Abstract
Background
The use of mandibular distraction osteogenesis (MDO) for tissue replacement after oncologic resection in head and neck cancer could have immense therapeutic ramifications. We have previously demonstrated significantly decreased mechanical and microdensitomeric metrics of our MDO regenerate after 36-Gy radiation. Quantitative histomorphometry, a third metric, would permit objective investigation of the effects of radiation on tissue and cellular composition. Our hypothesis is that radiation-induced cellular depletion and diminution in function impair optimal bone regeneration. Methods: Five rats received radiation to the left mandible; 5 received none. All animals underwent surgical placement of external fixators, creation of mandibular osteotomies, distraction to a 5.1-mm gap width, and consolidation. Point counting and color thresholding were performed.
Results
There was a significant increase in empty lacunae and a corresponding diminution in osteocytes after radiation. Whereas the volume fraction of mineralized, mature bone was not different, that of nonmineralized, immature osteoid was significantly increased in the radiated group compared with that in the nonradiated group.
Conclusions
Our findings confirm our prior 2 metrics. Actually, all 3 diverse metrics—microdensitometry, biomechanical analysis, and histomorphometry—corroborate our hypothesis of cellular depletion and diminution of function as the potential mechanism of radiation-induced attenuation in the distracted regenerate. Furthermore, our findings of tissue and cellular changes in the irradiated regenerate elucidate the pathophysiology of decreased bone quality when amalgamated with our previous results. Therapeutic agents may now be introduced, and their effects on the irradiated regenerate critically measured, so that MDO may be used as a viable reconstructive option in patients with head and neck cancer.
Keywords: Mandibular distraction osteogenesis, quantitative histomorphometry, radiation therapy
In 2008, there were an estimated 35,310 new cases of oral and pharyngeal cancers, resulting in 7590 expected deaths.1 A substantial number of these patients require surgical extirpation of their tumors with subsequent adjuvant radiotherapy and reconstruction. Reconstructive efforts must contend with the blight of adverse effects resulting from high-dose radiation (x-ray therapy [XRT]) while achieving structural and functional restoration of the uniquely complex anatomy. Free tissue transfer is the current reconstructive option but is a massive procedure characterized by multiple comorbidities.2,3 A less invasive reconstructive option would be both desirable and beneficial.
Mandibular distraction osteogenesis (MDO), the stimulation of new bone formation by the gradual separation of 2 osteogenic fronts, has been successfully established as a reconstructive tool for the correction of congenital mandibular hypoplasias,4,5 but its use after oncologic resection and adjuvant radiotherapy (XRT) is unclear. Animal studies6–9 and clinical case reports10–12 have reported both successes and failures of MDO in the irradiated mandible. Although outcomes may be inconsistent, it is well known that radiation to normal bone induces cellular and vascular injuries leading to hypoxic, hypovascular, and hypocellular environments that drastically impair tissue repair.13 Accordingly, the irradiated MDO regenerate would be expected to act in a similar fashion and conceivably worse because of new active bone formation.
Specifically, our hypothesis is that radiation induces cellular depletion and dimunition in cell function, impairing optimal bone regeneration. We developed a unique murine model of MDO after 36 Gy of preoperative fractionated radiation. Our model was tested and verified using micro—computed tomographic (microCT) analysis and mechanical testing. We demonstrated a significant increase in low-mineralized, immature bone and a corresponding decrease in highly mineralized, mature bone in the MDO regenerate after 36 Gy of XRT using microCT analysis.14 Using tension testing, a metric of mechanical strength, we showed a statistically significant decrease in breaking strength of the irradiated MDO regenerate compared with that of the nonradiated regenerate.15
Both microCT and mechanical testing are outcome measures that test the quality of the irradiated MDO regenerate. Neither metric examines the pathophysiology of healing, resulting in our observed attenuation of the irradiated MDO regenerate. Our goal, in this article, was to assess a third outcome measure quantitative histomorphometry (QHM). Quantitative histomorphometry serves as the criterion standard to objectively measure the effects of XRT on cellularity and tissue composition and will also permit investigation of the pathophysiology of radiation-induced injury. In particular, our QHM outcome measures will be osteocyte count (Oc) per high-power field (HPF), empty lacunae (EL) count per HPF, bone volume (BV), and osteoid volume (OV). Identifying the mechanisms resulting in decreased bone quality will allow us to formulate and then measure outcomes of therapeutic interventions designed to enhance MDO to improve treatment strategies for head and neck cancer patients experiencing the disastrous sequelae of radiation therapy.
