Abstract
Background
Insulin-like growth factor (IGF-1) is associated with chronic diseases such as diabetes, cardiovascular disease, and hypertension, as well as muscle dysfunction. Previous studies of exercise interventions yield controversial results regarding plasma IGF-1, IGFBP3, and IGF-1/IGFBP3 ratio. In this study, we examined whether 100 km walking exercise affects serum levels of IGF-1 and IGFBP3 and IGF-1/IGFBP3 ratio. We also investigated several metabolic-related blood parameters before and after walking.
Methods
Participants were 14 healthy middle aged men (41.0 ± 6.78 years of age). We assessed body composition and measured metabolic-related blood indicators, such as such as lipid profiles, glucose, renal and hepatic metabolic bio-markers before and after a 100 km walking race. Blood samples from all participants were taken before and immediately after the walkathon. We also analyzed serum levels of IGF-1 and IGFBP3, and calculated the IGF-1/IGFBP3 ratio.
Results
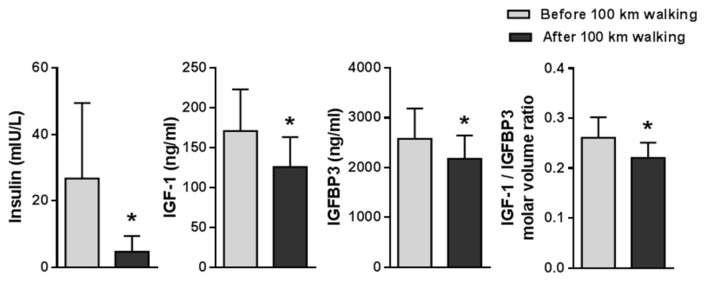
After participants completed a 100 km walking race, some of their metabolic profiles were markedly changed. Serum levels of IGF-1 and IGFBP3 were significantly decreased, and therefore the IGF-1/IGFBP3 ratio also decreased before and after 100 km of walking.
Conclusion
Our results indicate that intense walking exercise affects serum levels of IGF-1 and IGFBP3 as well as metabolic bio-markers including high density cholesterol, glucose and triglycerides.
Keywords: IGF-1, IGFBP3, Walkathon
INTRODUCTION
Sedentary lifestyles and lack of physical activity have led to increases in mortality and chronic diseases such as hypertension, type 2 diabetes, and obesity. Lack of activity is also closely associated with elevated incidences of cancers. Regular physical activity improves diverse health conditions such as diabetes and cardiovascular disease [1–4]. Walking is a relatively simple and safe type of exercise with many health benefits. It is known to reduce the risk of heart disease and can help prevent dementia, depression, and Alzheimer’s disease. Some findings suggest that walking exercises improve both mental health and quality of lifestyle [5]. However, the physiological and pathophysiological effects of extreme exercise are not fully understood. Some previous studies have reported that prolonged moderate intensity walking caused muscular injury and injury to the ankle and knee [6]. Several studies have demonstrated that prolonged strenuous physical exercise can induce hydration, severe electrolyte abnormalities, dysfunction of heart and kidney, and myocardial infarction causing sudden death [7].
Insulin-like growth factor (IGF-1), also called somatome-din C, is synthesized in the liver and is regulated by pituitary growth hormones which plays a major role in cell growth, cell development, and energy metabolism. IGF-1 secretion causes hypoglycemia by glucose uptake and is synthesized by the liver and pituitary hormone. A number of studies have shown that impaired liver function causes decreases in IGF-1 secretion [8,9]. IGF-1 affects growth hormones (GHs) whose deficiencies are related to kidney dysfunction. Various studies showed that IGF-1 levels are correlated with metabolic diseases such as type 2 diabetes, hypertension, and insulin resistance. Insulin-like growth factor binding protein 3 (IGFBP3) is a family of IGFBP encoded by the IGFBP3 gene. IGFBP3 binds to IGF-1in the blood. Recently, IGF-1 and IGFBP3 have been found to affect energy balance, body weight, and body fat mass. Decreased serum levels of IGF1 are associated with energy balance and body composition in rhythmic gymnastics athletes [10]. Cumulative studies argue the effects of exercise intervention associated with plasma levels of IGF-1 and IGFBP3. It has been known that exercise increase IGF-1 secretion [11,12] with inconsistent results with relevant studies [13,14].
