Abstract
Background
A specific electrocardiographic (ECG) marker of ischemia would greatly improve the speed and accuracy of detecting and treating non-ST elevation myocardial infarction (NSTEMI). We hypothesize that ischemia induces ventricular repolarization dispersion (VRD), altering the T-wave before any ST segment deviation. We sought to evaluate the clinical utility of VRD to (1) detect NSTEMI cases in the emergency department (ED) and (2) identify NSTEMI cases at high risk for in-hospital major adverse cardiac events (MACEs).
Methods and Results
We continuously recorded 12-lead Holter ECGs from chest pain patients upon their arrival to the ED. VRD was quantified using principal component analysis of the 12-lead ECG to compute a T-wave complexity ratio (ie, ratio of second to first eigenvectors of repolarization). Clinical outcomes were obtained from hospital records. The sample was composed mainly of older males (n=369; ages 63±12 years; 63% males), and 92 (25%) had NSTEMI and 26 (7%) had MACEs. Baseline T-wave complexity ratio modestly correlated with peak troponin levels (r=0.41; P<0.001) and was a good classifier of NSTEMI events (area under the curve=0.70). An increased T-wave complexity ratio on the presenting ECG was strongly associated with NSTEMI (odds ratio [OR]=3.8 [2.1 to 5.8]) and in-hospital MACE (OR=8.2 [3.1 to 21.5]).
Conclusions
A simple measure of global VRD on the presenting 12-lead ECG correlates with ischemic myocardial injury and can discriminate NSTEMI cases very early during evaluation. Prospective studies should validate these findings and test whether VRD can guide therapy.
Keywords: complications, ECG, ischemia, myocardial infarction, repolarization
Every day, more than 2500 Americans suffer an ischemic coronary event, and more than 1400 of them will die of subsequent complications.1 This makes acute myocardial infarction (MI) the leading cause of death in the United States; reducing damage associated with MI is a critical step in addressing the issue. The electrocardiogram (ECG) is an integral part of MI detection and treatment, with immediate decisions about acute coronary interventions based on the presence or absence of ST elevation (STE). Treatment guidelines for acute and ongoing care are based on ECG categorization of STEMI and non-STEMI (or NSTEMI).2–4 Treatment strategy in NSTEMI is debatable, with growing evidence suggesting long-term benefit with appropriate early invasive strategy (<2 hours) in selected high-risk NSTEMI patients (ie, high ischemic burden).5,6 However, there are currently no precise ECG patterns that can detect or quantify such ischemic burden in NSTEMI, and NSTEMI can only be recognized by a clinical scenario combined with the presence of serum biomarkers of myocardial necrosis (ie, troponin). A specific ECG marker of ischemia in the absence of STE would have the potential to improve the speed and accuracy of detecting NSTEMI and selecting proper candidates for immediate revascularization.7
Growing evidence suggests that myocardial ischemia alters spatial and temporal indices of ventricular repolarization dispersion (VRD).8–10 Findings from these studies demonstrate that indices of VRD are dynamic in response to sudden coronary artery occlusion and restoration of normal perfusion in human and animal experiments. We previously explored temporal and spatial repolarization indices in both healthy adults and patients with coronary artery disease (CAD).11,12 Our data also suggest that myocardial ischemia increases the spatial and temporal distribution of the ventricular repolarization gradient preceding any displacement of the ST segment, which we previously quantified using the principal repolarization components from the surface 12-lead ECG. The diagnostic accuracy of VRD has never been tested before.
In this study, we explore the clinical utility of a specific index of spatial VRD, the T-wave complexity ratio, to (1) detect NSTEMI among nontraumatic chest pain patients observed at emergency departments (EDs) and (2) quantify the severity of ischemic burden to identify high-risk NSTEMI patients who would benefit from early revascularization (ie, percutaneous coronary interventions [PCIs]). We hypothesized that (1) the T-wave complexity ratio would increase in patients with NSTEMI, relative to other chest pain patients, and (2) the absolute values of the T-wave complexity ratio would be associated with the risk of in-hospital major adverse cardiac events (MACEs).
Methods
This was a secondary analysis of the National Institutes of Health (NIH)-funded electrocardiographic evaluation of ischemia comparing invasive to pharmacological treatment (COMPARE) study. COMPARE was an observational study designed to assess the frequency and clinical consequences of transient myocardial ischemia in patients with NSTEMI or unstable angina treated with either early invasive (ie, PCI) or conservative approaches. The study complies with the Declaration of Helsinki; appropriate institutional review boards (IRBs) approved the parent study, and subjects provided informed consent before participation. The specific methods are reported elsewhere.13 The University of Pittsburgh’s IRB approved this secondary analysis.
In brief, the parent COMPARE study recruited patients from the ED of 2 hospitals in Nevada and California between 2010 and 2012 who presented with a chief complaint of nontraumatic chest pain. The study purposefully recruited symptomatic patients at high clinical suspicion of acute coronary syndrome (ie, prior CAD, MI, coronary intervention, angina-like pain, and so on). Documented CAD was defined by a previous coronary angiography at any time before the current admission, with at least a 50% stenosis in a major coronary artery. Patients with (1) STEMI or other conditions that are known to cause troponin elevation (ie, endocarditis) or (2) with severe hemodynamic instability upon admission were excluded. Eligible subjects underwent continuous 12-lead ECG monitoring using H12+ Holter recorders (v3.12; Mortara Instruments, Milwaukee, WI). Holter recordings using Mason-Likar configuration (ie, limb leads on body) were initiated within 6 hours of the onset of symptoms and before any revascularization therapy. Patients were followed up during hospitalization, and their clinical data were manually abstracted from the electronic medical records. The primary outcome was a final discharge diagnosis of NSTEMI, documented by a rise of cardiac troponin levels (ie, ≥0.04 ng/dL) without new ST-segment elevation. Unstable angina (UA) was defined as angina pectoris (or equivalent type of ischemic discomfort) occurring at rest for at least 20 minutes despite initial anti-ischemic therapy (ie, nitroglycerin) without biochemical evidence of necrosis (ie, rise of cardiac troponin).14 The secondary outcome was the occurrence of in-hospital MACE. MACE was defined as death or deterioration in hemodynamic status requiring transfer to critical care (eg, lethal ventricular arrhythmias, pulmonary edema, and so on). A total of 488 patients (age, 64±13 years; 60% male; 92% white) were included in the parent study. Nearly 19% of patients (n=96) did not have raw digital data required for high-fidelity signal processing, and after excluding those with pacing and/or left bundle branch block (n=23), the analysis reported in this study included 369 patients (age, 63±12 years; 63% male; 93% white).