METHODS
Experimental Groups and Model
Male Sprague-Dawley rats were randomly assigned preoperatively to 1 of 2 experimental groups: group 1, rats that would receive preoperative radiation followed by distraction (MDOx [n = 5]) or group 2, rats that would receive distraction alone (MDO [n = 5]).
Group 1 MDOx rats underwent fractionated radiation to the left hemimandible followed by a 2-week recovery period before surgery. Both experimental groups then had an external fixator placed followed by the performance of a surgical mandibular osteotomy. Both experimental groups were then subjected to a 4-day of latency period followed by distraction to a 5.1-mm total gap width. Finally, both experimental groups underwent 28 days of regenerate consolidation (Fig. 1A, B). This protocol has been performed and verified in the past,14 but briefly, it is described later.
FIGURE 1.
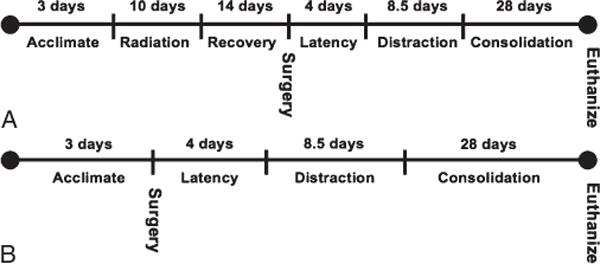
A, Group 1 MDOx timeline. B, Group 2 MDO timeline.
Preoperative Animal Care
Male Sprague-Dawley rats weighing approximately 400 g were housed 3 per cage in a pathogen-free, restricted-access facility upon arrival to our laboratory. All animal procedures were performed according to the National Institutes of Health Guide for Animal Care and approved by the University of Michigan Animal Care and Use Committee.
Radiation
x-Ray therapy was performed at the Irradiation Core of the University of Michigan Cancer Center. x-Ray therapy was delivered with a Pantak DXT 300 orthovoltage unit (250 kV, 15 mA; Kimtron Medical, Woodbury, CT). Dosimetry was carried out using an ionization chamber connected to an electrometer system. The 5 group 1 MDOx rats were radiated after being anesthetized with intraperitoneal ketamine (50 mg/kg) and xylazine (5 mg/kg). They were placed right side down to expose the left mandible. The radiation field was limited to 1 by 4 cm, and the distance to the source was 31.5 cm. A lead shield was used to protect the remainder of the animal (Fig. 2). A total of 36 Gy of radiation was delivered in 10 fractions per rat for 10 days at a rate of 3.6 Gy per fraction.
FIGURE 2.

Rat undergoing radiation. Window in lead shield permits radiation only to left posterior hemimandible.
Surgical Procedure
This surgical procedure has been described previously.14 Preoperatively, animals were given chloramphenicol (30 mg/kg subcutaneously [SQ]) prophylactically 1 hour before surgery, buprenorphine (0.15 mg/kg SQ), and 25-mL/kg lactating Ringer’s SQ. The surgical procedure was performed under sterile conditions and general anesthesia using an isoflurane/oxygen mixture.
A 2-cm midline incision was placed ventrally from the anterior submentum to the neck crease. Skin flaps were elevated exposing the anterolateral mandible. After predrilling (3/32 drill bit) 0.5 mm posterior to the symphysis, a 1.5-in. no. 0-80 stainless steel threaded rod was horizontally inserted across the anterior mandible, with the ends brought externally through the skin, creating the anterior portion of our modified external fixator. A 1-cm incision was made through the masseter, down to, and in-line with the inferior border of the mandible. Bilateral no. 0-80 threaded stainless steel pins were inserted buccal to lingual, after predrilling approximately 2 mm superior to the inferior border, and 6 mm anterior to the angle, and then secured with our custom titanium washer and nut. The pin ends were brought externally through the skin for the posterior fixator placement with titanium caps. The right side was rigidly fixed, whereas the left side received a distraction screw for postoperative manipulation. A vertical mandibular osteotomy was created approximately 2 mm anterior to the titanium washer on the left hemimandible using a 10-mm micro—reciprocating blade (Stryker, Portage, MI) attached to a power saw (Stryker, Portage, MI). The mandibular osteotomy extended from the inferior mandible border superiorly to the sigmoid notch along the anterior aspect of the coronoid process. The mandibular osteotomy edges were reduced and then resecured with the fixator. The wound was irrigated, hemostasis verified, tissues approximated using 4-0 Vicryl suture (Ethicon Inc, Somerville, NJ), and the midline incision closed with staples.