Long-distance (100 km) walking is an extreme sport that is becoming more popular. It is one of the most usable and accessible forms of physical activity. Nevertheless the effects of long-distance walking are not known. There are few previous studies regarding the effects of prolonged exercise, such as swimming and marathon running, and the associated physiological changes remain unclear. In the present study, we investigate the effect of intense exercise on metabolic hepatorenal indicators including serum IGF-1 and IGFBP3.
MATERIALS AND METHODS
1. Subjects and study protocol
The subjects were recruited among the participants of a 100 km walk festival in W city. All subjects were healthy middle aged men (Table 1). All participants completed an essential medical questionnaire, medical history check-up, and body composition analysis 1 hour prior to the start of the race. Only 14 of 20 subjects completed the race, while the remaining 6 failed to complete all 100 km. The race began at 2:00 p.m., and the average finishing time was 21 hours. The participants were allowed to ingest water and food freely on their own time during the event.
Table 1.
Physical characteristics of participants
| n | Age (yr) | Height (cm) | Weight (kg) | BMI (kg/m2) | %BF (%) | |
|---|---|---|---|---|---|---|
| Participants | 14 | 41.8 ± 6.8 | 165.5 ± 5.5 | 58.2 ± 11.2 | 23.7 ± 2.0 | 23.8 ± 8.5 |
Values are mean ± S.D., BMI: body mass index, %BF: percent body fat.
2. Blood sampling and analysis
Blood samples from all participants were obtained through the antecubital vein immediately before and after walking. Blood samples were centrifuged at 3,000 rpm for 10 min and metabolic profiles were measured with an automatic chemical blood analyzer (Samsung®IVD-A10A, Korea). The serum IGF-1 and IGFBP3 levels were also measured with IGF-1 and IGFBP3 ELISA kits.
3. Data analysis
All data were analyzed using the SPSS 19.0 software. Descriptive statistics were calculated to identify the means and standard deviations. Before and after prolonged moderate intensity walking, data analyses were performed using a Wilcoxon signed rank test. Statistical significance was set at p < 0.05. IGF-1 and IGFBP3 serum levels and IGF1/ IGFBP3 ratios were calculated as dependent outcomes. The molar ratio was obtained as follows: IGF-1/IGFBP3 = [IGF-1 (ng/mL) ×0.13] / [IGFBP3 (ng/mL) × 0.036] [15].
RESULTS
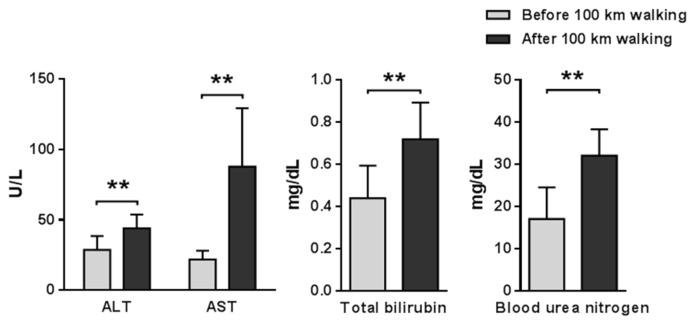
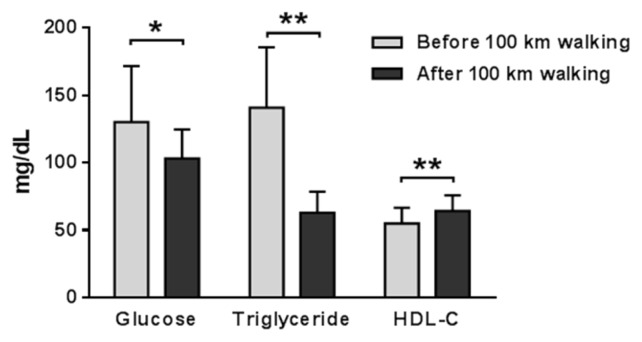
Data values were represented as the mean ± SD, before and after the 100 km walk. The subjects’ weights (66. 6 ± 5.1 vs 65.6 ± 5.0 kg; p < 0.001) and %body fat (23.9 ± 8.5 vs 18.8 ± 5.53%; p < 0.05) were significantly decreased after the 100 km walking. Serum biomarkers indicating liver function including ALT, AST, BUN and total bilirubin were increased after the 100 km walk (Fig. 1). Next, hemodynamic factors such as systolic blood pressure, diastolic blood pressure, and heart rate were examined before and after 100 km walking. Whereas systolic blood pressure (133.0 ± 13.7 vs 122.3 ± 9.8 mmHg; p < 0.05) and diastolic blood pressure (85.0 ± 8.9 vs 76.8 ± 4.2 mmHg; p < 0.05) were decreased, heart rate (75.1 ± 16.6 vs. 91.6 ± 13.4 beats/min; p < 0.001) was increased after the 100 km walking. In addition, we analyzed several metabolic-related blood factors. Blood glucose, insulin, and triglyceride were significantly decreased, while high-density lipoprotein was significantly increased after walking 100 km (Fig. 2 and 3). However, serum level of total protein, total and low-density cholesterol, γ-GTP, creatinine, and albumin were not altered after walking. IGF-1 and IGFBP3 and IGF-1/IGFBP3 ratio levels were significantly decreased after 100 km walking (Fig. 3).