Electrocardiographic Methods
Holter ECGs were downloaded to an encrypted server for offline analysis. After all medical treatments were completed, a researcher blinded to clinical data annotated the Holter ECGs using H-Scribe 5.11 (Mortara Instruments). Noise and artifacts were deleted from each recording, which resulted in an average monitoring period of 21 hours. Then, the high-fidelity ECG signal was preprocessed using Super-ECG (Mortara Instruments) to compute signal-average median beats for each minute of recording for every main ECG lead during the initial 60 minutes of Holter recording. T waves of median beats were then automatically filtered using a singular value decomposition technique to derive the main principal component analysis (PCA) beats for the initial 10-second recording (baseline) and each 5-minute segment for the first hour of recording.15 The PCA technique reduces the dimension of the 12-lead ECG into 3 principal components. These components mimic 3 mathematically orthogonal vectorocardiographic leads that contain the most significant information in the data. A simple ratio between the second to first PCA components (ie, eigenvalues of repolarization) constitutes a marker of global VRD.16 This PCA ratio is called the T-wave complexity (or repolarization complexity) ratio, and it has been linked previously to adverse cardiac events.16–18 T-wave complexity is a measure of how different the STT waveforms of all leads are. If all STT waveforms are identical, the T-wave complexity ratio would be zero, meaning that the first eigenvector is the only nonzero eigenvalue. As the difference between STT waveforms increase, the ratio increases to a maximum of 1, meaning that the second eigenvector is approximately as dominant as the first. Under normal conditions (Figure1, left), repolarization is a relatively smooth, regular process (eg, all upright T waves); therefore, most energy is expressed by the largest eigenvector of the first PCA component (Figure1A and 1B). This results in a noticeably diminished repolarization wave in the next 2 PCA components (Figure1C) and, consequently, a “thin” T loop when projected in the 2-dimensional plane with a small T-wave complexity ratio (Figure1D). Under pathological derangements (eg, ischemia; Figure1, right), injury currents disperse the repolarization energy into different eigenvectors (Figure1A and 1B). This increases the repolarization waves in all PCA components (Figure1C, red arrows) and results in a “fatter” T loop with a larger T-wave complexity ratio (Figure1D). Thus, we hypothesize that increased severity of ischemic injury increases VRD, which is measured as increased T-wave complexity values. It should be noted that, however, T-wave complexity is a global measure of spatial and temporal VRD and cannot localize ischemia; it can be sensitive to endo-mid, endo-epi, apex-base, epi-mid, or endocardial wall ischemia. Interestingly, given that NSTEMI represents a more progressive CAD with no localized wall ischemia, T-wave complexity—as a global measure—can be very sensitive to detect such global injury.
Figure 1.
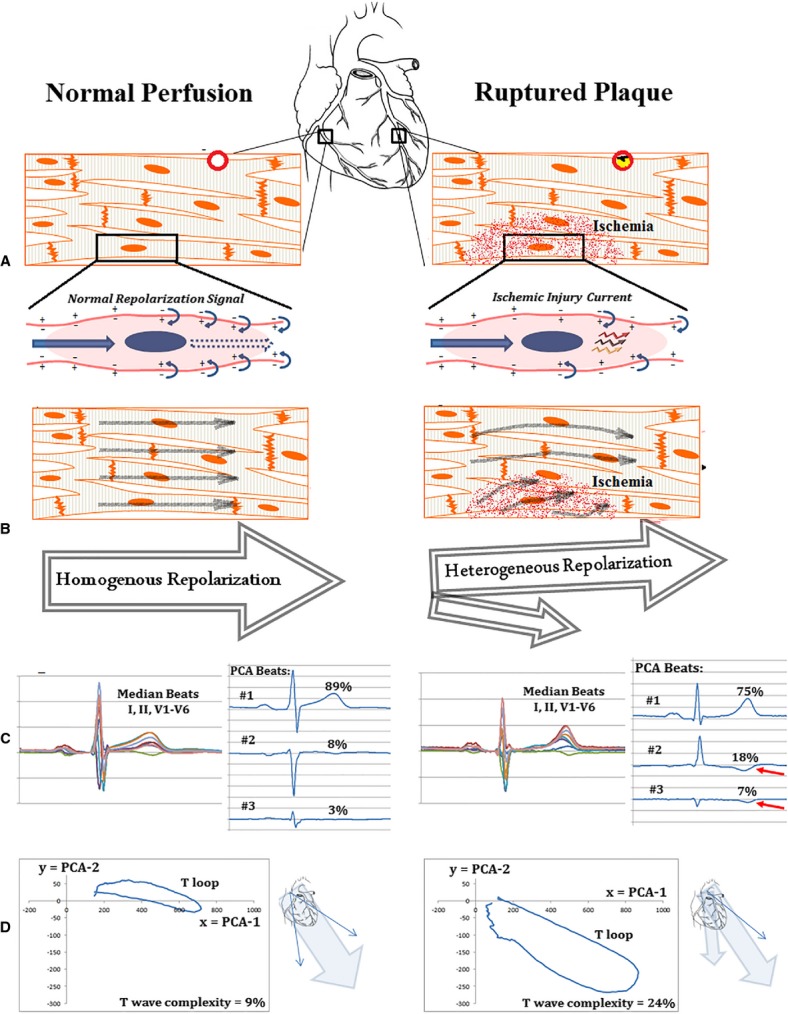
Illustration of ischemia-induced ventricular repolarization dispersion. Left side shows normal repolarization signal propagation and normal coronary perfusion with corresponding T-wave eigenvectors and loop morphology in a 54-year-old male with no coronary events. Right side shows a dispersed repolarization signal and diminished coronary perfusion with corresponding T-wave eigenvectors and loop morphology in a 72-year-old female with NSTEMI. A, Myocardial tissue. B, Normal repolarization wave and response to injury currents. C, PCA-derived ECG beats. D, Plot of T-wave amplitude for first PCA beat (x axis) versus second PCA beat (y axis). Ratio between these eigenvalues is T-wave complexity. ECG indicates electrocardiogram; NSTEMI, non-ST elevation myocardial infarction; PCA, principal component analysis.