Postoperative Animal Care
Animals were housed 1 per cage and fed moist chow with Hill’s high calorie diet (Columbus Serum, Columbus, OH) and water ad libitum. They were given 1 postoperative dose of given chloramphenicol (30 mg/kg SQ). Buprenorphine was continued (0.15 mg/kg) with 10-mL lactating Ringer’s SQ every 12 hours through postoperative day 4 and as needed thereafter. Pin care was performed with Silvadene (King Pharmaceuticals, Bristol, TN) every other day. Maxillary incisors were clipped weekly owing to overgrowth from crossbite and staples removed by postoperative day 10. Weights were monitored daily and diets adjusted as needed.
Distraction Protocol
All animals were distracted after 4 days of latency. One 180-degree clockwise turn of the distraction screw corresponded to a 0.3-mm separation of the mandibular osteotomy fronts (Fig. 3). A total of 17 half-turns were performed on every 12-hour interval, resulting in a maximum 5.1-mm distraction gap. No analgesic or sedation was required during the distraction.
FIGURE 3.
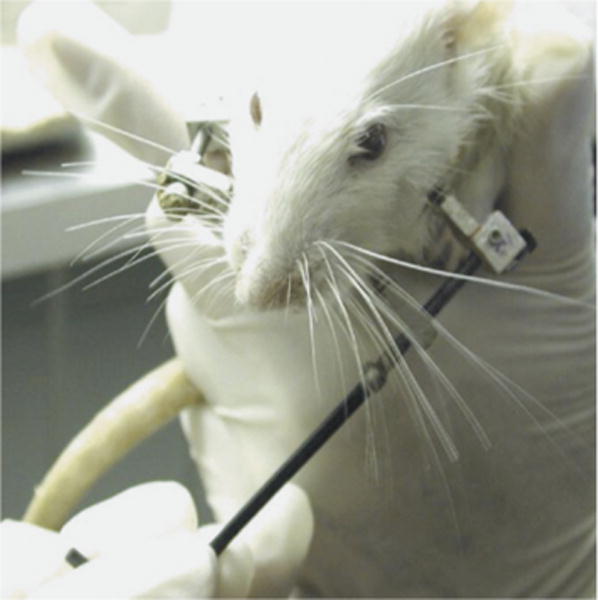
Rat’s left-sided mandibular osteotomy being distracted, no anesthesia required.
Tissue Processing
Mandibles were harvested. The left hemimandibles were fixed in 70% alcohol before being decalcified in formic acid, sodium citrate solution. Specimens were vacuum processed by dehydration and paraffin infiltration for 48 hours (Shandon Hypercenter XP, Pittsburgh, PA), reinfiltrated for 2 hours in a vacuum bath (Leica Embedding Center, model EG1160, Germany), and embedded in paraffin using Paraplast Plus (St Louis, MO). Peel-away embedding molds were used and refrigerated overnight (4-C). Blocks were then sectioned from buccal to lingual into 7-μm sagittal sections and mounted on glass slides. A total of 70 to 100 slides per block were obtained. Every 10th slide was selected to uniformly represent the distraction gap and stained with Gomori 1-step trichrome. Two midgap representative slides per specimen were chosen for continued evaluation by digital color analysis and point counting.
Threshold (Digital Color Analysis) Evaluation
Digital images of each slide were obtained and loaded onto the imaging analysis software Bioquant NOVA Osteo, version 7 (R&M Biometrics, Nashville, TN) for placement of a standard template over the region of interest (ROI) and color thresholding.
Three independent reviewers each obtained (1) the tissue volume (TV), the total volume of the ROI template; (2) the BV, the volume of mineralized bone; and (3) the OV, the volume of nonmineralized, immature bone and vacuoles. Mature, mineralized bone color thresholded green, and osteoid, immature bone color thresholded pink (Fig. 4A, B). After all 3 measurements were obtained, the software automatically calculated our prespecified ratios, namely, BV/TV and OV/TV.
