Abstract
Background:
At high altitude, patent arterial ducts tend to be larger and associated with pulmonary hypertension. Patent ductus arteriosus device closure in this background could be challenging.
Objectives:
We report our experience with percutaneous closure of patent arterial ducts using a variety of devices in patients residing in a high altitude.
Patients and Methods:
This is a retrospective review of the case records of 145 patients (age 9 months-20 years, mean 5.6 ± 3.9 years, and weight 7-54 kg, mean 17.7 ± 9.4) with duct sizes ranging between 2 and 21 mm, (mean, 5.8 ± 2.7) who underwent percutaneous closure of patent arterial ducts. One hundred thirty-six (93.8%) of the patients were from a geographic area of 2100-2800 m above sea level.
Results:
Successful device closure was achieved in 143 cases. It was difficult to achieve device stability in two patients with expansile ducts. Therefore, they were treated surgically. The devices used were various types of duct occluder devices in 131 patients, while atrial and ventricular septal occluders were used in eight patients. For the group, mean systolic pulmonary artery (PA) pressure decreased from 47.0 ± 16.7 mmHg before occlusion to 29.0 ± 7.4 mmHg after occlusion (P ≤ 0.001)., mean diastolic PA pressure from 25.0 ± 10.9 mmHg to 14.8 ± 6.0 mmHg and the average mean PA pressure decreased from 35.9 ± 13.5 mmHg to 21.1 ± 6.5 mmHg. Complications (4.8%) included device and coil embolization, bleeding, and pulse loss. On follow-up (mean duration of 36.1 ± 12.1 months, range 12-62 months), 137 patients were in functional class 1, 3 had residual shunt, 2 had device migration and one patient had persisting pulse loss.
Conclusions:
Successful duct closure was achieved in the vast majority of patients, even though the ducts were larger and significant number of them had pulmonary hypertension in this high altitude group. There was a relatively higher incidence of residual shunts and device migration in this series, generally due to the nonavailability of optimal device and surgical support. Long-term follow-up is required before we can draw conclusions with regard to the sustainability of drop in PA pressures. Septal Occluder devices may be a possible alternative for large tubular or window-type ducts with severe pulmonary hypertension, where there may be concerns about the size and stability of duct occluder devices.
Keywords: High altitude, percutaneous patent ductus arteriosus closure, pulmonary artery pressure, pulmonary hypertension
INTRODUCTION
Patent arterial duct constitutes 6-11% of all congenital heart defects. In preterm infants, the incidence is higher, at about 8/1000.[1] In patients living at high altitude, the ducts tend to be larger and increased pulmonary artery (PA) pressure more common.[2] The postnatal persistence of pulmonary hypertension in a hypoxic environment and delayed closure of ductus arteriosus are believed to be contributing factors in the high prevalence of patent arterial ducts at high altitude.[3] Those patients with moderate to large ducts, who are not treated, may develop congestive cardiac failure, pulmonary vascular disease, and may die as a result of prolonged exposure to the left-to-right shunt.[4,5] The short-term, mid-term, and long-term results of percutaneous closure of patent arterial ducts are excellent, both in children and in adults.[6,7,8,9] Transcatheter closure using the Amplatzer duct occluder I (ADO I) device is an effective and safe therapy for the majority of patients with patent arterial ducts.[10] In larger ducts, other devices such as Amplatzer muscular VSD occluder (AMVSDO), atrial septal occluder (ASO) devices or Amplatzer vascular plug have been used successfully.[11,12,13,14,15]
This paper reports our experience with percutaneous closure of patent arterial ducts using a variety of devices in patients with or without pulmonary hypertension, residing in a high altitude sub-Saharan country whose capital city and its surroundings have an altitude of 2100-2800 m above sea level.
PATIENTS AND METHODS
We retrospectively reviewed records of 145 patients with patent ductus arteriosus (PDA) treated percutaneously between January 2009 and May 2014. One hundred thirty-six (93.8%) of the patients were born and were living in a geographic area; 2100-2800 m above sea level. We reviewed demographic, clinical, radiographic, echocardiographic, and hemodynamic data of the patients. We also reviewed the follow-up data. The departmental research ethics and publication committee approved the study.