Statistical Analysis
Values are presented as mean plus or minus SDs or count (%). All analyses were completed using SPSS software (22.0; IBM Corp., Armonk, NY), and P values less than 0.05 were considered statistically significant. Baseline demographic and clinical differences between groups (ie, NSTEMI, UA, and others) were tested using chi-square for categorical variables and ANOVA for continuous variables. Magnitude of difference in T-wave complexity ratio between groups was analyzed at baseline and across time points.
First, given that clinical decisions are generally based on an initial 10-second 12-lead ECG, T-wave complexity from the initial ECG recording was computed. Baseline was defined as the first resting 10-second segment of the Holter ECG recording. The association between this baseline T-wave complexity and peak troponin levels was tested using Pearson’s r correlation coefficient, and the classification performance of baseline T-wave complexity to detect NSTEMI events was tested using the receiver operator characteristics (ROC) curve. To test the clinical value of baseline T-wave complexity ratio to predict primary (ie, NSTEMI: yes vs. no) and secondary (ie, MACE: yes vs. no) outcomes, other variables, significant at P<0.10 in univariate analyses, were entered simultaneously in a binary logistic regression model. Variables without significant associations in the multivariable model were removed one by one to create a parsimonious model. Goodness of fit was tested with a Hosmer-Lemeshow test. Odds ratios (ORs) with 95% confidence intervals (CIs) were presented for significant predictors in the final model. Then, ROC-optimized cut-off point for baseline T-wave complexity was used to compute sensitivity, specificity, and positive (PPV) and negative predictive values (NPV) to detect primary outcome. Then, we used the same cut-off point to compare time-to-MACE events rate between groups (normal vs. abnormal baseline T-wave complexity) using Kaplan-Meier events probability curves with log-rank test between curves.
Second, we wanted to test the significance of ambulatory T-wave complexity monitoring, so we computed T-wave complexity for every 5-minute segment of the initial 60 minutes of the Holter ECG recording (13 time points). We then compared groups (ie, NSTEMI, UA, and others) using repeated-measures ANOVA. Time-group interaction terms were tested using Tukey’s post-hoc adjustment to examine differences between groups in each time point and across different time points.
Results
Baseline ECG and Clinical Characteristics
The final sample included 369 chest pain patients who were mainly older white males (age, 63±12 years; 63% male; 93% white). Nearly one half of the sample (n=170; 46%) had documented CAD at baseline. Of those, 112 had previously experienced acute MI that was primarily managed by PCI (n=70) or bypass surgery (n=39). Moreover, more than half of the sample had hypertension (HTN) and dyslipidemia and were current or former smokers; nearly 25% were diabetic.
During hospitalization, 92 (25%) subjects were diagnosed with NSTEMI, which was documented by a rise in serum cardiac troponin (peak cTnI=1.54±3.46 ng/dL; range, 0.10 to 18.09). Of those, 65 (71%) were treated by PCI (n=55) or bypass surgery (n=10; Table1). Other pharmacological treatments included nitrates (64%), antiplatelet (97%), aspirin (97%), beta-blockers (85%), and angiotensin inhibition therapy (51%). During the course of hospitalization (length of stay ranged from 5 hours to 31 days), 26 patients (7%) experienced in-hospital MACE, including myocardial reinfarction (n=13), pulmonary edema (n=3), cardiogenic shock (n=2), lethal ventricular tachyarrhythmia (n=3), and external pacing or cardioversion (n=5).
Table 1.
Characteristics of Patients at Baseline
| Characteristic | All Patients (N=369) | NSTEMI (n=92) | UA (n=45) | Others (n=232) | P Value |
|---|---|---|---|---|---|
| Demographics (%) | |||||
| Age, y | 63±12 | 65±12* | 65±13 | 61±12 | 0.022† |
| Sex (female) | 138 (37) | 27 (29) | 16 (36) | 95 (41) | 0.145 |
| Race (white) | 342 (93) | 87 (95) | 43 (96) | 212 (91) | 0.447 |
| Past medical history and clinical risk factors (%) | |||||
| Documented CAD | 170 (46) | 46 (50) | 35 (78)* | 89 (38) | <0.001† |
| Previous myocardial infarction | 112 (30) | 25 (27) | 23 (51)* | 64 (28) | <0.01† |
| Previous PCI | 106 (29) | 27 (29) | 24 (53)* | 55 (24) | <0.001† |
| Previous CABG | 58 (16) | 17 (18) | 14 (31)* | 27 (12) | <0.01† |
| Hypertension | 247 (67) | 58 (63) | 32 (71) | 157 (68) | 0.596 |
| Diabetes mellitus | 95 (26) | 23 (25) | 17 (38) | 55 (24) | 0.140 |
| Dyslipidemia | 218 (59) | 60 (65) | 34 (76)* | 124 (53) | <0.01† |
| Current or former smoker | 208 (56) | 53 (58) | 25 (56) | 130 (56) | 0.782 |
| Known CHF | 24 (7) | 5 (5) | 6 (13) | 13 (6) | 0.141 |
| Current hospitalization (%) | |||||
| LDL, md/dL | 101±41 | 103±37 | 93±43 | 99±42 | 0.461 |
| HDL, mg/dL | 45±18 | 40±12* | 42±13 | 48±21 | 0.029† |
| Creatinine | 1.12±0.62 | 1.3±0.9* | 1.1±0.5 | 1.1±0.5 | <0.01† |
| Ejection fraction, % | 62±12 | 57±12* | 60±11 | 65±11 | <0.001† |
| Length of stay, days | 2.5±3.1 | 3.9±4.2* | 3.3±4.0* | 1.8±1.9 | <0.001† |
| MACE | 26 (7) | 20 (22)* | 4 (9) | 2 (1) | <0.001† |
| Treated with PCI/CABG | 116 (31) | 65 (71)* | 23 (51)* | 28 (12) | <0.001† |
| Presenting 12-lead electrocardiogram (%) | |||||
| Any ST depression | 46 (13) | 20 (22)* | 10 (22)* | 16 (7) | <0.001† |
| Any T wave inversion | 24 (7) | 10 (11) | 3 (7) | 11 (5) | 0.131 |
| T-wave complexity ratio | 22.2±14.6 | 0.27±0.12* | 0.24±0.16 | 0.20±0.13 | <0.001† |
P values are obtained using ANOVA. CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHF indicates congestive heart failure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MACE, major adverse cardiac events; NSTEMI, non-ST elevation myocardial infarction; PCI, percutaneous coronary interventions; UA, unstable angina.
