Abstract
The human body produces and removes 1011 platelets daily to maintain a normal steady state platelet count. Platelet production must be regulated to avoid spontaneous bleeding or arterial occlusion and organ damage. Multifaceted and complex mechanisms control platelet production and removal in physiological and pathological conditions. This review will focus on different mechanisms of platelet senescence and clearance with specific emphasis on the role of posttranslational modifications. It will also briefly address platelet transfusion and the role of glycans in the clearance of stored platelets.
Introduction
Although the primary function of platelets is hemostasis, platelets also participate in antimicrobial host defense, secrete cytokines that can induce inflammation, and growth factors that aid tissue repair. Chronic inflammation is often associated with reactive high platelet counts, and responses to acute infections may be accompanied by sudden reduction or increase of platelets (thrombocytopenia or thrombocytosis, respectively), placing platelets as reporters of disease progression or healing. A steady platelet supply is ensured by a continuous platelet clearance and production of 1011 platelets daily to maintain levels of 150 000 to 400 000 platelets per microliter of blood. Platelet clearance and production must therefore be regulated to avoid spontaneous bleeding or arterial occlusion and organ damage; however, both processes remain poorly understood. This review will focus on the current knowledge of platelet clearance and briefly address mechanisms of production.
Thrombopoiesis
A major milestone in understanding the molecular mechanisms of thrombopoiesis was the discovery of thrombopoietin (TPO), the primary regulator of thrombopoiesis, in 1994.1 TPO is the primary regulator of platelet production, supporting the survival, proliferation, and differentiation of the platelet precursors, the bone marrow (BM) megakaryocytes (MKs).2-4 Since the discovery of TPO, many molecular mechanisms of thrombopoiesis have been identified, including the development of polyploidy and proplatelet formation, the final fragmentation of the MK cytoplasm to yield blood platelets, and the regulation of this process.3,5-8
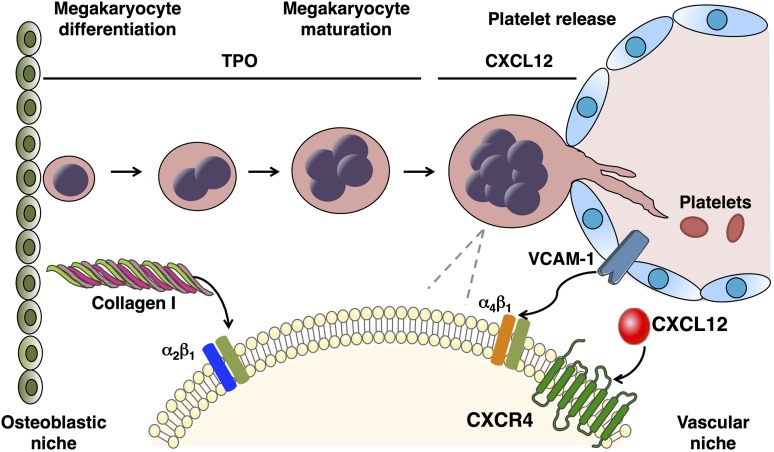
Platelet production is a complex process that requires differentiation of hematopoietic stem cells (HSCs) into specialized progenitors, and their organized interplay with the BM microenvironment and hematopoietic cytokines (Figure 1). Data support the existence of two anatomical and functional marrow microenvironmental “niches”: the osteoblastic niche and the vascular niche.9,10 MK maturation and platelet formation are dependent on cellular migration from the osteoblastic to the vascular niche, where once adequately mature, MKs extend pseudopodial projections, termed proplatelets, through or between cells of the sinusoidal endothelial layer and shed platelets into the bloodstream.11 Marrow stromal cells are an integral part of these local microenvironments through expression of soluble and surface-bound cytokines, counter-receptors for integrins and other adhesion molecules on the surface of hematopoietic cells, and the secretion of extracellular macromolecules.12
Figure 1.
Megakaryopoiesis and thrombopoiesis. HSCs reside in the BM osteoblastic niche and differentiate into MK progenitors and finally into mature polyploidy MKs. This process, called megakaryopoiesis, includes endomitotic cell cycles, leading to polyploidy and markedly enlarged cell size. TPO is the primary regulator of megakaryopoiesis, inducing differentiation and maturation of MKs. In the osteoblastic niche, collagen I inhibits platelet production through interaction with the MK integrin α2β1. Platelet production is dependent on MKs migration toward the vascular niche, where they interact with sinusoidal endothelial cells, produce long-branching transendothelial extensions called proplatelets, and release platelets into the circulation. Migration and localization of MKs in proximity to the BM sinusoids is regulated by several factors, among which the chemokine CXCL12 and its receptor CXCR4 that increase mobility of MK progenitors, facilitating their interaction with sinusoidal endothelial cells mediated by endothelial cell vascular cell adhesion molecule-1 and MK integrin α4β1.
In reference to MK development, BM stromal cells have been shown to secrete TPO and CXCL12 (also called stromal cell-derived factor 1), a primary chemokine that attracts MKs and other hematopoietic cells to the marrow microenvironment.13,14 Additionally, CXCL12 acts to stimulate MKs to express cell surface stem cell factor,15 which synergistically with TPO promotes MK growth,16 and to express vascular cell adhesion molecule-1 and fibronectin, which promote cell growth through their binding to the MK integrin α4β1.17,18 In addition to its effects on MK progenitors and mature cells, TPO affects HSCs, especially when used in combination with interleukin (IL)-3 or stem cell factor.19,20 HSCs express the TPO receptor, Mpl, on their surface, indicating that the stem cell effects of TPO are direct.21,22
The interaction of microenvironmental von Willebrand factor (VWF) and its MK receptor glycoprotein (GP) Ib-IX appears important for platelet formation and release, whereas in contrast, type I collagen, which localizes to the osteoblastic niche, prevents platelet formation.23 Recent studies point to the fact that glycans are key elements in hematopoiesis regulating HSC function and MK migration, specifically type 2 lactosaminoglycan (ie, LacNAc) synthesis by the β1,4-galactosyltransferase 1 (S.G. and K.M.H., unpublished data). Although much progress has been made toward the understanding of thrombopoiesis, multiple unanswered questions remain.
TPO regulation
One unanswered question is the regulation of TPO production under steady state and under pathological conditions. Multiple organs display TPO RNA transcripts, with hepatocytes having the highest levels and being the primary cells responsible for the production and secretion of TPO into the bloodstream (Figure 2). TPO production has long been thought to be constitutive, with TPO serum levels maintained solely by its uptake and metabolism by platelets and MKs.24-28 Circulating TPO levels are elevated in patients with congenital amegakaryocytic thrombocytopenia, caused by germline Mpl mutations,29,30 thrombocytopenia-absent radius syndrome,31 or acquired aplastic anemia.32,33 In these cases, circulating TPO levels are inversely correlated to platelet counts. Thus, the removal and destruction of TPO released into the bloodstream depends on the platelet and MK mass, and on expression of Mpl on the platelet and MK surface (Figure 2).