Fig. 1.
Changes in metabolic indicators of the liver and kidney before and after 100 km walking.
Fig. 2.
Changes in bio-markers of glucolipid-metabolism before and after 100 km walking.
Fig. 3.
Changes in insulin, IGF-1, IGFBP3 and IGF-1/IGFBP3 before and after 100 km walking.
DISCUSSION
In this study, we examined the effects of 100 km walking exercise on the serum concentration of IGF-1 and IGFBP3 and the IGF-1/IGFBP3 ratio. The IGF-1/IGFBP3 ratio is also an important factor for the assessment of IGF-1 and IGFBP3 [15]. Previous studies demonstrated that exercise influences circulating IGF-1 and IGFBP3 which are depend on exercise intensity and types. In most studies, exercise interventions led to increases in IGF-1 and IGFBP3 secretion [12,16], however, some reports argue that IGF-1, IGFBP3, and the IGF-1/IGFBP3 ratio were decreased or not affected by exercise interventions [14,17]. Circulating serum levels of IGF-1 and IGFBP3 are associated with both aging and exercise [18,19]. Moderate intensity treadmill exercise led to increases in IGF-1 in both young and old animals [19,20].
Interestingly, the serum IGF-1, IGFBP3, and IGF-1/ IGFBP3 ratio were significantly decreased in our study. Many studies have reported that long-duration moderate exercise results in the decrease of insulin, glucose, triglyceride, total cholesterol, and low-density lipoprotein, and the increase of high-density lipoprotein [21]. We also found that 100 km walking exercise caused decrease of insulin, glucose, and triglyceride, while high- density lipoprotein level was increased. We also observed decreased blood glucose and insulin and increased serum level of indicators for hepatorenal function such as ALT, AST, BUN and total bilirubin. IGF-1 is produced in the liver and function as an endocrine hormone that is regulated by glucose and insulin independently of caloric restriction such as fasting and protein supplementation [22]. Our results suggest that intense walking may decrease serum level of insulin and glucose induced by decrease of serum IGF-1 and IGFBP3. We allowed subjects ingestion of food and water freely during walking which may affect the IGF-1 and IGFBP3 levels. Energy intake was not analyzed in the present study that should be considered for prolonged walking exercise in future studies.
Exercise intensity and time are important factors governing the beneficial effects of exercise on body function. Most studies have detected negative effects of long-duration exercise such as marathons and ultra-marathons [23]. Generally, excessive exercise impairs body functions such as kidney and liver [24,25]. In our study, hepatic markers such as ALT and AST markedly increased in blood samples after completing a 100 km walking. Impaired liver function might lead to decrease of IGF1 and IGFBP3 levels through functional impairments of gluconeogenesis.
Generally, increased level of HDL-c is known to prevent obesity and enhance vascular function through improved transportation of LDL-c to the liver. In addition to exercise-induced decrease of triglyceride, other benefits have been observed in terms of ischemic vascular events [26]. Recent studies have demonstrated that decreased level of IGF-1 is associated with reduced body weight and fat mass. It should be noted that decreased IGF-1 level affects obesity after exercise regardless of intensity, type, or time of exercise. We found that 100 km walking exercise led to decreased and increased level of triglyceride and high- density lipoprotein, respectively, suggesting that IGF-1 and IGFBP3 associated with metabolic biomarkers with additional benefits. The effect of exercise on IGF-1 secretion in the context of energy supplementation and exercise intensity, type, and time awaits future investigation.
ACKNOWLEDGEMENTS
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF- 2013R1A1A2060764).