Significance against “others” subcategory using Tukey’s post-hoc adjustment.
Significant differences between groups using ANOVA.
Compared to other patients with similar symptoms, but without ischemic myocardial injury (n=232; 63%), those with NSTEMI (n=92; 25%) were more likely to (1) be older, (2) have a lower high-density lipoprotein (HDL) and ejection fraction, and (3) have higher creatinine values (Table1). On the presenting 12-lead ECGs, NSTEMI patients were more likely to have diagnostic ST depression (horizontal depression >1 mm in 2 contiguous leads) in at least 1 myocardial wall, but no specific T-wave inversion pattern. More interestingly, NSTEMI patients, but not UA patients, were more likely to have larger T-wave complexity on their baseline ECG.
The Clinical Utility of T-Wave Complexity
The T-wave complexity ratio demonstrated greater repolarization heterogeneity among NSTEMI subjects, compared to all other subjects, and this differential power was maintained throughout the initial 60-minute of ECG recording in the ED (Figure2). Of note, T-wave complexity could differentiate NSTEMI, but not UA, from others without coronary events, suggesting that this measure is sensitive to myocardial injury rather than myocardial ischemia. This finding is interesting given that UA currently constitutes a clinical challenge, and being able to isolate NSTEMI patients early during triage can help improve outcomes in this subcategory. Moreover, the baseline T-wave complexity ratio was positively correlated with peak troponin level (r=0.41; P<0.001; Figure3A), and the total area under the curve (AUC) for the baseline T-wave complexity ratio as a classifier of NSTEMI events was 70% (Figure3B). This important correlation again supports a role for T-wave complexity in ischemic injury detection and suggests that the larger the ischemic damage in the myocardium, the greater is this ratio. This finding is novel and suggests that measures of VRD can quantify myocardial ischemic burden in the absence of STE.
Figure 2.
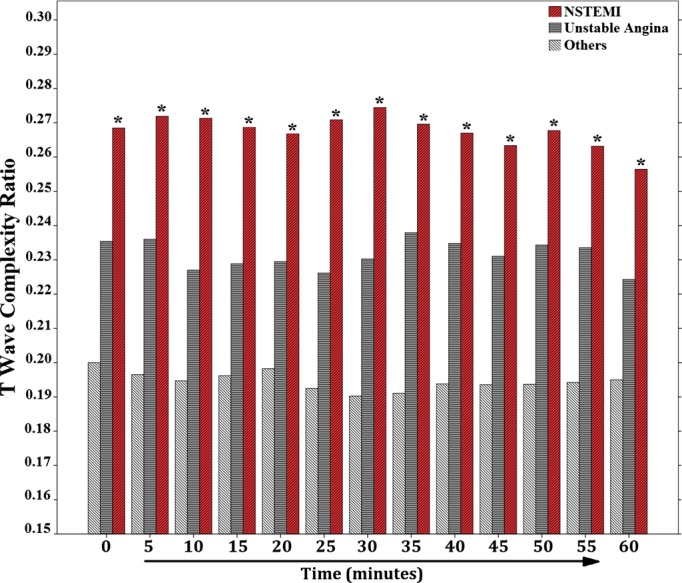
Magnitude of ventricular repolarization dispersion between groups. This figure shows the mean repolarization complexity between groups. Time zero represents the baseline 12-lead ECG, and other time points represent the 5-minute averages. *Significant against “others” subcategory during each time point using repeated-measures ANOVA with Tukey’s post-hoc adjustment. In any single group, there were no differences in T-wave complexity across different time points. ECG indicates electrocardiogram; NSTEMI, non-ST elevation myocardial infarction.
Figure 3.
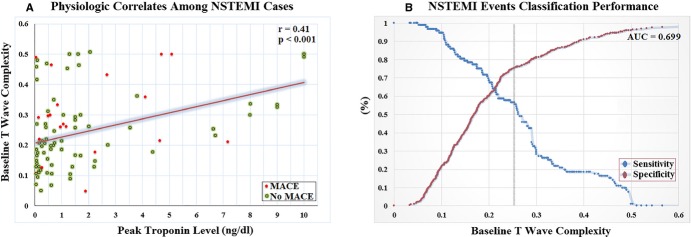
Physiological correlates and classification performance of VRD. A, Scatter plot and best-fit linear regression mean between peak troponing level at ED and baseline T-wave complexity from Holter ECG among NSTEMI cases (n=92). B, ROC showing point coordinates of T-wave complexity to classify NSTEMI events; vertical line shows ROC-optimized cut-off point. AUC indicates area under the curve; ECG, electrocardiogram; ED, emergency department; MACE, major adverse cardiac events; NSTEMI, non-ST elevation myocardial infarction; ROC, receiver operator characteristics; VRD, ventricular repolarization dispersion.