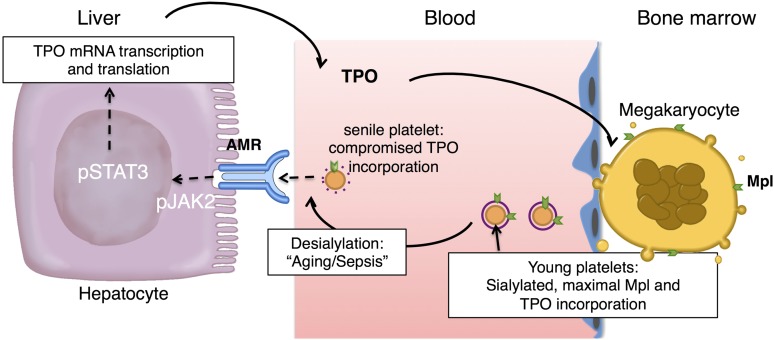
Figure 2.
Hepatic TPO production via JAK2-STAT3 signaling after desialylated platelet uptake by the AMR. Desialylated, senile platelets are recognized by the hepatic AMR to regulate hepatic TPO production, BM homeostasis, and thrombopoiesis. BM MKs produce and release young sialic acid (purple ring) containing platelets into the blood stream. Young platelets maximally internalize TPO through Mpl receptors. Circulating platelets become desialylated as they age by active blood-borne sialidases (dashed purple ring), become ligands for the AMR, and are ingested by hepatocytes. Desialylated platelet ingestion signaling positively stimulates hepatic TPO mRNA expression via activation of JAK2-STAT3 and TPO release into plasma, thereby regulating BM homeostasis and thrombopoiesis.
Support for this model also comes from mice generated to specifically lack in MKs and platelets Mpl or Mpl-regulatory proteins, ie, the Mpl-associated tyrosine kinase Janus kinase (JAK)2 and the large GTPase dynamin 2 (DNM2), which plays a critical role in MK demarcation membrane system formation and receptor-mediated endocytosis (eg, Mpl).6,34,35 Mplfl/fl Pf4-Cre mice and Jak2fl/fl Pf4-Cre mice displayed profound megakaryocytosis and thrombocytosis with a remarkable expansion of MK-committed and multipotential progenitor cells, the latter displaying biological responses and a gene expression signature indicative of chronic TPO overstimulation as the underlying causative mechanism.34,35 This is an intriguing finding as Mplfl/fl Pf4-Cre mice and Jak2fl/fl Pf4-Cre mice were obviously able to “bypass” the lack of Mpl and JAK2 in the MK lineage, respectively. These mice express Mpl and JAK2 normally in stem/progenitor cells. The studies conclude that TPO signaling in MKs is dispensable for platelet production, and that the key role of TPO signaling is in controlling platelet numbers via generation and stimulation of the bipotential MK precursors. On the other hand, Mpl expression in MKs and platelets is essential to prevent megakaryocytosis and myeloproliferation by restricting the amount of TPO available to stimulate the production of MKs from the progenitor cell pool. Even more surprising was the normal circulating TPO levels in these mice, presenting more evidence that circulating TPO levels are regulated in a complicated manner. Dnm2fl/fl Pf4-Cre mice have impaired Mpl-mediated endocytosis, resulting in elevated plasma TPO levels and constitutive phosphorylation of JAK2, although JAK2 expression is reduced in platelets lacking DNM2.6 Dnm2fl/fl Pf4-Cre mice develop MK hyperplasia, myelofibrosis, extramedullary hematopoiesis, and severe splenomegaly. However, Dnm2fl/fl Pf4-Cre mice develop macrothrombocytopenia, not thrombocytosis, as DNM2-dependent receptor-mediated endocytosis plays an additional critical role in the formation of the MK demarcation membrane system required for platelet formation. The low blood platelet numbers of Dnm2fl/fl Pf4-Cre mice and their inability to clear circulating TPO due to impaired Mpl-mediated endocytosis likely exacerbate their rapid and severe myelofibrosis.
A growing body of evidence lends credence to the assertion that platelet TPO metabolism is not the sole determinant of plasma TPO levels in humans. In contrast to the “autoregulation” model of blood TPO levels, serum TPO levels are lower than expected in patients with immune thrombocytopenia (ITP)36,37 and high in patients with essential thrombocythemia.38,39 In patients with thrombocytopenia, little of the hepatocyte-produced TPO is presumed to be removed by platelets and TPO blood levels rise. In contrast, thrombocytosis should be accompanied by low steady state levels of blood TPO, because platelet-mediated TPO destruction surpasses its production.24-28 However, Mpl expression levels on the membrane surface of platelets are strongly decreased in patients with essential thrombocythemia presenting the somatic JAK2 mutation V617F,40,41 which can explain the decreased TPO uptake and high circulation TPO levels.
The notion that TPO production is regulated, rather than autonomous, is further supported by data showing that marrow stromal cells produce TPO in response to thrombocytopenia in both mice and humans.1,42 Selective liver irradiation in mice stimulates hepatic TPO production.43 Further, a number of inflammatory states (eg, ulcerative colitis, rheumatoid arthritis, and ovarian cancer) are associated with increased blood TPO levels and thrombocytosis.1,38,44-51 This inflammation-induced increase in TPO expression is mediated by IL-6, which stimulates hepatic TPO messenger RNA (mRNA) expression and production both in hepatocytes in vivo and in hepatoma HepG2 and Hep3B cells in vitro.49,50,52,53 If hepatic TPO regulation by IL-6 is now well characterized, the ligand-receptor pair regulating hepatic TPO production at steady state has remained elusive. A new model detailed below furthers our understanding of the regulation of blood TPO levels and thrombopoiesis: desialylated, senescent platelet clearance via the hepatic Ashwell-Morrell receptor (AMR) enhances hepatic TPO production (Figure 2).
In summary, circulating TPO levels are regulated in a complicated manner by platelet and MK Mpl-mediated endocytosis and destruction, and hepatic TPO production, regulated by IL-6 and desialylated, senescent platelets. Further studies are required to determine the relative contribution of these regulatory mechanisms under physiological and pathological conditions.
Platelet clearance
Several mechanisms mediate platelet clearance. One mechanism appears to function via aging (senescence) induced signals, ie, via glycan degradation and apoptotic mechanisms. Platelets are also removed by immune (antibody mediated) responses.
Senescence-induced platelet clearance by the AMR
Studies have shown that platelet surface glycans mediate platelet clearance.54,55 Recently, loss of sialic acid has been identified as a determinant for the removal of senescent circulating platelets.56 Sialic acid loss is likely mediated by upregulation of platelet sialidases, ie, Neu1 and Neu3, expressed in granular compartments and on the plasma membrane, respectively.57 Platelet Neu1 and Neu3 have a preference for the sialic acid linkage found on glycans of the VWF receptor complex GPIbα subunit, exposing underlying galactose residues and priming GPIbα for metalloproteinase-mediated degradation during storage. Interestingly, platelet incubation with the sialidase inhibitor, 2-deoxy-2,3-dehydro-N-acetylneuraminic acid, enhances the recovery and survival of platelets stored at 4°C in mice.57 The sialidase inhibitor oseltamivir phosphate (Tamiflu), which is clinically used to treat influenza, has also been shown to increase platelet counts in 2 patients with ITP, as well as in 77 patients from the Erasmus Medical Center, Rotterdam, independently of influenza diagnosis.58-60 Clinical studies are required to determine whether preventing platelet desialylation by inhibition of platelet Neu1 and Neu3 with 2-deoxy-2,3-dehydro-N-acetylneuraminic acid and/or oseltamivir phosphate treatment improves platelet recovery and survival in transfusion settings.
