Non-ST-elevation myocardial infarction (NSTEMI) is lower on the severity spectrum of acute coronary syndromes than is myocardial infarction (MI), resulting from complete occlusion of a major coronary artery. As the name implies, it is a syndrome that does not exhibit the dramatic ST elevation observed in the standard 12-lead ECG in chest pain patients with confirmed acute MI. The important clinical significance of NSTEMI is that delay in diagnosis can lead to increased morbidity, risk of arrhythmia, and death. The article Clinical Utility of Ventricular Repolarization Dispersion for Real-Time Detection of Non-ST Elevation Myocardial Infarction in Emergency Departments in this issue of the Journal by Al-Zaiti et al1 is a timely presentation of a new approach to quickly diagnose NSTEMI and significantly reduce the time to treatment in these patients. The marker, ventricular repolarization dispersion (VRD), incorporates much more information in the ECG than that provided by measuring elevation of the ST segment and, thus, heralds the emergence of more powerful and robust methods of assessing ECG morphology and dynamics than can be provided by classical interval and amplitude measurements.
Non-ST-Elevation Myocardial Infarction
As the name states, NSTEMI is myocardial infarction in which the elevation—or for that matter, depression—of ST segments is not significantly different from normal and thus the reason that the condition is not identified in patients presenting with chest pain. Typically, chest-pain patients without significantly abnormal ST elevation are monitored, potentially for hours, until other tests confirm the presence of acute infarction (eg, elevated troponin levels). There are several explanations that account for the absence of abnormal ST elevation in acute MI. These include numerous possibilities: that the infarcts may be relatively small; that the location of the infarcts may be in locations only weakly sensed by the lead fields of the standard 12-lead ECG; or that the infarct is slowly developing. Importantly, this does not mean that the QRS and ST-T waves of the ECG do not change dynamically during the infarct’s time course. At the cellular level, acute ischemia results in changes in action potential amplitude, duration, triangularization or sloping of the plateau, and even reduction in action potential (AP) upstroke velocity in affected cells. Consequently, the ECG shows a dynamic progression of changes in ST level and slope as well as T wave amplitude and morphology, in addition to widening of the QRS as a consequence of conduction slowing—all with different time course and magnitude. In NSTEMI all of these may be present although much more subtly than in ST-segment myocardial infarction, in which the ST segments show definitive elevation. Thus, any method that is sensitive to and quantitates these changes in QRS and ST-T waveform should greatly improve the sensitivity of the test for infarction as well as drastically reduce the time required to diagnose the syndrome.
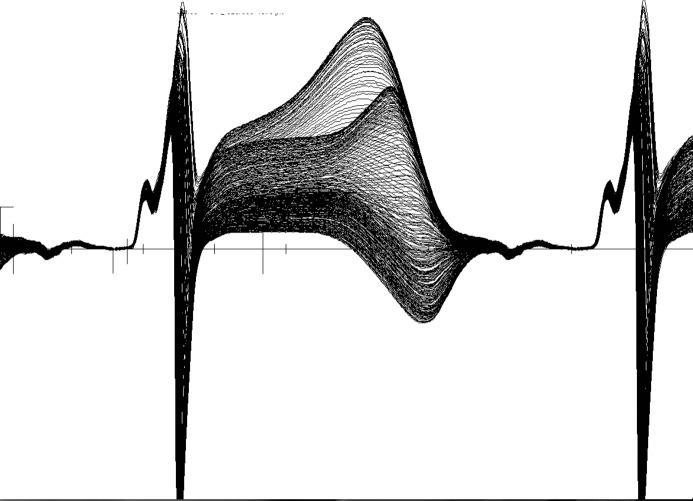
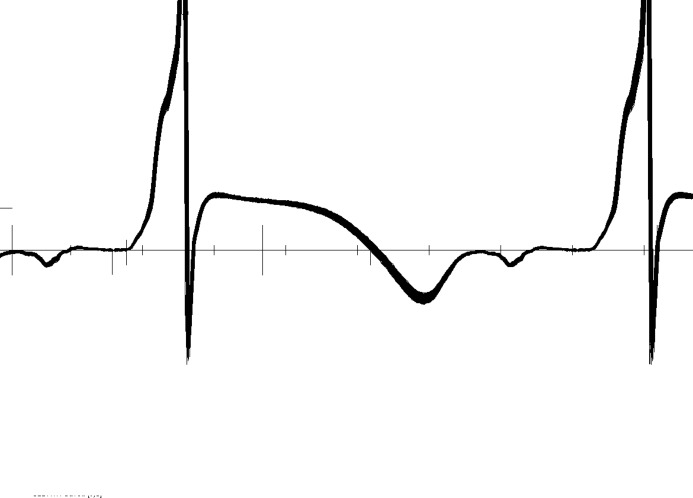