In regression analysis (Table2), an increased baseline T-wave complexity ratio predicted NSTEMI, independent of age and other ischemic ECG changes (ie, ST depression), and increased values (≥0.26) accounted for 4-fold (OR=3.8 [2.1 to 5.8]) increased likelihood of detecting subsequent rise in serum markers of myocardial necrosis early during triage. Compared to ST depression, the ROC-optimized cut-off point of the T-wave complexity ratio (ie, ≥0.26) had a better sensitivity (57% vs. 22%), while maintaining a relatively comparable specificity (76% vs. 90%) and other PPVs (43% vs. 57%) and NPVs (84% vs. 78%). This is better demonstrated in Figure 5, which shows a NSTEMI case of a 70-year-old female with no ST changes on the presenting ECG, but increased T-wave complexity throughout her initial ED evaluation.
Table 2.
Clinical and Electrocardiographic Predictors of NSTEMI and MACE
| Univariate OR (95% CI) | Multivariate OR (95% CI) | |
|---|---|---|
| Predictors of NSTEMI upon ED admission* | ||
| Baseline characteristics | ||
| Age (per 10-year increment) | 1.2 (1.0 to 1.5) | NS |
| Clinical diagnostics | ||
| HDL (per 5 unit increment) | 0.90 (0.77 to 0.95) | NS |
| Creatinine (per 0.1 unit increment) | 1.05 (1.01 to 1.09) | NS |
| Ejection fraction (per 10% increment) | 0.63 (0.51 to 0.79) | NS |
| Presenting 12-lead ECG | ||
| Any ST depression | 2.7 (1.4 to 5.1) | NS |
| T wave complexity (per 1% increment) | 1.03 (1.02 to 1.05) | 1.03 (1.01 to 1.05) |
| Predictors of MACE during hospitalization† | ||
| Baseline characteristics | ||
| Male sex | 2.7 (1.0 to 7.2) | NS |
| Known CHF | 4.1 (1.4 to 12.0) | NS |
| Clinical diagnostics | ||
| Creatinine (per 0.1 unit increment) | 1.05 (1.01 to 1.09) | NS |
| B-type natriuretic peptide (per 100 units) | 1.11 (1.05 to 1.12) | NS |
| Presenting 12-lead ECG | ||
| Any ST depression | 2.3 (0.8 to 5.9) | NS |
| T-wave complexity (per 1% increment) | 1.04 (1.01 to 1.06) | 1.04 (1.01 to 1.06) |
CHF indicates congestive heart failure; CI, confidence interval; ED indicates emergency department; HDL, high-density lipoprotein; MACE, major adverse cardiac events; NS, not significant; NSTEMI, non-ST elevation myocardial infarction; OR, odds ratio.
Hosmer–Lemshow chi-square=9.09; P=0.34.
Hosmer–Lemshow chi-square=10.87; P=0.21.
Finally, as expected, those who experienced MACEs were more likely to have greater extent of baseline repolarization complexity, compared to all others (T-wave complexity ratio 29.6±14.2 vs. 21.7±14.5, P=0.008). In the multivariate model (Table2), an increased baseline T-wave complexity ratio—but not ST depression—was the only significant and independent predictor of MACEs, and increased values (≥0.26) accounted for 8-fold (OR=8.2 [3.1 to 21.5]) increased risk of adverse events. Interestingly, increased T-wave complexity (≥0.26) was also associated with excess risk for time-to-MACE (Figure4), suggesting that T-wave complexity can help guide which patients can benefit from immediate revascularization.
Figure 4.
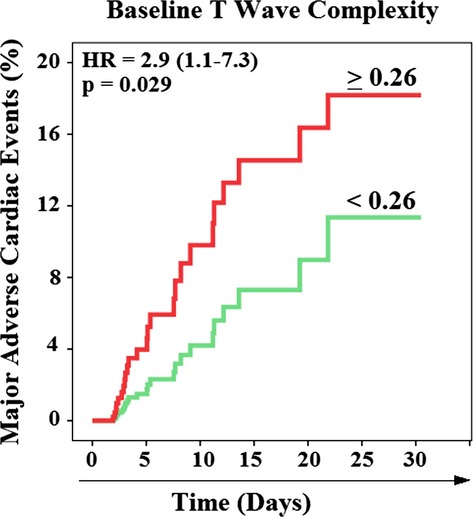
Kaplan-Meier events probability curves for in-hospital major adverse cardiac events (MACE). This figure compares time-to-MACE between those with T-wave complexity below 0.26 (n=250) and above or equal to 0.26 (n=119). HR indicates hazard ratio.
Discussion
This study demonstrates that T-wave complexity on baseline 12-lead ECG not only can differentiate NSTEMI patients from UA and other nonischemic chest pain patients very early during evaluation, but also correlate with the risk of adverse in-hospital outcomes. This is the first study to test the clinical utility of T-wave complexity to detect and risk stratify NSTEMI patients in the ED. Our findings are novel and support the notion that global myocardial ischemia, associated with ruptured plaques and partial coronary artery occlusion, can precipitate quantifiable T-wave distortions before any ST segment displacement.19 This makes sense because ischemic injury is most likely to alter local gradients of repolarization manifested in the T waves (ie, phase 3 of the action potentials) before distorting the ST segment (ie, phase 2 of the action potentials) of the ECG.20 As such, our data suggest that a simple measure of T-wave complexity is more sensitive for (1) detecting ischemic myocardial necrosis associated with NSTEMI and (2) quantifying the severity of ischemic burden to identify high-risk NSTEMI patients who would benefit from early revascularization.