The mean age at intervention was 5.6 ± 3.9 years (range 9 months-20 years). One hundred and six (73%) patients were females. The baseline characteristics of the patients are shown in Table 1. Figure 1 shows the age of the patients treated versus the narrowest duct diameters. The most common ductal morphology was conical (61%) followed by the tubular type (26%). Window type ducts accounted for 5% of the cases [Table 1]. Sixteen patients had other associated cardiac lesions, such as variable degree of mitral regurgitation with/without mitral valve prolapse in seven patients. Other associated lesions included atrial septal defect, ventricular septal defect, pulmonary stenosis, membranous subaortic stenosis, bicuspid aortic valve, supravalvar aortic stenosis, right aortic arch, hypoplasia of aortic isthmus, and interrupted inferior vena cava with azygos continuation. In one patient with a patent arterial duct and a secundum atrial septal defect, the atrial septal defect was closed at the same procedure. In another patient with patent arterial duct, atrial septal defect and pulmonary valvar stenosis, the pulmonary valve was dilated with a balloon and the patent arterial duct and atrial septal defect were closed 1-year later. In the other patients with associated cardiac lesions, no intervention was performed as the lesions were judged not to pose significant hemodynamic risks.
Table 1.
Baseline clinical, echocardiographic, and hemodynamic characteristics of patients treated for PDA
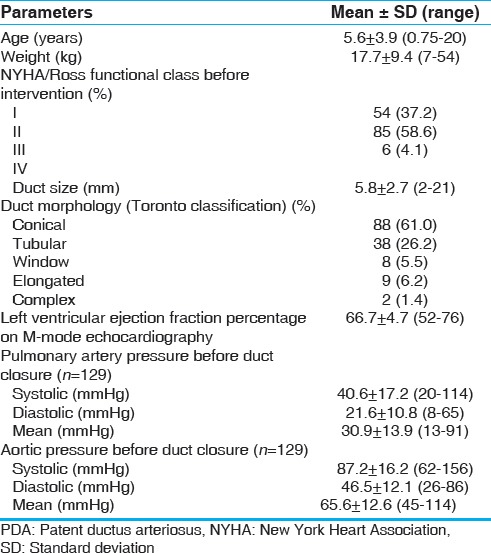
Figure 1.
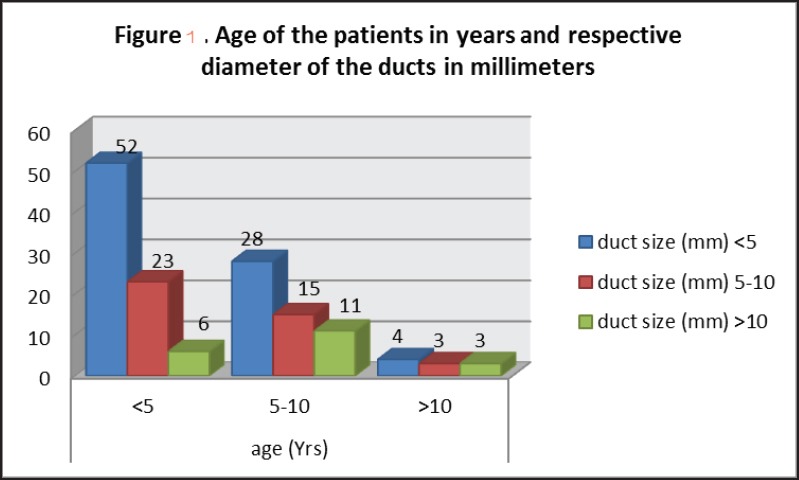
Age category of patients treated in relation to duct diameters (narrowest point)
The mean systolic PA pressure was 41 ± 17.2 mmHg (range 20-114 mmHg). The mean diastolic PA pressure was 22 ± 10.8 mmHg (range 8-65 mmHg). The average mean PA pressure was 31 ± 13.9 mmHg (range 13-91 mmHg). Sixty-eight (47%) patients had pulmonary hypertension, defined as resting mean PA pressure of >25 mmHg,[16] while 44 (30%) of the patients had systolic PA pressure >2/3 of simultaneously measured systolic aortic pressure.