FIGURE 4.
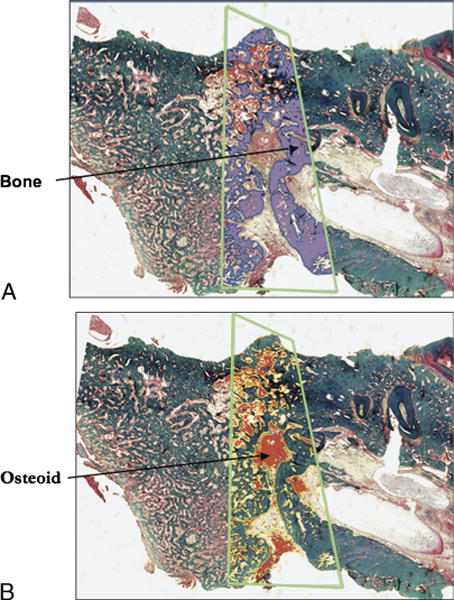
A, Sagittal section of midportion of rat mandible stained with Gomori trichome. Green trapezoid outlines distraction regenerate. Bone is highlighted in purple by color thresholding. B, Sagittal section of midportion of rat mandible stained with Gomori trichome. Green trapezoid outlines distraction regenerate. Nonmineralized matrix is highlighted in red by color thresholding.
Point Counting Osteocytes
In addition, osteocytes and EL were assessed using a light microscope interfaced with a digital camera connected to a computer at 16× magnification. Our ROI template as previously described was superimposed and placed over the ROI. The templates were aligned with the midpoint of the base corresponding to 0,0 and the midpoint of the apex to 0,+9000 on an x, y coordinate grid. The ROIs were then anatomically divided equally into inferior, middle, and superior sub-ROIs. Using the Microsoft Excel randomization function, we obtained coordinates for 3 HPF regions within each sub-ROI, for a total of 9 HPFs per ROI. We ensured that at least 4 of the 9 HPF sections per ROI had osteoid and/or bone present. Otherwise, randomization was repeated, and HPF areas were reimaged. Three independent reviewers each manually counted the number of osteocytes and EL within each HPF (Fig. 5).
FIGURE 5.
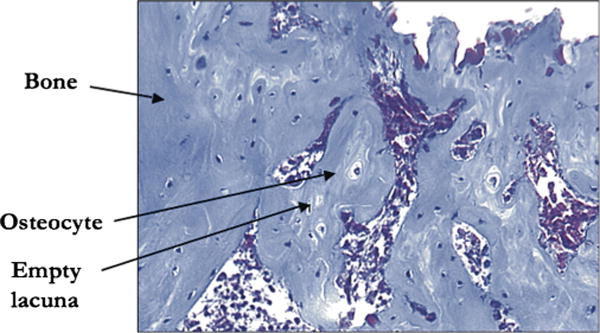
A single digital photograph at 16× magnification demonstrating bone stained green with Gomori trichome, osteocytes within lacunae, and EL.
Statistics
The SPSS for Windows, version 16.0 (SPSS Inc, Chicago, IL), was used to run all statistical tests when comparing group 1 MDOx with group 2 MDO, for a statistically significant difference. Independent samples t-test was used for analyzing the following metrics: osteocytes/HPF, EL count/HPF, BV/TV, and OV/TV. Results were accepted as statistically significant at a P < 0.05.
RESULTS
During radiation, no skin or mucosal changes occurred. Both experimental groups completed the surgical procedure without incident. Postoperatively, all animals gained weight and maintained normal cage activity. There were no wound infections, but localized and encapsulated abscesses were later found in some animals. None of the animals experienced device dislodgement, and the fixators remained stable until the animals were killed. Gross examination of the left hemimandibles did not demonstrate atrophy, and the distraction gaps appeared to be completely bridged.
Quantitative histomorphometry point counting of osteocytes demonstrated a significant decrease in the number of osteocytes per HPF in the MDOx group compared with that in the MDO group (27 vs 16, P < 0.05; Figs. 6 and 7A). In addition, there was a corresponding, statistically significant, 360-fold increase in EL count per HPF in the MDOx group compared with that in the MDO group (2.86 vs 0.78, P < 0.05; Fig. 7B).