VRD and Ischemic Myocardial Injury
Myocardial ischemia induces electrophysiological alterations in action potentials, causing repolarization dispersion between normal and ischemic fibers and between epicardium and endocardium, which can be detected on the surface 12-lead ECG.8,21 However, despite the ongoing electrical dispersion currents during infarction (whether STEMI or NSTEMI), ST changes mainly evolve when action potential gradients between ischemic and nonischemic zones exceeds 65 mV.19 This suggests that early ischemic gradients that alter action potential amplitude, repolarization time, excitability, and conduction would contribute to various repolarization changes—other than STE—on the surface 12-lead ECG very early during MI evolution (ie, myocardial injury). This means that examining STT waveforms (rather than ST segment alone) is a more promising approach in MI detection. Our results are congruent with these findings and show that T-wave complexity ratio demonstrates greater repolarization complexity among NSTEMI (ie, myocardial injury), but not UA (ie, ischemia), when compared to all other chest pain subjects.
To date, current guidelines do not precisely describe the evolution of ischemic ECG changes associated with NSTEMI, and there are few clinical studies on the topic.7 Notably, the work by Nikus et al. that demonstrates that ST depression—in conjunction with T-wave inversion—in NSTEMI correlates with significant left main coronary disease and subsequent MACE.22,23 This makes sense given that significant left main coronary disease is more likely to result in profound injury currents and subsequent ST changes. However, as demonstrated by our analysis, NSTEMI does not necessarily include significant left main involvement, and hence ST depression, with or without T-wave inversion, has poor sensitivity to detect myocardial injury (ie, NSTEMI) and is not an independent predictor of MACEs. A simple measure of repolarization heterogeneity (ie, T-wave complexity) that is sensitive to global changes in action potential amplitude, repolarization time, excitability, and conduction would then be more likely to detect NSTEMI.
Human data from experimental models support the finding that ischemic injury leads to VRD.9,10 In a recent experiment that examined the evolution of different temporal and spatial VRD indices during artificially induced transmural ischemia in 95 patients undergoing PCI, dynamic ECG changes were recorded during and after 5-minute balloon inflation in each of the dominant coronary arteries.10 Moreover, repolarization time and complexity were found to change significantly within 2 minutes after balloon inflation (and disappear immediately after balloon deflation), which indicates the transient nature of such changes.8 Our findings demonstrate that T-wave complexity changes persist in NSTEMI patients throughout the initial 60 minutes of ED admission, when patients are usually waiting for cardiac enzyme results. This is a time window in which treatments to restore coronary perfusion might be administered to those at greater risk of adverse events. There is an opportunity for this change in approach, given that only 25% of those with increased repolarization complexity in this study were treated by PCI.
In our data, overall repolarization complexity is higher (22% vs. 11%), compared to other general populations,11 perhaps because nearly 65% of our sample have some degree of cardiovascular disease. This is expected given that other physiological (eg, CAD) and psychological (eg, depression) factors besides ischemia can induce nonspecific STT changes.24 However, despite the nonspecific repolarization changes observed in our sample at baseline, myocardial ischemia resulted in clear increases in T-wave complexity ratio between groups for more than 60 minutes, confirming that ischemia-induced changes in repolarization complexity are significantly greater than other nonspecific factors in the context of chest pain.
Nevertheless, our data show a simple linear relationship between magnitude of T-wave complexity and peak troponin levels. Despite the modest correlation (R2=17%), there seems to be a logical association between magnitude of repolarization heterogeneity and degree of ischemic myocardial necrosis. Along with multiple other potential factors, this can explain the ability of T-wave complexity to identify inhospital MACE in our data. Further research is needed to fully understand the clinical utility of VRD in quantifying the ischemic burden and guiding the selection of therapy in high-risk NSTEMI patients.
Clinical Implications
The flow of electrical currents across the boundaries between the ischemic and nonischemic zones (ie, injury currents; Figure1B, right) seems to precipitate changes in repolarization time, excitability, and conduction in most patients with acute coronary events (phases 2 and 3 of action potential [or STT complex]).25 Our data suggest that T-wave complexity is sensitive to such ischemic repolarization changes, which explains why ST changes (phase 2 only of action potential) may not always evolve during nontransmural myocardial injury. For example, Figure5 shows a case of a 70-year-old Caucasian female with no known CAD who had no ST changes on the presenting ECG. However, owing to recurrent angina that precipitated transient ventricular arrhythmia, she underwent PCI (door-to-balloon time=4 hours) that showed 95% occlusion in the proximal LAD. These findings logically support the notion that distortion of the T wave occurs before any ST-segment displacement (ie, more sensitive to early myocardial injury), suggesting that T-wave complexity can play a significant role in detecting acute coronary events in chest pain patients.
Figure 5.