St3gal4−/− mice lacking the α2,3-sialyltransferase IV (ST3GalIV) develop thrombocytopenia due to increased platelet clearance.61,62 Desialylated platelets, either senescent, treated with neuraminidase, or isolated from St3gal4−/− mice, are cleared by the hepatic AMR, a transmembrane heteroligomeric GP complex composed of two ASGPR1 (CLEC4H1, hepatic lectin 1) and one ASGPR2 (CLEC4H2, hepatic lectin 2) subunits. The hepatocyte AMR, originally termed the hepatic asialo-GP receptor, was the first cellular receptor to be identified and isolated, and the first lectin to be detected in mammals. It is one of the multiple lectins of the C-type family involved in recognition, binding, and clearance of asialo-GPs. This highly conserved receptor has been largely regarded as an endocytic receptor, and since its discovery 4 decades ago, the regulatory role of the hepatic AMR has remained largely unclear.63 Specifically, mice lacking either ASGPR1 or ASGPR2 do not accumulate plasma proteins or lipids lacking sialic acid, which has been the predicted outcome of eliminating one of the AMR subunits. It has therefore been a surprising discovery that platelets with reduced α2,3-linked sialic acid during sepsis, after cold storage (in vitro aging), or in mice lacking ST3GalIV, are cleared by the hepatic endocytic AMR.61,62,64,65
These findings subsequently led to the discovery that removal of senescent, sialic acid deprived platelets drives hepatic TPO mRNA expression in vivo and in vitro via JAK2 and signal transducer and activator of transcription (STAT)3 to increase MK numbers and de novo platelet production.56 The notion that the loss of sialic acid determines platelet lifespan is not novel,61,62,64,66-68 however, the recent study elucidates that aged, desialylated platelets regulate hepatic TPO mRNA production in vivo via the AMR. This feedback mechanism presents the AMR-desialylated platelet pair as an important control point for TPO homeostasis and shows that TPO expression in hepatocytes is regulated and not constitutive (Figure 2).
The AMR–IL-6R connection
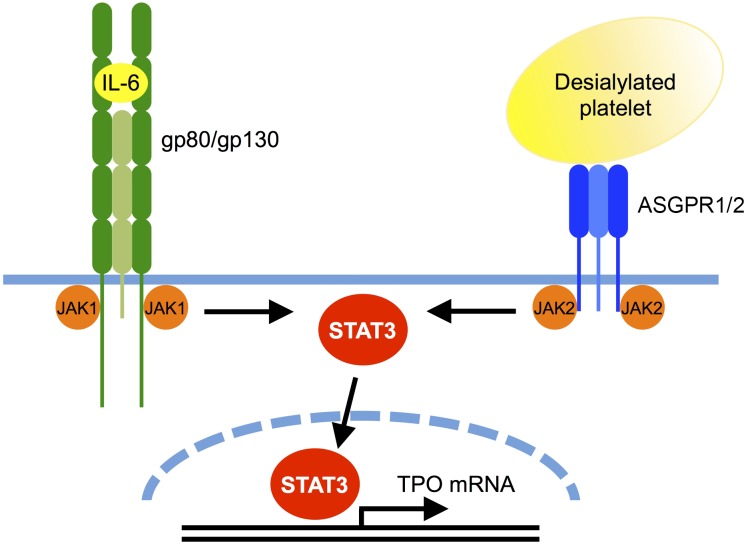
Interestingly, the AMR signaling cascade shares similarities with that of IL-6, as it involves JAK2 and STAT3 tyrosine phosphorylation, and STAT3 translocation to the nucleus (Figure 3).56,69 Binding of IL-6 to its hepatic receptor, IL-6R/gp80, engages the signal transducing subunit gp130, leading to STAT3 tyrosine phosphorylation and activation by gp130-associated JAK1, and to a lesser extent, JAK2. Thus, both desialylated platelets and IL-6 lead to STAT3-mediated hepatic TPO mRNA expression downstream of the AMR-JAK2 and IL-6R–JAK1 signaling cascades, respectively. Sequence analysis suggests that the TPO promoter region contains STAT3 binding sites. However, whether these are functional has not yet been investigated.
Figure 3.
Comparison between the AMR and IL-6R signaling pathways leading to TPO mRNA expression. Binding of desialylated platelets to the hepatic AMR composed of one ASGPR2 and two ASGPR1 subunits activates JAK2. IL-6 binding to its hepatic receptor composed of one gp80 and two gp130 subunits activates gp130-associated JAK1, and to a lesser extent JAK2. Both JAK1 and JAK2 phosphorylate STAT3, resulting in its translocation to the nucleus where it stimulates mRNA expression of TPO and acute phase response proteins. It is unclear whether JAK2 directly associates with ASGPR1 and whether STAT3 directly binds to the TPO promoter.
Importantly, the disruption of AMR-desialylated platelet signaling by the JAK1/2 inhibitors AZD1480, TG101348, and BMS911543 adversely affects hepatic TPO mRNA expression and secretion in mouse hepatocytes in vivo and in human HepG2 cells in vitro.56 Thrombocytopenia is a common adverse event of JAK1/2 inhibitor treatment, which is clinically used in myeloproliferative neoplasms.70,71 JAK1/2 inhibitors target hematopoietic stem and precursor cell mutant JAK2-V617F, as well as wild-type JAK2, the activation of which is essential for red blood cell and platelet production.72,73 This new study indicates that inhibition of TPO production downstream of the hepatic AMR-JAK2 signaling cascade could additionally contribute to the thrombocytopenia associated with JAK1/2 treatment. Clinical studies are necessary to investigate this notion, particularly to determine whether myeloproliferative neoplasm patients treated with JAK1/2 inhibitors have low circulating TPO levels.
Is there a crosstalk between the AMR and IL-6R? Whether JAK2 and STAT3 directly associate with the AMR or require gp130 remains to be determined. A tyrosine kinase of 127 kDa (possibly JAK2) constitutively associates with the AMR ASGPR1 subunit in HepG2 cells.74 It is therefore possible that both IL-6 and desialylated platelets lead to STAT3-mediated hepatic TPO mRNA expression downstream of JAK1 and JAK2. Hepatic STAT3 controls the transcription of mRNA for acute phase plasma proteins.75 Because both the AMR and IL-6R share signaling through STAT3, it is tempting to speculate that acute phase proteins are produced in response to AMR ligation, which would establish clearance of desialylated platelets as a component of the acute phase response. Consistent with this hypothesis, the AMR-mediated removal of desialylated platelets improves the probability of host survival during sepsis.62,65 The AMR modulates VWF homeostasis and is responsible for thrombocytopenia during systemic Streptococcus pneumoniae infection by eliminating platelets desialylated by the bacterium’s neuraminidase. It appears that hemostatic adaptation by the AMR moderates the onset and severity of disseminated intravascular coagulation during sepsis, and improves the probability of host survival. Separate studies have shown that liver regeneration following injury is promoted by platelets,76 and requires AMR and hepatic STAT3 function.77,78 Thus, the platelet-AMR-STAT3 signaling cascade may connect desialylated platelets to inflammatory responses.