PERCUTANEOUS CLOSURE TECHNIQUE
The procedure was performed under general anesthesia and intubation. Femoral vein and artery access were taken. Aortogram was performed in the lateral and/or right anterior oblique (30-45°) projections. We relied on the prior good quality echocardiographic assessment of ductal size in many cases, because of hardware/software problems of calibration and measurements with the angiographic equipment. In cases of large tubular/window type, expansile ducts, we performed balloon sizing before attempting catheter closure. In most cases where we used regular duct occluder devices, we oversized the devices by at least 2 mm for conical ducts and 4-6 mm for tubular and window type ducts. In some cases, we used transthoracic echocardiography for final confirmation of device position and stability because of shortage of contrast medium.
Statistical analysis
Data were entered into SPSS version 20 (Armonk, NY: IBM Corp.) and were analyzed. Mean ± SD were used to describe continuous variables among the study patients. Paired t-tests were used to compare baseline and post-intervention numerical variables. Chi-squared test was used to compare ordinal and categorical variables. Nonparametric tests were also used where appropriate.
RESULTS
Amplatzer duct occluder I device (St Jude Medical, St. Paul, MN, USA) was used in 66 (46%) of the patients and ADO II in 9 (6.2%) patients. Lifetech PDA device (Lifetech Scientific, Shenzhen, China) was used in 40 (28%) of the patients. Other devices and coils used are shown in Table 2. In six patients, with large tubular and expansile ducts measuring 14-21 mm, Amplatzer ASO devices (St. Jude Medical, St. Paul, MN, USA) ranging from 16 to 22 mm were used. In two other patients, AMVSDO devices (St. Jude Medical, St. Paul, MN, USA) were used. The mean fluoroscopy time was 9.0 ± 6.2 min (range 2.6-36.0 min). Device was successfully implanted in 143 (98.6%) of the patients. In two patients with expansile ducts, it was difficult to achieve stability. The patients were treated surgically as there were no atrial septal or ventricular septal occluder devices at those particular moments.
Table 2.
Postocclusion clinical and hemodynamic variables in patients treated for PDA
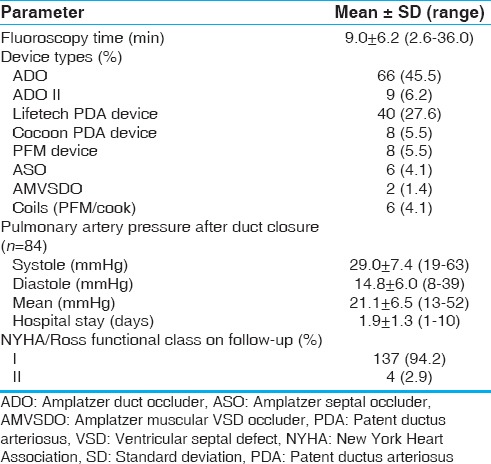
Data for PA pressures both before and after occlusion were available in 84 patients. In these patients, the mean systolic PA pressure decreased from 47 ± 16.7 mmHg before occlusion to 29 ± 7.4 mmHg after occlusion (P < 0.001). The mean diastolic PA pressure decreased from 25 ± 10.9 mmHg before occlusion to 15 ± 6.0 mmHg after occlusion (P < 0.001). The average mean PA pressure decreased from 36 ± 13.5 mmHg before occlusion to 21 ± 6.5 mmHg after occlusion (P < 0.001). The postocclusion and follow-up data of the patients is shown in Table 2.
Complete occlusion was achieved immediately at the end of the procedure in 120 (83%) of the patients, whilst residual shunting was present in the remaining 25 (17%) patients. Seven of the 8 patients, in whom a PFM device (PFM Medical AG, Köln, Germany) was used, and 3 of the 4 patients, in whom a Nit-Occlud coil was used, had residual shunting immediately at the end of the procedure. The residual shunt disappeared in 15 of the 25 patients on discharge.
