Presentation
Adverse Event
Our patient was 13 years and 2 months old, a white boy with type 1 diabetes diagnosed 2 years and 5 months before this adverse event (AE). He had no other significant medical history. His A1C was 7.6% 1 month before the insulin infusion needle break event. The patient had started on the t-slim insulin pump (Tandem Diabetes Care, San Diego, Calif.) 1 year and 6 days before the AE occurred. He was using the contact Detach (Unomedical, Inc., Bridgewater, N.J.) infusion set and lispro insulin. The contact Detach infusion set features a very fine, 29-gauge, 90° steel needle. With its additional adhesive pad, contact Detach provides extra security against needle dislodging. Its simplicity and security make it a good choice for active young children, pregnant women, and adults for whom soft cannula sets do not work well. The contact Detach set is available in 6- and 8-mm needle lengths and 23- and 32-inch tubing lengths (1). There were no reported previous pump AEs, emergency department visits, or diabetes ketoacidosis episodes related to continuous subcutaneous insulin infusion (CSII) therapy. The patient’s BMI was 18.65 kg/m2. The patient’s mother reported changing the pump site, per usual, on 16 July 2014. When she went to remove the infusion set, the needle was not attached to it. The parents could not find the needle. The patient did not feel any pain or discomfort where the infusion set had been placed. At the time the 8-mm needle broke off from the infusion site, it had been placed on the upper left buttock area at a 90° angle with IV3000 1-HAND adhesive dressing (Smith & Nephew, Inc., Austin, Tex.) for 2 days. The site was clean, dry, and intact at all times, with no trauma to the area.
Patient Examination
The patient was brought to clinic for further assessment, at which time only a slight elevation in the skin could be palpated. There was a red mark where the needle had been inserted. There was no evidence of lipohypertrophy at or around the infusion site. There was no pain or discomfort to the patient while manually manipulating the site. X-ray confirmed the needle remained within the soft tissues behind the mid-sacrum. An outpatient surgical consult was requested.
Surgical Removal
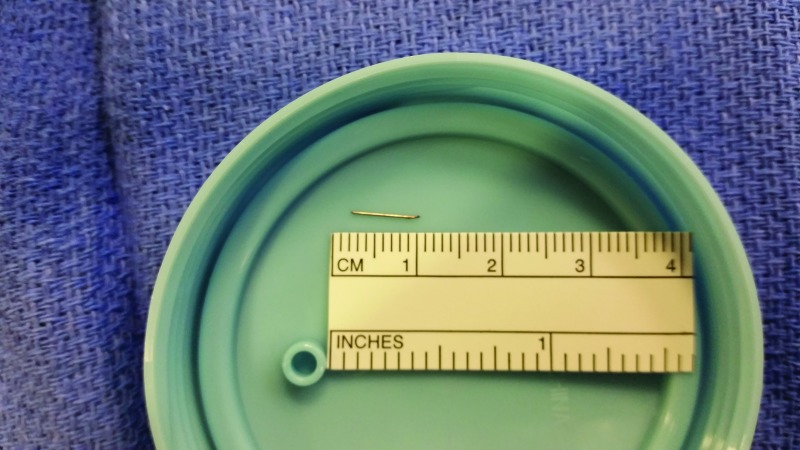
At the time of the operation, 12 days after the mother reported the broken needle from the infusion set, there was no visible scar in the patient’s left gluteal area. However, the patient was able to identify the estimated area where the infusion set had been placed. Once under anesthesia, as recommended by the surgeon for this pediatric patient, he was placed in a prone jack-knife position. No physical signs were noted on palpation. Fluoroscopy was used in an attempt to locate the foreign body. On anterior-posterior and lateral views, the surgeons were unable to locate the needle because of the bone density background. The fluoroscopy c-arm was then placed in an oblique position, and the surgeons were able to localize the needle. Once the needle was seen on fluoroscopy, a probe was used with gentle cutaneous pressure to visualize needle movement. A 2- to 3-cm incision was made on top of the area. The needle was found deep in the subcutaneous tissue lateral to the incision site and removed (Figure 1).
FIGURE 1.
Picture of the broken infusion set needle after surgical removal from the patient.
Analysis of Blood Glucose Data
Review of the blood glucose values obtained from the t:connect diabetes data management software (Tandem Diabetes Care, San Diego, Calif.) revealed no appreciable issues related to overall metabolic control. However, the patient’s blood glucose values on the day of the incident fluctuated from 200 mg/dL at 8:00 p.m. to 469 mg/dL at 10:00 p.m. The patient’s mother changed the site at 9:10 p.m., which was when she noticed the needle was no longer attached to the infusion set.
Questions
What are the optimal procedures for infusion set needle breaks?
What should be the recommended timeliness for needle removal?
