. 2001;73:523–531. doi: 10.1093/ajcn/73.3.523. [DOI] [PubMed] [Google Scholar]
- van den Berg R, de Groot S, Swart KMA, van der Woude LHV. Physical capacity after 7 weeks of low-intensity wheelchair training. Disabil Rehabil. 2010;32:2244–2252. doi: 10.3109/09638288.2010.535688. [DOI] [PubMed] [Google Scholar]
- Vicente-Campos D, Mora J, Castro-Pinero J, Gonzalez-Montesinos JL, Conde-Caveda J, Chicharro JL. Impact of a physical activity program on cerebral vasoreactivity in sedentary elderly people. J Sports Med Phys Fitness. 2012;52:537–544. [PubMed] [Google Scholar]
- Vincent KR, Braith RW. Resistance exercise and bone turnover in elderly men and women. Med Sci Sports Exerc. 2002;34:17–23. doi: 10.1097/00005768-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Vissers D, Verrijken A, Mertens I, Van Gils C, Van de Sompel A, Truijen S, Van Gaal L. Effect of long-term whole body vibration training on visceral adipose tissue: a preliminary report. Obes Facts. 2010;3:93–100. doi: 10.1159/000301785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello MV, Wilkinson CW, Merriam GR, Moe KE, Prinz PN, Ralph DD, Colasurdo EA, Schwartz RS. Successful 6-month endurance training does not alter insulin-like growth factor-I in healthy older men and women. J Gerontol A Biol Sci Med Sci. 1997;52:M149–M154. doi: 10.1093/gerona/52a.3.m149. [DOI] [PubMed] [Google Scholar]
- Volpe SL, Kobusingye H, Bailur S, Stanek E. Effect of diet and exercise on body composition, energy intake and leptin levels in overweight women and men. J Am Coll Nutr. 2008;27:195–208. doi: 10.1080/07315724.2008.10719691. [DOI] [PubMed] [Google Scholar]
- Waib PH, Goncalves MI, Barrile SR. Improvements in insulin sensitivity and muscle blood flow in aerobic-trained overweight-obese hypertensive patients are not associated with ambulatory blood pressure. J Clin Hypertens. 2011;13:89–96. doi: 10.1111/j.1751-7176.2010.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallman K, Plant LA, Rakimov B, Maiorana AJ. The effects of two modes of exercise on aerobic fitness and fat mass in an overweight population. Res Sports Med. 2009;17:156–170. doi: 10.1080/15438620903120215. [DOI] [PubMed] [Google Scholar]
- Wang J-S, Li Y-S, Chen J-C, Chen Y-W. Effects of exercise training and deconditioning on platelet aggregation induced by alternating shear stress in men. Arterioscler Thromb Vasc Biol. 2005;25:454–460. doi: 10.1161/01.ATV.0000151987.04607.24. [DOI] [PubMed] [Google Scholar]
- Wang J-S, Chen W-L, Weng T-P. Hypoxic exercise training reduces senescent T-lymphocyte subsets in blood. Brain Behav Immun. 2011;25:270–278. doi: 10.1016/j.bbi.2010.09.018. [DOI] [PubMed] [Google Scholar]
- Warner JG, Jr, Ullrich IH, Albrink MJ, Yeater RA. Combined effects of aerobic exercise and omega-3 fatty acids in hyperlipidemic persons. Med Sci Sports Exerc. 1989;21:498–505. [PubMed] [Google Scholar]
- Warren BJ, Nieman DC, Dotson RG, Adkins CH, O’Donnell KA, Haddock BL, Butterworth DE. Cardiorespiratory responses to exercise training in septuagenarian women. Int J Sports Med. 1993;14:60–65. doi: 10.1055/s-2007-1021147. [DOI] [PubMed] [Google Scholar]
- Watkins LL, Sherwood A, Feinglos M, Hinderliter A, Babyak M, Gullette E, Waugh R, Blumenthal JA. Effects of exercise and weight loss on cardiac risk factors associated with syndrome X. Arch Intern Med. 2003;163:1889–1895. doi: 10.1001/archinte.163.16.1889. [DOI] [PubMed] [Google Scholar]
- Wong DG, Rechnitzer PA, Cunningham DA, Howard JH. Effect of an exercise program on the perception of exertion in males at retirement. Can J Sport Sci. 1990;15:249–253. [PubMed] [Google Scholar]
- Woods JA, Ceddia MA, Wolters BW, Evans JK, Lu Q, McAuley E. Effects of 6 months of moderate aerobic exercise training on immune function in the elderly. Mech Ageing Dev. 1999;109:1–19. doi: 10.1016/s0047-6374(99)00014-7. [DOI] [PubMed] [Google Scholar]