Correcting platelet counts
Thrombocytopenia can arise from multiple factors including BM disorders, antineoplastic chemotherapy, or HSC transplantation, and is often prophylactically treated with platelet transfusion in the absence of actual bleeding. Platelet collection is performed in multiple ways, including platelet separation from individually donated whole blood or through apheresis procedures. In 1999, over 9 million platelets transfusions were performed.79 As with any therapy, platelet transfusions can be beneficial but are also accompanied by adverse reactions, such as fever, chills, rigor, and rarely life-threatening acute lung injury, which occurs in ∼0.3% of patients transfused with platelets.80,81 Allogenic platelet transfusions frequently result in the development of major histocompatibility complex-specific alloantibodies, which target platelets in subsequent transfusions and induce a state of refractoriness. Platelet refractoriness deprives the affected individual of the benefits (increase in platelet counts) expected after platelet transfusion. The role of the platelet ABO blood group system compatibility is also subject to discussions. ABO-incompatible platelet transfusions are associated with lower platelet count increments when compared with ABO-compatible platelet transfusions, which leads to decreased intervals between transfusions.82
Platelet storage
In vitro storage (in vitro induced senescence) of platelets for transfusion is also associated with changes in glycan composition.57 Specifically, platelets lose sialic acid during platelet storage. It is therefore tempting to speculate that transfusion of old, desialylated long-term stored platelets, although poorly functional, may have the benefit to stimulate hepatic TPO production.56 In support of this notion, Karpatkin et al demonstrated that injection of platelets desialylated by neuraminidase affected thrombopoiesis in rabbits.83 Early studies by Karpatkin et al concluded that asialo platelets stimulate MKs to produce more platelets. These studies suggested that the basal stimulus to thrombopoiesis might be regulated by desialylated “older” platelets, presumably by stimulating liver TPO secretion.
The cellular and molecular mechanism underlying the clearance of cold-stored platelets unraveled several “non-canonical” platelet glycan-lectin interactions. Cold-stored and room temperature platelets sequentially lose sialic acid and galactose (K.M.H., unpublished data), a process that leads to exposure of the underlying β-galactose and N-acetylglucosamine (GlcNAc), respectively. Cooling platelets furthers clustering of the platelet surface VWF receptor consisting of GPIbα, GPIbβ, GPIX, and GPV subunits, a process that may increase the density of N-linked glycans with terminal β-galacatose and βGlcNAc on GPIbα subunits. The cold storage induced an increase in β-galactose, and βGlcNAc density increases clearance by the hepatic AMR and the macrophage (Kupffer cell) αMβ2 integrin (also known as complement receptor 3, or macrophage-1 antigen, and CD11b), respectively (Figure 4).64,84 Beta-galactosidase expression and activity increases on platelet surfaces upon storage (K.M.H., unpublished data) presumably mediating surface galactose residue cleavage. Integrin αM is one protein subunit that forms the heterodimeric integrin αMβ2. The second chain of αMβ2 is the common integrin β2 subunit (CD18) and αMβ2 is expressed on the surface of many leukocytes involved in the innate immune system. The αM domain contains a cation-dependent ligand binding I-domain, which mediates inflammation by regulating leukocyte adhesion and migration, and has been implicated in several immune processes such as phagocytosis, cell-mediated cytotoxicity, chemotaxis, and cellular activation. It is involved in the complement system due to its capacity to bind inactivated complement component 3b (iC3b) and the αMβ2 integrin serves as a phagocytic receptor for the iC3b fragment of complement.85-87 The αM domain also binds to platelet GPIbα. Inhibition studies with monoclonal antibodies or receptor ligands showed that the interaction involves the macrophage-1 antigen domain (homologous to the VWF A1 domain), and the GPIbα leucine-rich repeat and carboxyl-terminal flanking regions. These observations provide a molecular target for disrupting leukocyte-platelet complexes that promote vascular inflammation in thrombosis, atherosclerosis.88
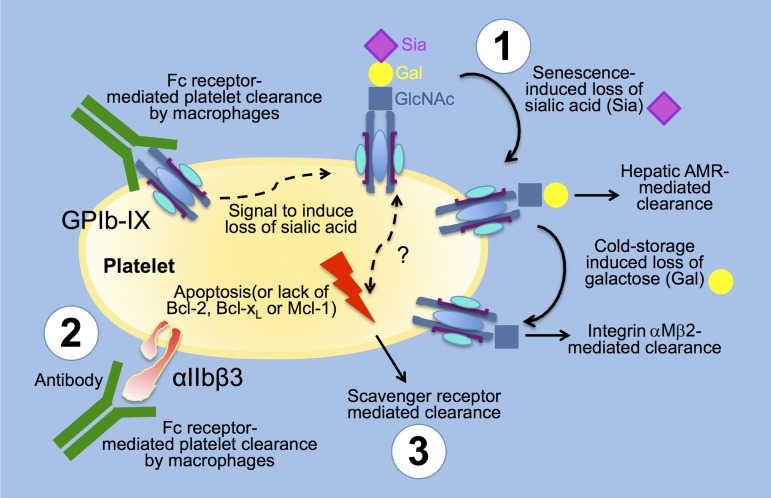
Figure 4.
Platelet clearance mechanisms. (1) Glycan-lectin mediated clearance: platelets lose Sia as they age, leading to their clearance by the C-type lectin AMR on hepatocytes. Stored platelets additionally lose Gal leading to exposure of GlcNAc and their clearance by the αMβ2 integrin on hepatic macrophages (Kupffer cells). (2) Autoantibody Fc receptor and lectin-mediated clearance: platelet clearance is mediated by autoreactive antibodies toward the integrin αIIbβ3 and the GP GPIbα as part of the von Willebrand receptor complex (GPIb-IX). Platelets are cleared via Fc receptors on macrophages and CD8+ cytotoxic T lymphocytes. Specific anti-GPIbα but not anti-αIIbβ3 antibodies induce platelet desialylation, thereby diverging platelet clearance to hepatic AMRs. (3) Programmed cell death (apoptosis) mediated clearance: platelet survival also depends on the interplay between prosurvival and proapoptotic members of the Bcl-2 family, which are critical regulators of the intrinsic apoptotic pathway. Platelet clearance via scavenger receptors is accelerated in mice lacking the prosurvival proteins Bcl-2, Bcl-xL, and Mcl-1. It is unclear if the apoptotic and glycan-lectin–mediated clearance converge at a certain stage of platelet “death” to induce platelet clearance. Gal, galactose; Sia, sialic acid.
The αM domain also contains a cation-independent lectin-site,86,89 which recognizes and binds GlcNAc on cold-stored platelets,84,90,91 and microbial surface polysaccharides and β-glucan.92 The carbohydrate-binding integrin αM selectively recognizes GlcNAc on clustered GPIbα subunits of the VWF receptor following short-term (hours) platelet cooling leading to phagocytosis and clearance of platelets independently of iC3.84,90,91 Although galactosylation of platelet GlcNAc residues with uridine 5′-diphosphogalactose normalizes the survival of short-term chilled isolated mouse platelets,93 the treatment failed to prevent the clearance of transfused autologous apheresis platelets stored for 48 hours at 4°C in a phase 1 feasibility study.94 In agreement with the human platelet study, uridine 5′-diphosphogalactose treatment of murine platelets stored for 48 hours at 4°C also did not prevent their rapid clearance, showing that different mechanisms of clearance exist for short- and long-term cold-stored platelets.94 Without subsequent sialylation, transfused galactosylated platelets are likely cleared by the hepatic AMR. It is noteworthy that in vitro stored platelets are cleared by receptors expressed in the liver (ie, the hepatocyte AMR and the Kupffer cell αMβ2 integrin), and not by the spleen, independently of increase of apoptotic markers.