Commentary
Intensive insulin regimens, which include CSII therapy, are now the standard of care for pediatric patients with type 1 diabetes. Limited data exist describing AEs related to CSII therapy, but, recently, the first prospective study to look at such events in modern insulin pumps was published (2). This study reported confirmed AEs in 11.1% of the pump patients, which was annualized to 40 AEs/100 person-years. Pump malfunctions were the most common AEs (54%), and infusion set/site failures were the second most common (36%). A hospital admission or emergency department visit was required as a consequence in 32% of the patients described in this report. The same study showed an increased risk of AEs in patients <10 years of age (odds ratio 3.2 [95% CI 1.7–6.1]), but no increased risk related to diabetes duration, duration of insulin pump therapy, sex, or A1C. A recent retrospective study using family- and self-reported survey data suggested an annual CSII therapy AE rate of 45%. Again, pump malfunction was the most common event, followed by infusion set/site failures (3).
Insulin infusion sites are clearly a vital component of CSII therapy and have been scrutinized in several studies. Wood et al. (4) reported that difficulties with sites were the precipitating reason for permanent pump discontinuation in 21% of patients. Renard et al. (5) reported that 8–9% of total insertions needed premature cannula replacements. Guilhem et al. (6) identified infusion set obstruction in the needle (maybe resulting from insulin precipitation) was the most commonly reported AE. Additional AEs included leakage from the infusion site where the needle was placed in the subcutaneous tissue.
When notified of this AE, Tandem Diabetes Care representatives reported that they had not had any reports of needle breaks with the contact Detach infusion set (J.K.B., personal communication, 27 August 2014). Unomedical, Inc., representatives also said there had been no reports of problems with the contact Detach (J.K.B., personal communication, 1 September 2014). Based on a literature review and inquiry to the manufacturers, to the best of our knowledge, there have been no reported AEs involving an infusion set needle breaking off in a pediatric patient.
Steel needle infusion sets are an integral component to CSII therapy. Recommendations call for changing infusion sets and infusion sites every 48–72 hours to avoid troublesome infusion site–related AEs. It has been shown that infusion sets left in place longer than the recommended 48–72 hours may cause bacterial contamination leading to skin inflammation and catheter occlusions (7,8).
In this case, the pump download revealed the infusion set was only in place for 2 days before the AE. This patient and both of his parents have a history of excellent adherence to all diabetes team and insulin pump manufacturer recommendations. It is difficult to determine whether the needle broke off during the removal process or before that patient’s mother started to change the site. Based on blood glucose data, it is most probable that the needle broke off some time in the hours before the attempted site change. The authors believe this incident was not the result of user error, but rather of a defect in the insulin infusion set product.
Because it was not urgent, removal of the broken needle was not given immediate surgical priority. However, removal of these types of needles can pose technical challenges to surgeons. Fluoroscopy is commonly used to retrieve metallic objects. However, some small objects are difficult to locate if a bone density background is present.
Clinical Pearls
To our knowledge, this is the first reported case of a contact Detach steel needle infusion set needle breaking off in a pediatric patient on CSII therapy.
Among other factors, the age of the patient and the depth of needle will guide decisions regarding the best clinical environment in which to remove the broken needle.
Prompt surgical removal is recommended so the puncture site might still be visible, enabling the surgeon to make a smaller, less invasive incision at the precise needle insertion site.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
References
- 1.Tandem Diabetes Care contact™ Detach infusion set. Available from http://www.tandemdiabetes.com/Products/Infusion-Sets/Contact-Detach. Accessed 27 September 2014
- 2.Wheeler BJ, Heels K, Donaghue KC, Reith DM, Ambler GR. Insulin pump-associated adverse events in children and adolescents: a prospective study. Diabetes Technol Ther 2014;16:558–562 [DOI] [PubMed] [Google Scholar]
- 3.Wheeler BJ, Donaghue KC, Heels K, Ambler GR. Family perceptions of insulin pump adverse events in children and adolescents. Diabetes Technol Ther 2014;16:204–207 [DOI] [PubMed] [Google Scholar]
- 4.Wood JR, Moreland EC, Volkening LK, Svoren BM, Butler DA, Laffel LM. Durability of insulin pump use in pediatric patients with type 1 diabetes. Diabetes Care 2006;29:2355–2360 [DOI] [PubMed] [Google Scholar]
- 5.Renard E, Guerci B, Leguerrier AM, Boizel R. Lower rate of initial failures and reduced occurrence of adverse events with a new catheter model for continuous subcutaneous insulin infusion: prospective, two-period, observational, multicenter study. Diabetes Technol Ther 2010;12:769–773 [DOI] [PubMed] [Google Scholar]
- 6.Guilhem I, Leguerrier AM, Lecordier F, Poirier JY, Maugendre D. Technical risks with subcutaneous insulin infusion. Diabetes Metab 2006;32:279–284 [DOI] [PubMed] [Google Scholar]
- 7.Kerr D, Morton J, Whately-Smith C, Everett J, Begley JP. Laboratory-based non-clinical comparison of occlusion rates using three rapid-acting insulin analogs in continuous subcutaneous insulin infusion catheters using low flow rates. J Diabetes Sci Technol 2008;2:450–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid V, Hohberg C, Borchert M, Forst T, Pfutzner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy; trouble starts on day 3. J Diabetes Sci Technol 2010;4:976–982 [DOI] [PMC free article] [PubMed] [Google Scholar]