- Wu Y-T, Hwang C-L, Chen C-N, Chuang L-M. Home-based exercise for middle-aged Chinese at diabetic risk: a randomized controlled trial. Prev Med. 2011;52:337–343. doi: 10.1016/j.ypmed.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M, Maeda S, Miyaki A, Misono M, Choi Y, Shimojo N, Ajisaka R, Tanaka H. Additive beneficial effects of lactotripeptides and aerobic exercise on arterial compliance in postmenopausal women. Am J Physiol Heart Circ Physiol. 2009;297:H1899–H1903. doi: 10.1152/ajpheart.00433.2009. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M, Maeda S, Miyaki A, Misono M, Saito Y, Tanabe K, Kuno S, Ajisaka R. Effect of 12 weeks of moderate-intensity resistance training on arterial stiffness: a randomised controlled trial in women aged 32-59 years. Br J Sports Med. 2009;43:615–618. doi: 10.1136/bjsm.2008.052126. [DOI] [PubMed] [Google Scholar]
- You T, Murphy KM, Lyles MF, Demons JL, Lenchik L, Nicklas BJ. Addition of aerobic exercise to dietary weight loss preferentially reduces abdominal adipocyte size. Int J Obes. 2006;30:1211–1216. doi: 10.1038/sj.ijo.0803245. [DOI] [PubMed] [Google Scholar]
- Ziemann E, Grzywacz T, Luszczyk M, Laskowski R, Olek RA, Gibson AL. Aerobic and anaerobic changes with high-intensity interval training in active college-aged men. J Strength Cond Res. 2011;25:1104–1112. doi: 10.1519/JSC.0b013e3181d09ec9. [DOI] [PubMed] [Google Scholar]
- Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69:373–380. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Crouse SF, Goodpaster B, Kelley D, Moyna N, Pescatello L. The acute versus the chronic response to exercise. Med Sci Sports Exerc. 2001;33:S438–S445. doi: 10.1097/00005768-200106001-00012. discussion S452-433. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Shepard RJ, Stephens T, Sutton JR, McPherson BD. Exercise, fitness, and health: a consensus of current knowledge. Med Sci Sports Exerc. 1991;23:643. [Google Scholar]
- Leaf DA. The effect of physical exercise on reverse cholesterol transport. Metabolism. 2003;52:950–957. doi: 10.1016/s0026-0495(03)00147-1. [DOI] [PubMed] [Google Scholar]
- Hamilton MT, Etienne J, McClure WC, Pavey BS, Holloway AK. Role of local contractile activity and muscle fiber type on LPL regulation during exercise. Am J Physiol. 1998;275:E1016–E1022. doi: 10.1152/ajpendo.1998.275.6.E1016. [DOI] [PubMed] [Google Scholar]
- Oscai LB, Tsika RW, Essig DA. Exercise training has a heparin-like effect on lipoprotein lipase activity in muscle. Can J Physiol Pharmacol. 1992;70:905–909. doi: 10.1139/y92-121. [DOI] [PubMed] [Google Scholar]
- Plaisance EP, Grandjean PW, Mahurin AJ. Independent and combined effects of aerobic exercise and pharmacological strategies on serum triglyceride concentrations: a qualitative review. Phys Sportsmed. 2009;37:11–19. doi: 10.3810/psm.2009.04.1678. [DOI] [PubMed] [Google Scholar]
- Kohl HW., III Physical activity and cardiovascular disease: evidence for a dose response. Med Sci Sports Exerc. 2001;33:S472–S483. doi: 10.1097/00005768-200106001-00017. discussion S493-474. [DOI] [PubMed] [Google Scholar]
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC, Kriska A, Leon AS, Marcus BH, Morris J, Paffenbarger RS, Jr, Patrick K, Pollock ML, Rippe JM, Sallis J, Wilmore JH. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Ogasawara J, Kizaki T, Sato S, Ishibashi Y, Takahashi M, Kobayashi O, Oh-Ishi S, Nagasawa J, Takahashi K, Ishida H, Izawa T, Ohno H. The effects of exercise training on obesity-induced dysregulated expression of adipokines in white adipose tissue. Int J Endocrinol. 2013;2013:801743. doi: 10.1155/2013/801743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14:741–751. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma Y, Shinoda-Tagawa T. Physical activity decreases cardiovascular disease risk in women: review and meta-analysis. Am J Prev Med. 2004;26:407–418. doi: 10.1016/j.amepre.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee Report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev. 2009;67:114–120. doi: 10.1111/j.1753-4887.2008.00136.x. [DOI] [PubMed] [Google Scholar]
- Kelley GA, Kelley KS, Tran ZV. Aerobic exercise and lipids and lipoproteins in women: a meta-analysis of randomized controlled trials. J Womens Health (Larchmt) 2004;13:1148–1164. doi: 10.1089/jwh.2004.13.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]