FIGURE 6.
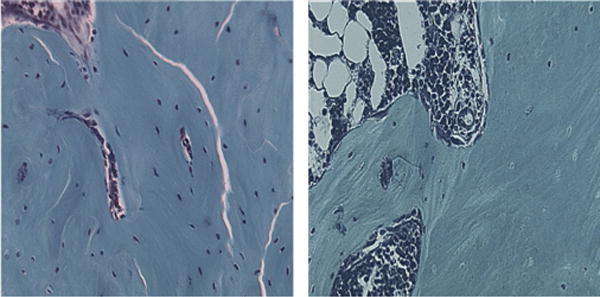
Side-by-side comparison of single digital photos at 16× magnification, of MDO and MDOx rats.
FIGURE 7.
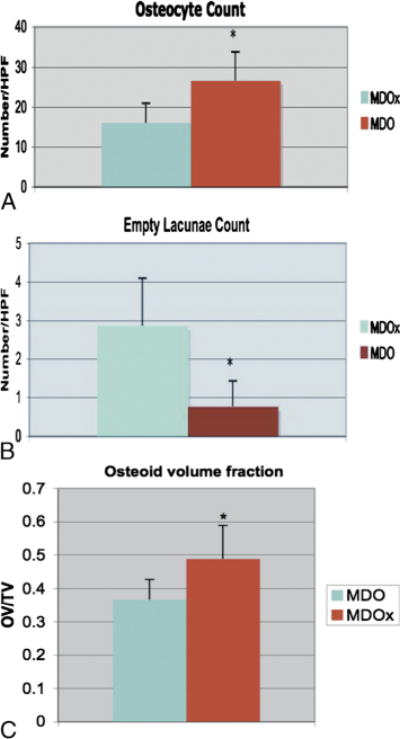
A, Graph of mean Oc/HPF in MDOx and MDO. B, Graph of mean EL count per HPF in MDOx and MDO. C, Graph of mean values of nonmineralized matrix/OV per total volume in MDOx and MDO.
Gross examination of irradiated and nonirradiated slides showed an increase in vacuoles in the irradiated group. Specifically, color thresholding of BV demonstrated no significant change in BV fraction of the irradiated group compared with that of the non-radiated group (0.41 vs 0.39, P > 0.05). There was, however, a significant increase in nonmineralized, osteoid matrix volume fraction in the irradiated mandibles compared with that in the nonradiated ones (0.32 vs 0.49, P < 0.05; Fig. 7C).
DISCUSSION
The role of MDO after oncologic resection and adjuvant XRT is unclear. Numerous animal studies6–9 and clinical case reports10–12 have described the use of MDO in the irradiated mandible. However, these authors report anecdotal, qualitative evaluations with minimal quantitative data regarding the quality of the distraction regenerate after radiation. There is a paucity of reproducible, impartial, quantitative outcome measures to dependably evaluate the quality of the irradiated regenerate. Our laboratory has developed a unique, reliable murine model of MDO after 36 Gy of fractionated radiation. We then validated the efficacy of the model with mechanical testing and microCT. We demonstrated a significantly decreased breaking load during tension testing15 and a significantly increased low-mineralized, immature bone with a corresponding decreased highly mineralized, mature bone using microdensitometry, in the radiated mandibles compared with those in the nonradiated.14 In this article, we evaluated and established a third reliable quantitative outcome measure. Using QHM, we documented quantitative changes in tissue and cell composition of the distraction regenerate after radiotherapy. We demonstrated increased immature, nonmineralized matrix and unchanged BV; we also show decreased Oc with a corresponding increase in EL count.
Interestingly, our histomorphometric tissue findings of no significant difference in BV fractions between the radiated and nonradiated groups support and substantiate our prior microdensitometric results.14 Furthermore, although the overall bone quantity was unchanged with both metrics; microdensitometry, like histomorphometry, also documented a decrease in bone quality, as evidenced by increased low-mineralized and decreased highly mineralized bones. Both metrics are in agreement, are complimentary, and importantly seem to be equally reliable.