Thirty-seven (26%) patients had left ventricular ejection fraction (LVEF) (measured by M-mode echocardiography) <56%[17] following closure of the duct. All these patients had ducts >5 mm. Twenty-three of these had left LVEF <45% and were started on treatment with captopril for left ventricular dysfunction. LVEF before and after closure of the duct in relation to the duct diameters is shown in Figure 2. However, the left ventricular function normalized in all of these patients within 3-6 weeks of the procedure.
Figure 2.
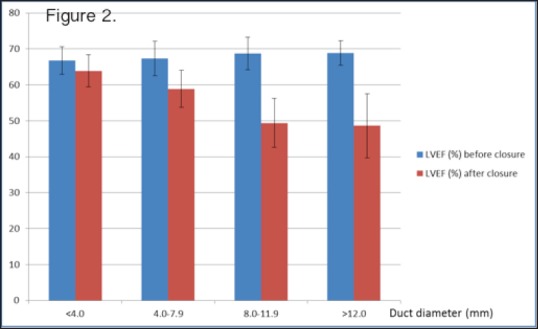
Change in left ventricular ejection fraction before and after duct occlusion compared with duct diameter
COMPLICATIONS
Complications occurred in a total of 7 (4.8%) patients. In two patients, a 20-month-old boy and an 18-month-old girl, both with elongated type of ducts, the devices embolized into the right PA, soon after release in one patient and after 12 h in the other. The devices used in both cases were ADO I, 6/4. Both required surgery for removal of the devices combined with duct ligation. In a third patient, a Nit-Occlud coil embolized into the left common iliac artery immediately after release, but the coil was successfully snared, retrieved, and repositioned in the duct.
In two patients, because of iatrogenic heparin overdosage, hemostasis was difficult and one of them required blood transfusion. There were two cases of pulse loss in the right lower limb and heparinization was not successful. Follow-up femoral artery Doppler study showed marked stenosis of the right femoral artery. One of these two patients regained good popliteal and dorsalis pedis pulsations after 3 years of the procedure, probably from collateral formation.
Follow-up
On follow-up (mean duration of 36.1 ± 12.1 months, range 12-62 months), one hundred and thirty-eight patients had completely occluded ducts, whilst three patients (two with PFM device and one with Nit-Occlud coil) had detectable residual shunts after 6 months of follow-up. In an 18-year-old girl, who had 21 mm tubular patent arterial duct closed with a 22 mm ASO device, there was a residual shunt through the device mesh, which resolved after 6 months. There was no evidence of hemolysis in this patient. One hundred and thirty-seven patients were in NYHA/Ross functional class I, and only four patients, who also had additional moderate or severe mitral regurgitation before and after closure of the ducts, were in functional class II. The left ventricular systolic function normalized in all patients on the 3 months follow-up assessment. None of the patients had echocardiographic evidence of pulmonary hypertension such as right ventricular hypertrophy/dilatation or tricuspid regurgitation.
In two patients, in whom successful deployment of the device and complete occlusion of the shunt was achieved, device embolization or migration was detected on follow-up. One of these patients was a 5-year-old boy, who had a 12 mm tubular duct closed with a 16/14 ADO I device. He was discharged after 48 h and the discharge echocardiogram showed good position of the device, with no residual shunt. However, review after 2 weeks revealed loud murmur over the left second intercostal space. Chest X-ray and echocardiogram showed that the device was in the right PA. There was no surgical backup at that time, so he was maintained on oral anticoagulation with warfarin. A surgical mission from overseas successfully removed the device surgically 3 months after the initial implantation. The second patient was a 22-month-old girl with 10 mm diameter tubular duct, and PA systolic pressure of 59 mmHg. The duct was closed with a 16/14 ADO I device. She was found to have slow migration of the device into the descending aorta on the 3 months follow-up visit, resulting in significant coarctation of the aorta. The device was removed surgically 6 months after implantation. There was no echocardiographic evidence of pulmonary hypertension on follow-up in this particular patient. Both of these patients had high PA systolic pressure before occlusion of the ducts.