Figures1 and 2 show data from an unpublished study investigating depolarization and repolarization changes induced by coronary occlusion in a canine model of acute ischemia (Lux RL, unpublished data, NIH SCOR in Sudden Cardiac Death, P50 HL52338, 1994–2005). Figure1 shows 272 consecutive superimposed beats from an electrogram recorded near the anterior base of the left ventricle (LV) during atrial pacing (320-ms cycle length) and in the first minutes after temporarily occluding the left anterior descending coronary artery (LAD). Note the near identical P, QRS, ST-T complexes in this electrogram. Figure2 shows the exact same beats but at a site 3 cm away and close to the left ventricular apex. Note the dramatic changes, not only in the ST elevation, but the ST-T and even QRS morphologies that confirm conduction (activation sequence) as well as repolarization changes. The point of these figures is that the changes during ischemia, regardless of how subtle, are regional and include the entire QRS, ST-T intervals. This underscores the importance of sampling more than a few leads and taking into account the entire QRST complex.
Figure 1.
Two hundred and seventy-two consecutive, superimposed beats from a unipolar electrogram recorded from the basal LV epicardium during atrial pacing (cycle length=320 ms) immediately following occlusion of a major branch of the LAD in a canine model of ischemia. LV indicates left ventricle; LAD, left anterior descending coronary artery.
Figure 2.
Superposition of the same sequence of 272 consecutive beats shown in Figure1 but from a unipolar electrogram positioned 3 cm distant from that in Figure1 and close to the LV apex. LV indicate left ventricle.
Ventricular Repolarization Dispersion (VRD)
The VRD metric presented in the Al-Zaiti article is based on Principal Component Analysis, a method closely related to factor analysis and Karhunen-Loeve data representation methods. These methods provide powerful, robust statistical methods of representing data of any number of variables or signals in a signal space of orthogonal (independent) dimensions derived directly from the data itself. Given N ECGs whether N P-QRS-T complexes from one lead, or N ECGs from different leads, one can calculate a set of independent (orthogonal) and normalized waveforms (principal components) that characterize the data from which they were derived. Each waveform has a magnitude (Eigenvalue) associated with it that signifies the importance of that waveform in representing the data set. Eigenvalues are ordered by magnitude and for data that are rich in variability (“nonuniform,” “complex,” etc), and Eigenvalue magnitudes decrease gradually with number. On the other hand, if there is significant redundancy in the data (eg, the waveforms are very similar), then only the first few Eigenvalues and associated principal components are significant.
The VRD metric is, in fact, identical to the complexity ratio published by Priori et al 2 and is defined as the ratio of the second to first Eigenvalue calculated from the Principal Component Analysis representation of each beat (QRS-ST-T). The acronym “VRD” is somewhat misleading given that the index, as defined, includes information in QRS as well as ST-T. In a normal ECG, there is considerable redundancy in the 12 waveforms, and this results in a low complexity ratio, where the first component carries a much higher information content than the second, leading to a small ratio. In the presence of acute ischemia, regional differences in action potential amplitudes and morphologies produce varying degrees of change across all 12 leads, but particularly in those leads that are most sensitive to the location of the ischemia. This leads to an increase in the complexity ratio as the second Eigenvalue will be slightly less in magnitude than the first. Thus, the magnitude and dynamics of this index over time are exquisitely sensitive to changes in amplitude and morphology changes of the QRST, no matter how subtle or in whichever leads.