REFERENCES
- 1.Dutheil F, Lac G, Lesourd B, Chapier R, Walther G, Vinet A, Sapin V, Verney J, Ouchchane L, Duclos M, Obert P, Courteix D. Different modalities of exercise to reduce visceral fat mass and cardiovascular risk in metabolic syndrome: the RESOLVE randomized trial. Int J Cardiol. 2013;168:3634–42. doi: 10.1016/j.ijcard.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Chang Q, Miao X, Ju X, Zhu L, Huang C, Huang T, Zuo X, Gao C. Effects of pulse current on endurance exercise and its anti-fatigue properties in the hepatic tissue of trained rats. PLoS One. 2013;8:e75093. doi: 10.1371/journal.pone.0075093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalzill C, Nigam A, Juneau M, Guilbeault V, Latour E, Mauriege P, Gayda M. Intensive lifestyle intervention improves cardiometabolic and exercise parameters in metabolically healthy obese and metabolically unhealthy obese individuals. Can J Cardiol. 2014;30:434–40. doi: 10.1016/j.cjca.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 4.Delussu AS, Morone G, Iosa M, Bragoni M, Traballesi M, Paolucci S. Physiological responses and energy cost of walking on the Gait Trainer with and without body weight support in subacute stroke patients. J Neuroeng Rehabil. 2014;11:54. doi: 10.1186/1743-0003-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunters A, Armstrong VJ, Zaman G, Kypta RM, Kawano Y, Lanyon LE, Price JS. Mechano-transduction in osteo-blastic cells involves strain-regulated estrogen receptor alpha-mediated control of insulin-like growth factor (IGF) I receptor sensitivity to Ambient IGF, leading to phosphatidylinositol 3-kinase/AKT-dependent Wnt/LRP5 receptor-independent activation of beta-catenin signaling. J Biol Chem. 2010;285:8743–58. doi: 10.1074/jbc.M109.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruso JR, Hoffman MD, Rogers IR, Lee L, Towle G, Hew-Butler T. Rhabdomyolysis and hyponatremia: a cluster of five cases at the 161-km 2009 Western States Endurance Run. Wilderness Environ Med. 2010;21:303–8. doi: 10.1016/j.wem.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 7.McCullough PA, Chinnaiyan KM, Gallagher MJ, Colar JM, Geddes T, Gold JM, Trivax JE. Changes in renal markers and acute kidney injury after marathon running. Nephrology (Carlton) 2011;16:194–9. doi: 10.1111/j.1440-1797.2010.01354.x. [DOI] [PubMed] [Google Scholar]
- 8.Volzke H, Nauck M, Rettig R, Dorr M, Higham C, Brabant G, Wallaschofski H. Association between hepatic steatosis and serum IGF1 and IGFBP-3 levels in a population-based sample. Eur J Endocrinol. 2009;161:705–13. doi: 10.1530/EJE-09-0374. [DOI] [PubMed] [Google Scholar]
- 9.Poehlman ET, Arciero PJ, Goran MI. Endurance exercise in aging humans: effects on energy metabolism. Exerc Sport Sci Rev. 1994;22:251–84. doi: 10.1249/00003677-199401000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Ahtiainen M, Pollanen E, Ronkainen PH, Alen M, Puolakka J, Kaprio J, Sipila S, Kovanen V. Age and estrogen-based hormone therapy affect systemic and local IL-6 and IGF-1 pathways in women. Age (Dordr) 2012;34:1249–60. doi: 10.1007/s11357-011-9298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bang P, Brandt J, Degerblad M, Enberg G, Kaijser L, Thoren M, Hall K. Exercise-induced changes in insulin-like growth factors and their low molecular weight binding protein in healthy subjects and patients with growth hormone deficiency. Eur J Clin Invest. 1990;20:285–92. doi: 10.1111/j.1365-2362.1990.tb01857.x. [DOI] [PubMed] [Google Scholar]
- 12.Eliakim A, Brasel JA, Mohan S, Wong WL, Cooper DM. Increased physical activity and the growth hormone-IGF-I axis in adolescent males. Am J Physiol. 1998;275:R308–14. doi: 10.1152/ajpregu.1998.275.1.R308. [DOI] [PubMed] [Google Scholar]