DISCUSSION
The ducts reported in our study are larger, predominantly conical and tubular, and similar to those reported from high altitude areas in the rest of the world.[18,19,20,21] Bialkowski et al. compared two groups of patients who have undergone percutaneous duct closure. One group included 696 patients living at a high altitude while the second group included 708 patients living at low altitude. Mean duct diameter for the high altitude group was 4.1 ± 1.2 mm compared to 2.3 ± 1.3 mm for the low altitude group (P < 0.001). Mean pulmonary arterial pressure was also significantly greater in the high altitude group.[2] The postnatal persistence of pulmonary hypertension in a hypoxic environment and delayed closure of the arterial ducts are contributing factors to the high prevalence of these large ducts at high altitude.[2,3] Although pulmonary hypertension was prevalent in this population, there was resolution of the pulmonary hypertension after duct occlusion, indicating that irreversible pulmonary hypertension probably was not common. However, this observation was based on echocardiographic evidence. Follow-up cardiac catheterization was not performed in any of these patients. There are some reports which suggest that high altitude might protect against irreversible pulmonary vascular disease.[19] Although the mechanism is not clearly defined, with increased altitude, there is a decrease in the partial pressure of oxygen, which serves as the stimulus for a number of physiologic changes, that include increased blood pressure, heart rate, plasma catecholamine, renin activity, and increased pulmonary vasoconstriction.[22,23,24] It is possible that this pulmonary vasoconstriction early in life may protect the pulmonary vasculature from over-circulation, thereby preventing the development of the irreversible pulmonary vascular disease.
In our patients, the most common ductal morphology was that of type A (conical), similar to the Bialkowski et al. study.[2] The proportion of window type (type B) ducts has accounted for 5% of the cases. This is significantly higher when compared with a report by Azhar et al. from Saudi Arabia.[25] This type of ductal morphology poses a significant technical challenge as there is a significant risk of device instability. In our patients, we tried to overcome this by significantly oversizing the device or using septal occluder devices.
In our setting, we have mostly used the ADO I, and Lifetech PDA devices, usually generously donated by the companies. They were equally effective in closing large ducts with pulmonary hypertension. We have also used ADO II and Cocoon devices in some of our patients and results were excellent. We mostly oversized devices to try and minimize the risk of device embolization.[8,11,12,20] Immediate and follow-up residual shunts were more common with the small number of PFM devices, used for small and moderate sized ducts. However, we cannot form any conclusions about their efficacy. The ASO and AMVSDO devices are also acceptable alternatives for closing large tubular or window-type ducts, in which the PDA devices are likely to be small or unstable. The ASO device, in particular, may be suitable for large window-type ducts as it has discs on both sides. Other authors have also reported its effectiveness in such circumstances.[13,14]
Transient left ventricular systolic dysfunction occurred in several of the patients with large ducts. This was reported to occur after both surgical and percutaneous closure of large ducts.[21,26] Although the mechanism underlying the transient left ventricular dysfunction following closure of the duct and its relatively quick recovery is not completely understood, some authors have speculated that it might be related to the sudden decrease of the preload following closure of the arterial duct.[27] Delayed device migration occurred in two of our patients with tubular duct morphology and pulmonary arterial hypertension. This is generally a rare complication that is reported in the literature.[28,29]
CONCLUSION