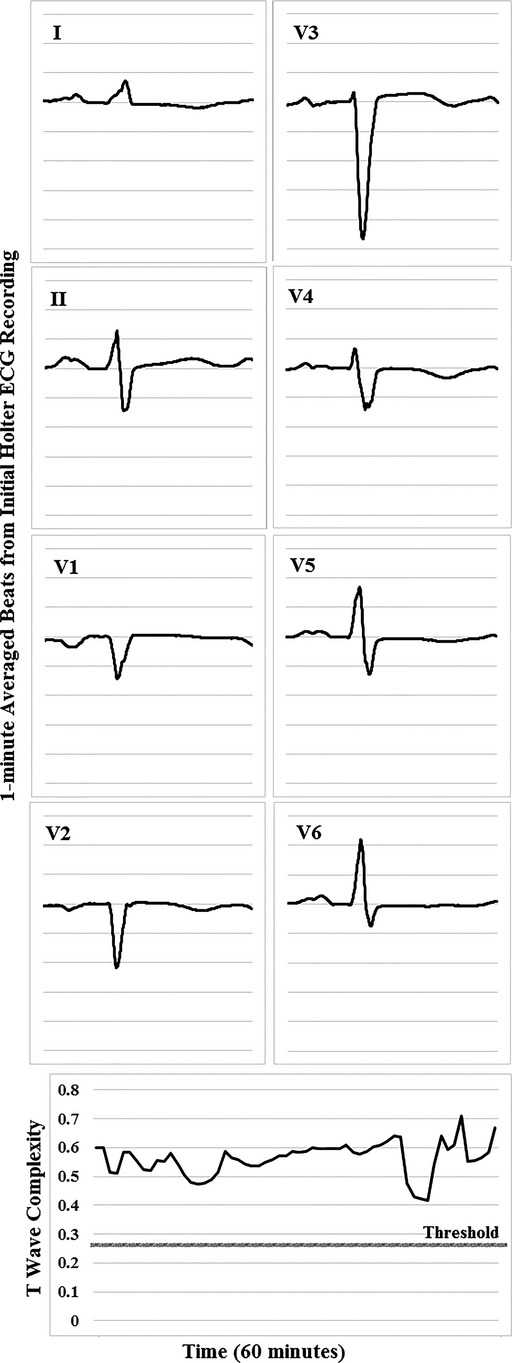
Baseline Holter ECG and corresponding VRD trend in a selected patient. A case of a 70-year-old female with no previous history of CAD, HTN, or DM. Initial troponin was 10.4 ng/dL, but there was no significant ST changes on admission ECG. She experienced nonsustained ventricular tachycardia and was sent to the catheterization lab 4 hours after her initial presentation. Angiography revealed 95%, 80%, and 30% occlusions in the LAD, LCx, and RCA coronary arteries. CAD indicates coronary artery disease; DM, diabetes mellitus; ECG, electrocardiogram; HTN, hypertension; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; RCA, right coronary artery; VRD, ventricular repolarization dispersion.
The issue of NSTEMI detection is complex. In contrast to STEMI, in which most patients have ongoing ischemia, in NSTEMI there are fluctuations. Actually, a large part of these patients present after resolution of symptoms and ECGs are recorded when the patient does not have active ischemia, which might explain the moderate sensitivity (57%) of T-wave complexity reported in this study. To make things even more complicated, patients may be observed during a short episode of ischemia that does not result in leak of troponin. This is demonstrated in our data where T-wave complexity correlates with myocardial injury (ie, NSTEMI), not myocardial ischemia alone (ie, UA), which explains the relatively stable trends in T-wave complexity during initial ED presentation (Figure2). Finally, it has been demonstrated that continuous ECG recording, especially when there are fluctuations in the patient’s symptoms, may detect subtle changes that otherwise could be overlooked.26 Our data support these recommendations and suggest that performing continuous ECG monitoring (≈60 minutes) can help rule out low-risk patients with a negative predictive value up to 84%.
Limitations and Future Research
Our findings are based on a single measure of global VRD in a selected high-risk patient population suspected of coronary ischemia and needs to be validated in larger or less-selected cohorts. We used our data as a testing set only (hypothesis generating); this approach should be validated for prospective identification of NSTEMI. Of note, T-wave complexity is not exclusively specific to ischemia. Other conditions that result in local or regional disparity of repolarization (eg, regional autonomic nerve excitement, local conduction block or slowing, localized necrosis, and so on) can also alter T-wave complexity. As such, future studies should (1) evaluate multiple VRD indices in prediction models to determine the best, most stable predictive tool; using multiple parameters that describe various aspects of spatial and temporal heterogeneity in ventricular repolarization may improve the sensitivity and specificity of this method to detect ischemia-induced changes in VRD; (2) explore the impact of medical interventions that can relieve symptoms (ie, oxygen and nitrates) on the evolution of VRD patterns; and (3) evaluate the clinical utility of VRD indices to prioritize selection of high-risk candidates for early PCI. Nevertheless, given the wide CIs in our backward elimination modeling technique, it is possible that some potentially important variables were removed owing to lack of significance, but this lack of significance may be owing to small sample size. Our results need to be validated in larger, prospective studies.
Conclusions
In this study, we report that, compared to ST deviation, a simple measure of global VRD on the presenting 12-lead ECG is more sensitive—yet still specific enough—to detect and quantify nonlocalized ischemic injury in NSTEMI, which suggests a potential benefit in detecting and risk stratifying chest pain patients very early during evaluation. Automated measures of T-wave complexity can be easily incorporated in manufacturer-specific devices for real-time display for immediate clinical decision support. This early detection not only has the potential to expedite treatment decisions, but also may reduce unnecessary loss of myocardial tissue in patients with chest pain and no STE.
Sources of Funding
This study was supported by grant R21NR011202 (principal investigator: Pelter) provided by the NIH. The authors have no relationships whatsoever with any interests of any kind in business or industry related to the planning, execution, and/or publication of this study.
Disclosures
None.