Apoptosis as a platelet clearance mechanism
Platelet survival also depends on the interplay between prosurvival and proapoptotic members of the Bcl-2 family, which are critical regulators of the intrinsic apoptotic pathway (Figure 4). Platelet survival is extended in mice lacking the proapoptotic proteins Bak and Bax, whereas platelet clearance is accelerated in mice lacking the prosurvival proteins Bcl-2, Bcl-xL, and Mcl-1, and in mice treated with the BH3 mimetic ABT-737 (inhibitor of prosurvival Bcl-2, Bcl-xL, and Bcl-w).95-97 Whether members of the Bcl-2 family alter platelet surface sialic acid content or whether these mechanisms converge at certain points during platelet lifetime is unclear. Interestingly, the primary platelet clearance site following administration of ABT-737 is the liver in dogs, presumably via scavenger receptors,98 whereas the spleen does not appear to regulate the platelet lifespan in mice.97 More data are needed to establish if glycan degradation in vivo (ie, sialic acid loss), triggers the intrinsic apoptotic machinery in platelets, linking glycan degradation and intrinsic apoptotic machinery in the clearance mechanisms regulating platelet survival. Interestingly, data show that newborn and adult mice have similar platelet production rates, but neonatal platelets survived 1 day longer in circulation.99 A study of proapoptotic and antiapoptotic Bcl-2 family proteins shows that neonatal platelets have higher levels of the antiapoptotic protein Bcl-2 and are more resistant to apoptosis induced by the Bcl-2/Bcl-xL inhibitor ABT-737 than adult platelets. However, genetic ablation or pharmacologic inhibition of Bcl-2 alone does not shorten neonatal platelet survival or reduce platelet counts in newborn mice, indicating the existence of redundant or alternative mechanisms mediating the prolonged lifespan of neonatal platelets.99 Whether glycans (increase in platelet surface sialic acid) play a role in the prolonged survival of neonatal platelets remains to be established.
Immune platelet clearance
ITP is a common bleeding disorder caused primarily by platelet autoantibodies that accelerate platelet destruction, alter platelet function, and/or inhibit platelet production.100 These autoantibodies are mainly directed against the two most abundant platelet GPs, ie, the integrin αIIbβ3 (GPIIb-IIIa) and/or the GPIb-IX complex (Figure 4). The prevailing model posits that antibody mediated platelet destruction occurs in the spleen,101 where the interaction between the Fc portion of platelet-associated immunoglobulin G antibodies and Fcγ receptors (FcγRs) on macrophages initiates phagocytosis. However, data show that, in contrast to anti–αIIbβ3-mediated ITP, anti–GPIbα-mediated ITP is often refractory to therapies targeting FcγR pathways or splenectomy. Recent findings show that certain anti-GPIbα antibodies trigger platelet desialylation, a process that deviates platelet clearance from splenic macrophage Fc receptors to the hepatic AMR, showing that FcγR-independent mechanisms of ITP exist.102,103 The mechanisms of how anti–GPIbα-antibody binding leads to desialylation remain to be established. It is likely that platelets secrete active Neu1 and Neu3 upon antibody binding and/or platelet activation.57 The notion that the AMR plays a significant role in the clearance of anti–GPIbα-opsonized and desialylated platelets provides a potential explanation for refractoriness to splenectomy, as well as to steroid and intravenous immunoglobulin therapies. Recent data also show that platelet destruction in ITP patients is mediated by CD8+ cytotoxic T lymphocytes.104
Conclusion
Platelet counts are controlled in a multifaceted, complex manner. Recent evidence shows that the AMR recognizes senescent, desialylated platelets under steady state conditions. Desialylated platelets and the AMR are the physiological ligand-receptor pair regulating hepatic TPO mRNA production, as the AMR-mediated removal of desialylated platelets regulates TPO synthesis in the liver by recruiting JAK2 and STAT3 to increase thrombopoiesis. Senescent platelets are also removed from the circulation by apoptotic signals. Platelets are cleared by antibody binding to platelets via macrophage FcγRs during pathological conditions. Recent findings suggest that anti-GPIbα antibodies can induce platelet desialylation, thereby converging signals for platelet removal with immune-mediated platelet removal. Many questions remain concerning the mechanisms governing platelet numbers. How do the above processes work together to maintain platelet numbers? Do clearance systems communicate with the BM environment to ensure adequate thrombopoiesis? Further studies will continue to elucidate the mechanisms regulating the 1011 platelets that are produced and cleared daily.
Acknowledgments
This study was supported by US National Institutes of Health, National Heart, Lung, and Blood Institute grant R01 HL089224 and the Program of Excellence in Glycosciences (P01 HL107146) (K.M.H.), and the Brigham Research Institute and American Society of Hematology bridge grant awards (H.F.).
Authorship
Contribution: K.M.H. and H.F. drafted the manuscript; and R.G. and S.G. assisted with writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karin M. Hoffmeister, Division of Hematology, Brigham and Women’s Hospital, One Blackfan Circle, Karp 6, Boston, MA 02115; e-mail: khoffmeister@bwh.harvard.edu; and Hervé Falet, Division of Hematology, Brigham and Women’s Hospital, One Blackfan Circle, Karp 6, Boston, MA 02115; e-mail: hfalet@bwh.harvard.edu.
References
- 1.McCarty JM, Sprugel KH, Fox NE, Sabath DE, Kaushansky K. Murine thrombopoietin mRNA levels are modulated by platelet count. Blood. 1995;86(10):3668–3675. [PubMed] [Google Scholar]
- 2.Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115(12):3339–3347. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaushansky K. Determinants of platelet number and regulation of thrombopoiesis. Hematology Am Soc Hematol Educ Program. 2009;2009:147–152. doi: 10.1182/asheducation-2009.1.147. [DOI] [PubMed] [Google Scholar]
- 4.Kuter DJ. Thrombopoietin and thrombopoietin mimetics in the treatment of thrombocytopenia. Annu Rev Med. 2009;60:193–206. doi: 10.1146/annurev.med.60.042307.181154. [DOI] [PubMed] [Google Scholar]
- 5.Machlus KR, Thon JN, Italiano JE., Jr Interpreting the developmental dance of the megakaryocyte: a review of the cellular and molecular processes mediating platelet formation. Br J Haematol. 2014;165(2):227–236. doi: 10.1111/bjh.12758. [DOI] [PubMed] [Google Scholar]