Patent arterial ducts in our patients were large and were associated with early development of pulmonary hypertension. In the majority of patients, the initial drop in PA pressure was sustained at follow-up, on echocardiographic evaluation. This is probably because of a protection offered by pulmonary vasoconstriction at high altitudes. Residual shunts and device embolization/migration was more frequent in this study and surgical retrieval was required in four cases. Percutaneous closure of large ducts with pulmonary hypertension at high altitudes is safe and effective in the majority of instances. However, patients need careful follow-up to detect any device migration and also to assess PA pressures. Septal Occluder devices may be a possible alternative for large tubular or window-type ducts with severe pulmonary hypertension, where there may be concerns about the size and stability of Duct Occluder devices.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Krichenko A, Benson LN, Burrows P, Möes CA, McLaughlin P, Freedom RM. Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol. 1989;63:877–80. doi: 10.1016/0002-9149(89)90064-7. [DOI] [PubMed] [Google Scholar]
- 2.Bialkowski J, Glowacki J, Zabal C, Garcia-Montes A, Bermudez-Canete R, Flores-Arizmendi R, et al. Patent ductus arteriosus at low and high altitudes: Anatomical and haemodynamic features and their implications for transcatheter closure. Kardiol Pol. 2011;69:431–6. [PubMed] [Google Scholar]
- 3.Penaloza D, Sime F, Ruiz L. Pulmonary hemodynamics in children living at high altitudes. High Alt Med Biol. 2008;9:199–207. doi: 10.1089/ham.2008.1004. [DOI] [PubMed] [Google Scholar]
- 4.Clyman RI, Chorne N. Patent ductus arteriosus: Evidence for and against treatment. J Pediatr. 2007;150:216–9. doi: 10.1016/j.jpeds.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood P. The Eisenmenger syndrome or pulmonary hypertension with reversed central shunt. Br Med J. 1958;2:755–62. doi: 10.1136/bmj.2.5099.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu ML, Huang XM, Wang JF, Qin YW, Zhao XX, Zheng X. Safety and efficacy of transcatheter closure of large patent ductus arteriosus in adults with a self-expandable occluder. Heart Vessels. 2009;24:440–5. doi: 10.1007/s00380-009-1150-5. [DOI] [PubMed] [Google Scholar]
- 7.Li JJ, Li YF, Zhang ZW, Qian MY, Wang HS. A follow-up study on transcatheter closure of patent ductus arteriosus with Amplatzer duct occluder in children. Zhonghua Er Ke Za Zhi. 2005;43:608–11. [PubMed] [Google Scholar]
- 8.Yan C, Zhao S, Jiang S, Xu Z, Huang L, Zheng H, et al. Transcatheter closure of patent ductus arteriosus with severe pulmonary arterial hypertension in adults. Heart. 2007;93:514–8. doi: 10.1136/hrt.2006.091215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JK, Wu MH, Lin MT, Chiu SN, Chen CA, Chiu HH. Transcatheter closure of moderate-to-large patent ductus arteriosus in infants using Amplatzer duct occluder. Circ J. 2010;74:361–4. doi: 10.1253/circj.cj-09-0473. [DOI] [PubMed] [Google Scholar]
- 10.Thanopoulos BD, Hakim FA, Hiari A, Goussous Y, Basta E, Zarayelyan AA, et al. Further experience with transcatheter closure of the patent ductus arteriosus using the Amplatzer duct occluder. J Am Coll Cardiol. 2000;35:1016–21. doi: 10.1016/s0735-1097(99)00626-9. [DOI] [PubMed] [Google Scholar]
- 11.Thanopoulos BD, Tsaousis GS, Djukic M, Al Hakim F, Eleftherakis NG, Simeunovic SD. Transcatheter closure of high pulmonary artery pressure persistent ductus arteriosus with the Amplatzer muscular ventricular septal defect occluder. Heart. 2002;87:260–3. doi: 10.1136/heart.87.3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zabal C, García-Montes JA, Buendía-Hernández A, Calderón-Colmenero J, Patiño-Bahena E, Juanico-Enriquez A, et al. Percutaneous closure of hypertensive ductus arteriosus. Heart. 2010;96:625–9. doi: 10.1136/hrt.2009.185025. [DOI] [PubMed] [Google Scholar]
- 13.Fatima NN. Closure of large patent ductus arteriosus by Amplatzer sepatal occluder (ASO): A case report. J Armed Forces Med Coll Bangladesh. 2009;5:46–8. [Google Scholar]