Other animal studies on the irradiated mandibular distraction regenerate have reported more cursory and less quantitative tissue changes of increased immature, nonmineralized matrix and unchanged BV.6,8 There have also been numerous animal studies on the effect of radiation on long bone fracture healing; these reports, like the aforementioned distraction studies, demonstrate a delay or lag in callous maturation.16–19 Although this delay in callous maturation has been well established, no animal model study has addressed in vivo cellular changes after radiation. To our knowledge, our study represents the first to objectively quantitate in vivo cellular changes after radiation. Only in vitro studies so far have shed some light on the cellular alterations associated with radiation. These studies report that radiotherapy causes unavoidable injury to cell populations and cytokine expression profiles.20–23 Our in vivo results of decreased Oc with a corresponding increase in EL count confirm that radiation-induced cell death occurs in vivo as well. Hence, QHM not only represents another reliable outcome measure but also provides us, within our unique and reliable in vivo model of distraction osteogenesis after radiation, valuable mechanistic information to further comprehend radiation-induced disturbances in distraction osteogenesis.
These findings of both tissue and cellular changes elucidate the pathophysiology of decreased bone quality when amalgamated with our previous documented microdensitometric and biomechanical findings. Furthermore, all 3 diverse metrics—microdensitometry, biomechanical analysis, and QHM—serve to corroborate our hypothesis of cellular depletion and diminution of function as the potential mechanism of radiation-induced attenuation of MDO. The benefits of confirming our hypothesis with reliable outcome measures are that specific therapeutic strategies can be directed at the root cause of the radiation damage to either mitigate the injury or assuage the harmful effects of radiation and subsequently reliably measure the efficacy of those strategies.
The role of MDO for reconstruction of mandibular defects after XRT will depend on determining the quality and the extent of regenerate attenuation as we have done here in a murine model. Although sporadic clinical case reports10–12 have described both successes and failures of MDO after irradiation, we feel it is important to investigate the effects of XRT on MDO within a reliable and reproducible model using objective quantitative metrics before moving blindly into the clinical realm. Using the data we have generated, therapeutic agents may now be introduced and their effects on healing of the irradiated regenerate critically evaluated and strategically optimized so that MDO may potentially be used as a viable reconstructive option in patients with head and neck cancer.
Acknowledgments
The authors thank Charles Roehm and John Baker for their technical assistance in the preparation of the external fixator/distractor and preparation of the tissues for histologic analysis, respectively.
Funding supported by the following grants: “Optimization of Bone Regeneration in the Irradiated Mandible,” National Institutes of Health RO1 no. CA 12587-01; PI: Steven R. Buchman, MD.
Footnotes
The authors report no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Nakamizo M, Yokoshima K, Yagi T. Use of free flaps for reconstruction in head and neck surgery: a retrospective study of 182 cases. Auris Nasus Larynx. 2004;31:269–273. doi: 10.1016/j.anl.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Ross DA, Hundal JS, Son YH, et al. Microsurgical free flap reconstruction outcomes in head and neck cancer patients after surgical extirpation and intraoperative brachytherapy. Laryngoscope. 2004;114:1170–1176. doi: 10.1097/00005537-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Molina F, Ortiz Monasterio F. Mandibular elongation and remodeling by distraction: a farewell to major osteotomies. Plast Reconstr Surg. 1995;96:825–840. [PubMed] [Google Scholar]
- 5.McCarthy JG, Stelnicki EJ, Grayson BH. Distraction osteogenesis of the mandible: a ten-year experience. Semin Orthod. 1999;5:3–8. doi: 10.1016/s1073-8746(99)80037-1. [DOI] [PubMed] [Google Scholar]