Interestingly, Principal Component Analysis and related methods have been used to represent ECGs, body surface potential maps, and QRST integral (ie, area) as means to diagnose heart disease and assess arrhythmia vulnerability. Horan et al used factor analysis to statistically characterize the QRS of the ECG.3 Urie et al applied principal component analysis to QRST integral maps—related to Wilson’s ventricular gradient4—to investigate its utility in identifying patients at risk of ventricular arrhythmia.5 Lux et al6 and Evans et al7 used the Karhunen-Loeve expansion to statistically represent the spatial and temporal information in body surface potential maps and showed the extent of redundancy in lead systems as well as ECG waveforms and body surface potential distributions. In each of these examples, the recorded electrocardiographic information—both waveforms and spatial potential distributions—were reduced to independent variables that allowed for substantial data reduction and simplification of statistical analysis.
Significance of the Method
The importance of this study is that it shows a significantly higher rate of detecting NSTEMI than the classical ST-elevation measurement in chest-pain patients with ischemia-related symptoms. This significantly reduces the time to treatment compared to the “wait and watch” approach necessitated by the time required to obtain serum enzyme results. Moreover, in addition to improved rapid detection of NSTEMI, the method shows promise in identifying NSTEMI patients at high risk for in-hospital major adverse cardiac events. The simplicity and ease with which the complexity ratio can be calculated for each beat over extended time periods provides valuable information on the time course and dynamics of ischemia that better support the diagnosis of NSTEMI than can be provided by hours of tracking the near-normal ST segments over standard 10-s ECG snapshots. The fact that the technology required to calculate the metric—both hardware and software—is already commercially available (Mortara Instruments, Inc, Milwaukee, WI) points to the potential ease of including this type of analysis in conventional ECG carts or Holter monitoring systems used in Emergency Departments.
In conclusion, the VRD metric provides a timely, powerful, and robust tool for early detection of NSTEMI in chest-pain patients and, if adopted, is likely to have a significant impact on reducing time to treatment and hence morbidity and mortality. Adoption of this diagnostic tool addresses a present need in a significant patient population.
Disclosures
The author has nothing to disclose.
References
- Al-Zaiti SS, Callaway CW, Kozik TM, Carey MG, Pelter MM. Clinical utility of ventricular repolarization dispersion for real-time detection of non-ST elevation myocardial infarction in emergency departments. J Am Heart Assoc. 2015;4:e002057. doi: 10.1161/JAHA.115.002057. doi: 10.1161/JAHA.115.002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori SG, Mortara DW, Napolitano C, Diehl L, Paganini V, Cantù F, Cantù G, Schwartz PJ. Evaluation of the spatial aspects of T-wave complexity in the long-QT syndrome. Circulation. 1997;96:3006–3012. doi: 10.1161/01.cir.96.9.3006. [DOI] [PubMed] [Google Scholar]
- Horan LG, Flowers NC, Brody DA. Principal factor waveforms of the thoracic QRS complex. Circ Res. 1964;15:131–145. doi: 10.1161/01.res.15.2.131. [DOI] [PubMed] [Google Scholar]
- Urie PM, Burgess MJ, Lux RL, Wyatt RF, Abildskov JA. The electrocardiographic recognition of cardiac states at high risk of ventricular arrhythmias. An experimental study in dogs. Circ Res. 1978;42:350–358. doi: 10.1161/01.res.42.3.350. [DOI] [PubMed] [Google Scholar]
- Wilson PF, MacLeod AG, Barker PS, Johnston FD. The determination and the significance of the areas of ventricular deflections of the electrocardiogram. Am Heart J. 1934;10:46–61. [Google Scholar]
- Lux RL, Evans AK, Burgess MJ, Wyatt RF, Abildskov JA. Redundancy reduction for improved display and analysis of body surface potential maps. I. Spatial compression. Circ Res. 1981;49:186–196. doi: 10.1161/01.res.49.1.186. [DOI] [PubMed] [Google Scholar]
- Evans AK, Lux RL, Burgess MJ, Wyatt RF, Abildskov JA. Redundancy reduction for improved display and analysis of body surface potential maps. II. Temporal compression. Circ Res. 1981;49:197–203. doi: 10.1161/01.res.49.1.197. [DOI] [PubMed] [Google Scholar]