- 6.Bender M, Giannini S, Grozovsky R, et al. Dynamin 2-dependent endocytosis is required for normal megakaryocyte development in mice. Blood. 2015;125(6):1014–1024. doi: 10.1182/blood-2014-07-587857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Orban M, Lorenz M, et al. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J Exp Med. 2012;209(12):2165–2181. doi: 10.1084/jem.20121090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaushansky K. Thrombopoiesis. Semin Hematol. 2015;52(1):4–11. doi: 10.1053/j.seminhematol.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 10.Kiel MJ, Morrison SJ. Maintaining hematopoietic stem cells in the vascular niche. Immunity. 2006;25(6):862–864. doi: 10.1016/j.immuni.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Junt T, Schulze H, Chen Z, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317(5845):1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 12.Malara A, Currao M, Gruppi C, et al. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen, and laminin. Stem Cells. 2014;32(4):926–937. doi: 10.1002/stem.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodohara K, Fujii N, Yamamoto N, Kaushansky K. Stromal cell-derived factor-1 (SDF-1) acts together with thrombopoietin to enhance the development of megakaryocytic progenitor cells (CFU-MK). Blood. 2000;95(3):769–775. [PubMed] [Google Scholar]
- 14.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90(4):1345–1364. [PubMed] [Google Scholar]
- 16.Broudy VC, Lin NL, Kaushansky K. Thrombopoietin (c-mpl ligand) acts synergistically with erythropoietin, stem cell factor, and interleukin-11 to enhance murine megakaryocyte colony growth and increases megakaryocyte ploidy in vitro. Blood. 1995;85(7):1719–1726. [PubMed] [Google Scholar]
- 17.Avraham H, Cowley S, Chi SY, Jiang S, Groopman JE. Characterization of adhesive interactions between human endothelial cells and megakaryocytes. J Clin Invest. 1993;91(6):2378–2384. doi: 10.1172/JCI116470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox NE, Kaushansky K. Engagement of integrin alpha4beta1 enhances thrombopoietin-induced megakaryopoiesis. Exp Hematol. 2005;33(1):94–99. doi: 10.1016/j.exphem.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Ku H, Yonemura Y, Kaushansky K, Ogawa M. Thrombopoietin, the ligand for the Mpl receptor, synergizes with steel factor and other early acting cytokines in supporting proliferation of primitive hematopoietic progenitors of mice. Blood. 1996;87(11):4544–4551. [PubMed] [Google Scholar]
- 20.Sitnicka E, Lin N, Priestley GV, et al. The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood. 1996;87(12):4998–5005. [PubMed] [Google Scholar]
- 21.Qian H, Buza-Vidas N, Hyland CD, et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1(6):671–684. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Arai F, Yoshihara H, Hosokawa K, et al. Niche regulation of hematopoietic stem cells in the endosteum. Ann N Y Acad Sci. 2009;1176:36–46. doi: 10.1111/j.1749-6632.2009.04561.x. [DOI] [PubMed] [Google Scholar]
- 23.Balduini A, Pallotta I, Malara A, et al. Adhesive receptors, extracellular proteins and myosin IIA orchestrate proplatelet formation by human megakaryocytes. J Thromb Haemost. 2008;6(11):1900–1907. doi: 10.1111/j.1538-7836.2008.03132.x. [DOI] [PubMed] [Google Scholar]
- 24.Kuter DJ, Rosenberg RD. The reciprocal relationship of thrombopoietin (c-Mpl ligand) to changes in the platelet mass during busulfan-induced thrombocytopenia in the rabbit. Blood. 1995;85(10):2720–2730. [PubMed] [Google Scholar]
- 25.Cohen-Solal K, Villeval JL, Titeux M, Lok S, Vainchenker W, Wendling F. Constitutive expression of Mpl ligand transcripts during thrombocytopenia or thrombocytosis. Blood. 1996;88(7):2578–2584. [PubMed] [Google Scholar]
- 26.Fielder PJ, Gurney AL, Stefanich E, et al. Regulation of thrombopoietin levels by c-mpl-mediated binding to platelets. Blood. 1996;87(6):2154–2161. [PubMed] [Google Scholar]
- 27.Shinjo K, Takeshita A, Nakamura S, et al. Serum thrombopoietin levels in patients correlate inversely with platelet counts during chemotherapy-induced thrombocytopenia. Leukemia. 1998;12(3):295–300. doi: 10.1038/sj.leu.2400946. [DOI] [PubMed] [Google Scholar]
- 28.Engel C, Loeffler M, Franke H, Schmitz S. Endogenous thrombopoietin serum levels during multicycle chemotherapy. Br J Haematol. 1999;105(3):832–838. doi: 10.1046/j.1365-2141.1999.01459.x. [DOI] [PubMed] [Google Scholar]
- 29.Ihara K, Ishii E, Eguchi M, et al. Identification of mutations in the c-mpl gene in congenital amegakaryocytic thrombocytopenia. Proc Natl Acad Sci USA. 1999;96(6):3132–3136. doi: 10.1073/pnas.96.6.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballmaier M, Germeshausen M, Schulze H, et al. c-mpl mutations are the cause of congenital amegakaryocytic thrombocytopenia. Blood. 2001;97(1):139–146. doi: 10.1182/blood.v97.1.139. [DOI] [PubMed] [Google Scholar]
- 31.Ballmaier M, Schulze H, Strauss G, et al. Thrombopoietin in patients with congenital thrombocytopenia and absent radii: elevated serum levels, normal receptor expression, but defective reactivity to thrombopoietin. Blood. 1997;90(2):612–619. [PubMed] [Google Scholar]
- 32.Schrezenmeier H, Griesshammer M, Hornkohl A, et al. Thrombopoietin serum levels in patients with aplastic anaemia: correlation with platelet count and persistent elevation in remission. Br J Haematol. 1998;100(3):571–576. doi: 10.1046/j.1365-2141.1998.00590.x. [DOI] [PubMed] [Google Scholar]
- 33.Gu J, Lu L, Xu R, Chen X. Plasma thrombopoietin levels in patients with aplastic anemia and idiopathic thrombocytopenic purpura. Chin Med J (Engl) 2002;115(7):983–986. [PubMed] [Google Scholar]
- 34.Ng AP, Kauppi M, Metcalf D, et al. Mpl expression on megakaryocytes and platelets is dispensable for thrombopoiesis but essential to prevent myeloproliferation. Proc Natl Acad Sci USA. 2014;111(16):5884–5889. doi: 10.1073/pnas.1404354111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer SC, Keller MD, Woods BA, et al. Genetic studies reveal an unexpected negative regulatory role for Jak2 in thrombopoiesis. Blood. 2014;124(14):2280–2284. doi: 10.1182/blood-2014-03-560441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kosugi S, Kurata Y, Tomiyama Y, et al. Circulating thrombopoietin level in chronic immune thrombocytopenic purpura. Br J Haematol. 1996;93(3):704–706. doi: 10.1046/j.1365-2141.1996.d01-1702.x. [DOI] [PubMed] [Google Scholar]
- 37.Ichikawa N, Ishida F, Shimodaira S, Tahara T, Kato T, Kitano K. Regulation of serum thrombopoietin levels by platelets and megakaryocytes in patients with aplastic anaemia and idiopathic thrombocytopenic purpura. Thromb Haemost. 1996;76(2):156–160. [PubMed] [Google Scholar]
- 38.Wang JC, Chen C, Novetsky AD, Lichter SM, Ahmed F, Friedberg NM. Blood thrombopoietin levels in clonal thrombocytosis and reactive thrombocytosis. Am J Med. 1998;104(5):451–455. doi: 10.1016/s0002-9343(98)00090-4. [DOI] [PubMed] [Google Scholar]
- 39.Griesshammer M, Hornkohl A, Nichol JL, et al. High levels of thrombopoietin in sera of patients with essential thrombocythemia: cause or consequence of abnormal platelet production? Ann Hematol. 1998;77(5):211–215. doi: 10.1007/s002770050445. [DOI] [PubMed] [Google Scholar]