- 14.Spies C, Ujivari F, Schräder R. Transcatheter closure of a 22 mm patent ductus arteriosus with an Amplatzer atrial septal occluder. Catheter Cardiovasc Interv. 2005;64:352–5. doi: 10.1002/ccd.20283. [DOI] [PubMed] [Google Scholar]
- 15.Onorato E, Mbala-Mukendi M, Casilli F, Girardi P, Canali G, Lanzoni L, et al. Amplatzer muscular VSD occluder for catheter closure of a 20 mm hypertensive patent ductus arteriosus. A case report and literature review. Minerva Cardioangiol. 2004;52:219–23. [PubMed] [Google Scholar]
- 16.Rosenzweig EB, Barst RJ. Clinical management of patients with pulmonary hypertension. In: Allen HD, Driscoll DJ, Shaddy RE, Feltes TF, editors. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents including the fetus and Young Adult. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. pp. 1355–66. [Google Scholar]
- 17.Myung KP. 5th ed. Philadelphia, PA: Mosby Elsevier; 2005. Special tools in evaluation of cardiac patients (Non-invasive techniques): Echocardiography. Pediatric Cardiology for Practitioners; p. 200. [Google Scholar]
- 18.Heath A, Lang N, Levi DS, Granja M, Villanueva J, Navarro J, et al. Transcatheter closure of large patent ductus arteriosus at high altitude with a novel nitinol device. Catheter Cardiovasc Interv. 2012;79:399–407. doi: 10.1002/ccd.23302. [DOI] [PubMed] [Google Scholar]
- 19.Heath A, Stewart K, Mendes J, Ramirez M, Freudenthal F. Does high altitude protect against irreversible pulmonary hypertension? PVRI Rev. 2010;2:131–3. [Google Scholar]
- 20.Szkutnik M, Menacho-Delgadillo R, Palmero-Zilveti E, Bialkowski J. Transcatheter closure of patent ductus arteriosus among native high-altitude habitants. Pediatr Cardiol. 2008;29:624–7. doi: 10.1007/s00246-007-9174-z. [DOI] [PubMed] [Google Scholar]
- 21.Tilahun B, Tefera E. Transient left ventricular systolic dysfunction following surgical closure of large patent ductus arteriosus among children and adolescents operated at the cardiac centre, Ethiopia. J Cardiothorac Surg. 2013;8:139. doi: 10.1186/1749-8090-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kollias J, Buskirk E. Exercise and altitude. In: Johnson W, Buskirk E, editors. Science and Medicine in Exercise and Sports. New York: Harper & Row; 1974. pp. 211–27. [Google Scholar]
- 23.Swenson ER, Robertson HT, Hlastala MP. Effects of inspired carbon dioxide on ventilation-perfusion matching in normoxia, hypoxia, and hyperoxia. Am J Respir Crit Care Med. 1994;149:1563–9. doi: 10.1164/ajrccm.149.6.8004314. [DOI] [PubMed] [Google Scholar]
- 24.Levine BD, Zuckerman JH, deFilippi CR. Effect of high-altitude exposure in the elderly: The Tenth Mountain Division study. Circulation. 1997;19;96:1224–32. doi: 10.1161/01.cir.96.4.1224. [DOI] [PubMed] [Google Scholar]
- 25.Azhar AS, Abd El-Azim AA, Habib HS. Transcatheter closure of patent ductus arteriosus: Evaluating the effect of the learning curve on the outcome. Ann Pediatr Cardiol. 2009;2:36–40. doi: 10.4103/0974-2069.52804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galal MO, Amin M, Hussein A, Kouatli A, Al-Ata J, Jamjoom A. Left ventricular dysfunction after closure of large patent ductus arteriosus. Asian Cardiovasc Thorac Ann. 2005;13:24–9. doi: 10.1177/021849230501300106. [DOI] [PubMed] [Google Scholar]
- 27.Noori S, Friedlich P, Seri I, Wong P. Changes in myocardial function and hemodynamics after ligation of the ductus arteriosus in preterm infants. J Pediatr. 2007;150:597–602. doi: 10.1016/j.jpeds.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 28.Tripathi RR, Agarwal R, Premsekar R. Late surgical removal of an embolized patent ductus arteriosus device causing erosion of the aortic wall. Pediatr Cardiol. 2012;33:1453–5. doi: 10.1007/s00246-012-0272-1. [DOI] [PubMed] [Google Scholar]
- 29.McMullan DM, Moulick A, Jonas RA. Late embolization of Amplatzer patent ductus arteriosus occlusion device with thoracic aorta embedment. Ann Thorac Surg. 2007;83:1177–9. doi: 10.1016/j.athoracsur.2006.07.062. [DOI] [PubMed] [Google Scholar]