- 13.Fontana L, Klein S, Holloszy JO. Long-term low-protein, low-calorie diet and endurance exercise modulate metabolic factors associated with cancer risk. Am J Clin Nutr. 2006;84:1456–62. doi: 10.1093/ajcn/84.6.1456. [DOI] [PubMed] [Google Scholar]
- 14.Bacon SL, Sherwood A, Hinderliter A, Blumenthal JA. Effects of exercise, diet and weight loss on high blood pressure. Sports Med. 2004;34:307–16. doi: 10.2165/00007256-200434050-00003. [DOI] [PubMed] [Google Scholar]
- 15.Morimoto LM, Newcomb PA, White E, Bigler J, Potter JD. Variation in plasma insulin-like growth factor-1 and insulin-like growth factor binding protein-3: genetic factors. Cancer Epidemiol Biomarkers Prev. 2005;14:1394–401. doi: 10.1158/1055-9965.EPI-04-0694. [DOI] [PubMed] [Google Scholar]
- 16.Koziris LP, Hickson RC, Chatterton RT, Jr, Groseth RT, Christie JM, Goldflies DG, Unterman TG. Serum levels of total and free IGF-I and IGFBP-3 are increased and maintained in long-term training. J Appl Physiol (1985) 1999;86:1436–42. doi: 10.1152/jappl.1999.86.4.1436. [DOI] [PubMed] [Google Scholar]
- 17.Vitiello MV, Wilkinson CW, Merriam GR, Moe KE, Prinz PN, Ralph DD, Colasurdo EA, Schwartz RS. Successful 6-month endurance training does not alter insulin-like growth factor-I in healthy older men and women. J Gerontol A Biol Sci Med Sci. 1997;52:M149–54. doi: 10.1093/gerona/52A.3.M149. [DOI] [PubMed] [Google Scholar]
- 18.Goldspink G. Age-related loss of muscle mass and strength. J Aging Res. 2012;2012:158279. doi: 10.1155/2012/158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willis PE, Chadan SG, Baracos V, Parkhouse WS. Restoration of insulin-like growth factor I action in skeletal muscle of old mice. Am J Physiol. 1998;275:E525–30. doi: 10.1152/ajpendo.1998.275.3.E525. [DOI] [PubMed] [Google Scholar]
- 20.Owino V, Yang SY, Goldspink G. Age-related loss of skeletal muscle function and the inability to express the autocrine form of insulin-like growth factor-1 (MGF) in response to mechanical overload. FEBS Lett. 2001;505:259–63. doi: 10.1016/S0014-5793(01)02825-3. [DOI] [PubMed] [Google Scholar]
- 21.Al-Jiffri O, Al-Sharif FM, Abd El-Kader SM, Ashmawy EM. Weight reduction improves markers of hepatic function and insulin resistance in type-2 diabetic patients with non-alcoholic fatty liver. Afr Health Sci. 2013;13:667–72. doi: 10.4314/ahs.v13i3.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clemmons DR. The relative roles of growth hormone and IGF-1 in controlling insulin sensitivity. J Clin Invest. 2004;113:25–7. doi: 10.1172/JCI20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peter L, Rust CA, Knechtle B, Rosemann T, Lepers R. Sex differences in 24-hour ultra-marathon performance--a retrospective data analysis from 1977 to 2012. Clinics (Sao Paulo) 2014;69:38–46. doi: 10.6061/clinics/2014(01)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Junglee NA, Di Felice U, Dolci A, Fortes MB, Jibani MM, Lemmey AB, Walsh NP, Macdonald JH. Exercising in a hot environment with muscle damage: effects on acute kidney injury biomarkers and kidney function. Am J Physiol Renal Physiol. 2013;305:F813–20. doi: 10.1152/ajprenal.00091.2013. [DOI] [PubMed] [Google Scholar]
- 25.Romagnoli M, Alis R, Aloe R, Salvagno GL, Basterra J, Pareja-Galeano H, Sanchis-Gomar F, Lippi G. Influence of training and a maximal exercise test in analytical variability of muscular, hepatic, and cardiovascular biochemical variables. Scand J Clin Lab Invest. 2014;74:192–8. doi: 10.3109/00365513.2013.873948. [DOI] [PubMed] [Google Scholar]
- 26.Pikula A, Beiser AS, Wang J, Himali JJ, Kelly-Hayes M, Kase CS, Yang Q, Seshadri S, Wolf PA. Lipid and lipoprotein measurements and the risk of ischemic vascular events: Framingham Study. Neurology. 2015;84:472–9. doi: 10.1212/WNL.0000000000001202. [DOI] [PMC free article] [PubMed] [Google Scholar]