- 40.Besancenot R, Chaligné R, Tonetti C, et al. A senescence-like cell-cycle arrest occurs during megakaryocytic maturation: implications for physiological and pathological megakaryocytic proliferation. PLoS Biol. 2010;8(9):e1000476. doi: 10.1371/journal.pbio.1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pecquet C, Diaconu CC, Staerk J, et al. Thrombopoietin receptor down-modulation by JAK2 V617F: restoration of receptor levels by inhibitors of pathologic JAK2 signaling and of proteasomes. Blood. 2012;119(20):4625–4635. doi: 10.1182/blood-2011-08-372524. [DOI] [PubMed] [Google Scholar]
- 42.Sungaran R, Chisholm OT, Markovic B, Khachigian LM, Tanaka Y, Chong BH. The role of platelet alpha-granular proteins in the regulation of thrombopoietin messenger RNA expression in human bone marrow stromal cells. Blood. 2000;95(10):3094–3101. [PubMed] [Google Scholar]
- 43.Mouthon MA, Vandamme M, Gourmelon P, Vainchenker W, Wendling F. Preferential liver irradiation enhances hematopoiesis through a thrombopoietin-independent mechanism. Radiat Res. 1999;152(4):390–397. [PubMed] [Google Scholar]
- 44.Sungaran R, Markovic B, Chong BH. Localization and regulation of thrombopoietin mRNa expression in human kidney, liver, bone marrow, and spleen using in situ hybridization. Blood. 1997;89(1):101–107. [PubMed] [Google Scholar]
- 45.Qian S, Fu F, Li W, Chen Q, de Sauvage FJ. Primary role of the liver in thrombopoietin production shown by tissue-specific knockout. Blood. 1998;92(6):2189–2191. [PubMed] [Google Scholar]
- 46.Wolber EM, Ganschow R, Burdelski M, Jelkmann W. Hepatic thrombopoietin mRNA levels in acute and chronic liver failure of childhood. Hepatology. 1999;29(6):1739–1742. doi: 10.1002/hep.510290627. [DOI] [PubMed] [Google Scholar]
- 47.McIntosh B, Kaushansky K. Transcriptional regulation of bone marrow thrombopoietin by platelet proteins. Exp Hematol. 2008;36(7):799–806. doi: 10.1016/j.exphem.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolber EM, Fandrey J, Frackowski U, Jelkmann W. Hepatic thrombopoietin mRNA is increased in acute inflammation. Thromb Haemost. 2001;86(6):1421–1424. [PubMed] [Google Scholar]
- 49.Kaser A, Brandacher G, Steurer W, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98(9):2720–2725. doi: 10.1182/blood.v98.9.2720. [DOI] [PubMed] [Google Scholar]
- 50.Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer [published correction appears in N Engl J Med. 2012;367(18):1768]. N Engl J Med. 2012;366(7):610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cerutti A, Custodi P, Duranti M, Noris P, Balduini CL. Thrombopoietin levels in patients with primary and reactive thrombocytosis. Br J Haematol. 1997;99(2):281–284. doi: 10.1046/j.1365-2141.1997.3823196.x. [DOI] [PubMed] [Google Scholar]
- 52.Wolber EM, Jelkmann W. Interleukin-6 increases thrombopoietin production in human hepatoma cells HepG2 and Hep3B. J Interferon Cytokine Res. 2000;20(5):499–506. doi: 10.1089/10799900050023915. [DOI] [PubMed] [Google Scholar]
- 53.Burmester H, Wolber EM, Freitag P, Fandrey J, Jelkmann W. Thrombopoietin production in wild-type and interleukin-6 knockout mice with acute inflammation. J Interferon Cytokine Res. 2005;25(7):407–413. doi: 10.1089/jir.2005.25.407. [DOI] [PubMed] [Google Scholar]
- 54.Rumjantseva V, Hoffmeister KM. Novel and unexpected clearance mechanisms for cold platelets. Transfus Apheresis Sci. 2010;42(1):63–70. doi: 10.1016/j.transci.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoffmeister KM. The role of lectins and glycans in platelet clearance. J Thromb Haemost. 2011;9(suppl 1):35–43. doi: 10.1111/j.1538-7836.2011.04276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grozovsky R, Begonja AJ, Liu K, et al. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat Med. 2015;21(1):47–54. doi: 10.1038/nm.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jansen AJ, Josefsson EC, Rumjantseva V, et al. Desialylation accelerates platelet clearance after refrigeration and initiates GPIbα metalloproteinase-mediated cleavage in mice. Blood. 2012;119(5):1263–1273. doi: 10.1182/blood-2011-05-355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alioglu B, Tasar A, Ozen C, Selver B, Dallar Y. An experience of oseltamivir phosphate (tamiflu™) in a pediatric patient with chronic idiopathic thrombocytopenic purpura: a case report. Pathophysiol Haemost Thromb. 2010;37(2-4):55–58. doi: 10.1159/000321379. [DOI] [PubMed] [Google Scholar]
- 59.Shao L, Wu Y, Zhou H, et al. Successful treatment with oseltamivir phosphate in a patient with chronic immune thrombocytopenia positive for anti-GPIb/IX autoantibody. Platelets. 2015;26(5):495–497. doi: 10.3109/09537104.2014.948838. [DOI] [PubMed] [Google Scholar]
- 60.Jansen AJ, Peng J, Zhao HG, Hou M, Ni H. Sialidase inhibition to increase platelet counts: a new treatment option for thrombocytopenia. Am J Hematol. 2015;90(5):E94–E95. doi: 10.1002/ajh.23953. [DOI] [PubMed] [Google Scholar]
- 61.Sørensen AL, Rumjantseva V, Nayeb-Hashemi S, et al. Role of sialic acid for platelet life span: exposure of beta-galactose results in the rapid clearance of platelets from the circulation by asialoglycoprotein receptor-expressing liver macrophages and hepatocytes. Blood. 2009;114(8):1645–1654. doi: 10.1182/blood-2009-01-199414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grewal PK, Uchiyama S, Ditto D, et al. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med. 2008;14(6):648–655. doi: 10.1038/nm1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grewal PK. The Ashwell-Morell receptor. Methods Enzymol. 2010;479:223–241. doi: 10.1016/S0076-6879(10)79013-3. [DOI] [PubMed] [Google Scholar]
- 64.Rumjantseva V, Grewal PK, Wandall HH, et al. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat Med. 2009;15(11):1273–1280. doi: 10.1038/nm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grewal PK, Aziz PV, Uchiyama S, et al. Inducing host protection in pneumococcal sepsis by preactivation of the Ashwell-Morell receptor. Proc Natl Acad Sci USA. 2013;110(50):20218–20223. doi: 10.1073/pnas.1313905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crook M. Platelet sialic acid and its significance to platelet life-spans. Platelets. 1990;1(3):167. doi: 10.3109/09537109009005484. [DOI] [PubMed] [Google Scholar]
- 67.Crook M. Sialic acid: its importance to platelet function in health and disease. Platelets. 1991;2(1):1–10. doi: 10.3109/09537109109005496. [DOI] [PubMed] [Google Scholar]
- 68.Reimers HJ, Greenberg J, Cazenave JP, Packham MA, Mustard JF. Experimental modification of platelet survival. Adv Exp Med Biol. 1977;82:231–233. doi: 10.1007/978-1-4613-4220-5_48. [DOI] [PubMed] [Google Scholar]