- 6.Shao Z, Liu B, Liu Y, et al. Distraction osteogenesis in the irradiated rabbit mandible. J Plast Reconstr Aesthet Surg. 2006;59:181–187. doi: 10.1016/j.bjps.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 7.Gantous A, Phillips JH, Catton P, et al. Distraction osteogenesis in the irradiated canine mandible. Plast Reconstr Surg. 1994;93:164–168. doi: 10.1097/00006534-199401000-00025. [DOI] [PubMed] [Google Scholar]
- 8.Muhonen A, Muhonen J, Lindholm TC, et al. Osteodistraction of a previously irradiated mandible with or without adjunctive hyperbaric oxygenation: an experimental study in rabbits. Int J Oral Maxillofac Surg. 2002;31:519–524. doi: 10.1054/ijom.2002.0257. [DOI] [PubMed] [Google Scholar]
- 9.Clark CL, Strider J, Hall C, et al. Distraction osteogenesis in irradiated rabbit mandibles with adjunctive hyperbaric oxygen therapy. J Oral Maxillofac Surg. 2006;64:589–593. doi: 10.1016/j.joms.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 10.Holmes SB, Lloyd T, Coghlan KM, et al. Distraction osteogenesis of the mandible in the previously irradiated patient. J Oral Maxillofac Surg. 2002;60:305–309. doi: 10.1053/joms.2002.30581. [DOI] [PubMed] [Google Scholar]
- 11.Raghoebar GM, Jansma J, Vissink A, et al. Distraction osteogenesis in the irradiated mandible. A case report. J Craniomaxillofac Surg. 2005;33:246–250. doi: 10.1016/j.jcms.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Sawaki Y, Hagino H, Yamamoto H, et al. Trifocal distraction osteogenesis for segmental mandibular defect: a technical innovation. J Craniomaxillofac Surg. 1997;25:310–315. doi: 10.1016/s1010-5182(97)80032-7. [DOI] [PubMed] [Google Scholar]
- 13.Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41:283–288. doi: 10.1016/0278-2391(83)90294-x. [DOI] [PubMed] [Google Scholar]
- 14.Fregene A, Jing XL, Monson LA, et al. Alteration in volumetric bone mineralization density gradation patterns in mandibular distraction osteogenesis following radiation therapy. Plast Reconstr Surg. 2009;124:1237–1244. doi: 10.1097/PRS.0b013e3181b5a42f. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz DA, Jamali AM, Kakwan MS, et al. Biomechanical assessment of regenerate integrity in radiated mandibular distraction osteogenesis. Plast Reconstr Surg. 2009;123(2 suppl):114S–122S. doi: 10.1097/PRS.0b013e318191c5d2. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi S, Sugimoto M, Kotoura Y, et al. The effects of intraoperative radiotherapy on bone-healing ability in relation to different doses and postradiotherapy intervals. Int J Radiat Oncol Biol Phys. 1994;30:1147–1152. doi: 10.1016/0360-3016(94)90322-0. [DOI] [PubMed] [Google Scholar]
- 17.Arnold M, Kummermehr J, Trott KR. Radiation-induced impairment of osseous healing: quantitative studies using a standard drilling defect in rat femur. Radiat Res. 1995;143:77–84. [PubMed] [Google Scholar]
- 18.Arnold M, Stas P, Kummermehr J, et al. Radiation-induced impairment of bone healing in rat femur: effects of radiation dose, sequence and interval between surgery and irradiation. Radiother Oncol. 1998;48:259–265. doi: 10.1016/s0167-8140(98)00039-5. [DOI] [PubMed] [Google Scholar]
- 19.Pelker RR, Friedlaender GE. The Nicolas Andry Award—1995. Fracture healing. Radiation induced alterations. Clin Orthop Relat Res. 1997;341:267–282. [PubMed] [Google Scholar]
- 20.Dudziak ME, Saadeh PB, Mehrara BJ, et al. The effects of ionizing radiation on osteoblast-like cells in vitro. Plast Reconstr Surg. 2000;106:1049–1061. doi: 10.1097/00006534-200010000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Dare A, Hachisu R, Yamaguchi A, et al. Effects of ionizing radiation on proliferation and differentiation of osteoblast-like cells. J Dent Res. 1997;76:658–664. doi: 10.1177/00220345970760020601. [DOI] [PubMed] [Google Scholar]
- 22.Gevorgyan A, Sukhu B, Alman BA, et al. Radiation effects and radioprotection in MC3T3-E1 mouse calvarial osteoblastic cells. Plast Reconstr Surg. 2008;122:1025–1035. doi: 10.1097/PRS.0b013e3181845931. [DOI] [PubMed] [Google Scholar]
- 23.Gevorgyan A, La Scala GC, Sukhu B, et al. An in vitro model of radiation-induced craniofacial bone growth inhibition. J Craniofac Surg. 2007;18:1044–1050. doi: 10.1097/scs.0b013e31814c916f. [DOI] [PubMed] [Google Scholar]