References
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the ACC/AHA Task Force on Practice Guidelines. J Am Coll Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary. A report of the ACC/AHA Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:485–510. doi: 10.1016/j.jacc.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Macfarlane PW. The continuing renaissance of the resting 10s ECG. J Electrocardiol. 2013;46:383–384. doi: 10.1016/j.jelectrocard.2013.06.029. [DOI] [PubMed] [Google Scholar]
- Mehta SR, Cannon CP, Fox KA, Wallentin L, Boden W, Spacek R, Widimsky P, McCullough P, Hunt D, Braunwald E, Yusuf S. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta-analysis of randomized trials. JAMA. 2005;293:2908–2917. doi: 10.1001/jama.293.23.2908. [DOI] [PubMed] [Google Scholar]
- Hamm CW, Bassand J-P, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- Gorgels APM. ST-elevation and non-ST-elevation acute coronary syndromes: should the guidelines be changed? J Electrocardiol. 2013;46:318–323. doi: 10.1016/j.jelectrocard.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Nash MP, Bradley CP, Paterson DJ. Imaging electrocardiographic dispersion of depolarization and repolarization during ischemia: simultaneous body surface and epicardial mapping. Circulation. 2003;107:2257–2263. doi: 10.1161/01.CIR.0000065602.78328.B5. [DOI] [PubMed] [Google Scholar]
- Arini PD, Baglivo FH, Martinez JP, Laguna P. Ventricular repolarization dispersion during ischemia course measured by temporal and spatial electrocardiographic parameters. Comput Cardiol. 2008 . Available at: http://ieeexplore.ieee.org/xpl/login.jsp?tp=&arnumber=4749043&url=http%3A%2F%2Fieeexplore.ieee.org%2Fxpls%2Fabs_all.jsp%3Farnumber%3D4749043. Accessed June 23, 2015. [Google Scholar]
- Arini P, Baglivo F, Martínez J, Laguna P. Evaluation of ventricular repolarization dispersion during acute myocardial ischemia: spatial and temporal ECG indices. Med Biol Eng Comput. 2014;52:375–391. doi: 10.1007/s11517-014-1136-z. [DOI] [PubMed] [Google Scholar]
- Al-Zaiti S, Runco K, Carey M. Increased T-wave complexity can indicate subclinical myocardial ischemia in asymptomatic adults. J Electrocardiol. 2011;44:684–688. doi: 10.1016/j.jelectrocard.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zaiti S, Fallavollita J, Carey M. Is the QRS-T angle a more sensitive marker of ischemia than ST-segment deviation? J Electrocardiol. 2010;43:640. [Google Scholar]
- Pelter MM, Kozik TM, Loranger DL, Carey MG. A research method for detecting transient myocardial ischemia in patients with suspected acute coronary syndrome using continuous ST-segment analysis. J Vis Exp. 2012;70:e50124–e50134. doi: 10.3791/50124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, Flaherty JT, Harrington RA, Krumholz HM, Simoons ML. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes: a report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, American College of Emergency Physicians, American Heart Association, Cardiac Society of Australia & New Zealand, National Heart Foundation of Australia, Society for Cardiac Angiography and Interventions, and the Taiwan Society of Cardiology. J Am Coll Cardiol. 2001;38:2114–2130. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- Acar B, Yi G, Hnatkova K, Malik M. Spatial, temporal and wavefront direction characteristics of 12-lead T-wave morphology. Med Biol Eng Comput. 1999;37:574–584. doi: 10.1007/BF02513351. [DOI] [PubMed] [Google Scholar]
- Priori SG, Mortara DW, Napolitano C, Diehl L, Paganini V, Cantu F, Cantu G, Schwartz PJ. Evaluation of the spatial aspects of T-wave complexity in the long-QT syndrome. Circulation. 1997;96:3006–3012. doi: 10.1161/01.cir.96.9.3006. [DOI] [PubMed] [Google Scholar]
- Okin PM, Devereux RB, Lee ET, Galloway JM, Howard BV. Electrocardiographic repolarization complexity and abnormality predict all-cause and cardiovascular mortality in diabetes. Diabetes. 2004;53:434–440. doi: 10.2337/diabetes.53.2.434. [DOI] [PubMed] [Google Scholar]
- Yi G, Prasad K, Elliott P, Sharma S, Guo X, McKenna WJ, Malik M. T wave complexity in patients with hypertrophic cardiomyopathy. Pacing Clin Electrophysiol. 1998;21:2382–2386. doi: 10.1111/j.1540-8159.1998.tb01186.x. [DOI] [PubMed] [Google Scholar]
- Di Diego JM, Antzelevitch C. Acute myocardial ischemia: cellular mechanisms underlying ST segment elevation. J Electrocardiol. 2014;47:486–490. doi: 10.1016/j.jelectrocard.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G-X, Lankipalli RS, Burke JF, Musco S, Kowey PR. Ventricular repolarization components on the electrocardiogram: cellular basis and clinical significance. J Am Coll Cardiol. 2003;42:401–409. doi: 10.1016/s0735-1097(03)00713-7. [DOI] [PubMed] [Google Scholar]
- Lukas A, Antzelevitch C. Differences in the electrophysiological response of canine ventricular epicardium and endocardium to ischemia. Role of the transient outward current. Circulation. 1993;88:2903–2915. doi: 10.1161/01.cir.88.6.2903. [DOI] [PubMed] [Google Scholar]
- Nikus KC, Eskola MJ, Virtanen VK, Vikman S, Niemelä KO, Huhtala H, Sclarovsky S. ST-depression with negative T waves in leads V4–V5—a marker of severe coronary artery disease in non-ST elevation acute coronary syndrome: a prospective study of angina at rest, with troponin, clinical, electrocardiographic, and angiographic correlation. Ann Noninvasive Electrocardiol. 2004;9:207–214. doi: 10.1111/j.1542-474X.2004.93545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikus KC, Sclarovsky S, Huhtala H, Niemelä K, Karhunen P, Eskola MJ. Electrocardiographic presentation of global ischemia in acute coronary syndrome predicts poor outcome. Ann Med. 2012;44:494–502. doi: 10.3109/07853890.2011.585345. [DOI] [PubMed] [Google Scholar]
- Friedberg CK, Zager A. “Nonspecific” ST and T-wave changes. Circulation. 1961;23:655–661. doi: 10.1161/01.cir.23.5.655. [DOI] [PubMed] [Google Scholar]
- Rautaharju PM, Surawicz B, Gettes LS. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval. J Am Coll Cardiol. 2009;53:982–991. doi: 10.1016/j.jacc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Drew BJ, Califf RM, Funk M, Kaufman ES, Krucoff MW, Laks MM, Macfarlane PW, Sommargren C, Swiryn S, Van Hare GF. AHA scientific statement: practice standards for electrocardiographic monitoring in hospital settings: an AHA Scientific Statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized electrocardiology and the American Association of Critical-Care Nurses. J Cardiovasc Nurs. 2005;20:76–106. doi: 10.1097/00005082-200503000-00003. [DOI] [PubMed] [Google Scholar]