- 69.Eulenfeld R, Dittrich A, Khouri C, et al. Interleukin-6 signalling: more than Jaks and STATs. Eur J Cell Biol. 2012;91(6-7):486–495. doi: 10.1016/j.ejcb.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 70.Levine RL, Gilliland DG. Myeloproliferative disorders. Blood. 2008;112(6):2190–2198. doi: 10.1182/blood-2008-03-077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.LaFave LM, Levine RL. JAK2 the future: therapeutic strategies for JAK-dependent malignancies. Trends Pharmacol Sci. 2012;33(11):574–582. doi: 10.1016/j.tips.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 72.Park SO, Wamsley HL, Bae K, et al. Conditional deletion of Jak2 reveals an essential role in hematopoiesis throughout mouse ontogeny: implications for Jak2 inhibition in humans. PLoS One. 2013;8(3):e59675. doi: 10.1371/journal.pone.0059675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grisouard J, Hao-Shen H, Dirnhofer S, Wagner KU, Skoda RC. Selective deletion of Jak2 in adult mouse hematopoietic cells leads to lethal anemia and thrombocytopenia. Haematologica. 2014;99(4):e52–e54. doi: 10.3324/haematol.2013.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fallon RJ, Danaher M, Saxena A. The asialoglycoprotein receptor is associated with a tyrosine kinase in HepG2 cells. J Biol Chem. 1994;269(43):26626–26629. [PubMed] [Google Scholar]
- 75.Alonzi T, Maritano D, Gorgoni B, Rizzuto G, Libert C, Poli V. Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene inactivation [correction of activation] in the liver. Mol Cell Biol. 2001;21(5):1621–1632. doi: 10.1128/MCB.21.5.1621-1632.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lesurtel M, Graf R, Aleil B, et al. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312(5770):104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 77.Moh A, Iwamoto Y, Chai GX, et al. Role of STAT3 in liver regeneration: survival, DNA synthesis, inflammatory reaction and liver mass recovery. Lab Invest. 2007;87(10):1018–1028. doi: 10.1038/labinvest.3700630. [DOI] [PubMed] [Google Scholar]
- 78.Dalton SR, Lee SM, King RN, et al. Carbon tetrachloride-induced liver damage in asialoglycoprotein receptor-deficient mice. Biochem Pharmacol. 2009;77(7):1283–1290. doi: 10.1016/j.bcp.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sullivan MT, Wallace EL. Blood collection and transfusion in the United States in 1999. Transfusion. 2005;45(2):141–148. doi: 10.1111/j.1537-2995.2004.03288.x. [DOI] [PubMed] [Google Scholar]
- 80.Heddle NM, Klama L, Frassetto R, O’Hoski P, Leaman B. A retrospective study to determine the risk of red cell alloimmunization and transfusion during pregnancy. Transfusion. 1993;33(3):217–220. doi: 10.1046/j.1537-2995.1993.33393174447.x. [DOI] [PubMed] [Google Scholar]
- 81.Silliman CC, Boshkov LK, Mehdizadehkashi Z, et al. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood. 2003;101(2):454–462. doi: 10.1182/blood-2002-03-0958. [DOI] [PubMed] [Google Scholar]
- 82.Dunbar NM, Ornstein DL, Dumont LJ. ABO incompatible platelets: risks versus benefit. Curr Opin Hematol. 2012;19(6):475–479. doi: 10.1097/MOH.0b013e328358b135. [DOI] [PubMed] [Google Scholar]
- 83.Karpatkin S, Shulman S. Asialo platelets enhance thrombopoiesis. Trans Assoc Am Physicians. 1980;93:244–250. [PubMed] [Google Scholar]
- 84.Hoffmeister KM, Felbinger TW, Falet H, et al. The clearance mechanism of chilled blood platelets. Cell. 2003;112(1):87–97. doi: 10.1016/s0092-8674(02)01253-9. [DOI] [PubMed] [Google Scholar]
- 85.Petty HR, Todd RF., III Receptor-receptor interactions of complement receptor type 3 in neutrophil membranes. J Leukoc Biol. 1993;54(5):492–494. doi: 10.1002/jlb.54.5.492. [DOI] [PubMed] [Google Scholar]
- 86.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 87.Xia Y, Borland G, Huang J, et al. Function of the lectin domain of Mac-1/complement receptor type 3 (CD11b/CD18) in regulating neutrophil adhesion. J Immunol. 2002;169(11):6417–6426. doi: 10.4049/jimmunol.169.11.6417. [DOI] [PubMed] [Google Scholar]
- 88.Simon DI, Chen Z, Xu H, et al. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J Exp Med. 2000;192(2):193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thornton BP, Vĕtvicka V, Pitman M, Goldman RC, Ross GD. Analysis of the sugar specificity and molecular location of the β-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J Immunol. 1996;156(3):1235–1246. [PubMed] [Google Scholar]
- 90.Josefsson EC, Gebhard HH, Stossel TP, Hartwig JH, Hoffmeister KM. The macrophage alphaMbeta2 integrin alphaM lectin domain mediates the phagocytosis of chilled platelets. J Biol Chem. 2005;280(18):18025–18032. doi: 10.1074/jbc.M501178200. [DOI] [PubMed] [Google Scholar]
- 91.Badlou BA, Spierenburg G, Ulrichts H, Deckmyn H, Smid WM, Akkerman JW. Role of glycoprotein Ibalpha in phagocytosis of platelets by macrophages. Transfusion. 2006;46(12):2090–2099. doi: 10.1111/j.1537-2995.2006.01034.x. [DOI] [PubMed] [Google Scholar]
- 92.Ross GD. Role of the lectin domain of Mac-1/CR3 (CD11b/CD18) in regulating intercellular adhesion. Immunol Res. 2002;25(3):219–227. doi: 10.1385/IR:25:3:219. [DOI] [PubMed] [Google Scholar]
- 93.Hoffmeister KM, Josefsson EC, Isaac NA, Clausen H, Hartwig JH, Stossel TP. Glycosylation restores survival of chilled blood platelets. Science. 2003;301(5639):1531–1534. doi: 10.1126/science.1085322. [DOI] [PubMed] [Google Scholar]
- 94.Wandall HH, Hoffmeister KM, Sørensen AL, et al. Galactosylation does not prevent the rapid clearance of long-term, 4 degrees C-stored platelets. Blood. 2008;111(6):3249–3256. doi: 10.1182/blood-2007-06-097295. [DOI] [PubMed] [Google Scholar]
- 95.Mason KD, Carpinelli MR, Fletcher JI, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128(6):1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 96.Debrincat MA, Josefsson EC, James C, et al. Mcl-1 and Bcl-x(L) coordinately regulate megakaryocyte survival. Blood. 2012;119(24):5850–5858. doi: 10.1182/blood-2011-12-398834. [DOI] [PubMed] [Google Scholar]
- 97.Josefsson EC, James C, Henley KJ, et al. Megakaryocytes possess a functional intrinsic apoptosis pathway that must be restrained to survive and produce platelets. J Exp Med. 2011;208(10):2017–2031. doi: 10.1084/jem.20110750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang H, Nimmer PM, Tahir SK, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007;14(5):943–951. doi: 10.1038/sj.cdd.4402081. [DOI] [PubMed] [Google Scholar]
- 99.Liu ZJ, Hoffmeister KM, Hu Z, et al. Expansion of the neonatal platelet mass is achieved via an extension of platelet lifespan. Blood. 2014;123(22):3381–3389. doi: 10.1182/blood-2013-06-508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113(26):6511–6521. doi: 10.1182/blood-2009-01-129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McMillan R. The pathogenesis of chronic immune thrombocytopenic purpura. Semin Hematol. 2007;44(4 suppl 5):S3–S11. doi: 10.1053/j.seminhematol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 102.Li J, Callum JL, Lin Y, Zhou Y, Zhu G, Ni H. Severe platelet desialylation in a patient with glycoprotein Ib/IX antibody-mediated immune thrombocytopenia and fatal pulmonary hemorrhage. Haematologica. 2014;99(4):e61–e63. doi: 10.3324/haematol.2013.102897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li J, van der Wal DE, Zhu G, et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat Commun. 2015;6:7737. doi: 10.1038/ncomms8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]